Abstract
It is well recognized that the brain uses sensory information to accurately produce motor commands. Indeed, most research into the relationship between sensory and motor systems has focused on how sensory information modulates motor function. In contrast, recent studies have begun to investigate the reverse: how sensory and perceptual systems are tuned based on motor function, and specifically motor learning. In the present study we investigated changes to human proprioceptive acuity following recent motor learning. Sensitivity to small displacements of the hand was measured before and after 10 min of motor learning, during which subjects grasped the handle of a robotic arm and guided a cursor to a series of visual targets randomly located within a small workspace region. We used a novel method of assessing proprioceptive acuity that avoids active movement, interhemispheric transfer, and intermodality coordinate transformations. We found that proprioceptive acuity improved following motor learning, but only in the region of the arm's workspace explored during learning. No proprioceptive improvement was observed when motor learning was performed in a different location or when subjects passively experienced limb trajectories matched to those of subjects who actively performed motor learning. Our findings support the idea that sensory changes occur in parallel with changes to motor commands during motor learning.
Keywords: sensorimotor plasticity, human motor learning, arm movements, reaching
the central nervous system receives information from a wide range of sense organs that are characterized by differential sensitivity across their respective domains. In this study we investigated changes to the sense of proprioception after spatially localized motor learning and found that estimates of limb position are flexibly improved in the region of motor learning.
Relatively little is known about how proprioception changes with motor learning (Ostry et al. 2010), and indeed, few studies have been reported that assess how proprioception may vary across the workspace of the limb (Fuentes and Bastian 2010; van Beers et al. 1998; Wilson et al. 2010). In contrast, we know a great deal about other sensory systems that are characterized by differential anatomic sensor density, resulting in greater visual (Wald 1945), acoustic (Davis and Kranz 1964), and haptic sensitivity (Bolanowski et al. 1988; Verrillo 1963; Weinstein 1968) across a subset of the input domain.
Sensory sensitivity is modulated dynamically via descending signals from the brain. Efferent innervation of sense organs occurs in many human sensory systems, including those associated with proprioception. Efferent innervation of semicircular canals (Purcell and Perachio 1997; Warr 1975) and retinal cells (Honrubia and Elliott 1970) modulate the signals about head orientation and the visual field. Muscle spindles, widely regarded to be the primary source of information about the position of the limbs, also receive modulating efferent signals. It has been proposed that such signals may account for the sensory consequences of self-generated action (Bays et al. 2005; Blakemore et al. 1998; Wolpert and Flanagan 2001) and augment the functional dynamic range of the sensor (Scott and Loeb 1994; Windhorst 2007).
Recent studies of human muscle spindle afferents have shown that attention can modify afferent signals. Directing a subject's attention to the passive rotation occurring at a joint can cause changes to mean spike rate and range (Hospod et al. 2007). This suggests that proprioception may be modulated to provide greater acuity for changes in limb position that are behaviorally relevant. Such changes may accompany motor learning, to enhance proprioception for newly learned motor behaviors.
The literature cited above shows that anatomic substrates of perception support differential sensitivity, efferent neural signals provide top-down modulation of sensitivity, and behavioral context may play a role in this descending modulation. In this study we further explored the nature of behaviorally relevant modulations of perceptual sensitivity and specifically test the extent to which motor learning is accompanied by changes in proprioceptive acuity. We did this by assessing changes to the psychophysics of proprioception following motor learning. We observed spatially selective improvements in proprioceptive acuity, which occurred only in limb positions experienced during motor learning. Additional experiments showed that this effect specifically depends on active, rather than passive, movement and does not occur for subjects who perform motor training without learning. Our results support the idea that proprioceptive acuity is tuned in a spatially selective manner during motor learning.
MATERIALS AND METHODS
Subjects
One hundred eighty-six subjects between 17 and 40 yr of age (97 females; mean age = 20.9 yr) were randomly assigned to one of seven groups. All subjects reported no history of visual, neurological, or musculoskeletal disorder. Twenty-five subjects were assigned per group, except for the two groups involving passive kinematics, which were composed of 18 subjects. Written informed consent was obtained from each subject before participation. The University of Western Ontario Research Ethics Board approved all procedures.
Apparatus
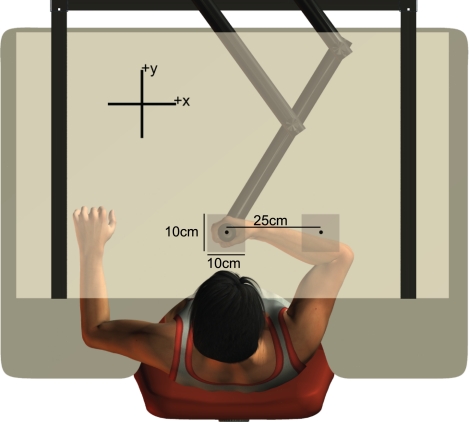
Subjects performed reaching movements and tests of proprioceptive acuity while grasping the handle of a robotic manipulandum (InMotion Technologies) in the right hand. A six-axis force transducer (ATI Industrial Automation, Apec, NC; resolution: 0.05 N), located inside the handle, measured forces at the hand. All subjects were seated at a desk and interacted with the experimental robot in the horizontal plane at shoulder height (see Fig. 1). A custom air sled, placed beneath the subject's right elbow, supported the arm against gravity and minimized friction between the arm and the desk. During motor learning, visual information was displayed via a mirror and LCD monitor display system (Kistemaker et al. 2010). The horizontal mirror was placed just below chin height and occluded the subject's view of his or her arm. Visual feedback of hand position was provided on the mirror in real time using the LCD display. Proprioception tests took place in the dark, and subjects were asked to close their eyes.
Fig. 1.
Experimental apparatus. Subjects grasped a robotic linkage and performed perceptual tests and reaching movements while seated at a desk. Perceptual tests were performed in darkness, with the eyes closed. A semisilvered mirror and black curtains blocked vision of the arm and handle. The 2 shaded boxes indicate the 2 workspace regions in which subjects performed reaching movements during the motor task.
Experimental Protocol
At the start of each experiment we measured baseline proprioceptive acuity. This perceptual test was followed by motor learning (explained below), during which reaching movements were made to visual targets (except for control groups, as noted). Finally, subjects performed a proprioception test immediately following learning. For control subjects, the learning phase was replaced with a control task (reading quietly) of the same duration.
Movement Task
Subjects moved their hand to 5-mm (diameter; circular) targets presented pseudorandomly, within a 10 × 10-cm workspace centered either on the location of proprioceptive testing or in a location 25 cm to the right (Fig. 1). A cursor (small filled circle, diameter 4 mm) was displayed in real time to represent the position of the hand. Motor learning consisted of 4 blocks of 100 movements (400 movements total). Subject instructions were told to “move your hand to the target as quickly and accurately as possible.” Once the hand was within 2 mm of the target's center, the current target was extinguished and the next target appeared. A number of kinematic measures were computed to characterize learning (see results). Movement time was recorded for each trial, beginning when the target appeared and ending when the hand arrived within 2 mm of the target's center. Subjects were provided with their total movement time after each block and were encouraged to improve this time over the course of learning.
Proprioception Measurement
The proprioception measurement procedure was performed in the absence of vision with subjects' eyes closed and an opaque mirror resting at shoulder height to block vision of the hand and robot. The subject's unseen right hand was moved by the robot along a left-right axis, 18 cm in front of the body. Subjects made two-alternative forced-choice judgments about whether they perceived their right hand to be left or right of a previous reference location. The reference location was in the center of the 10 × 10-cm movement area. Each perceptual judgment was composed of three phases: a 2-s hold phase during which the hand was held stationary at the reference position, a randomized passive movement that brought the hand indirectly to the test location, and the subject's response (“left” or “right”). During the passive movement phase, the subject's hand was moved along a line, first from the reference location to a peripheral location positioned at least 6 cm away from the reference, and then back to the test location (near the reference). To reduce the possibility that subjects might use cues related to passive movement speed or direction to aid their judgments of arm position, the passive movement between each reference and judgment position was randomized in terms of duration (between 1,000 and 1,600 ms, square distribution), total distance travelled (14 ± 2 cm, SD normal distribution), and direction (left/right). The passive movements were designed to be smooth, using a bell-shaped velocity profile. After the subject's response, the limb was again moved passively with random distance, speed, and direction to a peripheral location, before the hand was returned to the reference location to start the next trial. This passive movement prevented the subject from receiving any trial-to-trial feedback about the accuracy of their responses and hence minimized the possibility of adaptation over the course of perceptual testing.
We used the method of constant stimuli to present subjects with proprioceptive judgment locations. Subjects were tested at 7 different distances (0 ± 0.7, 1.3, and 3.0 cm) from the reference location for a total of 74 judgments, requiring ∼8 min to complete. The test locations more distant from the reference location were tested fewer times because subjects performed at 100% accuracy on these positions. Each judgment location was approached via leftward and rightward distractor movements an equal number of times.
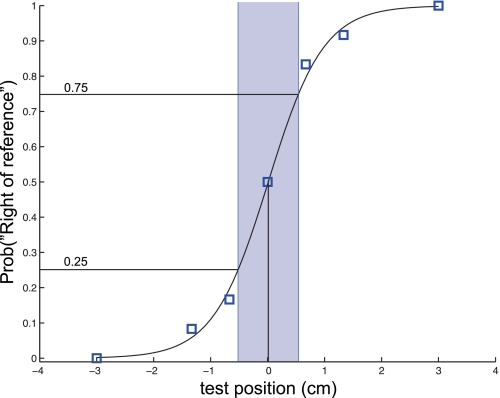
A logistic function was fit to the set of binary response data across test locations. Proprioceptive acuity was quantified as the distance spanning the 25th and 75th percentiles of the logistic function (Fig. 2). This measure, sometimes called uncertainty range (Henriques and Soechting 2003), is inversely related to sensitivity, and thus smaller values represent greater perceptual acuity. Statistical analysis of changes in proprioception and of kinematic measures were assessed using analysis of variance and Bonferroni or Tukey post hoc tests.
Fig. 2.
Example psychometric function. Open squares denote the probability with which a subject reported a given hand position to be right of the reference location, as a function of the actual hand location. Subjects' responses were fit to a binomial model using a cumulative normal distribution function. The shaded region represents the uncertainty range of the fit, and the vertical dashed line denotes the perceptual bias.
RESULTS
We measured proprioceptive acuity at baseline and again following ∼10 min of motor learning. We examined a number of kinematic measures to characterize how subjects improved performances over the course of learning.
Movement Speed
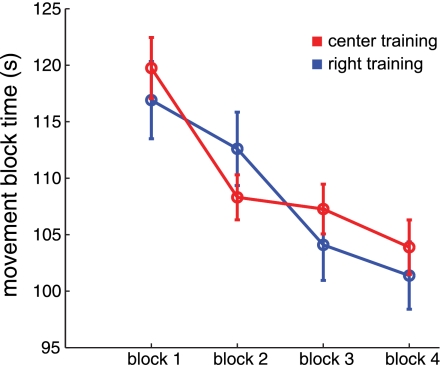
Figure 3 (red line) shows total movement time recorded in each of four movement blocks. Clear decreases in total movement time were observed as subjects (n = 25) learned to reach the targets more quickly over the course of practice in blocks 1 through 4. An analysis of variance was performed with one within-subjects measure (block: levels 1–4). A significant effect of movement block was found on total movement time [F(3,54) = 21.975, P < 0.0001]. Post hoc tests showed that movement time in block 4 was significantly less than in block 1 (P < 0.001);, thus subjects significantly improved performance on the reaching task over four blocks. Similarly, we also examined measures of movement speed and found reliable increases in tangential velocity across movement blocks (block 1: 19 ± 3.4 cm/s; block 4: 21.5 ± 4.1 cm/s; paired 2-tailed t-test, P < 0.01). A similar pattern was seen for a second group of subjects who were trained in a separate region of the workspace, 25 cm to the right (see Spatial Sensitivity).
Fig. 3.
Motor learning: movement time. Mean (±SE) movement time to reach to 100 randomly placed targets within a 10 × 10-cm region in each of 4 blocks is shown. The red line denotes subjects performing reaches in a central region aligned with the perceptual test location, 18 cm from the sternum; the blue line denotes subjects performing reaches in a region located 25 cm to the right of the center location.
Movement Accuracy
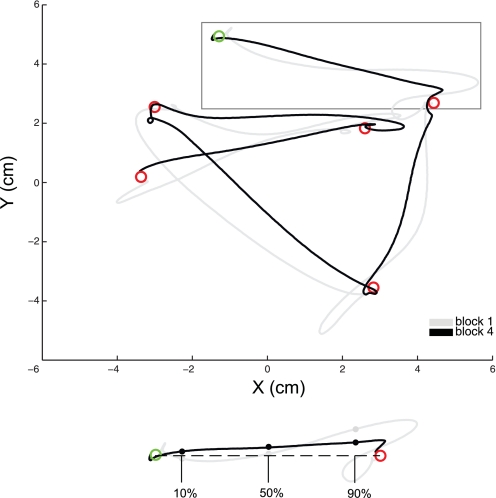
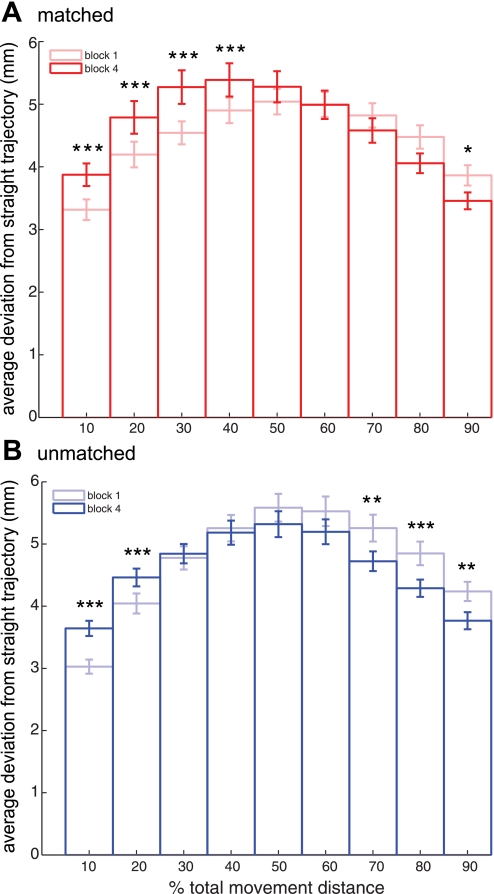
We also measured changes in the positional accuracy of reaching movements across motor learning. If subjects were learning to be more precise when reaching to targets, the perpendicular distance between the position of the hand and a straight line connecting the two targets might decrease across the motor learning period (Brashers-Krug et al. 1996; Caithness et al. 2004; Cothros et al. 2006a, 2006b, 2009). Figure 4 displays measurement of absolute perpendicular deviation (PD) for each movement. Although this movement task does not include a perturbing force field, the goal of reaching targets “as quickly and accurately as possible” still provides strong incentive to reduce PD, particularly as the hand nears the target, because deviations from a straight line would necessitate corrective movements to prevent missing, or even overshooting, the target, both of which represent considerable costs to task performance. Figure 5 shows absolute PD from a straight trajectory at 10% increments of the normalized movement distance between the previous target and the current target. Differences between initial (block 1) and final (block 4) motor performance are apparent. Deviations of the hand from the straight trajectory are reduced specifically where the hand approaches the target. To determine whether these differences are statistically reliable, we performed a split-plot repeated-measures analysis of variance (2 within-subjects factors: block, levels 1 and 4, and %movement length, levels 10–90; 1 between-subjects factor: group; see Spatial Specificity). Post hoc tests (Bonferroni corrected) revealed statistically reliable differences between blocks 1 and 4 at several points along the movement trajectory, including at 90% of movement length (see Fig. 5 for all pairwise comparisons). Thus subjects learned to move to the targets both more quickly and with greater accuracy.
Fig. 4.
Movement accuracy measurements. Overhead view shows 5 movements from an example subject, during blocks 1 and 4, depicting measurement of movement accuracy. Absolute perpendicular deviation (PD) from the straight trajectory was calculated for each movement, at 10% intervals of normalized movement length.
Fig. 5.
Motor learning: movement accuracy. Mean (±SE) PD at 10% intervals along the straight line linking start and end targets is shown, averaged across subjects for movement blocks 1 and 4. A: matched group data (red). B: unmatched group data (blue). Bonferroni-corrected pairwise comparisons between blocks are noted for significance: *P < 0.05; **P < 0.01; ***P < 0.001.
Proprioceptive Acuity
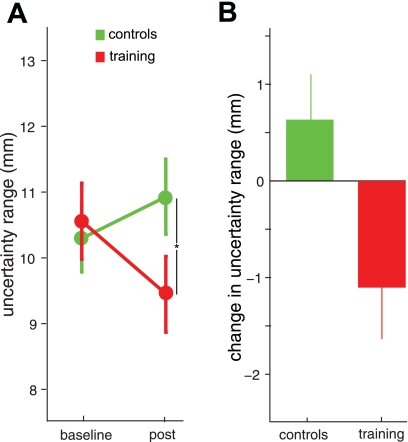
To determine whether motor learning results in changes to proprioceptive acuity, we estimated uncertainty range at baseline and again following motor learning. To control for the possibility that observed changes in proprioceptive acuity might be due to the passage of time and not motor learning per se, we tested a second group of subjects (n = 25) who did not perform the motor learning task but read quietly for a matched duration of time. The uncertainty range (mean ± SE) for the control subjects who did not undergo learning was 10.27 ± 0.51 mm at baseline and 10.9 ± 0.56 mm on postlearning retesting (Fig. 6). In contrast, subjects who performed the motor learning task demonstrated uncertainty ranges at baseline of 10.53 ± 0.58 mm that decreased to 9.43 ± 0.56 mm following learning, representing an 11% improvement in acuity. To test for differences in proprioceptive acuity as a function of learning, we performed a split-plot repeated-measures analysis of variance on uncertainty range, with one between-subjects factor (learning: control vs. learning) and one within-subjects factor (time: baseline vs. postlearning). A significant interaction was found (P = 0.018). Post hoc Tukey tests revealed that the control group that did not experience motor learning and the group that did showed the same acuity at baseline (P = 0.95); however, subjects who underwent learning had significantly smaller uncertainty ranges postlearning (P < 0.05) relative to control subjects. These results suggest that motor learning results in an improvement in proprioceptive acuity.
Fig. 6.
Proprioceptive acuity is improved following motor learning. A: mean (±SE) uncertainty range measured at baseline and following motor learning for subjects who performed movements at the perceptual test location and for control subjects who performed no motor learning. Uncertainty range is inversely related to proprioceptive acuity. B: change in uncertainty range between baseline and retest. The motor learning group (training, red) demonstrates an improvement in proprioceptive acuity relative to control subjects. Statistically significant post hoc Tukey comparisons are noted: *P < 0.05.
Spatial Specificity
We next investigated the spatial specificity of this effect. Is the sense of limb position tuned locally, only in the region of motor learning, or is position sense improved more globally? To examine the degree of spatial generalization, we tested an additional group of subjects (n = 25) who performed motor learning in a different workspace location, positioned to the right of the position at which proprioceptive tests were performed. The same motor learning task as described above was performed in a 10 × 10-cm area, centered 25 cm to the right of the subject's midline. Proprioceptive tests were performed at the central workspace location as described above. Two additional groups of subjects were tested in the opposite configuration: one group (n = 25) had spatially matched motor learning (right location), whereas another (n = 25) had unmatched motor learning (center) and proprioceptive testing (right). We were also interested to see whether even more spatially focused motor learning results in a greater perceptual effect, and thus for these groups we restricted movements such that visual targets appeared only along a 10-cm left/right (transverse) line, rather than within a 100-cm2 patch. Like the group of subjects trained and tested in the central workspace location (Fig. 3, red line, and Fig. 5A), the group of subjects undergoing unmatched training improved both movement speed (Fig. 3, blue line) and accuracy (Fig. 5B) over the course of learning.
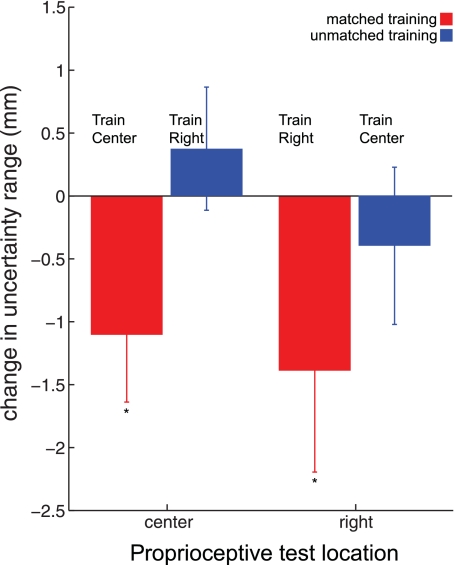
Figure 7 shows the change in proprioceptive acuity following learning for the two matched and two unmatched groups. Subjects receiving matched motor learning (red) showed decreased uncertainty range. In contrast, unmatched learning had apparently little effect on the measured uncertainty range. Averaged together, the two matched groups had a mean (±SE) baseline uncertainty range of 11.45 ± 0.47 mm that decreased to 10.14 ± 0.49 mm following learning, representing an ∼11% improvement in acuity. In contrast, subjects who performed unmatched learning demonstrated no change in acuity (baseline, 11.056 ± 0.48 mm; following learning, 11.051 ± 0.45 mm).
Fig. 7.
Tuning of proprioception as a function of learning location. Mean (±SE) change in uncertainty range is given as a function of motor learning location (center vs. right) and the location of proprioceptive tests (center vs. right). A decrease in uncertainty range (and thus an improvement in proprioceptive acuity) was observed only in the workspace locations at which motor learning was completed (matched training, red): *P < 0.05. No change in uncertainty range occurred in workspace locations distant to the location of motor learning (unmatched training, blue).
A split-plot analysis of variance was performed with two between-subjects factors (proprioception test: right vs. center, and learning: matched vs. unmatched) and one within-subjects factor (time: baseline vs. post). A significant interaction effect of learning location and time was found [F(7,91) = 3.974; P < 0.05]. Post hoc tests showed a significant difference between perceptual acuity at baseline vs. following learning (P < 0.05) for subjects who performed matched learning. Subjects who performed unmatched learning showed no such change (P > 0.9). For the subject groups who underwent matched learning, no difference was found in the acuity improvement when subjects who performed movements in the 100-cm2 patch were compared with those who moved along a left-right transverse line. Our results suggest that proprioceptive acuity is not improved broadly across the workspace but is only modified in the region in which motor learning occurred.
Control Tests
Sensory signals.
It should be noted that motor learning also provides subjects with a specific set of sensory signals related to the movement trajectories experienced over the course of learning. The possibility exists that the pattern of changes in proprioceptive acuity observed may be due to this sensory experience alone and do not specifically depend on motor learning per se. To assess this possibility, we performed a control study in which subjects did not actively move their limb but nevertheless experienced the same movement kinematics as those who underwent active movement. Subjects (n = 18) grasped the handle of the robotic linkage, which was programmed to move their passive limb through the same trajectories recorded from a previous subject in experiment 1.
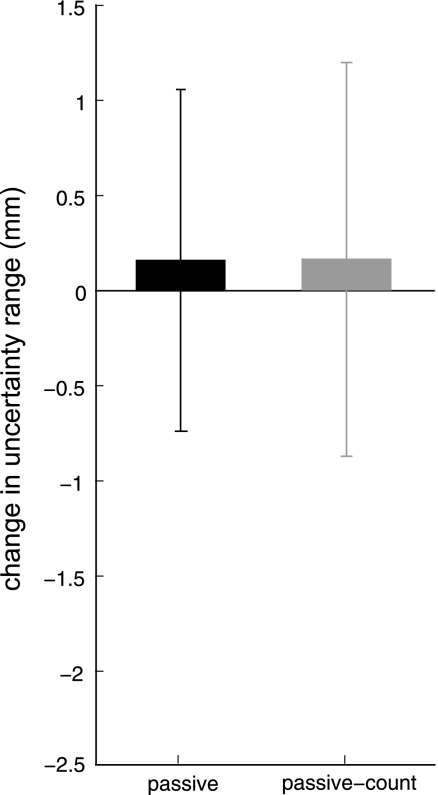
Figure 8, solid bar, shows changes in proprioceptive acuity for subjects in the passive control. These subjects did not show any change in acuity (P = 0.57, paired 1-tailed t-test). One potential concern with this control test is that subjects may not have dedicated the same amount of attention to the task as subjects who performed active movement (Hospod et al. 2007). To control for this possibility, we tested an additional group (n = 18; Fig. 8, shaded bar) who were asked to attend to the passive movements of their limb. These subjects were instructed to pay attention to the direction of the passive movements and to count the number of times their limb was moved in a leftward direction. This task proved to be at least difficult enough so that no subjects performed at ceiling; thus it is likely that this task was providing significant attentional demands on the subject. As before, no reliable changes in proprioceptive acuity were observed for this group (P = 0.56), suggesting that passive sensory experience in the absence of active motor learning does not result in changes to proprioceptive acuity.
Fig. 8.
Passive controls. Mean (±SE) change in uncertainty range for subjects in the passive controls. Subjects did not perform active motor learning but experienced the same kinematic trajectories as subjects who did (passive, solid). The robot was programmed to move each subject's passive arm through the same kinematic trajectories as a subject chosen from the main study, who performed active motor learning. Passive-count (shaded) gives mean change in uncertainty range for subjects who were specifically asked to attend to the passive movements by counting the number of times the robot moved their hand in a leftward direction.
Subjects were instructed to remain passive throughout proprioceptive tests and to grasp the handle in a consistent fashion throughout the study. Despite these instructions, it is possible that subjects changed the way they grasped the robot handle, for example, by applying differential amounts of force for different testing positions or for proprioceptive testing at baseline vs. following learning. Differences such as these could conceivably influence their responses during proprioceptive testing (Allen and Proske 2006; Ribot-Ciscar et al. 1991; Walsh et al. 2006, 2009). To assess this possibility, we examined the measured force applied to the handle before and after proprioceptive tests. We measured force for at all test locations (0 ± 0.7, 1.3, and 3.0 cm) during baseline and compared those with measurements following motor learning. We found no statistically reliable difference in either the direction or the magnitude of this force (P > 0.3 in all cases). These results suggest that grasping behavior did not change as a result of learning and thus presumably did not affect estimates of proprioceptive acuity.
Learning.
The possibility exists that motor learning during the movement task may not be necessary for the observed changes in sensory acuity. Perhaps any spatially matched arm movements are sufficient for sensory acuity improvements, even those resulting in no changes to movement accuracy.
To assess this possibility we ran an additional control study. Subjects (n = 25) performed reaching movements and perceptual testing in the central workspace location. Movements were made to the same visual targets as in the main experiment, but we modified the task to minimize the opportunity for learning. The cursor representing hand position was not displayed, preventing subjects from learning about movement errors incurred throughout the motor task. We also changed the display of visual targets such that instead of remaining on the screen until the subject reached the target, each target was displayed for a duration equal to the mean presentation time for experimental subjects and then extinguished. Thus target presentation was independent of subject behavior, but subjects in the control were exposed to a similar set of visual stimuli as subjects in the main study. Control subjects were instructed to move to the targets as quickly and as accurately as possible. Thus subjects in the control group produced the same kind of movements (similar in speed and amplitude) as subjects in the main experiment but did not receive feedback that would be required to improve their performance over time.
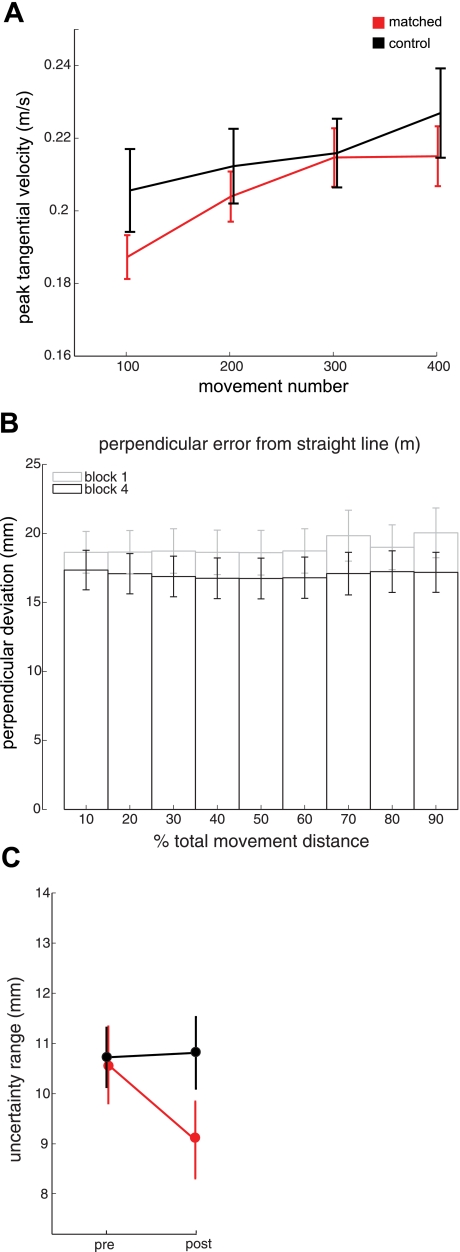
We examined movement performance for control subjects to verify that a range of kinematic features was similar to that for the experimental group and to test for any sign of learning. Figure 9A shows mean peak tangential velocity across the motor learning session for control subjects and for subjects in the main experiment who performed motor learning in the center workspace location, matched to the central perceptual test location. It can be seen that movement speeds for the control group were in the same range as those for subjects in the main experiment. Statistical tests showed that mean peak tangential velocity did not reliably differ between controls and subjects in the main experiment for any of the blocks, including the final training block (means: experimental, 0.215 ± 0.008 m/s; control, 0.228 ± 0.012 m/s; t-tests, P > 0.05 in all cases).
Fig. 9.
Learning controls. A: mean (±SE) peak tangential velocity of movements made during the motor task for the matched experimental group (red) and the control group (black). B: PD from a straight trajectory throughout normalized movement distance for blocks 1 and 4. No pairwise comparisons at any point in movement distance were significant (P > 0.4 for all comparisons, paired t-tests). C: uncertainty range at baseline (pre) and following the motor task (post) for the matched experimental group (red) and the control group (black).
Some have reported proprioceptive drift over time in the absence of any visual feedback (Brown et al. 2003a, 2003b). To test for the possibility that movements of control subjects drifted over the course of the movement task, we measured mean x and y position for each of the four training blocks. No reliable differences were observed (P > 0.05 in all cases). We also measured total distance traveled and found that the average movement distance did not differ between groups in any of the blocks (block 4 mean distance per movement: experimental, 10.5 ± 0.32 cm; control, 9.95 ± 0.61 cm; t-tests, P > 0.05 for all pairwise tests on the 4 blocks). Thus movement kinematics were similar for experimental and control groups.
Movement accuracy for control subjects was measured several different ways across the motor task, and in each case, no statistically reliable differences were found. Figure 9B shows movement accuracy (absolute PD) along the normalized movement length. A two-factor within-subjects analysis of variance was performed to test for any differences in PD. Neither factor (blocks 1 and 4) nor percent distance (10–90) was significant (P > 0.4 in both cases). In addition, none of the nine pairwise comparisons along the trajectory, made between the first and last training block, were statistically reliable (paired t-tests, P > 0.4 in all cases). We also measured for each movement 1) the smallest distance achieved between the subject's hand and target and 2) the distance between the hand and target at the end of each movement trial. Neither of these measures were significantly different when the first motor block was compared with the fourth and final block (P > 0.05 in both cases). To summarize, control subjects performed movements with qualitatively similar kinematics over the course of their motor training, but in contrast to subjects in the main experiment, controls did not improve movement accuracy.
Figure 9C shows sensory acuity before and after the movement task for controls (black) and for subjects in the main experiment (red). In contrast to subjects in the main experiment, who showed a reliable improvement in proprioceptive acuity (a decrease in uncertainty range), the uncertainty range for control subjects following the motor task was not statistically different from baseline. These results support the idea that improvements in proprioceptive acuity seen in the main experiment are dependent on motor learning.
DISCUSSION
We investigated the effects of motor learning on human proprioception and found that proprioceptive acuity is improved following motor learning. Improvements to proprioceptive acuity occurred only for limb configurations matching those experienced during motor learning and depended specifically on active motor learning. No improvement in proprioceptive acuity was observed for subjects who did not undergo active motor learning but who experienced the same movement kinematics as those who did. In addition, no improvement in acuity was observed for subjects who performed similar movements but who did not improve motor performance over time. In the present study, proprioceptive acuity was improved by ∼11% following motor learning, representing a sizable proportional improvement from baseline levels of acuity. Although this effect size is modest in absolute terms, it is on the same order of magnitude as the improvement in movement accuracy. Our findings are consistent with the idea that motor learning is a process of sensorimotor adaptation consisting not only of changes to motor signals but also modulation to sensory systems (Ostry et al. 2010). The present findings represent the first report of a spatially localized tuning of proprioceptive acuity following recent motor learning.
In a recent study (Ostry et al. 2010), it was observed that the directional bias of perceived hand position changed with motor adaptation to a viscous force field, and the perceptual bias varied with the direction of the experienced load. This is in contrast to the improved acuity of sensed hand position in the current study, observed when subjects learned to move accurately to targets in the absence of a novel, external load. Together, these data suggest that the nature of perceptual change is coupled to the particular type of motor adaptation. Future studies may clarify the degree to which motor learning and perceptual change are causally linked.
The changes in proprioceptive acuity observed in the present study occurred after only 10 min of motor training and were measured during 8 min of perceptual testing. These effects therefore represent a relatively rapid adaptation of the sensorimotor system. Future studies may determine how long the observed proprioceptive changes persist over time and whether they remain coupled with the retention of motor learning (e.g., Ostry et al. 2010).
It should be noted that although perceptual changes were found following active movement but not passive movement, this may not necessarily imply that passive movements are less salient. Indeed, a recent study found that the direction of passive hand movements is more accurately perceived than that of self-generated movement (Scheidt et al. 2010).
To investigate sensory changes due to motor learning, we developed a novel paradigm that avoided active movement during the perceptual response and avoided the sort of interhemispheric and intermodal coordinate transformations involved in other methods of assessing proprioceptive function (Adamo and Martin 2009; Desmurget et al. 2000; Goble and Brown 2008, 2007; Leibowitz et al. 2008; Sittig et al. 1985; van Beers et al. 1998; Wann and Ibrahim 1992). This technique does require subjects to remember the reference position of their limb while their hand is brought to the test location, so it should be noted that there is a memory component involved in their response. However, the impact of this is likely minimal, because the duration between the presentation of the reference position and the subject's response is short, between 800 and 1,500 ms.
The specific neurophysiological basis for the modulation of proprioceptive acuity we observed has not been determined, although there are a number of possibilities, including peripheral modulation of sensory afferents and cortical changes in sensory-motor processing. For example, it is known that changes in spindle afferent signals are mediated by alpha-gamma coactivation during active movement (Ribot-Ciscar et al. 2009). However, it is not clear how this mechanism could explain the change in proprioceptive acuity we observed, since the perceptual test does not involve active movement. Studies using the microneurographic technique to measure spindle afferent signals in vivo have found modulations of spindle afferent signals when subjects attended to and classified passive rotations of their ankle joint (Hospod et al. 2007). The authors have shown in a subsequent study (Ribot-Ciscar et al. 2009) that attention to different aspects of the passive joint rotations, to either position changes or changes in movement velocity, produced different kinds of modulation of spindle afferent firing. Isometric muscle contraction also has been shown to increase spindle sensitivity immediately following motor activity (Ribot-Ciscar et al. 1991; Walsh et al. 2006, 2009). Together, these studies support the idea that the central nervous system modulates the sensitivity of the primary proprioceptive sensors.
Studies of brain areas related to proprioception do not suggest clear putative neural mechanisms underlying the acuity changes we observed. Studies of adult cortical plasticity in animal models of the somatosensory system have assessed changes to intracortical depression and excitation in barrel cortex in rats following whisker removal and resulting sensory deprivation (Finnerty et al. 1999; Fox and Wong 2005). S1 hand representations in monkeys have demonstrated changes to the size of neural representations in S1 following peripheral nerve stimulation (Recanzone et al. 1990) or surgical syndactyly (Blake et al. 2005; Clark et al. 1988; Jenkins et al. 1990). Similar adaptive changes have been shown to occur in primary motor cortex (Kleim et al. 1998; Nudo et al. 1996). These findings, however, depend on fundamentally different timescales to elicit behavioral changes, involving days or weeks of sensorimotor learning. In contrast, the effects reported in the present study are the result of ∼10 min of motor learning and therefore represent highly dynamic changes to perceived limb position. Use-dependent changes to cortical excitability have been found to occur over short timescales in primary motor cortex and presumably reflect increased excitability of cortical areas related to the recently practiced movement (Classen et al. 1998; Pascual-Leone et al. 1995). Our findings therefore may reflect a sensory component of short-term sensorimotor plasticity during which parallel changes to motor and sensory areas occur throughout motor learning (Ostry et al. 2010).
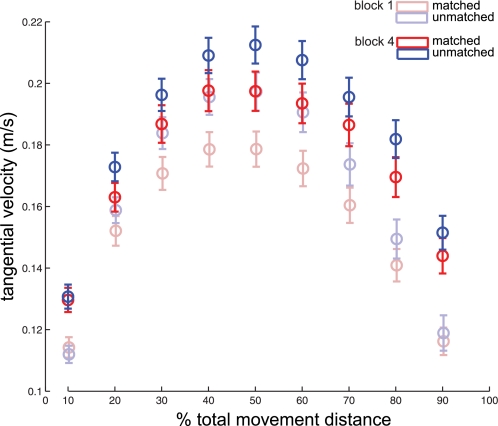
Interestingly, our analysis of kinematic accuracy found that subjects not only moved with greater precision as their hand approached the target but also were more variable at the beginning of movement. We hypothesize that this results from increased movement speed as a result of motor learning. Figure 10 shows peak tangential velocities for the kinematic data sets shown in Fig. 5. Tangential velocity increased across the movement trajectory as a whole, but subjects only increased movement accuracy as their hand neared the target. These data might be interpreted within a motor control theory that postulates active reduction of motor variability strictly in task-relevant domains while admitting motor variability increases within task-irrelevant areas (Domkin et al. 2005; Scholz et al. 2003; Scholz and Schoner 1999; Todorov and Jordan 2002; Valero-Cuevas et al. 2009; Verrel 2010).
Fig. 10.
Tangential velocity. Mean (±SE) tangential velocity throughout the movement is shown for matched (red) and unmatched subjects (blue). Block 1 data are denoted by shaded lines; block 4 data are denoted by solid lines.
We attempted to determine whether motor learning itself is required for the perceptual acuity effect by conducting a control experiment in which subjects were not provided with visual feedback of any kind. We showed that although these subjects produced movements that were kinematically similar to those produced by subjects in the main experiment, the control subjects did not show motor improvement over training. Importantly, control subjects also did not show changes in proprioceptive acuity, suggesting that motor learning is a necessary condition for this effect. It should be noted that on the basis of this control study alone, one cannot exclude the possibility that visually guided movements, by providing an opportunity to calibrate vision and proprioception, may also result in proprioceptive change. Although strictly speaking we cannot rule out this possibility, the fact that the perceptual test in the present study occurs in the absence of any vision mitigates its likelihood.
The findings reported in the present study are important because they demonstrate that proprioception is not simply used by the motor system as a static map of the position of our limbs. Rather, the sense of body position is modulated over the course of motor learning. Motor commands not only generate movement but also may result in modulation of the sensitivity of our proprioceptive sense. This relationship may reflect one way in which the sensorimotor system optimally recruits sensory areas for use in motor tasks.
More generally, our findings support the idea that motor learning modifies not only motor areas of the brain but also affects somatosensory systems (Ostry et al. 2010). This new way of thinking about motor learning could lead to novel approaches to rehabilitation that specifically exploit the link between sensory change and motor learning.
GRANTS
This work was supported by the National Institutes of Health, the Canadian Institutes of Health Research, and the Natural Sciences and Engineering Research Council of Canada.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Nicholas Cothros and Dinant Kistemaker for helpful comments.
REFERENCES
- Adamo DE, Martin BJ. Position sense asymmetry. Exp Brain Res 192: 87–95, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen TJ, Proske U. Effect of muscle fatigue on the sense of limb position and movement. Exp Brain Res 170: 30–38, 2006 [DOI] [PubMed] [Google Scholar]
- Bays PM, Wolpert DM, Flanagan JR. Perception of the consequences of self-action is temporally tuned and event driven. Curr Biol 15: 1125–1128, 2005 [DOI] [PubMed] [Google Scholar]
- Blake DT, Strata F, Kempter R, Merzenich MM. Experience-dependent plasticity in S1 caused by noncoincident inputs. J Neurophysiol 94: 2239–2250, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore SJ, Goodbody SJ, Wolpert DM. Predicting the consequences of our own actions: the role of sensorimotor context estimation. J Neurosci 18: 7511–7518, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolanowski SJ, Jr, Gescheider GA, Verrillo RT, Checkosky CM. Four channels mediate the mechanical aspects of touch. J Acoust Soc Am 84: 1680–1694, 1988 [DOI] [PubMed] [Google Scholar]
- Brashers-Krug T, Shadmehr R, Bizzi E. Consolidation in human motor memory. Nature 382: 252–255, 1996 [DOI] [PubMed] [Google Scholar]
- Brown LE, Rosenbaum DA, Sainburg RL. Limb position drift: implications for control of posture and movement. J Neurophysiol 90: 3105–3118, 2003a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LE, Rosenbaum DA, Sainburg RL. Movement speed effects on limb position drift. Exp Brain Res 153: 266–274, 2003b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caithness G, Osu R, Bays P, Chase H, Klassen J, Kawato M, Wolpert DM, Flanagan JR. Failure to consolidate the consolidation theory of learning for sensorimotor adaptation tasks. J Neurosci 24: 8662–8671, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark SA, Allard T, Jenkins WM, Merzenich MM. Receptive fields in the body-surface map in adult cortex defined by temporally correlated inputs. Nature 332: 444–445, 1988 [DOI] [PubMed] [Google Scholar]
- Classen J, Liepert J, Wise SP, Hallett M, Cohen LG. Rapid plasticity of human cortical movement representation induced by practice. J Neurophysiol 79: 1117–1123, 1998 [DOI] [PubMed] [Google Scholar]
- Cothros N, Kohler S, Dickie EW, Mirsattari SM, Gribble PL. Proactive interference as a result of persisting neural representations of previously learned motor skills in primary motor cortex. J Cogn Neurosci 18: 2167–2176, 2006a [DOI] [PubMed] [Google Scholar]
- Cothros N, Wong J, Gribble PL. Visual cues signaling object grasp reduce interference in motor learning. J Neurophysiol 102: 2112–2120, 2009 [DOI] [PubMed] [Google Scholar]
- Cothros N, Wong JD, Gribble PL. Are there distinct neural representations of object and limb dynamics? Exp Brain Res 173: 689–697, 2006b [DOI] [PubMed] [Google Scholar]
- Davis H, Kranz F. The international audiometric zero. J Acoust Soc Am 36: 1450–1454, 1964 [PubMed] [Google Scholar]
- Desmurget M, Vindras P, Grea H, Viviani P, Grafton ST. Proprioception does not quickly drift during visual occlusion. Exp Brain Res 134: 363–377, 2000 [DOI] [PubMed] [Google Scholar]
- Domkin D, Laczko J, Djupsjobacka M, Jaric S, Latash ML. Joint angle variability in 3D bimanual pointing: uncontrolled manifold analysis. Exp Brain Res 163: 44–57, 2005 [DOI] [PubMed] [Google Scholar]
- Finnerty GT, Roberts LS, Connors BW. Sensory experience modifies the short-term dynamics of neocortical synapses. Nature 400: 367–371, 1999 [DOI] [PubMed] [Google Scholar]
- Fox K, Wong RO. A comparison of experience-dependent plasticity in the visual and somatosensory systems. Neuron 48: 465–477, 2005 [DOI] [PubMed] [Google Scholar]
- Fuentes CT, Bastian AJ. Where is your arm? Variations in proprioception across space and tasks. J Neurophysiol 103: 164–171, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goble DJ, Brown SH. The biological and behavioral basis of upper limb asymmetries in sensorimotor performance. Neurosci Biobehav Rev 32: 598–610, 2008 [DOI] [PubMed] [Google Scholar]
- Goble DJ, Brown SH. Task-dependent asymmetries in the utilization of proprioceptive feedback for goal-directed movement. Exp Brain Res 180: 693–704, 2007 [DOI] [PubMed] [Google Scholar]
- Henriques DY, Soechting JF. Bias and sensitivity in the haptic perception of geometry. Exp Brain Res 150: 95–108, 2003 [DOI] [PubMed] [Google Scholar]
- Honrubia FM, Elliott JH. Efferent innervation of the retina. II. Morphologic study of the monkey retina. Invest Ophthalmol 9: 971–976, 1970 [PubMed] [Google Scholar]
- Hospod V, Aimonetti JM, Roll JP, Ribot-Ciscar E. Changes in human muscle spindle sensitivity during a proprioceptive attention task. J Neurosci 27: 5172–5178, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins WM, Merzenich MM, Ochs MT, Allard T, Guic-Robles E. Functional reorganization of primary somatosensory cortex in adult owl monkeys after behaviorally controlled tactile stimulation. J Neurophysiol 63: 82–104, 1990 [DOI] [PubMed] [Google Scholar]
- Kistemaker DA, Wong JD, Gribble PL. The central nervous system does not minimize energy cost in arm movements. J Neurophysiol 104: 2985–2994, 2010 [DOI] [PubMed] [Google Scholar]
- Kleim JA, Barbay S, Nudo RJ. Functional reorganization of the rat motor cortex following motor skill learning. J Neurophysiol 80: 3321–3325, 1998 [DOI] [PubMed] [Google Scholar]
- Leibowitz N, Levy N, Weingarten S, Grinberg Y, Karniel A, Sacher Y, Serfaty C, Soroker N. Automated measurement of proprioception following stroke. Disabil Rehabil 30: 1829–1836, 2008 [DOI] [PubMed] [Google Scholar]
- Nudo RJ, Milliken GW, Jenkins WM, Merzenich MM. Use-dependent alterations of movement representations in primary motor cortex of adult squirrel monkeys. J Neurosci 16: 785–807, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostry DJ, Darainy M, Mattar AA, Wong J, Gribble PL. Somatosensory plasticity and motor learning. J Neurosci 30: 5384–5393, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Leone A, Nguyet D, Cohen LG, Brasil-Neto JP, Cammarota A, Hallett M. Modulation of muscle responses evoked by transcranial magnetic stimulation during the acquisition of new fine motor skills. J Neurophysiol 74: 1037–1045, 1995 [DOI] [PubMed] [Google Scholar]
- Purcell IM, Perachio AA. Three-dimensional analysis of vestibular efferent neurons innervating semicircular canals of the gerbil. J Neurophysiol 78: 3234–3248, 1997 [DOI] [PubMed] [Google Scholar]
- Recanzone GH, Allard TT, Jenkins WM, Merzenich MM. Receptive-field changes induced by peripheral nerve stimulation in SI of adult cats. J Neurophysiol 63: 1213–1225, 1990 [DOI] [PubMed] [Google Scholar]
- Ribot-Ciscar E, Hospod V, Roll JP, Aimonetti JM. Fusimotor drive may adjust muscle spindle feedback to task requirements in humans. J Neurophysiol 101: 633–640, 2009 [DOI] [PubMed] [Google Scholar]
- Ribot-Ciscar E, Tardy-Gervet MF, Vedel JP, Roll JP. Post-contraction changes in human muscle spindle resting discharge and stretch sensitivity. Exp Brain Res 86: 673–678, 1991 [DOI] [PubMed] [Google Scholar]
- Scheidt RA, Lillis KP, Emerson SJ. Visual, motor and attentional influences on proprioceptive contributions to perception of hand path rectilinearity during reaching. Exp Brain Res 204: 239–254, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz JP, Kang N, Patterson D, Latash ML. Uncontrolled manifold analysis of single trials during multi-finger force production by persons with and without Down syndrome. Exp Brain Res 153: 45–58, 2003 [DOI] [PubMed] [Google Scholar]
- Scholz JP, Schoner G. The uncontrolled manifold concept: identifying control variables for a functional task. Exp Brain Res 126: 289–306, 1999 [DOI] [PubMed] [Google Scholar]
- Scott SH, Loeb GE. The computation of position sense from spindles in mono- and multiarticular muscles. J Neurosci 14: 7529–7540, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sittig AC, Denier van der Gon JJ, Gielen CC. Separate control of arm position and velocity demonstrated by vibration of muscle tendon in man. Exp Brain Res 60: 445–453, 1985 [DOI] [PubMed] [Google Scholar]
- Todorov E, Jordan MI. Optimal feedback control as a theory of motor coordination. Nat Neurosci 5: 1226–1235, 2002 [DOI] [PubMed] [Google Scholar]
- Valero-Cuevas FJ, Venkadesan M, Todorov E. Structured variability of muscle activations supports the minimal intervention principle of motor control. J Neurophysiol 102: 59–68, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beers RJ, Sittig AC, Denier van der Gon JJ. The precision of proprioceptive position sense. Exp Brain Res 122: 367–377, 1998 [DOI] [PubMed] [Google Scholar]
- Verrel J. Distributional properties and variance-stabilizing transformations for measures of uncontrolled manifold effects. J Neurosci Methods 191: 166–170, 2010 [DOI] [PubMed] [Google Scholar]
- Verrillo RT. Effect of contactor area on the vibrotactle threshold. J Acoust Soc Am 35: 1962–1966, 1963 [Google Scholar]
- Wald G. Human vision and the spectrum. Science 101: 653–658, 1945 [DOI] [PubMed] [Google Scholar]
- Walsh LD, Allen TJ, Gandevia SC, Proske U. Effect of eccentric exercise on position sense at the human forearm in different postures. J Appl Physiol 100: 1109–1116, 2006 [DOI] [PubMed] [Google Scholar]
- Walsh LD, Smith JL, Gandevia SC, Taylor JL. The combined effect of muscle contraction history and motor commands on human position sense. Exp Brain Res 195: 603–610, 2009 [DOI] [PubMed] [Google Scholar]
- Wann JP, Ibrahim SF. Does limb proprioception drift? Exp Brain Res 91: 162–166, 1992 [DOI] [PubMed] [Google Scholar]
- Warr WB. Olivocochlear and vestibular efferent neurons of the feline brain stem: their location, morphology and number determined by retrograde axonal transport and acetylcholinesterase histochemistry. J Comp Neurol 161: 159–181, 1975 [DOI] [PubMed] [Google Scholar]
- Weinstein S. (editor). Intensive and Extensive Aspects of Tactile Sensitivity as a Function of Body Part, Sex, and Laterality. Springfield, IL: Thomas, 1968 [Google Scholar]
- Wilson ET, Wong J, Gribble PL. Mapping proprioception across a 2D horizontal workspace. PLoS One 5: e11851, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windhorst U. Muscle proprioceptive feedback and spinal networks. Brain Res Bull 73: 155–202, 2007 [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Flanagan JR. Motor prediction. Curr Biol 11: R729–R732, 2001 [DOI] [PubMed] [Google Scholar]