Abstract
The dorsolateral striatum (DLS) receives extensive projections from primary somatosensory cortex (SI), but very few studies have used somesthetic stimulation to characterize the sensory coding properties of DLS neurons. In this study, we used computer-controlled whisker deflections to characterize the extracellular responses of DLS neurons in rats lightly anesthetized with isoflurane. When multiple whiskers were synchronously deflected by rapid back-and-forth movements, whisker-sensitive neurons in the DLS responded to both directions of movement. The latency and magnitude of these neuronal responses displayed very little variation with changes in the rate (2, 5, or 8 Hz) of whisker stimulation. Simultaneous recordings in SI barrel cortex and the DLS revealed important distinctions in the neuronal responses of these serially connected brain regions. In contrast to DLS neurons, SI neurons were activated by the initial deflection of the whiskers but did not respond when the whiskers moved back to their original position. As the rate of whisker stimulation increased, SI responsiveness declined, and the latencies of the responses increased. In fact, when whiskers were deflected at 5 or 8 Hz, many neurons in the DLS responded before the SI neurons. These results and earlier anatomic findings suggest that a component of the sensory-induced response in the DLS is mediated by inputs from the thalamus. Furthermore, the lack of sensory adaptation in the DLS may represent a critical part of the neural mechanism by which the DLS encodes stimulus-response associations that trigger motor habits and other stimulus-evoked behaviors that are not contingent on rewarded outcomes.
Keywords: adaptation, barrel cortex, basal ganglia, corticostriate, somatosensory, tactile stimulation, thalamostriate
the striatum has a complex functional organization that is defined by the topography of its corticostriatal inputs (Gerfen 2004). In rats, the dorsolateral striatum (DLS) receives convergent projections from multiple sites in primary somatosensory cortex (SI) and other sensorimotor cortical areas (Alloway et al. 2000, 2006; Brown et al. 1998; Hoffer and Alloway 2001). These and other findings suggest that the resulting combinatorial maps in the striatum may represent the permutations of different sensory inputs needed to execute specific patterns of somesthesis-guided movements (Aldridge and Berridge 1998; Brown 1992).
Despite substantial interest in the sensorimotor mechanisms of whisking and its functional role in guiding behavioral movements (Alloway 2008; Kleinfeld et al. 2006), very few studies have analyzed whisker-related responses in the rat striatum. One study has shown that DLS neurons in the awake rat discharge in rhythmic patterns that appear to correspond to the frequency of whisker motion during behavioral exploration (Carelli and West 1991). In a subsequent study, anesthetized rats were used to demonstrate that electrical stimulation of the peripheral whisker pad is effective in evoking neuronal discharges in the contralateral striatum (Wright et al. 2001).
Although many investigators have used whisker deflections in anesthetized rats to characterize the coding properties of neurons in the thalamus and related cortical areas (Bruno et al. 2003; Chakrabarti et al. 2008; Kwegyir-Afful and Keller 2004), the anesthetized rat preparation has not been useful for characterizing whisker-related neuronal responses in the DLS. The first study that examined striatal activity in the anesthetized rat preparation concluded that “unlike SI, striatal neurons stop firing in response to innocuous somatosensory stimulation during anesthesia” (West 1998). Unfortunately, that early study did not test the effects of whisker stimulation even though projections from SI barrel cortex represent the predominant source of corticostriatal inputs from SI (Hoover et al. 2003). In a more recent study, air puff stimulation of the whiskers evoked subthreshold excitatory potentials in the DLS of anesthetized rats (Pidoux et al. 2011), but very few neurons actually discharged, and the latencies of the responses were exceptionally long, ranging from 20 to 30 ms after stimulus onset.
In the present study, we used repetitive whisker stimulation to characterize the sensory coding properties of DLS neurons in the anesthetized rat. In contrast to other studies, rats were continuously ventilated with low concentrations of isoflurane (≤1%) that maintained them in a stable, lightly anesthetized plane. Furthermore, we simultaneously deflected multiple whiskers by using a mechanical stimulator that enabled precise, rapid back-and-forth movements at different frequencies. In most experiments, pairs of neurons were recorded simultaneously in both SI barrel cortex and the DLS to enable comparisons of neuronal responsiveness and the rate of sensory adaptation in these serially connected brain regions.
Our findings demonstrate that whisker deflections can activate extensive populations of neurons in the DLS of isoflurane-anesthetized rats. In contrast to neuronal responses in SI, neurons in the DLS showed little sensory adaptation when the whiskers were repetitively deflected at several different frequencies. These results are significant because whisking behavior is a highly repetitive motor activity, and growing evidence indicates that the dorsolateral part of the striatum is needed to execute stereotyped motor habits and other well-learned behaviors (Balleine and O'Doherty 2010; Graybiel 2008).
MATERIALS AND METHODS
Animals.
Anatomic and electrophysiological data were obtained from male Sprague-Dawley rats ranging from 250 to 550 g. All procedures complied with National Institutes of Health (NIH) guidelines and were approved by the Penn State Institutional Animal Care and Use Committee.
Surgery.
Before surgery, each rat was anesthetized with an intramuscular injection of ketamine (40 mg/kg) and xylazine (12 mg/kg). Atropine methyl nitrate (0.05 mg/kg) was injected intramuscularly to reduce bronchial secretions, and dexamethasone (0.5 mg/kg) was injected intramuscularly to prevent tissue inflammation. All rats were intubated through the oral cavity, placed in a stereotaxic frame (David Kopf Instruments, Tujunga, CA), and artificially ventilated with oxygen. Body temperature was maintained at 37°C with a heated water pad (under the trunk) and a homeothermic heating blanket (over the trunk). Ophthalmic ointment was applied to the eyes to prevent corneal irritation, and both heart rate and end-tidal CO2 were continuously monitored throughout all surgical procedures. Before exposing the cranium, lidocaine was injected subcutaneously into the scalp. As the effects of ketamine and xylazine subsided, isoflurane gas was administered at concentrations (0.5–1.0%) sufficient to prevent muscular reflexes.
Anterograde tracing.
In two rats, the anterograde tracer biotinylated dextran amine (BDA) was injected into SI barrel cortex. After exposing the cranium and making a craniotomy over SI barrel cortex, tracer injection sites were identified by using low-impedance electrodes to record multiunit responses to manual stimulation of the contralateral whiskers. Four whisker-sensitive sites, in which each site represented the corner of a square millimeter of SI, were identified for tracer injections. A glass pipette containing a 15% solution of BDA (D-7135; Molecular Probes, Eugene, OR) in 0.01 M PBS (pH 7.3) was placed sequentially in each of these sites, and the BDA was iontophoretically deposited at three depths (1.2, 0.8, and 0.4 mm) below the pia. Positive current (5 μA) pulses were applied in alternating on-off intervals of 7 s for 11–13 min at each depth. Following the BDA injections, the wound margin was sutured, and the animal was returned to its home cage.
One week later, each rat was deeply anesthetized with Nembutal (>100 mg/kg) and perfused transcardially with saline followed by 4% cold paraformaldehyde and 10% sucrose. The brain was extracted, immersed in fixative for 2 days, and sectioned coronally (60 μm). Tissue sections were processed for BDA as described previously (Alloway et al. 1998; Kincaid and Wilson 1996). The sections were agitated in 0.3% H2O2 and then in 0.1 M PBS with 0.3% Triton X-100 (pH 7.4) before being incubated in an activated avidin-biotinylated horseradish peroxidase solution (Vector Novocastra Laboratories, Burlingame, CA) for 2–4 h. Following several rinses in PBS, the sections were incubated in a solution of 0.05% diaminobenzidine, 0.005% H2O2, and 0.04% NiCl2 in 0.1 M Tris buffer (pH 7.1) for 9–12 min and were briefly washed in a final rinse of PBS. The sections were mounted serially on gel-coated slides, dried overnight, and dipped in alcohol and xylene before being coverslipped with Cytoseal.
Extracellular recordings.
Neuronal discharges and their responses to mechanical whisker stimulation were recorded from 23 rats in which the head was immobilized by means of an acrylic headstage mounted on the posterior cranium. After the cranium was exposed, holes were drilled at sites that enabled electrode penetrations into the DLS and SI. In addition, two holes were drilled at the occipital ridge to accommodate the insertion of small machine screws. After applying dental acrylic (Hygenic, Akron, OH) over the screws, two bolts were placed in the acrylic, ∼10 mm apart. A goose-neck manipulator (Flexbar, Long Island, NY) was attached to each bolt while the head was in the stereotaxic instrument. Subsequently, the stereotaxic ear bars were withdrawn to remove nociceptive activation originating from the external auditory meatus. Consequently, low concentrations of isoflurane (0.5–1.0%) were sufficient to maintain each rat in a stable anesthetic plane without spontaneous movements, and these low levels of anesthesia facilitated the detection of whisker-evoked neuronal responses in the DLS. Isoflurane produces a dose-dependent suppression of thalamocortical transmission, but the concentration of isoflurane used in the present study was below the level (>1%) that begins to exert a significant effect on evoked potentials in SI (Detsch et al. 1999, 2002; Masamoto et al. 2009). Although we did not record EEG or electrocorticogram activity, heart rate was generally in the range of 290–300 beats/min, which is noticeably higher than the rate that we observed when isoflurane was increased to levels that suppressed reflexive responses to intense nociceptive stimulation. Furthermore, we found that raising the concentration of isoflurane to 1.5% produced a suppression of neuronal responsiveness in the DLS.
For striatal recordings, a small craniotomy was made 2 mm lateral to midline and 0.4–2.0 mm caudal to bregma, which is medial to the SI hindlimb representation. A tungsten electrode having an impedance between 2 and 4 MΩ (Frederick Haer, Brunswick, ME) was advanced into the brain at an angle of 25° to the parasagittal plane. This angular approach enabled recordings from the DLS without damaging the overlying SI barrel cortex. A single striatal penetration was made in most rats, and isolated neuronal discharges were recorded during whisker pad stimulation at successive depths throughout the DLS. Electrical or mechanical stimulation of the contralateral whiskers was administered as the electrode was slowly advanced through the striatum while searching for isolated neuronal responses. Stimulus-triggered extracellular waveforms were visualized on a digital oscilloscope (Tektronix DPO4034; Tektronix, Beaverton, OR) while listening to the discharges on an acoustic speaker.
In 15 rats, neuronal responses in SI and DLS were recorded simultaneously. To record SI neuronal activity, a 2nd craniotomy was made 5.5 mm lateral and 3 mm caudal to bregma. A dural slit was made, and a tungsten electrode (1–2 MΩ) was advanced into the cortex at an angle orthogonal to the pial surface. Stimulus-induced responses in SI were recorded at depths of 350–1,000 μm, which includes neurons in layers III, IV, and V, and preference was given to isolating large-amplitude regular-spiking (RS) neurons.
Extracellular neuronal discharges having a signal-to-noise ratio of 3 or more were band-pass filtered (300 Hz to 3 kHz) and amplified (model 2200; Dagan, Minneapolis, MN). Stimulus control, data acquisition and storage, and waveform sorting of the extracellular discharges were managed by a SciWorks data acquisition system (version 6.0; DataWave Technologies, Broomfield, CO). For each computer-controlled trial, a continuous stream of time-varying voltage information was sampled at 25 kHz from each electrode channel by an analog-digital board (DT2839; Data Translation, Marlboro, MA). This trial-based continuous data stream was stored on hard disk and subsequently was replayed to enable sorting of isolated neuronal discharges according to spike amplitude, spike width, spike height, peak time, and valley time.
Neuronal discharges were time-stamped to a resolution of 0.1 ms and were displayed as peristimulus-timed histograms (PSTHs), autocorrelation histograms (ACGs), and interspike interval histograms using NeuroExplorer software (version 3.0; Nex Technologies, Littleton, MA). Statistical criteria were used to classify responsive neurons. Based on the mean rate of spontaneous activity, 99% confidence limits were constructed and displayed on the PSTH of each neuron. Responses to electrical or mechanical stimulation were considered statistically significant if they exceeded the 99% confidence limits for two contiguous bins. The time of the first bin that marked a significant response was defined as the response latency.
Electrical whisker pad stimulation.
In a few experiments, electrical stimulation was applied to the peripheral whisker pad to facilitate the search for whisker-sensitive neurons in DLS and to compare the response latencies of the neuronal responses in DLS and SI. For this purpose, two stainless steel needles were inserted into skin sites located at opposite edges of the whisker pad. Current pulses, 1 or 2 ms long, were passed through these leads using a constant-current source (BAK Electronics, Mount Airy, MD). Current pulse amplitude was adjusted to levels (250–350 μA) that evoked twitches of the whisker pad. Neuronal responses to electrical stimulation were recorded for 100 trials at each recording site. Each trial consisted of a 1,000-ms prestimulus period, a 1- or 2-ms electrical stimulus, and a 2,000-ms poststimulus period.
Mechanical whisker deflections.
Mechanical whisker stimulation was administered by a galvanometer obtained from a polygraph machine. A small square of window screen attached to the end of the ink pen was positioned 10–12 mm from the base of the whisker pad so that multiple whiskers protruded through the holes of the window screen. The window screen was curved to enable simultaneous stimulation of all whiskers located in the 5 most caudal arcs of all rows in the whisker pad. Hence, the galvanometer stimulated all whiskers in the Greek arc as well as whiskers in arcs 1–4 of rows A–E, thereby deflecting 24–25 whiskers simultaneously. Before data acquisition, the resting position of the screen was adjusted so that all of the whiskers protruding through the screen contacted the rostral edge of a hole, thereby ensuring simultaneous deflections of all whiskers at the onset of screen movement in the caudal direction.
The motion of the galvanometer and its attached screen was controlled by a 50-ms sawtooth signal delivered by a waveform generator (model LW420; LeCroy, Chestnut Ridge, NY) for which output was initiated by the data acquisition system. For each stimulus, the screen moved 1,500 μm caudally during the 1st 25 ms of the stimulus and then moved to the original resting position over the next 25-ms period. This represents a velocity of 60 mm/s in each direction. For each trial, a prestimulus period of 2–4 s was followed by 3 blocks of whisker deflections administered at 2, 5, or 8 Hz. For each stimulus block, a total of 4 whisker deflections were administered. Each block was separated by a 1-s interval, and the last block of whisker stimulation at 8 Hz was followed by a poststimulus period lasting 2 s. Neuronal responses to mechanical whisker deflections were recorded for 200 trials at each recording site. Methods similar to this were used previously in our laboratory to evoke neuronal responses in the SI and MI whisker regions (Chakrabarti et al. 2008; Chakrabarti and Alloway 2009; Zhang and Alloway 2004).
Statistical analysis.
The effect of stimulus frequency on the latency and magnitude of stimulus-induced responses in SI and the DLS was analyzed by statistical modules in Origin 8 (OriginLab, Northampton, MA). For cases in which analysis of variance revealed that stimulus frequency had a significant effect on neuronal responsiveness, matched-sample and unpaired t-tests were used to determine which groups were significantly different.
Histology.
In the 23 rats used for electrophysiology, coronal sections through the brain were histologically examined to visualize the electrode tracks and verify that the electrodes entered the DLS. In some of these rats (n = 9), electrolytic lesions (10 μA for 10–15 s) were made at the last recording site in the DSL so that interpolation of recording depths could be used to reconstruct the sequential locations of DLS recordings. In these 9 cases, electrolytic lesions were also made at the last recording site in SI barrel cortex.
All rats were killed with Nembutal (>100 mg/kg im) and transcardially perfused with saline followed by 4% paraformaldehyde containing 10% sucrose. After the brain was removed and placed in fixative for 1 or 2 days, it was sectioned coronally on a freezing microtome (60 μm). Tissue sections were mounted in serial order on gel-dipped slides and dried overnight before being stained with thionin and coverslipped with Cytoseal. The striatum and SI were visualized with a light microscope (Olympus BH2), and selected sections were photographed with a Retiga EX CCD digital camera (QImaging, Surrey, British Columbia, Canada).
RESULTS
Results are reported from a total of 25 rats. In 2 of these animals, the anterograde tracer BDA was injected into SI barrel cortex to reveal the spatial extent of the striatal region that receives dense projections from the SI whisker representation. The results from these 2 animals, together with previous tracing studies (Alloway et al. 1999; Brown et al. 1998; Hoffer et al. 2005; Hoover et al. 2003), guided our insertion of recording electrodes into the DLS of the other 23 animals. In these 23 cases, we recorded the responses of DLS neurons to mechanical whisker stimulation. In 15 of these 23 cases, we simultaneously recorded neuronal responses in both SI barrel cortex and the DLS.
Corticostriatal projections from SI barrel cortex.
The striatal territory from which we sampled neuronal responses was largely determined by two experiments in which multiple deposits of BDA were placed in SI barrel cortex. In these two cases, BDA was iontophoretically injected at three laminar depths in each of four cortical sites. As shown in Fig. 1, BDA infiltrated widespread parts of barrel cortex and produced dense labeling in layers III and V, which represent the origin of corticostriatal projections (Alloway et al. 2006; Reiner et al. 2003).
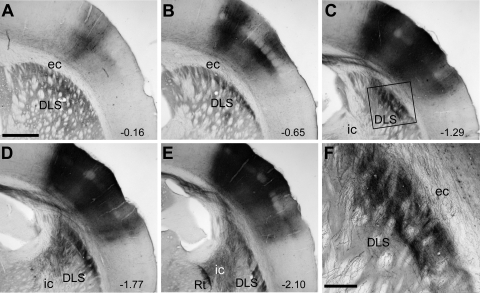
Fig. 1.
Projections from somatosensory (SI) barrel cortex to the dorsolateral striatum (DLS). A–E: coronal sections showing biotinylated dextran amine (BDA) injections in SI barrel cortex and the terminal labeling in the DLS. Caudal distance from bregma (in millimeters) is shown in the bottom right of each section. Rectangle in C indicates the region in F. Scale bar in A–E, 1 mm. F: magnified view of dense labeling in the DLS. Scale bar, 250 μm. ec, External capsule; ic, internal capsule; Rt, thalamic reticular nucleus.
Labeled corticostriatal projections from SI barrel cortex terminated in lamellar-shaped regions in the dorsolateral part of the DLS. Inspection of these regions at high magnification (×200) revealed dense collections of BDA-labeled terminals with beaded varicosities that represent presynaptic contacts (Kincaid and Wilson 1996). The most densely labeled regions were located immediately medial to the external capsule and formed curved lamellae approximately 500–800 μm wide and 2–3 mm in dorsoventral length. As shown by Fig. 1, the densest labeling appeared in coronal sections located 0.6–2.1 mm caudal to bregma. Based on these results and our previous studies of the topography of corticostriatal projections from SI (Alloway et al. 1999; Hoffer et al. 2005; Hoover et al. 2003), our electrode penetrations in the striatum were aimed at these dorsolateral regions.
Incidence of whisker-related responses in the striatum.
Striatal recording electrodes entered the brain at a 25° angle to match the “tilt” of the lamellar-shaped regions revealed by our neuronal tracing experiments (Fig. 1). As shown previously (Wright et al. 2001), this angled approach maximizes the probability of encountering whisker-sensitive striatal neurons.
Furthermore, we stimulated the peripheral whisker pad (mechanically or electrically) as we searched for neurons in the DLS because this increases the likelihood of detecting neurons that are involved in processing whisker-related information.
After passing through the external capsule, which was noticeably “quiet” during whisker stimulation, our electrodes encountered whisker-sensitive neurons at depths located 3–6 mm below the pial surface. Although many “silent” neurons may have been intermingled with these whisker-sensitive neurons, the use of peripheral whisker stimulation during our search process revealed responsive neurons at almost every depth of each electrode penetration in the DLS. In fact, the difficulty was not in detecting whisker-sensitive neurons but in adjusting the electrode position to isolate the extracellular discharges of one or two neurons at each recording site.
In 23 rats, we tested the effects of whisker deflections on 245 neurons in the DLS. In this sample, 231 isolated neurons in the DLS displayed significant extracellular responses that exceeded the 99% confidence limits. These whisker-sensitive neurons were recorded at 141 sites located throughout the DLS, which represents an average of 1.64 responsive neurons at each of these recording sites.
Histological inspection of the electrode tracks confirmed that all electrode penetrations traversed the DLS, and in nine cases an electrolytic lesion confirmed that the last recording site in the penetration was in the DLS neuropil. We encountered whisker-sensitive neuronal discharges in the DLS at multiple depths that ranged from 3 to 6 mm below the pial surface. After discounting the first 3 mm of each penetration, in which the electrode traversed the cortex and external capsule, the average length of each electrode path in DLS was 1.5 mm. On average, whisker-sensitive neurons in the DLS were recorded at five different depths in each penetration before the electrode was withdrawn from the brain. Hence, whisker-sensitive responses in the DLS were usually characterized at intervals of 300 μm.
Previous studies have characterized striatal neurons electrophysiologically and then labeled them, thereby correlating their discharge properties with cellular morphology (Inokawa et al. 2010; Kawaguchi 1993; Mallet et al. 2005; Wilson and Groves 1981; Wilson et al. 1990). Based on the consistent waveform properties associated with medium spiny (MS), fast-spiking (FS), and other neuronal subtypes in the striatum, several studies have relied on discharge properties alone to distinguish different types of striatal neurons (Barnes et al. 2005; Schmitzer-Torbert and Redish 2008; Sharott et al. 2009).
Consistent with these reports, we found that whisker-sensitive neurons in the DLS were characterized by distinct waveforms and other discharge properties that correspond to well-known categories of striatal neurons. Among the criteria used to classify our striatal neurons, the distribution of interspike intervals was the most reliable parameter for distinguishing FS and MS neurons. Virtually all DLS neurons with high firing rates (>5 Hz) had very few interspike intervals >1 s, and, because they were characterized by brief waveform durations (1 ms or lower), these cells were classified as FS neurons. By comparison, DLS neurons with long-duration waveforms (1–3 ms) were characterized by low firing rates (<5 Hz) and spike trains that contained many interspike intervals >1 s. These types of whisker-sensitive striatal neurons were classified as MS cells. Statistical analysis revealed that the mean (± SE) waveform durations of the MS (1.63 ± 0.03 ms; range 0.95–2.72 ms) and FS neurons (0.86 ± 0.01 ms; range 0.7–1.0 ms) were significantly different (t = 7.65; P < 0.001).
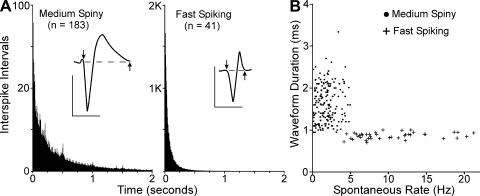
Using these criteria, our sample of 231 whisker-sensitive neurons in the DLS contained 41 FS and 183 MS neurons. As indicated by Fig. 2, these 2 populations of neurons were easily distinguished by the proportion of interspike intervals that lasted 1 s or longer. In addition, the spontaneous discharge rates of MS and FS neurons were noticeably different, averaging 1.99 or 9.89 discharges/s, respectively (Fig. 2B). Although MS neurons comprise >90% of the neurons in the striatum (Gerfen 2004), we probably recorded a disproportionate number of FS neurons because it is easier to detect neurons with higher discharge rates.
Fig. 2.
Discharge properties of medium spiny (MS) and fast-spiking (FS) whisker-sensitive neurons in the DLS. A: interspike interval (ISI) histograms illustrating distinctions in the temporal structure of activity recorded from MS and FS neurons. Each histogram represents the mean of all ISI histograms for whisker-sensitive neurons classified as MS or FS cells. Insets illustrate typical waveforms of each neuronal subtype, including waveform durations as indicated by the arrows. ISI bin widths, 1 ms; waveform scales, 200 μV, 1 ms. B: scatterplot illustrating the differential clustering of MS and FS cells when their waveform durations and spontaneous discharge rates are plotted.
One whisker-sensitive neuron in our DLS sample was classified as a giant tonically active neuron because analysis revealed a wide trough in its ACG, which indicates the total absence of short interspike intervals (Barnes et al. 2005). In addition, we were unable to classify six whisker-sensitive neurons for which waveform durations and other discharge properties were inconsistent with the conventional characteristics of FS and MS neuronal subtypes. Along with the giant tonically active neuron, these ambiguous neuronal responses were discarded from the pool of neurons that are analyzed in subsequent sections of this report.
Striatal response to mechanical whisker stimulation.
After isolating one or two DLS neurons at a recording site, we usually stimulated the whiskers manually with a wooden dowel rod or a hand-held air jet as we monitored the evoked discharges on the oscilloscope and acoustic speaker. This ensured that at least some of the whiskers deflected by the galvanometer were within the receptive fields of the DLS neurons. Although many neurons could be activated by manually deflecting a single whisker, simultaneous deflection of multiple whiskers was usually more effective at evoking a clear response.
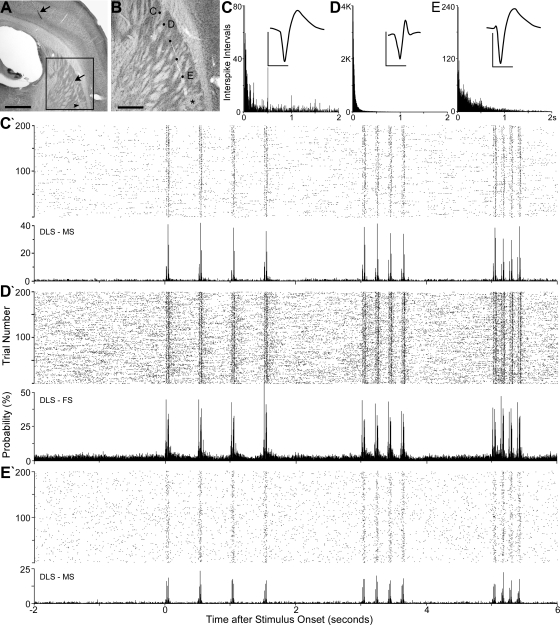
Examples of striatal responses to controlled mechanical stimulation of the whiskers are shown in Fig. 3. As Fig. 3B indicates, we characterized the responses of whisker-sensitive neurons at six successive sites in the DLS of this particular rat. Although spontaneous activity and stimulus-induced responsiveness varied substantially across different neurons, both FS and MS neurons responded to each frequency of whisker stimulation that we tested. More importantly, DLS neurons displayed very little adaptation as the whiskers were repetitively deflected at progressively higher frequencies.
Fig. 3.
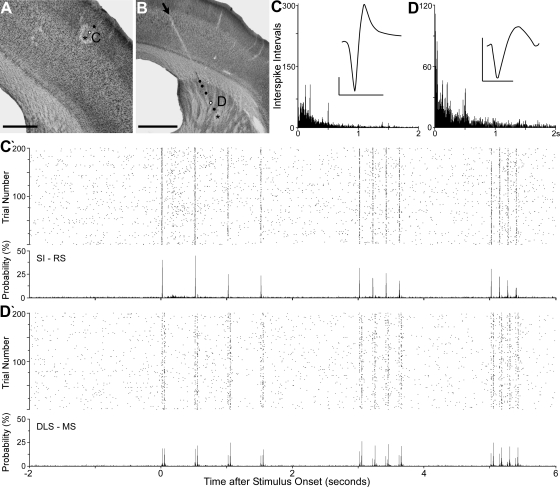
Examples of DLS neuronal responses during repetitive deflections of the peripheral whiskers. A: coronal section located 1.6 mm caudal to bregma shows the track (arrows) of an electrode that recorded 11 whisker-sensitive neurons at 6 sites in the DLS during controlled whisker deflections. Square indicates the region in B; arrowhead indicates a microlesion at the last electrode recording site. Scale, 1 mm. B: recording sites marked by black dots or an asterisk (lesion site); letters indicate recording sites of responses shown in subsequent panels. Scale, 0.5 mm. C–E: ISI histograms and waveforms used to classify the neurons as MS (C and E) or FS (D). Bin widths, 1 ms; waveform scales, 200 μV, 1 ms. C′, D′, and E′: responses to 200 trials of whisker stimulation at 2, 5, and 8 Hz depicted by dot raster and peristimulus-timed histograms (PSTHs). Dot raster and PSTH bin widths, 2 ms.
The mean responses of whisker-sensitive neurons in the DLS are illustrated in Fig. 4. As the population PSTHs indicate, both MS and FS neurons responded to each frequency of whisker stimulation. Consistent with the responses displayed by individual neurons (Fig. 3), the mean responses of the MS and FS neurons showed little adaptation during repetitive whisker stimulation (Fig. 4A).
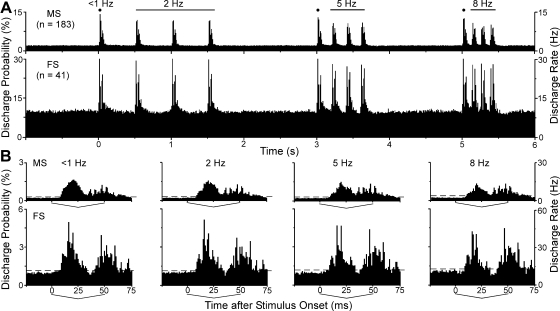
Fig. 4.
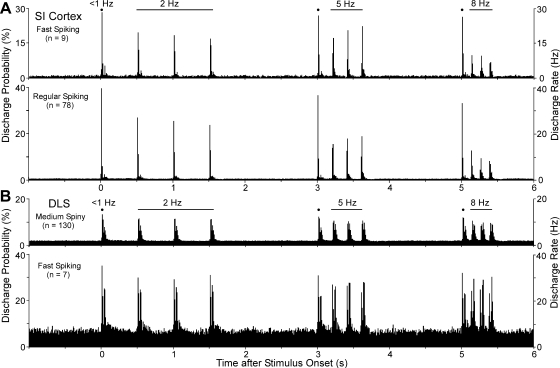
Mean population response of whisker-sensitive neurons in the DLS. A: population PSTHs depicting the mean response pattern of MS and FS neurons activated repetitively by synchronous deflections of multiple whiskers. Each PSTH illustrates the mean neuronal response to 1 trial of repetitive stimulation at 2, 5, and 8 Hz. Note that the 1st stimulus in each frequency-specific block is equivalent to stimulation at <1 Hz. Responsiveness is depicted both by the probability of a discharge at each bin time (left axis) and by the discharge rate (right axis). Bin widths, 10 ms. B: frequency-specific PSTHs showing the mean stimulus-induced response of MS and FS neurons. The response to the 1st stimulus in each block was averaged to illustrate responsiveness at <1 Hz; the remaining PSTHs represent the mean response to the last 3 stimuli in each frequency block. Sawtooth brackets from 0 to 50 ms indicate the duration of the back-and-forth whisker stimulation. Horizontal dashed lines indicate 99% confidence intervals; bin widths, 1 ms.
To illustrate the mean response to each stimulus administered at 2, 5, and 8 Hz, we constructed high-resolution PSTHs that were based on the responses to the last three whisker deflections in each frequency-specific block (Fig. 4B). The mean response to the first whisker deflection in each block was illustrated separately because it occurred after an interstimulus interval >1 s and, therefore, represents the response to stimulation at a rate <1 Hz.
Inspection of the mean response to each stimulus frequency (Fig. 4B) revealed two peaks of activity for both MS and FS neurons: one that occurred in response to the onset of the whisker deflection and a second response that occurred as the whiskers returned to their original position. At each frequency, the response to the initial onset of whisker movement was stronger than the response to the second phase of whisker movement, probably because the return of the whiskers to their original position was not directly controlled by the motion of the galvanometer but represents movement due to the elastic properties of the whisker pad. Nonetheless, the fact that well-defined responses to each phase of whisker motion could be identified in the average response of our neuronal sample indicates a high degree of temporal fidelity among whisker-sensitive neurons in the DLS.
Simultaneous recordings in SI barrel cortex and the striatum.
As our neuronal tracing results indicate (Fig. 1), the SI barrel cortex provides a major source of inputs to the DLS. The dense projections from SI to the DLS prompted us to compare the temporal pattern and magnitude of the neuronal responses in both regions. Therefore, SI and DLS neurons were recorded simultaneously in the last 15 rats used in this study. In these animals, 1 electrode penetrated the striatum as described earlier while a 2nd electrode was inserted into SI barrel cortex.
In these 15 rats, a total of 87 neurons (9 FS and 78 RS) were recorded from 77 sites in the middle layers of SI barrel cortex, while a total of 137 whisker-sensitive neurons (7 FS and 130 MS) were recorded from 80 sites in the DLS. For these simultaneous recordings in SI and DLS, preference was given to isolating and recording the output neurons in both structures. In the DLS, we preferentially recorded MS neurons because these cells comprise >90% of all neurons in the striatum and provide all of its efferent projections. In SI barrel cortex, we searched for RS neurons with large waveforms because these properties reflect pyramidal neurons, which convey the output of SI barrel cortex to the striatum and other postsynaptic targets. Pyramidal neurons in SI and other cortical areas are easily identified because they have long-duration waveforms that are easily distinguished from FS neurons, which usually represent local inhibitory neurons (Bruno and Simons 2002; Gibson et al. 1999; McCormick et al. 1985; Porter et al. 2001). Depth measurements and subsequent histological examination indicated that all neurons recorded in SI were located in cortical layers III, IV, and V.
Comparison of SI and striatal responses to repetitive whisker stimulation.
Figure 5 illustrates a pair of neuronal responses that were recorded concurrently in SI and the DLS during whisker stimulation. Consistent with data presented earlier (Figs. 3 and 4), the neuronal response in the DLS was relatively constant across different frequencies of whisker stimulation. By comparison, the neuronal response in SI displayed a high degree of adaptation as indicated by the decrease in responsiveness as stimulus frequency increased across successive blocks of stimulation.
Fig. 5.
Simultaneous responses of a MS neuron in the DLS and a regular-spiking (RS) neuron in SI barrel cortex during whisker stimulation. Recording sites and neuronal responses shown as in Fig. 3. A and B: white circles indicate the pair of sites that generated the responses in subsequent panels. Arrow shows electrode track in the DLS; scales, 0.5 mm (A), 1 mm (B). C and D: ISI histograms and waveform characteristics of the SI (C) and DLS (D) neurons. Bin widths, 1 ms; waveform scales, 200 mV, 1 ms. C′ and D′: simultaneous responses of both neurons during whisker stimulation at 2, 5, and 8 Hz; dot raster and PSTH bin widths, 2 ms.
The population responses obtained during simultaneous recordings in SI and the DLS indicate that sensory adaptation is much stronger in SI than in the DLS. As shown by the population PSTHs in Fig. 6, both neuronal subtypes in SI (i.e., RS and FS) were characterized by progressively smaller responses as the frequency of whisker stimulation increased. By contrast, both neuronal subtypes in the DLS (MS and FS) displayed similar responses at all stimulation frequencies. In general, the population PSTHs for SI contained brief responses that declined in amplitude as stimulation frequency increased, whereas the population PSTHs for the DLS contained longer-duration responses for which amplitudes were relatively constant for each block of whisker stimulation.
Fig. 6.
Mean response patterns of SI (A) and DLS (B) neurons in 15 rats in which neuronal activity was recorded simultaneously in both brain regions during repetitive whisker stimulation. PSTHs are illustrated as in Fig. 4A. Bin widths, 10 ms.
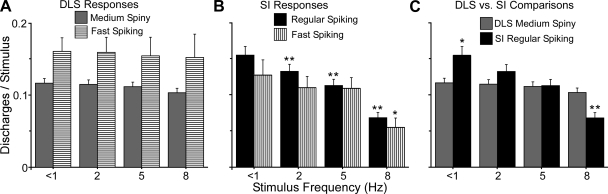
Quantitative analysis confirmed that the frequency of whisker stimulation had a significant effect on neuronal responsiveness in SI barrel cortex but not on the whisker-sensitive neurons in the DLS. As shown by Fig. 7, the mean response was similar at each frequency for both FS and MS neurons in the DLS (Fig. 7A). Response magnitude was expressed as the number of discharges occurring during each stimulus presentation (10–60 ms after stimulus onset) after subtracting discharges due to spontaneous activity. Analysis of variance failed to detect an effect of stimulus frequency on the response magnitudes recorded in the DLS (F = 0.47; P < 0.65). By comparison, as shown in Fig. 7B, stimulus frequency had a significant effect on neuronal responsiveness in SI (F = 12.9; P < 0.00001), and the magnitude of the RS responses to 1-Hz stimulation was significantly larger than the responses evoked at all other frequencies (Fig. 7B).
Fig. 7.
Magnitude of stimulus-induced responses recorded simultaneously in DLS and SI. A: magnitude of responses in the DLS during different frequencies of whisker stimulation. Each bar represents the mean number of discharges produced by each stimulus frequency after subtracting spontaneous activity. Analysis of variance failed to detect an effect of stimulus frequency on response magnitude in the DLS. B: magnitude of responses in SI barrel cortex during different frequencies of whisker stimulation. Stimulus frequency had a significant effect on responses in SI, and asterisks indicate the groups that were significantly different from the response evoked by 1-Hz stimulation (matched-sample t-tests, *P < 0.01; **P < 0.001). C: comparison of response magnitudes for the output neurons of DLS and SI. Asterisks indicate frequencies at which the responses of the SI-RS neurons were significantly different from the responses of the DLS-MS neurons (unpaired t-tests, *P < 0.01; **P < 0.001).
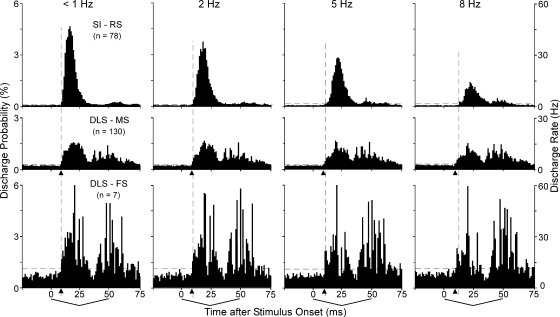
Analysis of the stimulus-induced responses at high temporal resolution revealed additional distinctions between the whisker-sensitive neurons in SI and the DLS. For this analysis, the responses of the DLS neurons were compared with the RS neurons in SI because these are most likely to represent the output responses of the cortex. As shown by Fig. 8, the DLS neurons responded to both phases of each whisker deflection, whereas the RS neurons in SI responded primarily to the first 25-ms phase of whisker motion. The absence of a response among RS neurons during the second phase of whisker motion suggests that the DLS responses during this time period are probably not due to corticostriatal inputs from SI. Furthermore, the RS responses in the SI showed adaptation at higher frequencies of stimulation, whereas those in the DLS were relatively constant for both phases of the tested frequencies. Consequently, when the whiskers were stimulated at 8 Hz, the stimulus-induced responses in the DLS were larger than the RS responses recorded concurrently in SI (Fig. 7C).
Fig. 8.
Frequency-specific responses of DLS neurons compared with the RS neurons in SI. Each PSTH represents the mean frequency-specific response. Vertical dashed lines indicate the earliest response of the RS population in SI; arrowheads below the abscissa indicate the earliest response recorded from the MS and FS neurons in the DLS. Note that the earliest response in the DLS occurred before the earliest SI response at most frequencies. Bin widths, 1 ms.
Comparison of response latencies in SI and the DLS.
To determine the relative sequence of stimulus-induced activity in SI and the striatum, we compared the response latencies of neurons recorded simultaneously in both regions. In 3 rats, response latencies in SI and DLS were measured during both electrical and mechanical stimulation of the peripheral whisker pad. In the remaining 12 rats, responses in both brain regions were activated by mechanical stimulation only. In both instances, response latencies were operationally defined by constructing PSTHs with 1-ms bin widths and then identifying the 1st of 2 contiguous bins that exceeded the 99% confidence limits. The onset of the response was distributed across contiguous bins because of temporal variations in response latency across individual trials; the presence of activity in contiguous bins did not represent bursting activity.
Response latencies evoked by electrical stimulation.
Electrical stimulation of the whisker pad was used to characterize response latencies for 13 SI and 17 DLS neurons. This included 9 RS and 4 FS neurons in SI; all DLS neurons activated by electrical stimulation were classified as MS cells. As indicated by Fig. 9, electrical stimulation activated neurons in both regions at virtually the same time. In several instances, however, an electrical stimulus evoked a discharge in the DLS before activating a concurrently recorded neuron in SI (Fig. 9A). Furthermore, MS neurons frequently discharged more than once in response to brief electrical stimulation, but discharges evoked in RS neurons were often followed by FS discharges, which probably represent the activity of nearby inhibitory interneurons. This SI response pattern is consistent with evidence indicating that evoked responses in SI are characterized by brief excitation followed by prolonged inhibition (Carvell and Simons 1988; Moore and Nelson 1998; Simons and Carvell 1989).
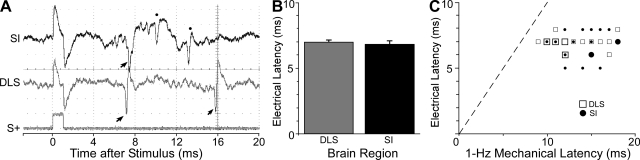
Fig. 9.
Response latency of SI and DLS neurons tested with both electrical and mechanical stimulation. A: oscilloscopic image of simultaneous neuronal responses evoked in SI and DLS by electrical stimulation (1-ms pulse, 350 μA) of the contralateral whisker pad. Arrows indicate discharges in a pair of MS (middle trace) and RS (top trace) neurons after stimulation (S+); dots indicate FS discharges that, presumably, represent inhibitory interneurons in SI (top trace). B: bar graph depicting the mean latency of SI and DLS neuronal responses to electrical stimulation. Brackets indicate SE. C: scatterplot comparing the latency of responses to electrical and mechanical (1 Hz) stimulation for those neurons in SI and DLS that were tested with both types of stimulation. Small symbols indicate 1 neuronal response, and larger symbols indicate where 2 neuronal responses are superimposed.
Across different neuron pairs, electrical stimulation of the whisker pad consistently activated responses in SI and the DLS at approximately the same time. As indicated by Fig. 9B, neuronal responses to electrical stimulation had similar latencies in SI (6.7 ± 0.26 ms) and the DLS (6.9 ± 0.13 ms), and the differences were insignificant (t = 0.59; P > 0.1). This suggests that the fastest routes by which somatosensory information reaches SI or the DLS are equivalent in terms of distance and velocity.
Among neurons tested with both electrical and mechanical stimulation, response latencies in both SI and the DLS were always longer for mechanical than for electrical stimulation. As indicated by Fig. 9C, electrical stimulation evoked responses within 5–8 ms in both brain regions, but the response latencies of the same neurons varied from 9 to 18 ms during mechanical stimulation at <1 Hz. Surprisingly, the earliest response to electrical stimulation was recorded in SI (5-ms latency), but the earliest response to mechanical stimulation for the same set of neurons was in the DLS (9 ms).
Response latencies evoked by mechanical stimulation.
As shown by the mean PSTHs in Fig. 8, the population responses in SI and DLS showed variations in latency as the frequency of whisker stimulation increased. Whereas the latency of the population remained fairly constant for neurons in the DLS, the population response in SI was noticeably delayed as the frequency of whisker stimulation increased. The latency of the population, however, is useful only for indicating the earliest response in the neuronal sample. Hence, for the purpose of statistical analysis, we measured the response latencies of individual neurons in both brain regions to determine whether stimulus frequency affected the latency of evoked discharges in SI and the DLS.
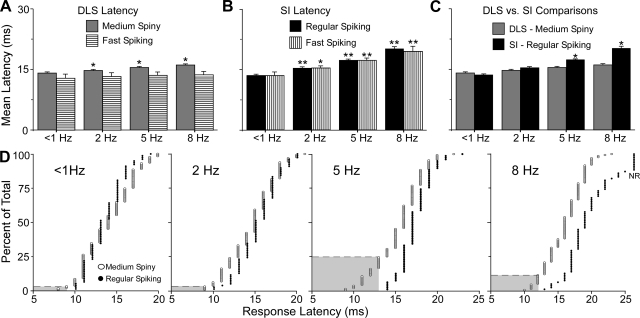
Statistical analysis indicated that the frequency of whisker stimulation had a significant effect on response latencies in both regions, but the effect was larger in SI barrel cortex (F = 82.5; P < 0.0001) than in the DLS (F = 12.7; P < 0.01). As shown by Fig. 10, increases in the rate of whisker stimulation did not alter the response latency of FS neurons in the DLS but did cause small increases in the latency of MS responses (Fig. 10A). By comparison, increases in the frequency of whisker stimulation produced larger effects on the response latencies of both FS and RS neurons in SI barrel cortex (Fig. 10B). Direct comparisons between the MS and RS neurons in the DLS and SI, respectively, indicated that response latencies for these groups were similar during stimulation at <1 or 2 Hz, but RS neurons had significantly longer latencies than MS neurons during stimulation at 5 or 8 Hz (Fig. 10C). In fact, cumulative distributions of the response latencies for these neurons revealed separate, nonoverlapping distributions when whisker stimulation was administered at 5 or 8 Hz (Fig. 10D). Furthermore, when the whiskers were stimulated at 5 Hz, one-fourth of the MS neurons responded before any response was evoked in the RS neurons of SI (see gray regions in Fig. 10D). By itself, this result suggests that some somesthetic information reaches the DLS by a route that does not involve SI.
Fig. 10.
Comparison of response latencies in SI and the DLS during mechanical whisker stimulation at different frequencies. A: response latencies in the DLS. Stimulus frequency had a significant effect on MS neurons in the DLS; asterisks indicate significant difference from the 1-Hz response (matched-sample t-tests, *P < 0.01). B: stimulus frequency had significant effects on response latencies of both RS and FS neurons in SI (matched-sample t-tests, *P < 0.01, **P < 0.00). C: comparison of response latencies for the output neurons of DLS and SI. Asterisks indicate frequencies at which RS latencies were significantly different from the MS neurons (unpaired t-tests, *P < 0.01). D: cumulative distributions illustrating differences in the response latencies of MS and RS neurons at different frequencies of stimulation. Gray areas indicate the proportion of MS neurons that discharged before the 1st RS response in SI. Nonresponsive neurons during 8-Hz stimulation are indicated as NR.
DISCUSSION
This study demonstrates that whisker-sensitive neurons in the DLS show little adaptation to repetitive stimulation despite the fact that the same stimulation produces strong adaptation in SI. This suggests that invariant responses in the DLS mediate the stimulus-response associations that underlie motor habits and other highly repetitive behaviors that have been linked to this brain region. In further contrast to SI, DLS neurons responded to both phases of whisker motion and displayed shorter response latencies at high frequencies of whisker stimulation. These three findings indicate that neuronal responses in the DLS do not depend entirely on inputs from SI. Although corticostriatal projections undoubtedly have a major influence on DLS responses, thalamostriate pathways probably play an important role in transmitting sensory information to the striatum.
Functional significance of whisker-induced responses in the DLS.
Exploratory whisking consists of successive epochs of repetitive whisking movements in which the back-and-forth sweeping motions of the whiskers are emitted across a wide range of frequencies, predominately in the range of 4–12 Hz (Berg and Kleinfeld 2003; Welker 1964). Whisking frequency is fairly constant within each epoch (1–2 s) but shifts to higher or lower values in successive epochs. After contacting an external object, bilateral whisker movements are altered in specific ways (Mitchinson et al. 2007). The sequential shifts in whisking frequency, as well as the stereotyped responses following whisker contacts with external stimuli, resemble certain aspects of the sequences of syntactic grooming behaviors that have been linked to the DLS (Aldridge et al. 2004; Aldridge and Berridge 1998; Cromwell and Berridge 1996). In fact, the striatal site that mediates syntactic grooming is near the whisker-sensitive DLS regions that we recorded in the present study.
In contrast to SI barrel cortex, which adapts during repetitive whisker stimulation in both anesthetized and awake rats (Chakrabarti and Alloway 2009; Melzer et al. 2006), the latency and magnitude of the DLS responses in the present study remained fairly constant across all stimulus frequencies that we tested. Such invariance in the responses of DLS neurons may represent the neural correlate of the stimulus-response associations that mediate highly repetitive motor habits and other behaviors that are triggered in specific circumstances. These invariant response patterns are noteworthy because the DLS is necessary for the development of stimulus-response associations and other well-learned behaviors that are not contingent on rewarded outcomes (Balleine and O'Doherty 2010; Graybiel 2008; Packard and Knowlton 2002; Yin et al. 2006).
Anesthetic influences on striatal responses.
Previous work indicated that anesthetics suppress stimulus-induced activity in the striatum. After testing several anesthetics on striatal responsiveness, including pentobarbital, urethane, ketamine, choral hydrate, and Metofane, one study concluded that striatal neurons do not respond to innocuous somatosensory stimulation when the animal is anesthetized (West 1998).
Although anesthesia can suppress sensory activation of the striatum, our results demonstrate the feasibility of characterizing striatal responses in lightly anesthetized rats. We administered isoflurane continuously at low concentrations (0.5–1.0%) that effectively maintained each animal in a shallow plane of anesthesia. By reducing nociceptive stimulation associated with stereotaxic immobilization, we minimized the amount of isoflurane needed to anesthetize the rat. We also optimized the probability of encountering whisker-sensitive neurons in the DLS by sampling striatal regions that receive dense inputs from SI barrel cortex.
Substantial evidence indicates that isoflurane has dose-dependent inhibitory effects on the transmission of tactile information to SI. With increasing concentrations, isoflurane suppresses the stimulus-induced responses of thalamocortical relay neurons and their cortical targets, but its effects on trigeminal inputs to the thalamus are less apparent (Detsch et al. 1999, 2002; Vahle-Hinz and Detsch 2002). The suppression of cortical responsiveness is consistent with evidence that low concentrations of isoflurane reduce glutamate release and that higher concentrations enhance neuronal inhibition by multiple GABA-dependent mechanisms (Joksovic and Todorovic 2010; Langmoen et al. 1995; Liachenko et al. 1999; Wakamori et al. 1991; Ying et al. 2009). Isoflurane also hyperpolarizes thalamocortical relay neurons by increasing the conductance of potassium leak channels (Langmoen et al. 1995; Ries and Puil 1999a,b). Hence, isoflurane suppresses neuronal activity by multiple mechanisms, and this effect becomes more pronounced as the concentration of isoflurane is increased.
When isoflurane is administered at low concentrations, somatosensory stimulation evokes neuronal responses in the rat thalamus that resemble the responses in the awake rat (Detsch et al. 1999; Montagne-Clavel and Oliveras 1995). Furthermore, our recordings from SI barrel cortex revealed a pattern of sensory adaptation that has been observed in the barrel field of awake rats (Melzer et al. 2006). Thus, regardless of whether rats are awake or lightly anesthetized with isoflurane, neurons in SI barrel cortex display sensory adaptation during repetitive whisker stimulation.
Assessing the effects of isoflurane on the activity of DLS neurons depends on several considerations. Neuronal activity varies across different parts of the striatum and is influenced by the sensorimotor activities of the animal (Aldridge et al. 2004; Aldridge and Berridge 1998; Sandstrom and Rebec 2003). Relevant to this, only whisker-sensitive neurons in the DLS were characterized in the present study, and their spontaneous discharge rates were measured between blocks of powerful whisker stimulation. Like other studies that recorded striatal activity in awake rats (Barnes et al. 2005; Burkhardt et al. 2009), we found that spontaneous activity was much higher in FS than in MS neurons. Although our sample of MS neurons displayed more spontaneous activity than in other studies (Chen et al. 2001; Dejean et al. 2007; Kish et al. 1999), our data are consistent with work showing that intracellularly labeled MS neurons in the unanesthetized rat may discharge spontaneously up to 10 Hz and that 40% of these neurons have a spontaneous rate above 1 Hz (Wilson and Groves 1981). Given the evidence that isoflurane inhibits neural activity, we must conclude that our low concentrations of isoflurane had minimal effects and that our results reflect the circuit connections and intrinsic properties of neurons in the DLS.
Striatal responsiveness to whisker stimulation.
In a recent study, whisker stimulation was tested on cortical and striatal neurons that were recorded in rats anesthetized by intraperitoneal injections of pentobarbital or fentanyl (Pidoux et al. 2011). Compared with neurons in SI barrel cortex, only a small fraction of striatal neurons (∼20%) discharged in response to whisker stimulation even though half of their striatal sample displayed subthreshold excitatory postsynaptic potential responses. This prompted the investigators to conclude that “the propagation of sensory flow through the corticostriatal pathway following whisker deflection results in a partial loss or a refinement of sensory information in the striatum.”
Our methods and results contrast with this recent report in a number of ways. First, because a low concentration of isoflurane was administered continuously per orally in our study, the depth of anesthesia was probably lower and less likely to vary compared with a protocol that relied on intermittent intraperitoneal injections of pentobarbital or fentanyl. Second, our whisker deflections were implemented by a controlled mechanical stimulator that was in direct physical contact with each whisker in the five most caudal arcs of the whisker pad. This difference may explain why we observed much lower response latencies in both SI and the DLS. Third, we specifically tested the effects of repetitive whisker stimulation at different frequencies. In combination with our anesthetic protocol, this stimulation paradigm revealed no impairment of neuronal responsiveness in the DLS at frequencies up to 8 Hz. In fact, we routinely encountered stimulus-induced neuronal discharges throughout the DLS regions that receive dense projections from SI barrel cortex.
Comparison of neuronal responses in SI and the DLS.
We compared the simultaneous responses of whisker-sensitive neurons in the DLS and SI barrel cortex because this cortical region sends dense excitatory projections to the DLS. In response to back-and-forth whisker movements, the neurons in SI responded to the first phase of whisker motion but did not respond when the whiskers moved back to their original position. The absence of a secondary response, as well as the adaptation produced by high-frequency stimulation, is probably mediated by prolonged cortical inhibition that follows the brief excitation evoked by sensory stimulation (Carvell and Simons 1988; Moore and Nelson 1998; Simons and Carvell 1989). Several studies have reported frequency-dependent adaptation in SI barrel cortex (Ahissar et al. 2001; Chakrabarti and Alloway 2009; Khatri et al. 2004; Melzer et al. 2006). Although we did not label the cells recorded in SI and cannot verify that our cortical sample contained corticostriatal neurons, other investigators have shown that corticostriatal neurons in mouse SI are characterized by strong frequency adaptation (Hattox and Nelson 2007).
By contrast, whisker-sensitive neurons in the DLS responded to both phases of whisker motion, and these responses were relatively constant across the stimulus frequencies that we tested. These findings are consistent with the fact that when striatal neurons are sufficiently excited, their membrane potentials shift to an “up state” that enhances the probability that a subsequent input will produce additional neuronal discharges (Wilson 1995). Furthermore, compared with SI, neurons in the DLS displayed smaller increases in response latencies as the frequency of whisker stimulation increased. In fact, when whiskers were stimulated at 5 or 8 Hz, many DLS neurons responded before the neurons in SI. Collectively, these distinctions in the magnitude and temporal patterns of neuronal responsiveness indicate that SI is not the only structure that transmits somatosensory information to the DLS.
In addition to well-known projections from the intralaminar nuclei of the thalamus, the rat DLS also receives projections from whisker-specific regions in the thalamus. Both the medial posterior (POm) and ventroposteromedial (VPM) nuclei project to the dorsolateral part of the striatum (Alloway et al. 2006; Deschenes et al. 1995; Erro et al. 2001, 2002). Although the POm may encode the kinematics of whisker movements, the VPM processes spatiotemporal information linked to whisker contacts with external objects (Alloway 2008; Sosnik et al. 2001). Regardless of their precise functional roles, projections from POm and VPM converge on focal sites in the DLS (Alloway et al. 2006), and this indicates that these thalamic nuclei could transmit whisker-related information directly to the DLS.
Neurons in the VPM display far less adaptation than neurons in SI (Hartings et al. 2003; Khatri et al. 2004), and modality-specific projections from VPM to the DLS could explain why sensory adaptation was less prominent among whisker-sensitive neurons in the DLS than in SI. In this context, it is noteworthy that corticostriatal neurons in layer V receive very few thalamocortical synaptic contacts on their apical dendrites and display greater adaptation than other classes of cortical neurons in layer V (Hattox and Nelson 2007; Hersch and White 1982). Although the basis for our findings and the relative functions of the inputs from the thalamus and cortex must await further research, our results are consistent with suggestions that thalamostriate projections cooperate or, in some circumstances, compete with corticostriatal projections in activating striatal targets and, presumably, in selecting specific motor behaviors (McHaffie et al. 2005).
GRANTS
This work was supported by NIH Grant NS-37532 awarded to K. D. Alloway.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Jared B. Smith for help with the corticostriatal tracing experiments.
REFERENCES
- Ahissar E, Sosnik R, Bagdasarian K, Haidarliu S. Temporal frequency of whisker movement. II. Laminar organization of cortical representations. J Neurophysiol 86: 354–367, 2001 [DOI] [PubMed] [Google Scholar]
- Aldridge JW, Berridge KC. Coding of serial order by neostriatal neurons: a “natural action” approach to movement sequence. J Neurosci 18: 2777–2787, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldridge JW, Berridge KC, Rosen AR. Basal ganglia neural mechanisms of natural movement sequences. Can J Physiol Pharmacol 82: 732–739, 2004 [DOI] [PubMed] [Google Scholar]
- Alloway KD. Information processing streams in rodent barrel cortex: the differential functions of barrel and septal circuits. Cereb Cortex 18: 979–989, 2008 [DOI] [PubMed] [Google Scholar]
- Alloway KD, Crist J, Mutic JJ, Roy SA. Corticostriatal projections from rat barrel cortex have an anisotropic organization that correlates with vibrissal whisking movements. J Neurosci 19: 10908–10922, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloway KD, Lou L, Nwabueze-Ogbo F, Chakrabarti S. Topography of cortical projections to the dorsolateral neostriatum in rats: multiple overlapping sensorimotor pathways. J Comp Neurol 499: 33–49, 2006 [DOI] [PubMed] [Google Scholar]
- Alloway KD, Mutic JJ, Hoffer ZS, Hoover JE. Overlapping corticostriatal projections from the rodent vibrissal representations in primary and secondary somatosensory cortex. J Comp Neurol 426: 51–67, 2000 [PubMed] [Google Scholar]
- Alloway KD, Mutic JJ, Hoover JE. Divergent corticostriatal projections from a single cortical column in the somatosensory cortex of rats. Brain Res 785: 341–346, 1998 [DOI] [PubMed] [Google Scholar]
- Balleine BW, O'Doherty JP. Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology 35: 48–69, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes TD, Kubota Y, Hu D, Jin DZ, Graybiel AM. Activity of striatal neurons reflects dynamic encoding and recording of procedural memories. Nature 437: 1158–1161, 2005 [DOI] [PubMed] [Google Scholar]
- Berg RW, Kleinfeld D. Rhythmic whisking by rat: retraction as well as protraction of the vibrissae is under active muscular control. J Neurophysiol 89: 104–117, 2003 [DOI] [PubMed] [Google Scholar]
- Brown LL. Somatotopic organization in rat striatum: evidence for a combinational map. Proc Natl Acad Sci USA 89: 7403–7407, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LL, Smith DM, Goldbloom LM. Organizing principles of cortical integration in the rat neostriatum: corticostriate map of the body surface is an ordered lattice of curved laminae and radial points. J Comp Neurol 392: 468–488, 1998 [PubMed] [Google Scholar]
- Bruno RM, Khatri V, Land PW, Simons DJ. Thalamocortical angular tuning domains within individual barrels of rat somatosensory cortex. J Neurosci 23: 9565–9574, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno RM, Simons DJ. Feedforward mechanisms of excitatory and inhibitory cortical receptive fields. J Neurosci 22: 10966–10975, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhardt JM, Jin X, Costa RM. Dissociable effects of dopamine on neuronal firing rate and synchrony in the dorsal striatum. Front Integr Neurosci 3: 1–12, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carelli RM, West MO. Representation of the body by single neurons in the dorsolateral striatum of the awake, unrestrained rat. J Comp Neurol 309: 231–249, 1991 [DOI] [PubMed] [Google Scholar]
- Carvell GE, Simons DJ. Membrane potential changes in rat SmI cortical neurons evoked by controlled stimulation of mystacial vibrissae. Brain Res 448: 186–191, 1988 [DOI] [PubMed] [Google Scholar]
- Chakrabarti S, Alloway KD. Differential response patterns in the SI barrel and septal compartments during mechanical whisker stimulation. J Neurophysiol 102: 1632–1646, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti S, Zhang M, Alloway KD. MI neuronal responses to peripheral whisker stimulation: relationship to neuronal activity in SI barrels and septa. J Neurophysiol 100: 50–63, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MT, Morales M, Woodward DJ, Hoffer BJ, Janak PH. In vivo extracellular recording of striatal neurons in the awake rat following unilateral 6-hydroxydopamine lesions. Exp Neurol 171: 73–83, 2001 [DOI] [PubMed] [Google Scholar]
- Cromwell HC, Berridge KC. Implementation of action sequences by a neostriatal site: a lesion mapping study of grooming syntax. J Neurosci 16: 3444–3458, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejean C, Gross CE, Bioulac B, Borand T. Synchronous high-voltage spindles in the cortex-basal ganglia network of awake and unrestrained rats. Eur J Neurosci 25: 772–784, 2007 [DOI] [PubMed] [Google Scholar]
- Deschenes M, Bourassa J, Parent A. Two different types of thalamic fibers innervate the rat neostriatum. Brain Res 701: 288–292, 1995 [DOI] [PubMed] [Google Scholar]
- Detsch O, Kochs E, Siemers M, Bromm B, Vahle-Hinz C. Differential effects of isoflurane on excitatory and inhibitory synaptic inputs to thalamic neurons in vivo. Br J Anaesth 89: 294–300, 2002 [DOI] [PubMed] [Google Scholar]
- Detsch O, Vahle-Hinz C, Kochs E, Siemers M, Bromm B. Isoflurane induces dose-dependent changes of thalamic somatosensory information transfer. Brain Res 829: 77–89, 1999 [DOI] [PubMed] [Google Scholar]
- Erro E, Lanciego JL, Arribas J, Gimenez-Amaya JM. Striatal input from the ventrobasal complex of the rat thalamus. Histochem Cell Biol 115: 447–454, 2001 [DOI] [PubMed] [Google Scholar]
- Erro E, Lanciego JL, Gimenez-Amaya JM. Re-examination of the thalamostriatal projections in the rat with retrograde tracers. Neurosci Res 42: 45–55, 2002 [DOI] [PubMed] [Google Scholar]
- Gerfen CR. Basal ganglia. In: The Rat Nervous System (3rd ed.), edited by Paxinos G. New York: Elsevier, 2004, p. 455–508 [Google Scholar]
- Gibson JR, Beierlein M, Connors BW. Two networks of electrically coupled inhibitory neurons in neocortex. Nature 402: 75–79, 1999 [DOI] [PubMed] [Google Scholar]
- Graybiel AM. Habits, rituals, and the evaluative brain. Annu Rev Neurosci 31: 359–387, 2008 [DOI] [PubMed] [Google Scholar]
- Hartings JA, Temereanca S, Simons DJ. Processing of periodic whisker deflections by neurons in the ventroposterior medial and thalamic reticular nuclei. J Neurophysiol 90: 3087–3094, 2003 [DOI] [PubMed] [Google Scholar]
- Hattox AM, Nelson SB. Layer V neurons in mouse cortex projecting to different targets have distinct physiological properties. J Neurophysiol 98: 3330–3340, 2007 [DOI] [PubMed] [Google Scholar]
- Hersch SM, White EL. A quantitative study of the thalamocortical and other synapses in layer IV of pyramidal cells projecting from mouse SmI cortex to the caudate-putamen nucleus. J Comp Neurol 211: 217–225, 1982 [DOI] [PubMed] [Google Scholar]
- Hoffer ZS, Alloway KD. Organization of corticostriatal projections from the vibrissal representations in the primary motor and somatosensory cortical areas in rodents. J Comp Neurol 439: 87–103, 2001 [DOI] [PubMed] [Google Scholar]
- Hoffer ZS, Arantes HB, Roth RL, Alloway KD. Functional circuits mediating sensorimotor integration: quantitative comparisons of projections from rodent barrel cortex to primary motor cortex, neostriatum, superior colliculus, and the pons. J Comp Neurol 488: 82–100, 2005 [DOI] [PubMed] [Google Scholar]
- Hoover JE, Hoffer ZS, Alloway KD. Projections from primary somatosensory cortex to the neostriatum: the role of somatotopic continuity in corticostriatal convergence. J Neurophysiol 89: 1576–1587, 2003 [DOI] [PubMed] [Google Scholar]
- Inokawa H, Yamada H, Matsumoto N, Muranishi M, Kimura M. Juxtacellular labeling of tonically active neurons and phasically active neurons in the rat striatum. Neuroscience 168: 395–404, 2010 [DOI] [PubMed] [Google Scholar]
- Joksovic PM, Todorovic SM. Isoflurane modulates neuronal excitability of the nucleus reticularis thalami in vitro. Ann NY Acad Sci 1199: 36–42, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y. Physiological, morphological, and histochemical characterization of three classes of interneurons in rat neostriatum. J Neurosci 13: 4908–4923, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatri V, Hartings JA, Simons DJ. Adaptation in thalamic barreloid and cortical barrel neurons to periodic whisker deflections varying in frequency and velocity. J Neurophysiol 92: 3244–3254, 2004 [DOI] [PubMed] [Google Scholar]
- Kincaid AE, Wilson CJ. Corticostriatal innervation of the patch and matrix in the rat neostriatum. J Comp Neurol 374: 578–592, 1996 [DOI] [PubMed] [Google Scholar]
- Kish LJ, Palmer MR, Gerhardt GA. Multiple single-unit recordings in the striatum of freely moving animals: effects of apomorphine and d-amphetamine in normal and unilateral 6-hydroxydopamine-lesioned rats. Brain Res 833: 58–70, 1999 [DOI] [PubMed] [Google Scholar]
- Kleinfeld D, Ahissar E, Diamond ME. Active sensation: insights from the rodent vibrissa sensorimotor system. Curr Opin Neurobiol 16: 1–10, 2006 [DOI] [PubMed] [Google Scholar]
- Kwegyir-Afful EE, Keller A. Response properties of whisker-related neurons in rat second somatosensory cortex. J Neurophysiol 92: 2083–2092, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmoen IA, Larsen M, Berg-Johnsen J. Volatile anaesthetics: cellular mechanisms of action. Eur J Anaesthesiol 12: 51–58, 1995 [PubMed] [Google Scholar]
- Liachenko S, Tang P, Somogyi GT, Xu Y. Concentration dependent isoflurane effects on depolarization-evoked glutamate and GABA outflows from mouse brain slices. Br J Pharmacol 127: 131–138, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet N, Le Moine C, Charpier S, Gonon F. Feedforward inhibition of projection neurons by fast-spiking GABA interneurons in the rat striatum in vivo. J Neurosci 25: 3857–3869, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masamoto K, Fukuda M, Vazquez A, Kim S. Dose-dependent effect of isoflurane on neurovascular coupling in rat cortex. Eur J Neurosci 30: 242–250, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Connors BW, Lighthall JW, Prince DA. Comparative electrophysiology of pyramidal and sparsely spiny stellate neurons of the neocortex. J Neurophysiol 54: 782–806, 1985 [DOI] [PubMed] [Google Scholar]
- McHaffie JG, Stanford TR, Stein BE, Coizet W, Redgrave P. Subcortical loops through the basal ganglia. Trends Neurosci 28: 401–407, 2005 [DOI] [PubMed] [Google Scholar]
- Melzer P, Sachdev RN, Jenkinson N, Ebner FF. Stimulus processing in awake rat barrel cortex. J Neurosci 26: 12198–12205, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchinson B, Martin CJ, Grant RA, Prescott TJ. Feedback control in active sensing: rat exploratory whisking is modulated by environmental contact. Proc Biol Sci 274: 1035–1041, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagne-Clavel J, Oliveras JL. Does barbiturate anesthesia modify the neuronal properties of the somatosensory thalamus? A single-unit study related to nociception in the awake-pentobarbital-treated rat. Neurosci Lett 196: 69–72, 1995 [DOI] [PubMed] [Google Scholar]
- Moore CL, Nelson SB. Spatio-temporal subthreshold receptive fields in the vibrissa representation of rat primary somatosensory cortex. J Neurophysiol 80: 2882–2892, 1998 [DOI] [PubMed] [Google Scholar]
- Packard MG, Knowlton BJ. Learning and memory functions of the basal ganglia. Annu Rev Neurosci 25: 563–593, 2002 [DOI] [PubMed] [Google Scholar]
- Pidoux M, Mahon S, Deniau JM, Charpier S. Integration and propagation of somatosensory responses in the corticostriatal pathway: an intracellular study in vivo. J Physiol 589: 263–281, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter JT, Johnson CK, Agmon A. Diverse types of interneurons generate thalamus-evoked feedforward inhibition in the mouse barrel cortex. J Neurosci 21: 2699–2710, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner A, Jiao Y, Del Mar N, Laverghetta AV, Lei WL. Differential morphology of pyramidal tract-type and intratelencephalically projecting-type corticostriatal neurons and their intrastriatal terminals in rats. J Comp Neurol 457: 420–440, 2003 [DOI] [PubMed] [Google Scholar]
- Ries CR, Puil E. Mechanism of anesthesia revealed by shunting actions of isoflurane on thalamocortical neurons. J Neurophysiol 81: 1795–1801, 1999a [DOI] [PubMed] [Google Scholar]
- Ries CR, Puil E. Ionic mechanism of isoflurane's action on thalamocortical neurons. J Neurophysiol 81: 1802–1809, 1999b [DOI] [PubMed] [Google Scholar]
- Sandstrom MI, Rebec GV. Characterization of striatal activity in conscious rats: contribution of NMDA and AMPA/kainate receptors to both spontaneous and glutamate-driven firing. Synapse 47: 91–100, 2003 [DOI] [PubMed] [Google Scholar]
- Schmitzer-Torbert NC, Redish AD. Task-dependent encoding of space and events by striatal neurons is dependent on neural subtype. Neuroscience 153: 349–360, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharott A, Moll CK, Engler G, Denker M, Grun S, Engel AK. Different subtypes of striatal neurons are selectively modulated by cortical oscillations. J Neurosci 29: 4571–4585, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons DJ, Carvell GE. Thalamocortical response transformation in the rat vibrissa/barrel system. J Neurophysiol 61: 311–330, 1989 [DOI] [PubMed] [Google Scholar]
- Sosnik R, Haidarliu S, Ahissar E. Temporal frequency of whisker movement. I. Representations in brain stem and thalamus. J Neurophysiol 86: 339–353, 2001 [DOI] [PubMed] [Google Scholar]
- Vahle-Hinz C, Detsch O. What can in vivo electrophysiology in animal models tell us about the mechanisms of anaesthesia? Br J Anaesth 89: 123–142, 2002 [DOI] [PubMed] [Google Scholar]
- Wakamori M, Ikemoto Y, Akaike N. Effects of two volatile anesthetics and a volatile convulsant on the excitatory and inhibitory amino acid responses in dissociated CNS neurons of the rat. J Neurophysiol 66: 2014–2021, 1991 [DOI] [PubMed] [Google Scholar]
- Welker WI. Analysis of sniffing of the albino rat. Behaviour 12: 223–244, 1964 [Google Scholar]
- West MO. Anesthetics eliminate somatosensory-evoked discharges of neurons in the somatotopically organized sensorimotor striatum of the rat. J Neurosci 18: 9055–9068, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CJ. The contribution of cortical neurons to the firing patterns of striatal spiny neurons. In: Models of Information Processing in the Basal Ganglia, edited by Houk JC, Davis JL, Beiser DG. Cambridge, MA: MIT Press, 1995, p. 29–50 [Google Scholar]
- Wilson CJ, Chang HT, Kitai ST. Firing patterns and synaptic potentials of identified giant aspiny interneurons in the rat neostriatum. J Neurosci 10: 508–519, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CJ, Groves PM. Spontaneous firing patterns of identified spiny neurons in the rat striatum. Brain Res 220: 67–80, 1981 [DOI] [PubMed] [Google Scholar]
- Wright AK, Ramanathan S, Arbuthnott GW. Identification of the source of the bilateral projections system from cortex to somatosensory neostriatum and an exploration of its physiological actions. Neuroscience 103: 87–96, 2001 [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ, Balleine BW. Inactivation of dorsolateral striatum enhances sensitivity to changes in the action-outcome contingency in instrumental conditioning. Behav Brain Res 166: 189–196, 2006 [DOI] [PubMed] [Google Scholar]
- Ying SW, Werner DF, Homanics GE, Harrison NL, Goldstein PA. Isoflurane modulates excitability in the mouse thalamus via GABA-dependent and GABA-independent mechanisms. Neuropharmacology 56: 438–447, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Alloway KD. Stimulus-induced intercolumnar synchronization of neuronal activity in rat barrel cortex: a laminar analysis. J Neurophysiol 92: 1464–1478, 2004 [DOI] [PubMed] [Google Scholar]