Abstract
Fast glutamatergic transmission via ionotropic receptors is critical for the generation of locomotion by spinal motor networks. In addition, glutamate can act via metabotropic glutamate receptors (mGluRs) to modulate the timing of ongoing locomotor activity. In the present study, we investigated whether mGluRs also modulate the intensity of motor output generated by spinal motor networks. Application of the group I mGluR agonist (S)-3,5-dihydroxyphenylglycine (DHPG) reduced the amplitude and increased the frequency of locomotor-related motoneuron output recorded from the lumbar ventral roots of isolated mouse spinal cord preparations. Whole cell patch-clamp recordings of spinal motoneurons revealed multiple mechanisms by which group I mGluRs modulate motoneuron output. Although DHPG depolarized the resting membrane potential and reduced the voltage threshold for action potential generation, the activation of group I mGluRs had a net inhibitory effect on motoneuron output that appeared to reflect the modulation of fast, inactivating Na+ currents and action potential parameters. In addition, group I mGluR activation decreased the amplitude of locomotor-related excitatory input to motoneurons. Analyses of miniature excitatory postsynaptic currents indicated that mGluRs modulate synaptic drive to motoneurons via both pre- and postsynaptic mechanisms. These data highlight group I mGluRs as a potentially important source of neuromodulation within the spinal cord that, in addition to modulating components of the central pattern generator for locomotion, can modulate the intensity of motoneuron output during motor behavior. Given that group I mGluR activation reduces motoneuron excitability, mGluRs may provide negative feedback control of motoneuron output, particularly during high levels of glutamatergic stimulation.
Keywords: spinal cord, motor control, neuromodulation
neuromodulation is an important determinant of neuronal activity, endowing networks with the functional flexibility that is critical to the generation of complex and adaptable behaviors. Within motor systems, neuromodulation is important for the control of rhythmic motor behaviors, such as locomotion, that must be adapted to suit the varied biomechanical and metabolic demands of different states, developmental stages, or environments. During locomotion, the basic timing and pattern of motoneuron activity are set by networks of local interneurons called central pattern generators (CPGs; Goulding 2009; Grillner 2006; Kiehn et al. 2010). Motor commands originating from locomotor CPGs are sent to motoneurons via premotor interneurons that utilize fast-acting ionotropic receptor-mediated neurotransmission (Cazalets et al. 1996; Hochman and Schmidt 1998; Orsal et al. 1986; Shefchyk and Jordan 1985). In contrast, neuromodulatory signaling affecting the output of the locomotor CPG typically involves the activation of slower-acting metabotropic receptors (Grillner 2006). Neuromodulatory inputs originate from well-characterized “extrinsic” supraspinal systems (e.g., brainstem raphe nuclei and locus coeruleus, reviewed by Heckman et al. 2009; Rekling et al. 2000; Schmidt and Jordan 2000) and less studied “intrinsic” intraspinal neuromodulatory systems (Alaburda and Hounsgaard 2003; Dale and Gilday 1996; El Manira et al. 2008; Katz and Frost 1996; Zagoraiou et al. 2009).
Within spinal motor circuitry, glutamate is best known for mediating fast, excitatory synaptic transmission between CPG interneurons (Grillner 2006) and for transmitting locomotor drive from last order interneurons to motoneurons via the activation of ionotropic receptors (Cazalets et al. 1996; Fetcho et al. 2008; Hochman and Schmidt 1998; Orsal et al. 1986; Roberts et al. 2008; Shefchyk and Jordan 1985). However, glutamate may also act as an important intrinsic modulator of locomotor CPGs due to parallel activation of metabotropic receptors (Alaburda and Hounsgaard 2003; El Manira et al. 2002; Nistri et al. 2006). Metabotropic glutamate receptors (mGluRs) are G-protein-coupled receptors that exist as eight different subtypes (mGluR1–8). These subtypes can be categorized into three groups (I-III) based on sequence homology, pharmacological profiles, and coupling to intracellular signaling pathways (Pin and Duvoisin 1995). mGluRs regulate neuronal activity throughout the central nervous system (CNS) by modulating both the intrinsic properties of neurons and synaptic transmission. Group I mGluRs generally facilitate neuronal activity via postsynaptic mechanisms while group II and III mGluRs commonly inhibit activity by acting as presynaptic autoreceptors (Anwyl 2009, 1999; Pin and Duvoisin 1995).
In the spinal cord, the activation of mGluRs, particularly group I mGluRs, has been shown to modulate locomotor networks that control swimming in lampreys (Krieger et al. 1998) and Xenopus tadpoles (Chapman and Sillar 2007) and walking in rats (Taccola et al. 2004). The role of mGluRs in locomotion is best described for the lamprey where group I mGluR activation leads to both short- and long-term increases in the frequency of locomotor activity (Krieger et al. 1998; Kyriakatos and El Manira 2007). These effects involve the modulation of synaptic transmission within the locomotor CPG (Kettunen et al. 2005; Kyriakatos and El Manira 2007), the depolarization of spinal neurons via the blockade of a leak current (Kettunen et al. 2003), and the enhancement of N-methyl-d-aspartic acid (NMDA)-mediated currents (Krieger et al. 2000; Nanou et al. 2009). Similarly, activation of group I mGluRs increases the frequency of fictive swimming in Xenopus tadpoles via a reduction in inhibitory transmission (Chapman et al. 2008; Chapman and Sillar 2007).
Before the present study, analyses of the effects of mGluR activation during mammalian locomotion were limited to studies of isolated neonatal rat spinal cord preparations. These studies have revealed complex roles for group I mGluRs in mammals where the application of group I mGluR agonists either disrupts locomotor activity completely or slows it down (Taccola et al. 2004), while group I specific, but not general, mGluR antagonists also decrease the frequency of locomotor activity (Taccola et al. 2003, 2004). The cellular and synaptic effects of group I mGluR activation are also diverse in the rat with data supporting both inhibition (Marchetti et al. 2003) and enhancement (Marchetti et al. 2005) of synaptic transmission and with studies reporting motoneuron depolarization associated with either no change (Marchetti et al. 2003) or an increase in input resistance (Marchetti et al. 2005). Given the complexities and unresolved roles of mGluR-mediated modulation in the mammalian spinal cord, further studies of the effects of group I mGluR activation on the function of spinal networks and spinal neurons are needed. Furthermore, although it has been demonstrated that mGluRs can modulate locomotor rhythm generation, it remains to be determined whether mGluRs also modulate motoneuron firing and hence the intensity of locomotor-related output, as has recently been shown for other intrinsic modulatory systems (Miles et al. 2007; Zagoraiou et al. 2009). In the present study, we have therefore investigated the effects of group I mGluR activation on the output of motoneurons in isolated mouse spinal cord preparations. We first demonstrate that the activation of group I mGluRs can modulate motoneuron output during locomotor activity. We then reveal cellular mechanisms by which activation of group I mGluRs can modulate both the synaptic excitation and intrinsic excitability of motoneurons and hence regulate the strength of motor outflow from the CNS.
METHODS
In vitro whole spinal cord preparation.
All methods required to obtain tissue for in vitro experiments were conducted in accordance with the UK Animals (Scientific Procedures) Act 1986. Spinal cord preparations were obtained from postnatal day 2 (P2)-P6 C57BL/6 mice using techniques similar to those described previously (Jiang et al. 1999). In brief, animals were killed via cervical dislocation, decapitated, and eviscerated before spinal cords were isolated from the mid-cervical to upper sacral segments in a chamber containing artificial cerebral spinal fluid (aCSF; equilibrated with 95% O2-5% CO2, ∼4°C). For experiments in which whole cell patch-clamp recordings were performed, following the removal of dura matter, a thin line of pia matter was scraped from the ventral surface of the spinal cord above the motor columns at the level of the first or second lumbar (L1-L2) roots to allow access for patch pipettes (Miles et al. 2002).
Ventral root recordings.
Glass suction electrodes were attached to L1 or L2 ventral roots of isolated spinal cord preparations. N-methyl-d-aspartic acid (NMDA; 5 μM), 5-hydroxytryptamine hydrochloride (5-HT; 10 μM), and 3,4-dihydroxyphenethylamine hydrochloride (dopamine; 50 μM) were added to the aCSF to induce rhythmic, left-right alternating bursts of locomotor-related ventral root activity (Jiang et al. 1999; Miles et al. 2007). Locomotor-related activity was left to stabilize (∼1 h) before subsequent drug applications. Signals were amplified, filtered (30–3,000 Hz), rectified, and integrated (Qjin Design, Ontario, Canada) before being acquired at ≥1 kHz using a Digidata 1440A A/D board and AxoScope software (Molecular Devices, Sunnyvale, CA).
Whole cell patch-clamp recordings.
Whole cell patch-clamp recordings were made from motoneurons visualized under infrared differential interference contrast microscopy. Most recorded cells were confirmed to be motoneurons by the presence of antidromic action potentials in response to ventral root stimulation (<100 μA; ISO-Flex stimulator; A.M.P.I., Jerusalem, Israel). Patch electrodes (3–4 MΩ) were pulled on a horizontal puller (Sutter Instrument, Novato, CA) from borosilicate glass (World Precision Instruments, Sarasota, FL). Patch-clamp signals were amplified and filtered (4-kHz low-pass Bessel filter) with a MultiClamp 700B amplifier (Molecular Devices) and acquired at ≥10 kHz using a Digidata 1440A A/D board and pClamp software (Molecular Devices). Details of voltage and current-clamp protocols appear in Results. Series resistance compensation (60%) was used during all voltage-clamp protocols.
Data analysis.
Data from ventral root recordings were analyzed offline using Dataview software (courtesy of W. J. Heitler, University of St Andrews). Whole cell patch-clamp recordings were analyzed using either Clampfit software (Molecular Devices) or, for analyses of miniature excitatory postsynaptic currents (mEPSCs), the Mini Analysis Program (Synaptosoft, Fort Lee, NJ). Na+ current activation and inactivation curves were fit with a Boltzmann function of the form: 1/[1 + exp (V1/2 − V)/k], where V1/2 is the half activation or half inactivation voltage, V is the test or conditioning voltage, and k is the slope of the fitted curve at V1/2. Boltzmann fits were performed using Microsoft Excel as described by Brown (2001). Data are reported as means ± SE. Differences in means were compared using Student's t-test. The Kolmogorov-Smirnov test was used to test for differences in mEPSC amplitude or inter-event interval. Values of P < 0.05 were considered significant.
Solution and drugs.
The standard aCSF solution used for dissecting and recording contained 127 mM NaCl, 3 mM KCl, 1.3 mM NaH2PO4, 1 mM MgCl2, 2 mM CaCl2, 26 mM NaHCO3, and 10 mM d-glucose (equilibriated with 95% O2-5% CO2). The standard patch-clamp pipette solution contained 140 mM potassium methane sulfonate, 10 mM NaCl, 1 mM CaCl2, 10 mM HEPES, 1 mM EGTA, 3 mM Mg-ATP, and 0.4 mM GTP-Na2 (pH 7.2–7.3, adjusted with KOH).
For experiments investigating Na+ currents in isolation, external and pipette solutions were designed to eliminate Ca2+ and K+ currents and reduce Na+ current amplitude to help minimize voltage-clamp errors. The modified aCSF contained 10 mM NaCl, 105 mM choline chloride, 3 mM KCl, 30 mM TEA-Cl, 10 mM HEPES, 1 mM MgCl2, 2 mM CaCl2, 10 mM d-glucose, 4 mM 4-aminopyridine (4-AP), 1.5 mM kynurenic acid, 10 μM bicuculline, 5 μM strychnine, and 0.5 mM CdCl2 (gassed with 100% O2, pH 7.3–7.4 adjusted with NaOH). The pipette solution contained 100 mM caesium methane sulfonate, 30 mM TEA-Cl, 0.5 or 10 mM NaCl, 1 mM CaCl2, 10 mM HEPES, 1 mM EGTA, 3 mM ATP-Mg, and 0.4 mM GTP-Na2 (pH 7.2–7.3 adjusted with KOH, osmolarity adjusted to ∼290 mosM with sucrose).
Pharmacological agents used included the following: NMDA, 5-HT, dopamine, strychnine [(−)-strychnine], and bicuculline [1(S),9(R)-(−)-bicuculline methiodide] purchased from Sigma-Aldrich (St Louis, MO); and DHPG [(S)-3,5-dihydroxyphenylglycine], LY367385 [(S)-(+)-a-amino-4-carboxy-2-methylbenzeneacetic acid], MPEP [2-methyl-6-(phenylethynyl)pyridine hydrochloride], and tetrodotoxin (TTX) purchased from Tocris Bioscience (Bristrol, UK).
RESULTS
Effects of group I mGluR activation on locomotor-related motoneuron output.
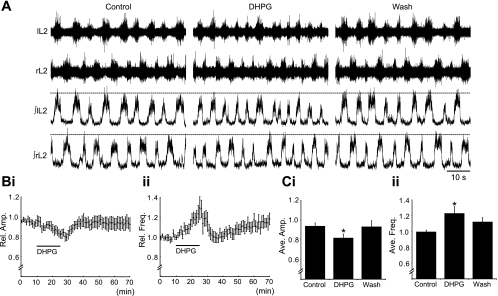
To investigate whether group I mGluRs modulate locomotor-related motoneuron output in mice, the group I mGluR agonist DHPG (5–50 μM) was bath applied to in vitro spinal cord preparations in which rhythmic, left-right alternating bursts of locomotor-related activity were induced pharmacologically (5 μM NMDA, 10 μM 5-HT, and 50 μM dopamine; Fig. 1A). Application of DHPG (5 μM; 15 min) led to a significant decrease in the amplitude of bursts of locomotor-related activity recorded from lumbar ventral roots (Fig. 1, A, Bi, and Ci). The amplitude of locomotor bursts decreased gradually, reaching a minimum near the end of the 15-min application of DHPG (18 ± 4.0% reduction, n = 11; Fig. 1Ci), before returning to control levels within ∼10 min of drug washout (Fig. 1Bi). Analyses of ventral root activity between locomotor bursts (interburst activity) revealed no change in baseline activity when DHPG was applied (data not shown). These data suggest that the modulatory effects of group I mGluRs on motoneuron output are specific to activity driven by locomotor circuitry.
Fig. 1.
Group I metabotropic glutamate receptor (mGluR)-mediated modulation of locomotor-related motoneuron output. A: raw (top) and rectified and integrated (bottom) traces showing the effects of the group I mGluR agonist (S)-3,5-dihydroxyphenylglycine (DHPG; 5 μM) on pharmacologically induced (5 μM NMDA, 10 μM 5-HT, and 50 μM dopamine) locomotor activity recorded from the lumbar ventral roots of an isolated mouse spinal cord preparation. B: time-course plots showing a decrease in locomotor burst amplitude (i) and an increase in burst frequency (ii) in response to DHPG application (5 μM, 15 min; n = 11). Each point represents 1 min worth of recording, normalized to control. C: pooled data, averaged from 5 min worth of recording in each condition, showing a significant decrease in locomotor burst amplitude (i) and increase in burst frequency (ii) following the application of DHPG (n = 11). *Significantly different from control.
Application of DHPG also caused a significant increase in the frequency of locomotor-related activity (Fig. 1, A, Bii, and Cii). The frequency of locomotor bursts increased gradually, peaking near the end of the 15-min application of DHPG (23 ± 8.8% increase; n = 11; Fig. 1Cii), before returning to control levels within ∼10 min of drug washout (Fig. 1Bii). Given the apparent excitatory effects of group I mGluRs on the locomotor CPG network, we also investigated whether DHPG (10 μM) alone or DHPG in combination with NMDA (5 μM) could elicit locomotor activity. However, locomotor activity was never observed under these conditions (n = 3).
Given that group I mGluR activation has long-term effects on locomotor activity recorded from isolated lamprey spinal cord preparations (Kyriakatos and El Manira 2007), we assessed whether higher doses of DHPG might also have long-term effects in isolated mouse spinal cord preparations. Application of 10 μM DHPG (n = 9) again led to a significant decrease in locomotor burst amplitude and a significant increase in locomotor frequency (data not shown), with both effects of equivalent magnitude to those seen following the application of 5 μM DHPG. Although the effects of 10 μM DHPG outlasted those of 5-μM applications, no long-term effects were observed. At higher doses (20–50 μM), DHPG also decreased the amplitude of locomotor-related bursts. However, this was accompanied by a rapid disruption in locomotor activity, with rhythmic activity ceasing several minutes after drug onset before returning after drug washout (data not shown).
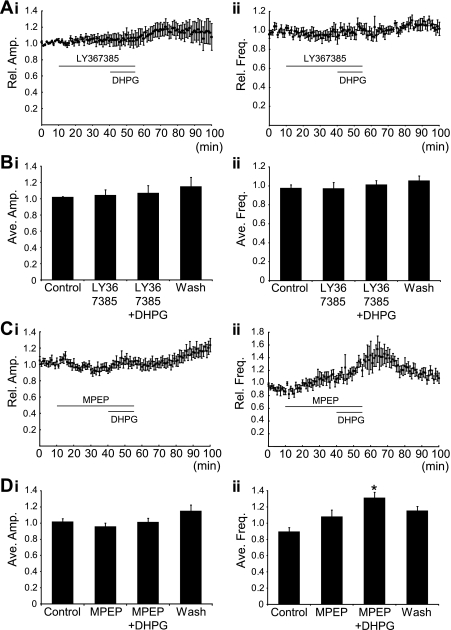
DHPG activates both mGluR1 and mGluR5 subtypes of group I mGluRs. Thus the activation of either receptor subtype could be responsible for the effects of DHPG on locomotor activity. To determine which receptor subtypes are involved and to assess whether there is endogenous activation of mGluRs in our preparation, we utilized mGluR1 (LY367385)- and mGluR5 (MPEP)-specific antagonists. Antagonists were applied for 30 min before, and then during, the application of DHPG (5 μM, 15 min; Fig. 2, A and C). Application of the mGluR1 antagonist LY367385 (50 μM) alone had no significant effects on the amplitude or frequency of locomotor bursts (n = 7; Fig. 2, A and B). However, LY367385 blocked the decrease in burst amplitude and increase in burst frequency normally induced by DHPG (Fig. 2, A and B). Application of the mGluR5 antagonist MPEP (50 μM) also had no significant effect on locomotor activity when applied alone (n = 6; Fig. 2, C and D). MPEP did, however, block the DHPG-induced decrease in locomotor burst amplitude but had no effect on DHPG-induced increases in locomotor frequency (Fig. 2, Cii and Dii).
Fig. 2.
Receptor subtype-specific effects of group I mGluR activation on locomotor-related motoneuron output. A: time course plots showing that the mGluR1 antagonist LY367385 (50 μM) blocked DHPG (5 μM)-mediated effects on locomotor burst amplitude (i) and frequency (ii) (n = 7). B: pooled data, averaged over 5 min worth of recording in each condition, again showing that DHPG had no effect on locomotor burst amplitude (i) or frequency (ii) in the presence of LY367385 (n = 7). C: time-course plots showing that the mGluR5 antagonist MPEP (50 μM) blocked DHPG-mediated effects on locomotor burst amplitude (i). MPEP did not, however, block the effect of DHPG on locomotor burst frequency (ii) (n = 6). D: pooled data, averaged from 5 min worth of recording in each condition, also show no change in burst amplitude when DHPG is applied in the presence of MPEP (i) but that burst frequency (ii) was significantly increased by DHPG application in the presence of MPEP (n = 6). *Significantly different from control.
Given that LY367385 is a competitive mGluR1 antagonist, it is possible that we were unable to uncover an endogenous role for these receptors due to weak competition of the antagonist with endogenous glutamate. To address this, we preincubated preparations in LY367385 (50 μM) before the induction of locomotor activity and then subsequently washed out LY367385 while locomotor activity was ongoing. In these experiments, LY367385 again had no effect on locomotor frequency or burst amplitude (n = 5).
Together our data suggest that group I mGluRs are not activated during fictive locomotor activity recorded in vitro from the isolated mouse spinal cord. However, once stimulated, group I mGluRs will modulate locomotor-related motoneuron output.
Effects of group I mGluR activation on motoneuron properties.
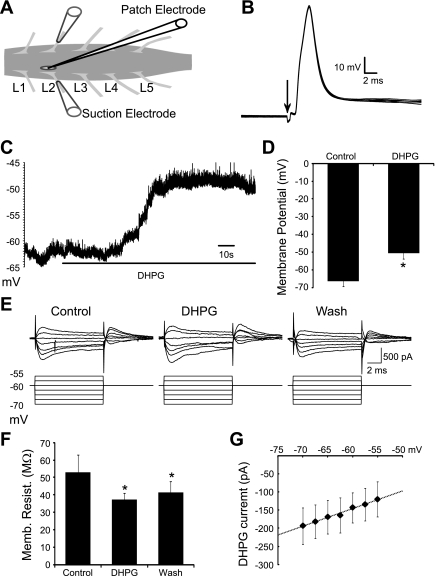
To investigate the cellular mechanisms that underlie group I mGluR-mediated modulation of locomotor-related motoneuron output, we analyzed the effects of group I mGluR activation on individual motoneurons. Whole cell patch-clamp recordings were established from motoneurons in isolated spinal cord preparations (Fig. 3A) under infrared differential interference microscopy. Most recorded cells were confirmed as motoneurons by the presence of antidromic action potentials in response to stimulation of segmentally aligned ipsilateral ventral roots (<100 μA, duration 0.5 ms; Fig. 3B). The effects of group I mGluR activation were assessed using bath applications of DHPG from doses of 10 μM up to 50 μM to maximize the likelihood of revealing the full range of mGluR-mediated modulatory effects on motoneurons.
Fig. 3.
Subthreshold effects of group I mGluR activation on individual motoneurons. A: schematic showing the experimental setup with a patch pipette targeting a spinal motoneuron and glass suction electrodes attached to the second lumbar (L2) ventral roots. B: antidromic action potentials recorded from a motoneuron in response to stimulation of the ipsilateral ventral root. Arrow indicates stimulus artefact. C and D: resting membrane potential of motoneurons was depolarized in response to DHPG (50 μM) application (n = 11). E: current recorded in response to brief sub-threshold voltage steps (10 ms, −70 to −55 mV) in a motoneuron held at −60 mV in control conditions, in the presence of DHPG, and following drug washout. F: membrane resistance was significantly decreased by DHPG application. G: DHPG-induced currents appeared to have a linear current-voltage relationship, with an extrapolated reversal potential of approximately −30 mV (n = 11). *Significantly different from control.
We started by investigating the subthreshold effects of group I mGluR activation on motoneurons. Application of DHPG significantly depolarized the resting membrane potential of motoneurons (10 μM, 11.4 ± 1.5 mV, n = 10; 50 μM, 16.0 ± 3.1 mV, n = 11; Fig. 3, C and D). The membrane potential began to repolarize during the course of the drug application (15–20 min; data not shown), indicating desensitization of receptors or other components of the group I mGluR signaling pathway, as seen in rat motoneurons (Marchetti et al. 2003). To investigate the currents responsible for the DHPG-induced depolarization, we measured the input resistance of motoneurons using small voltage steps (−70 to −55 mV, 2.5-mV increments, 10-ms duration) delivered in voltage-clamp mode (Fig. 3E). We were unable to reveal a significant change in input resistance with applications of 10 μM DHPG (n = 9; data not shown). However, at the higher dose of DHPG (50 μM) a significant decrease in input resistance was revealed (52.8 ± 10.1 to 37.3 ± 3.6 MΩ, n = 11; Fig. 3F). This effect was incompletely reversed following drug washout, suggesting that mGluR activation is associated with long-term effects on some conductances or that high doses of DHPG cannot be fully removed over the time course of our recordings. Current-voltage (I-V) relationships were examined for DHPG-induced currents by subtracting I-V relationships in control from those in the presence of DHPG (50 μM). I-V relationships obtained from individual motoneurons were then pooled to reveal a linear I-V relationship for DHPG-induced currents, with an approximate reversal potential of −30 mV (n = 11; Fig. 3G). Finally, DHPG-induced currents remained in the presence of TTX (50 μM DHPG, 0.5 μM TTX, n = 13; data not shown). These data suggest that the activation of group I mGluRs leads to the activation or facilitation of a mixed-cation current in motoneurons.
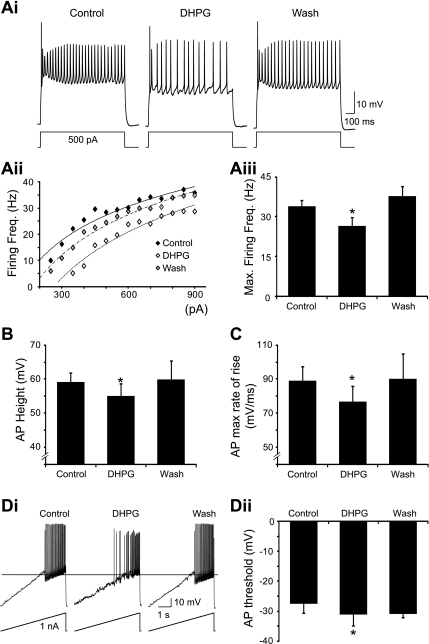
The group I mGluR-mediated depolarization of motoneurons is in apparent opposition to the decrease in locomotor-related motor output observed in ventral root recordings. Thus we next investigated the effects of group I mGluR activation on motoneuron firing. Repetitive firing of action potentials was evoked in motoneurons by the injection of square wave depolarizing current pulses (1-s duration). To study the effects of DHPG on motoneuron firing in isolation from its depolarizing effects, repolarizing bias currents were applied to bring motoneuron resting potentials back to their control levels. Application of DHPG led to a reduction in the frequency of motoneuron firing in response to current injection (Fig. 4Ai). This reduction in motoneuron excitability was evident as a rightward shift in the steady-state firing frequency vs. injected current (f-I) relationships of 6 out of 8 motoneurons in response to 10 μM DHPG and 7 out of 11 motoneurons in response to 50 μM DHPG (Fig. 4Aii). In the remaining cells, DHPG either had no clear effect on the f-I relationship or caused a leftward shift. Reduced excitability upon application of DHPG was also demonstrated by a significant reduction in the maximum firing frequency of motoneurons (10 μM, 26.3 ± 7.6% reduction, n = 8; 50 μM, 16.1 ± 4.9% reduction, n = 11; Fig. 4Aiii). Furthermore, reduced firing was observed in the presence of DHPG (50 μM) even when repolarizing bias currents were not injected (n = 11).
Fig. 4.
Group I mGluR-mediated effects on motoneuron firing. A: repetitive firing in a motoneuron in response to brief (1 s) square current pulses in control conditions, in the presence of DHPG (50 μM), and after drug washout (i). Firing frequency vs. injected current (f-I) relationship for this motoneuron was shifted to the right by DHPG application (ii). Maximum firing frequency of motoneurons was significantly reduced by DHPG application (iii) (n = 11). B: height of the first action potential (AP) evoked by a square current pulse was significantly decreased by DHPG application. C: maximum rate of rise of action potentials was also significantly reduced by DHPG. D: voltage threshold for action potential generation, measured by injecting a current ramp (i), was significantly hyperpolarized by DHPG application (ii). *Significantly different from control.
To reveal mechanisms that may underlie the group I mGluR-mediated decrease in motoneuron excitability, we measured action potential parameters in control and in the presence of DHPG. Measurements of the height and maximum rate of rise of the first action potential evoked by depolarizing pulses revealed a significant reduction in action potential height (10 μM, 5.5 ± 2.4 mV reduction, n = 9; 50 μM, 4.2 ± 1.8 mV reduction, n = 9; Fig. 4B) and a significant slowing of the maximum rate of rise (10 μM, 16.2 ± 5.0% reduction, n = 9; 50 μM, 13.8 ± 5.9% reduction, n = 9; Fig. 4C) upon the application of DHPG. Changes in these two parameters were evident whether the measurements were taken from the same current pulses in control and in the presence of DHPG or from current pulses that elicited similar firing frequencies in the two conditions. We also investigated whether group I mGluR activation affects the voltage threshold for the generation of action potentials in motoneurons. The voltage threshold was measured from the first action potential evoked by a ramp of depolarizing current (100–500 pA/s; Fig. 4Di). Surprisingly, the voltage threshold for action potential generation was significantly hyperpolarized during the application of DHPG (10 μM, 2.1 ± 0.7 mV hyperpolarization, n = 8; 50 μM, 5.0 ± 1.6 mV hyperpolarization, n = 9; Fig. 4D). Despite hyperpolarization of the action potential threshold, a reduction in motoneuron firing was still evident in ramp protocols during DHPG application (Fig. 4Di). Of note, there also appears to be a reduction in the action potential after hyperpolarization in the recording depicted in Fig. 4Di. However, this was not a consistent observation across our recordings. Finally, hyperpolarization of the action potential threshold was also apparent in recordings of motoneurons firing in response to square current pulses (Fig. 4Ai).
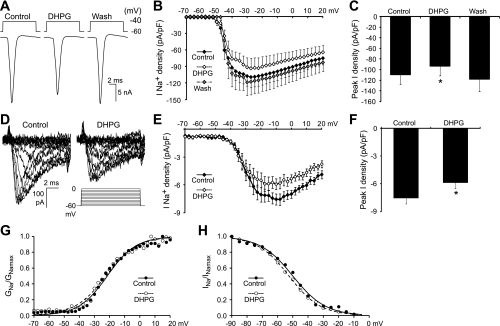
Given that group I mGluR activation leads to reductions in the height and maximum rate of rise of action potentials in motoneurons, we hypothesized that group I mGluR activation modulates Na+ channels that mediate the fast, inactivating Na+ current. This was tested by eliciting Na+ currents in motoneurons in voltage-clamp mode using a series of voltage steps (−70 to +20 mV, 2.5-mV increments, 10-ms duration) from a holding potential of −60 mV in control and in the presence of DHPG (Fig. 5A). Analyses performed on leak subtracted traces, recorded in standard external and pipette solutions, revealed that the density of fast, inactivating Na+ currents was significantly reduced during the application of DHPG (Fig. 5, B and C). The peak current density, elicited by steps to −27.5 mV, was decreased by DHPG application (10 μM, 19.4 ± 8.4% reduction, n = 9; 50 μM, 18.7 ± 5.3% reduction, n = 11; Fig. B and C).
Fig. 5.
Group I mGluR activation modulates the fast, inactivating Na+ current. A: fast, inactivating Na+ currents elicited in standard recording solutions by brief (10 ms) depolarizing voltage steps (−70 to +20 mV) in a motoneuron held at −60 mV in control conditions, in the presence of DHPG (50 μM), and after drug washout. Plots of current-voltage relationships (B) and average peak current density (C) for fast, inactivating Na+ currents demonstrate a DHPG-mediated reduction in Na+ current density (n = 11). D: Na+ currents recorded in control conditions and in the presence of DHPG (50 μM) in modified solutions designed to block Ca2+ channels, K+ channels, and synaptic transmission [TEA, 4-aminopyridine (4-AP), Cs+, CdCl2, bicuculline, strychnine, and kynurenic acid], and to reduce Na+ current magnitude by lowering the concentration of extracellular Na+ (10 mM). Plots of current-voltage relationships (E) and average peak current density (F) again demonstrate a DHPG-mediated reduction in Na+ current density (n = 6). Normalized activation and steady-state inactivation curves plotted in control conditions and in the presence of DHPG demonstrate no change in the voltage dependence of activation (G) or steady-state inactivation (H). *Significantly different from control.
When recording large currents from large neurons there is a high likelihood of voltage- and space-clamp errors. Although these errors may be equivalent in control conditions and in the presence of DHPG, it is also possible that changes in cell properties induced by DHPG may differentially affect the voltage control in the two conditions. We therefore also assessed whether DHPG modulated Na+ currents when other channel types (Ca2+ and K+) and synaptic transmission were blocked and Na+ currents were reduced by using an external solution with lowered Na+ concentration (10 mM NaCl; Carlier et al. 2006). In these modified solutions, Na+ currents elicited by depolarizing steps (−70 to +20 mV, 2.5-mV increments, 10-ms duration) from a holding potential of −60 mV were >10-fold smaller than those recorded in standard solutions (Fig. 5D). With Na+ currents reduced and other channels and synaptic transmission blocked, the application of DHPG (50 μM) still led to a significant decrease in the peak density of Na+ currents (20.3 ± 7.8% reduction, n = 6; Fig. 5, E and F).
To investigate the mechanisms by which group I mGluR activation modulates Na+ currents, we also investigated the voltage dependence of Na+ current activation and inactivation in control conditions and in the presence of DHPG (50 μM; using modified solutions). Na+ current activation was measured using depolarizing steps from a holding potential of −60 mV (−70 to +20 mV, 2.5-mV increments, 10-ms duration; Fig. 5G). Steady-state inactivation of Na+ currents was investigated using steps to 0 mV (10-ms duration) from prepulses (50-ms duration) ranging from −90 to −5 mV (5-mV increments; Fig. 5H). The half-maximal activation voltage of Na+ currents was unchanged by DHPG (−31.4 ± 1.1 mV in control, −33.2 ± 1.7 mV in DHPG, n = 6; Fig. 5G). There were also no significant changes in the half maximal voltage of steady-state inactivation in the presence of DHPG (−51.0 ± 0.8 mV in control, −51.1 ± 0.9 mV in DHPG, n = 6; Fig. 5H). These data indicate that group I mGluRs regulate Na+ current density by means other than modulating the voltage dependence of activation or inactivation of Na+ currents.
Taken together, our results demonstrate that the activation of group I mGluRs has a range of effects on the intrinsic properties of motoneurons. Although the effects include depolarization of the resting membrane potential and hyperpolarization of the action potential threshold, the net effect is a reduction in motoneuron output, most likely due to the modulation of Na+ channels.
Effects of group I mGluR activation on synaptic transmission to motoneurons.
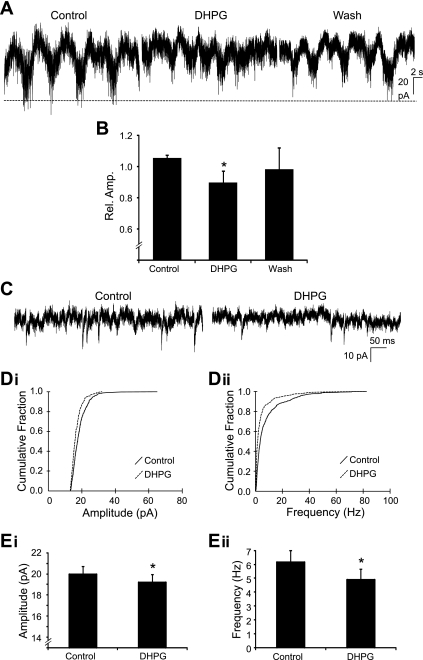
Modulation of locomotor-related motoneuron output by the activation of group I mGluRs could also involve regulation of the excitatory synaptic input received by motoneurons from spinal locomotor networks. We therefore performed voltage-clamp experiments to analyze the effects of group I mGluR activation on locomotor-related excitatory synaptic input to motoneurons. In pharmacologically activated (5 μM NMDA, 10 μM 5-HT, and 50 μM dopamine) in vitro spinal cord preparations, rhythmic locomotor-related input was recorded from motoneurons held at −60 mV (Fig. 6A). The rhythmic locomotor drive received by motoneurons was in phase with bursts of activity recorded from segmentally aligned ventral roots (data not shown). The amplitude of locomotor drive was significantly reduced by 10.4 ± 7.6% when DHPG (10 μM) was bath applied (n = 10; Fig. 6, A and B). Thus group I mGluR activation reduces the excitatory synaptic drive transmitted to motoneurons to stimulate bursts of locomotor-related output.
Fig. 6.
Effects of group I mGluR activation on synaptic input to motoneurons. A: Rhythmic locomotor-related drive currents recorded from a motoneuron held at −60 mV in control conditions, in the presence of DHPG (10 μM), and after drug washout. B: amplitude of locomotor-related drive was significantly decreased by DHPG (10 μM) application (n = 10). C: miniature excitatory postsynaptic currents (mEPSCs) recorded from a motoneuron in the presence of tetrodotoxin (TTX; 0.5 μM), bicuculline (10 μM), and strychnine (5 μM). D: cumulative probability plots showing a significant decrease in mEPSC amplitude (i) and frequency (ii) following DHPG (50 μM) application. E: pooled data demonstrating that DHPG caused a significant decrease in both mEPSC amplitude (i) and frequency (ii) (n = 13). *Significantly different from control.
Next, we investigated whether the activation of group I mGluRs modulates excitatory synaptic transmission to motoneurons via pre- or postsynaptic mechanisms. mEPSCs were recorded in voltage-clamp mode while motoneurons were held at −60 mV (Fig. 6C). To isolate mEPSCs, TTX (0.5 μM), strychnine (5 μM), and bicuculline (10 μM) were added to the recording solution. Upon the application of DHPG (50 μM), both the amplitude and frequency of mEPSCs were decreased (Fig. 6, C, D, and E). DHPG-mediated decreases in mEPSC amplitude and frequency were evident as leftward shifts in cumulative frequency plots generated from data collected from individual motoneurons (Fig. 6D). DHPG application lead to a significant reduction in mEPSC amplitude in 7 out of 13 motoneurons and a decrease in mEPSC frequency in 8 out of 13 motoneurons. Average mEPSC amplitude across all motoneurons decreased significantly from 20.0 ± 0.8 to 19.2 ± 0.8 pA (n = 13; Fig. 6Ei), while average mEPSC frequency decreased significantly from 6.2 ± 0.8 to 4.9 ± 0.7 Hz (n = 13; Fig. 6Eii). Together these results indicate that the activation of group I mGluRs reduces excitatory synaptic transmission to motoneurons via both pre- and postsynaptic mechanisms.
DISCUSSION
The activation of ionotropic glutamate receptors is essential for the functioning of the locomotor CPG (Beato et al. 1997; Talpalar and Kiehn 2010; Whelan et al. 2000) and for the transmission of locomotor drive from the CPG to motoneurons (Cazalets et al. 1996; Hochman and Schmidt 1998; Orsal et al. 1986; Shefchyk and Jordan 1985). However, glutamate can also control locomotor activity via the activation of metabotropic receptors that modulate the properties of spinal neurons and their connectivity (El Manira et al. 2002; Nistri et al. 2006). In the present study, we demonstrate that group I mGluRs can modulate the intensity of locomotor-related motoneuron output. We also elucidate several mechanisms by which activation of group I mGluRs can alter motoneuron excitability and excitatory synaptic transmission to motoneurons and hence modulate motoneuron output. Interestingly, our data demonstrating a net reduction in motoneuron output upon activation of group I mGluRs represent a deviation from the typical facilitatory role reported for these receptors (Anwyl 2009; 1999; Delgado-Lezama et al. 1997; Dong and Feldman 1999; Pin and Duvoisin 1995; Svirskis and Hounsgaard 1998).
Although the focus of the present study was on the effects of group I mGluRs on motoneuron output, we also observed an increase in the frequency of locomotor-related activity that suggests that group I mGluRs modulate the activity of spinal interneurons involved in locomotor rhythm generation. These data are consistent with findings in lampreys (Krieger et al. 1998; Kyriakatos and El Manira 2007) and Xenopus tadpoles (Chapman et al. 2008; Chapman and Sillar 2007). The only previous data regarding the effects of group I mGluRs on mammalian locomotion, obtained from isolated rat spinal cord preparations, are less clear with both group I mGluR agonists and antagonists reducing the frequency of locomotor activity (Taccola et al. 2004). To fully understand the role of mGluRs in locomotor rhythm generation in mammals, it will be important for future studies to investigate the effects of group I mGluRs on locomotor-related interneurons. Given that the interneuronal components of the mammalian locomotor CPG remain poorly understood compared with simpler vertebrates (Grillner 2006; Roberts 2000), the ongoing classification of distinct populations of locomotor-related interneurons in mammals will be critical toward future studies of mGluR-mediated modulation (Goulding 2009; Kiehn et al. 2010; Zagoraiou et al. 2009).
Our study represents the first report of modulation of the strength of locomotor-related motoneuron output following the activation of group I mGluRs. This modulation presents as a gradual, reversible decrease in the amplitude of bursts of locomotor-related motoneuron activity recorded from ventral roots. Our recordings of individual motoneurons highlight several mechanisms that may underlie group I mGluR-mediated modulation of motoneuron output including: subthreshold effects on membrane potential; effects on fast, inactivating Na+ channels and motoneuron action potentials; and effects on synaptic input to motoneurons.
First, we found that the activation of group I mGluRs transiently depolarizes the resting membrane potential of mouse motoneurons. These data are consistent with reports of group I mGluR-mediated depolarization of motoneurons in lamprey (Kettunen et al. 2003; Krieger et al. 1998), turtles (Svirskis and Hounsgaard 1998), and rats (Del Negro and Chandler 1998; Dong and Feldman 1999; Marchetti et al. 2005; 2003), but not Xenopus tadpoles (Chapman et al. 2008). mGluR-mediated depolarizations in motoneurons are typically associated with increases in input resistance, most often thought to reflect the blockade of leak K+ currents (Del Negro and Chandler 1998; Dong and Feldman 1999; Kettunen et al. 2003; Marchetti et al. 2005; Svirskis and Hounsgaard 1998). However, we found that in mouse motoneurons group I mGluR-mediated depolarizations were associated with a decrease in input resistance, with the underling current reversing near −30 mV. These data are consistent with the opening of nonselective cationic channels that has been reported in response to activation of group I mGluRs in a range of neuronal types (Congar et al. 1997; Dong et al. 2009; Kolaj and Renaud 2010). In addition, other currents modulated by group I mGluRs that cannot be ruled out include those mediated by Na+-Ca2+ exchangers (Jian et al. 2010; Keele et al. 1997; Lee and Boden 1997) and Na+-K+-2Cl− cotransporters (Schomberg et al. 2001).
The depolarization of motoneurons suggests group I mGluRs should increase motoneuron excitability. However, we observed a reduction in locomotor-related motoneuron output upon activation of group I mGluRs. Although membrane depolarization could lead to a depolarizing block of action potentials, this was unlikely based on the magnitude of the depolarization induced. Furthermore, the time course of the mGluR-mediated depolarization differed from that of the reduction in the amplitude of locomotor-related motoneuron output. Instead, the net inhibitory effect of group I mGluRs on motoneuron output appeared to involve the modulation of action potentials and repetitive firing of mouse motoneurons.
We found that the frequency of repetitive firing was reduced in mouse motoneurons upon the activation of group I mGluRs. This is in contrast to the only other detailed studies of the effects of group I mGluRs on the firing of individual vertebrate motoneurons, performed in turtles, that demonstrated that activation of group I mGluRs enhances sustained firing or plateau potentials via the facilitation of voltage-activated Ca2+ channels (Delgado-Lezama et al. 1997; Svirskis and Hounsgaard 1998). In addition to demonstrating mGluR-mediated reductions in repetitive firing in mouse motoneurons, we have demonstrated mGluR-mediated reductions in action potential height and maximum rate of rise that are consistent with reduced Na+ channel availability (Miles et al. 2005). In support of this, voltage-clamp analysis of fast, inactivating Na+ currents suggests a reduction in Na+ current density upon activation of group I mGluRs. Although the conclusions from data concerning the effects of group I mGluRs on Na+ current density may be complicated by voltage- and space-clamp errors, we believe that we have significantly reduced these errors through the use of modified recording solutions in which other channels types and synaptic transmission are blocked and Na+ currents are reduced (Cantrell et al. 1997; Carlier et al. 2006; Carr et al. 2003; Miles et al. 2005). Thus our data indicate that the modulation of Na+ channel properties and subsequent inhibition of motoneuron firing are likely to contribute to group I mGluR-mediated reductions in locomotor-related motoneuron output.
We also found that the voltage threshold for action potential initiation was hyperpolarized following the activation of group I mGluRs in mouse motoneurons. Interestingly, it has recently been shown that an undefined intraspinal system hyperpolarizes the voltage threshold for action potentials during fictive scratch in cats (Power et al. 2010). Given our findings in mice and previous work in rats showing modulation of the voltage threshold for spike initiation by protein kinase C (Dai et al. 2009), a downstream target of group I mGluRs (Pin and Duvoisin 1995), it seems plausible that group I mGluRs form part of the intraspinal system defined in the cat (Power et al. 2010) that controls action potential threshold in a state-dependent manner. Although the mechanisms underlying mGluR-mediated modulation of action potential threshold remain unclear, our data do not support a role for the modulation of the activation properties of fast, inactivating Na+ currents. Other potential mechanisms include the modulation of persistent Na+ currents (Carlier et al. 2006) and delayed rectifier K+ channels (Dai et al. 2002).
Although this is the first report of mGluR-mediated modulation of Na+ channels and action potential shape in motoneurons, similar findings have been reported in cortical pyramidal neurons (Carlier et al. 2006). In these neurons, group I mGluR activation hyperpolarizes the inactivation of transient Na+ currents, which in turn decreases Na+ current amplitude, reduces the height and maximum rate of rise of action potentials, and lowers the frequency of repetitive firing (Carlier et al. 2006). In contrast to these results in pyramidal neurons, in motoneurons we found that activation of group I mGluRs reduces Na+ current density without changing the voltage dependence of steady-state inactivation. Our findings are similar to previous reports in hippocampal neurons where phosphorylation of Na+ channels, following the activation of either dopaminergic or muscarinic receptors, decreases peak Na+ current without affecting the voltage dependence of activation or steady-state inactivation (Cantrell et al. 1996; Cantrell et al. 1997). In such cases, reduced Na+ channel availability may instead reflect modulation of slow inactivation (Carr et al. 2003), altered rates of inactivation, or a decrease in the unitary conductance of single channels.
In addition to decreased motoneuron excitability, we uncovered an mGluR-mediated inhibition of excitatory locomotor drive to motoneurons that is likely to contribute to reduced locomotor-related motoneuron output. The modulation of synaptic transmission (both excitatory and inhibitory) by mGluRs is commonly reported in spinal neurons (Chapman et al. 2008; Kettunen et al. 2005; Kyriakatos and El Manira 2007; Marchetti et al. 2005, 2003). We chose to concentrate on the effects of group I mGluRs on excitatory transmission because modulation of rhythmic excitatory drive to motoneurons seemed most likely to underlie decreases in locomotor-related motoneuron output. It could be argued that modulation of inhibition, should it occur concurrent with rhythmic excitation (Berg et al. 2007) or contribute to recurrent inhibitory circuitry, could contribute to the reduced amplitude of locomotor-related motoneuron output observed upon activation of group I mGluRs. Although we cannot rule out this possibility, it seems unlikely given that concurrent inhibition and excitation of motoneurons during locomotion appear not to be a feature of mammalian locomotion (Endo and Kiehn 2008) and previous data in the rat suggest that group I mGluRs actually depress recurrent inhibition to motoneurons (Marchetti et al. 2005).
Our data indicate that mGluR-mediated modulation of excitatory synaptic drive to mouse motoneurons involves both pre- and postsynaptic mechanisms. Although the exact processes involved remain to be investigated, one possible presynaptic mechanism, based on our current findings, is that group I mGluR activation inhibits transient Na+ currents in presynaptic terminals. Other potential mechanisms include those revealed in the lamprey spinal cord where modulation of both excitatory and inhibitory transmission involves activation of postsynaptic group I mGluRs followed by the release and presynaptic actions of endocannabinoids and nitric oxide (Kettunen et al. 2005; Kyriakatos and El Manira 2007; Kyriakatos et al. 2009). In addition, data demonstrating modulation of both NMDA and AMPA receptor-mediated currents by group I mGluRs (Krieger et al. 2000; Nanou and El Manira 2010; Nanou et al. 2009) provide potential mechanisms for postsynaptic modulation of transmission.
Taken together, data from our study and previous research demonstrate that the actions of group I mGluRs on motoneurons are complicated by the existence of multiple effects including the modulation of intrinsic neuronal properties and synaptic transmission. This may allow considerable flexibility in the actions of group I mGluRs if the multiple effects they mediate are controlled by pathways that are separable, for example, based on the group I mGluR subtype activated (mGluR1 vs. mGluR5) or the second messenger signaling involved. Although it was not analyzed in the present study, previous work supports the separation of some of the cellular effects of group I mGluRs. For example, in rat motoneurons mGluR1 activation is responsible for membrane potential depolarization while mGluR5 activation triggers membrane oscillations (Marchetti et al. 2003). In the lamprey spinal cord separate intracellular pathways downstream of mGluR1 receptor activation mediate blockade of leak channels vs. the potentiation of NMDA currents (Nanou et al. 2009). Interestingly, in opposition to the independence of separate aspects of group I mGluR-mediated signaling, studies of striatal cholinergic neurons have demonstrated an interaction between group I mGluRs with mGluR5 modulating mGluR1 activity (Bonsi et al. 2005).
Although endogenous activation of group I mGluRs has been reported in lampreys (Krieger et al. 1998), Xenopus tadpoles (Chapman and Sillar 2007), and rats (Taccola et al. 2004), under our experimental conditions we were unable to detect an endogenous role for group I mGluRs in the control of locomotor activity in mice. However, it is possible that our inability to detect an endogenous role for mGluRs reflects compensatory activation of serotonergic and dopaminergic receptors upon the antagonism of mGluRs. In addition, the reduced nature and simple state of our preparation do not preclude an involvement of mGluRs in the control of the locomotor CPG in the whole animal. Glutamatergic inputs to the CPG that are lost or inactive in our preparation, such as descending inputs implicated in the initiation of locomotion (Hagglund et al. 2010; Jordan et al. 2008), might activate both ionotropic and metabotropic glutamate receptors (Delgado-Lezama et al. 1997).
In brainstem motoneurons, where mGluR activation also depresses excitatory inputs and mediates membrane depolarization, mGluRs have been suggested to play a role in sculpting the final output of motoneurons by favoring strong synaptic inputs and amplifying their effects (Del Negro and Chandler 1998). Our data suggest that group I mGluR activation might mediate similar enhancement of the signal-to-noise ratio of synaptic inputs to spinal motoneurons by reducing excitatory inputs but depolarizing the resting membrane potential and hyperpolarizing the action potential threshold. However, the inhibition of repetitive firing via the modulation of Na+ channels may be inconsistent with this. Alternatively, modulation of Na+ currents and repetitive firing may represent a separate role for group I mGluRs in limiting the output of motoneurons, perhaps in the presence of intense glutamatergic input, to provide homeostatic control of motoneuron output (Carlier et al. 2006; Desai 2003). This might also serve to protect motoneurons from excitotoxic damage. Data indeed support a neuroprotective role for group I mGluRs in amyotrophic lateral sclerosis (Anneser et al. 1999, 2004; Aronica et al. 2001; Ma et al. 2006) where glutamate-mediated excitotoxicity is thought to contribute to motoneuron death (Boillee et al. 2006). Further analyses of the role of mGluRs in motoneurons may therefore be of importance, not only for understanding the control of motor outflow from the CNS, but also toward the design of new treatments for degenerative diseases that afflict spinal motor systems.
GRANTS
We are grateful for support from Motor Neurone Disease Scotland and Biotechnology and Biological Sciences Research Council United Kingdom.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Keith Sillar and Emily Witts for helpful comments on the manuscript.
REFERENCES
- Alaburda A, Hounsgaard J. Metabotropic modulation of motoneurons by scratch-like spinal network activity. J Neurosci 23: 8625–8629, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anneser JM, Borasio GD, Berthele A, Zieglgansberger W, Tolle TR. Differential expression of group I metabotropic glutamate receptors in rat spinal cord somatic and autonomic motoneurons: possible implications for the pathogenesis of amyotrophic lateral sclerosis. Neurobiol Dis 6: 140–147, 1999 [DOI] [PubMed] [Google Scholar]
- Anneser JM, Ince PG, Shaw PJ, Borasio GD. Differential expression of mGluR5 in human lumbosacral motoneurons. Neuroreport 15: 271–273, 2004 [DOI] [PubMed] [Google Scholar]
- Anwyl R. Metabotropic glutamate receptor-dependent long-term potentiation. Neuropharmacology 56: 735–740, 2009 [DOI] [PubMed] [Google Scholar]
- Anwyl R. Metabotropic glutamate receptors: electrophysiological properties and role in plasticity. Brain Res Brain Res Rev 29: 83–120, 1999 [DOI] [PubMed] [Google Scholar]
- Aronica E, Catania MV, Geurts J, Yankaya B, Troost D. Immunohistochemical localization of group I and II metabotropic glutamate receptors in control and amyotrophic lateral sclerosis human spinal cord: upregulation in reactive astrocytes. Neuroscience 105: 509–520, 2001 [DOI] [PubMed] [Google Scholar]
- Beato M, Bracci E, Nistri A. Contribution of NMDA and non-NMDA glutamate receptors to locomotor pattern generation in the neonatal rat spinal cord. Proc Biol Sci 264: 877–884, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg RW, Alaburda A, Hounsgaard J. Balanced inhibition and excitation drive spike activity in spinal half-centers. Science 315: 390–393, 2007 [DOI] [PubMed] [Google Scholar]
- Boillee S, Vande Velde C, Cleveland DW. ALS: a disease of motor neurons and their nonneuronal neighbors. Neuron 52: 39–59, 2006 [DOI] [PubMed] [Google Scholar]
- Bonsi P, Cuomo D, De Persis C, Centonze D, Bernardi G, Calabresi P, Pisani A. Modulatory action of metabotropic glutamate receptor (mGluR) 5 on mGluR1 function in striatal cholinergic interneurons. Neuropharmacology 49, Suppl 1: 104–113, 2005 [DOI] [PubMed] [Google Scholar]
- Brown AM. A step-by-step guide to non-linear regression analysis of experimental data using a Microsoft Excel spreadsheet. Comput Methods Programs Biomed 65: 191–200, 2001 [DOI] [PubMed] [Google Scholar]
- Cantrell AR, Ma JY, Scheuer T, Catterall WA. Muscarinic modulation of sodium current by activation of protein kinase C in rat hippocampal neurons. Neuron 16: 1019–1026, 1996 [DOI] [PubMed] [Google Scholar]
- Cantrell AR, Smith RD, Goldin AL, Scheuer T, Catterall WA. Dopaminergic modulation of sodium current in hippocampal neurons via cAMP-dependent phosphorylation of specific sites in the sodium channel alpha subunit. J Neurosci 17: 7330–7338, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlier E, Sourdet V, Boudkkazi S, Deglise P, Ankri N, Fronzaroli-Molinieres L, Debanne D. Metabotropic glutamate receptor subtype 1 regulates sodium currents in rat neocortical pyramidal neurons. J Physiol 577: 141–154, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr DB, Day M, Cantrell AR, Held J, Scheuer T, Catterall WA, Surmeier DJ. Transmitter modulation of slow, activity-dependent alterations in sodium channel availability endows neurons with a novel form of cellular plasticity. Neuron 39: 793–806, 2003 [DOI] [PubMed] [Google Scholar]
- Cazalets JR, Borde M, Clarac F. The synaptic drive from the spinal locomotor network to motoneurons in the newborn rat. J Neurosci 16: 298–306, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman RJ, Issberner JP, Sillar KT. Group I mGluRs increase locomotor network excitability in Xenopus tadpoles via presynaptic inhibition of glycinergic neurotransmission. Eur J Neurosci 28: 903–913, 2008 [DOI] [PubMed] [Google Scholar]
- Chapman RJ, Sillar KT. Modulation of a spinal locomotor network by metabotropic glutamate receptors. Eur J Neurosci 26: 2257–2268, 2007 [DOI] [PubMed] [Google Scholar]
- Congar P, Leinekugel X, Ben-Ari Y, Crepel V. A long-lasting calcium-activated nonselective cationic current is generated by synaptic stimulation or exogenous activation of group I metabotropic glutamate receptors in CA1 pyramidal neurons. J Neurosci 17: 5366–5379, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y, Jones KE, Fedirchuk B, McCrea DA, Jordan LM. A modelling study of locomotion-induced hyperpolarization of voltage threshold in cat lumbar motoneurones. J Physiol 544: 521–536, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y, Jordan LM, Fedirchuk B. Modulation of transient and persistent inward currents by activation of protein kinase C in spinal ventral neurons of the neonatal rat. J Neurophysiol 101: 112–128, 2009 [DOI] [PubMed] [Google Scholar]
- Dale N, Gilday D. Regulation of rhythmic movements by purinergic neurotransmitters in frog embryos. Nature 383: 259–263, 1996 [DOI] [PubMed] [Google Scholar]
- Del Negro CA, Chandler SH. Regulation of intrinsic and synaptic properties of neonatal rat trigeminal motoneurons by metabotropic glutamate receptors. J Neurosci 18: 9216–9226, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado-Lezama R, Perrier JF, Nedergaard S, Svirskis G, Hounsgaard J. Metabotropic synaptic regulation of intrinsic response properties of turtle spinal motoneurones. J Physiol 504: 97–102, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai NS. Homeostatic plasticity in the CNS: synaptic and intrinsic forms. J Physiol (Paris) 97: 391–402, 2003 [DOI] [PubMed] [Google Scholar]
- Dong HW, Hayar A, Callaway J, Yang XH, Nai Q, Ennis M. Group I mGluR activation enhances Ca(2+)-dependent nonselective cation currents and rhythmic bursting in main olfactory bulb external tufted cells. J Neurosci 29: 11943–11953, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong XW, Feldman JL. Distinct subtypes of metabotropic glutamate receptors mediate differential actions on excitability of spinal respiratory motoneurons. J Neurosci 19: 5173–5184, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Manira A, Kettunen P, Hess D, Krieger P. Metabotropic glutamate receptors provide intrinsic modulation of the lamprey locomotor network. Brain Res Brain Res Rev 40: 9–18, 2002 [DOI] [PubMed] [Google Scholar]
- El Manira A, Kyriakatos A, Nanou E, Mahmood R. Endocannabinoid signaling in the spinal locomotor circuitry. Brain Res Rev 57: 29–36, 2008 [DOI] [PubMed] [Google Scholar]
- Endo T, Kiehn O. Asymmetric operation of the locomotor central pattern generator in the neonatal mouse spinal cord. J Neurophysiol 100: 3043–3054, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetcho JR, Higashijima S, McLean DL. Zebrafish and motor control over the last decade. Brain Res Rev 57: 86–93, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulding M. Circuits controlling vertebrate locomotion: moving in a new direction. Nat Rev Neurosci 10: 507–518, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillner S. Biological pattern generation: the cellular and computational logic of networks in motion. Neuron 52: 751–766, 2006 [DOI] [PubMed] [Google Scholar]
- Hagglund M, Borgius L, Dougherty KJ, Kiehn O. Activation of groups of excitatory neurons in the mammalian spinal cord or hindbrain evokes locomotion. Nat Neurosci 13: 246–252, 2010 [DOI] [PubMed] [Google Scholar]
- Heckman CJ, Mottram C, Quinlan K, Theiss R, Schuster J. Motoneuron excitability: the importance of neuromodulatory inputs. Clin Neurophysiol 120: 2040–2054, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochman S, Schmidt BJ. Whole cell recordings of lumbar motoneurons during locomotor-like activity in the in vitro neonatal rat spinal cord. J Neurophysiol 79: 743–752, 1998 [DOI] [PubMed] [Google Scholar]
- Jian K, Cifelli P, Pignatelli A, Frigato E, Belluzzi O. Metabotropic glutamate receptors 1 and 5 differentially regulate bulbar dopaminergic cell function. Brain Res 1354: 47–63, 2010 [DOI] [PubMed] [Google Scholar]
- Jiang Z, Carlin KP, Brownstone RM. An in vitro functionally mature mouse spinal cord preparation for the study of spinal motor networks. Brain Res 816: 493–499, 1999 [DOI] [PubMed] [Google Scholar]
- Jordan LM, Liu J, Hedlund PB, Akay T, Pearson KG. Descending command systems for the initiation of locomotion in mammals. Brain Res Rev 57: 183–191, 2008 [DOI] [PubMed] [Google Scholar]
- Katz PS, Frost WN. Intrinsic neuromodulation: altering neuronal circuits from within. Trends Neurosci 19: 54–61, 1996 [DOI] [PubMed] [Google Scholar]
- Keele NB, Arvanov VL, Shinnick-Gallagher P. Quisqualate-preferring metabotropic glutamate receptor activates Na(+)-Ca2+ exchange in rat basolateral amygdala neurones. J Physiol 499: 87–104, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettunen P, Hess D, El Manira A. mGluR1, but not mGluR5, mediates depolarization of spinal cord neurons by blocking a leak current. J Neurophysiol 90: 2341–2348, 2003 [DOI] [PubMed] [Google Scholar]
- Kettunen P, Kyriakatos A, Hallen K, El Manira A. Neuromodulation via conditional release of endocannabinoids in the spinal locomotor network. Neuron 45: 95–104, 2005 [DOI] [PubMed] [Google Scholar]
- Kiehn O, Dougherty KJ, Hagglund M, Borgius L, Talpalar A, Restrepo CE. Probing spinal circuits controlling walking in mammals. Biochem Biophys Res Commun 396: 11–18, 2010 [DOI] [PubMed] [Google Scholar]
- Kolaj M, Renaud LP. Metabotropic glutamate receptors in median preoptic neurons modulate neuronal excitability and glutamatergic and GABAergic inputs from the subfornical organ. J Neurophysiol 103: 1104–1113, 2010 [DOI] [PubMed] [Google Scholar]
- Krieger P, Grillner S, El Manira A. Endogenous activation of metabotropic glutamate receptors contributes to burst frequency regulation in the lamprey locomotor network. Eur J Neurosci 10: 3333–3342, 1998 [DOI] [PubMed] [Google Scholar]
- Krieger P, Hellgren-Kotaleski J, Kettunen P, El Manira AJ. Interaction between metabotropic and ionotropic glutamate receptors regulates neuronal network activity. J Neurosci 20: 5382–5391, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakatos A, El Manira A. Long-term plasticity of the spinal locomotor circuitry mediated by endocannabinoid and nitric oxide signaling. J Neurosci 27: 12664–12674, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakatos A, Molinari M, Mahmood R, Grillner S, Sillar KT, El Manira A. Nitric oxide potentiation of locomotor activity in the spinal cord of the lamprey. J Neurosci 29: 13283–13291, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Boden PR. Characterization of the inward current induced by metabotropic glutamate receptor stimulation in rat ventromedial hypothalamic neurones. J Physiol 504: 649–663, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Ostrovsky H, Miles G, Lipski J, Funk GD, Nicholson LF. Differential expression of group I metabotropic glutamate receptors in human motoneurons at low and high risk of degeneration in amyotrophic lateral sclerosis. Neuroscience 143: 95–104, 2006 [DOI] [PubMed] [Google Scholar]
- Marchetti C, Taccola G, Nistri A. Activation of group I metabotropic glutamate receptors depresses recurrent inhibition of motoneurons in the neonatal rat spinal cord in vitro. Exp Brain Res 164: 406–410, 2005 [DOI] [PubMed] [Google Scholar]
- Marchetti C, Taccola G, Nistri A. Distinct subtypes of group I metabotropic glutamate receptors on rat spinal neurons mediate complex facilitatory and inhibitory effects. Eur J Neurosci 18: 1873–1883, 2003 [DOI] [PubMed] [Google Scholar]
- Miles GB, Dai Y, Brownstone RM. Mechanisms underlying the early phase of spike frequency adaptation in mouse spinal motoneurones. J Physiol 566: 519–532, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles GB, Hartley R, Todd AJ, Brownstone RM. Spinal cholinergic interneurons regulate the excitability of motoneurons during locomotion. Proc Natl Acad Sci USA 104: 2448–2453, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles GB, Parkis MA, Lipski J, Funk GD. Modulation of phrenic motoneuron excitability by ATP: consequences for respiratory-related output in vitro. J Appl Physiol 92: 1899–1910, 2002 [DOI] [PubMed] [Google Scholar]
- Nanou E, El Manira A. Mechanisms of modulation of AMPA-induced Na+-activated K+ current by mGluR1. J Neurophysiol 103: 441–445, 2010 [DOI] [PubMed] [Google Scholar]
- Nanou E, Kyriakatos A, Kettunen P, El Manira A. Separate signalling mechanisms underlie mGluR1 modulation of leak channels and NMDA receptors in the network underlying locomotion. J Physiol 587: 3001–3008, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nistri A, Ostroumov K, Sharifullina E, Taccola G. Tuning and playing a motor rhythm: how metabotropic glutamate receptors orchestrate generation of motor patterns in the mammalian central nervous system. J Physiol 572: 323–334, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsal D, Perret C, Cabelguen JM. Evidence of rhythmic inhibitory synaptic influences in hindlimb motoneurons during fictive locomotion in the thalamic cat. Exp Brain Res 64: 217–224, 1986 [DOI] [PubMed] [Google Scholar]
- Pin JP, Duvoisin R. The metabotropic glutamate receptors: structure and functions. Neuropharmacology 34: 1–26, 1995 [DOI] [PubMed] [Google Scholar]
- Power KE, McCrea DA, Fedirchuk B. Intraspinally mediated state-dependent enhancement of motoneurone excitability during fictive scratch in the adult decerebrate cat. J Physiol 588: 2839–2857, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekling JC, Funk GD, Bayliss DA, Dong XW, Feldman JL. Synaptic control of motoneuronal excitability. Physiol Rev 80: 767–852, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A. Early functional organization of spinal neurons in developing lower vertebrates. Brain Res Bull 53: 585–593, 2000 [DOI] [PubMed] [Google Scholar]
- Roberts A, Li WC, Soffe SR, Wolf E. Origin of excitatory drive to a spinal locomotor network. Brain Res Rev 57: 22–28, 2008 [DOI] [PubMed] [Google Scholar]
- Schmidt BJ, Jordan LM. The role of serotonin in reflex modulation and locomotor rhythm production in the mammalian spinal cord. Brain Res Bull 53: 689–710, 2000 [DOI] [PubMed] [Google Scholar]
- Schomberg SL, Su G, Haworth RA, Sun D. Stimulation of Na-K-2Cl cotransporter in neurons by activation of non-NMDA ionotropic receptor and group-I mGluRs. J Neurophysiol 85: 2563–2575, 2001 [DOI] [PubMed] [Google Scholar]
- Shefchyk SJ, Jordan LM. Excitatory and inhibitory postsynaptic potentials in alpha-motoneurons produced during fictive locomotion by stimulation of the mesencephalic locomotor region. J Neurophysiol 53: 1345–1355, 1985 [DOI] [PubMed] [Google Scholar]
- Svirskis G, Hounsgaard J. Transmitter regulation of plateau properties in turtle motoneurons. J Neurophysiol 79: 45–50, 1998 [DOI] [PubMed] [Google Scholar]
- Taccola G, Marchetti C, Nistri A. Effect of metabotropic glutamate receptor activity on rhythmic discharges of the neonatal rat spinal cord in vitro. Exp Brain Res 153: 388–393, 2003 [DOI] [PubMed] [Google Scholar]
- Taccola G, Marchetti C, Nistri A. Modulation of rhythmic patterns and cumulative depolarization by group I metabotropic glutamate receptors in the neonatal rat spinal cord in vitro. Eur J Neurosci 19: 533–541, 2004 [DOI] [PubMed] [Google Scholar]
- Talpalar AE, Kiehn O. Glutamatergic mechanisms for speed control and network operation in the rodent locomotor CpG. Front Neural Circuits 4: 19, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan P, Bonnot A, O'Donovan MJ. Properties of rhythmic activity generated by the isolated spinal cord of the neonatal mouse. J Neurophysiol 84: 2821–2833, 2000 [DOI] [PubMed] [Google Scholar]
- Zagoraiou L, Akay T, Martin JF, Brownstone RM, Jessell TM, Miles GB. A cluster of cholinergic premotor interneurons modulates mouse locomotor activity. Neuron 64: 645–662, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]