Abstract
In vitro and in vivo traumatic brain injury (TBI) alter the function and expression of glutamate receptors, yet the combined effect of these alterations on cortical excitatory synaptic transmission is unclear. We examined the effect of in vitro mechanical injury on excitatory synaptic function in cultured cortical neurons by assaying synaptically driven intracellular free calcium ([Ca2+]i) oscillations in small neuronal networks as well as spontaneous and miniature excitatory postsynaptic currents (mEPSCs). We show that injury decreased the incidence and frequency of spontaneous neuronal [Ca2+]i oscillations for at least 2 days post-injury. The amplitude of the oscillations was reduced immediately and 2 days post-injury, although a transient rebound at 4 h post-injury was observed due to increased activity of N-methyl-d-aspartate (NMDARs) and calcium-permeable α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate receptors (CP-AMPARs). Increased CP-AMPAR function was abolished by the inhibition of protein synthesis. In parallel, mEPSC amplitude decreased immediately, 4 h, and 2 days post-injury, with a transient increase in the contribution of synaptic CP-AMPARs observed at 4 h post-injury. Decreased mEPSC amplitude was evident after injury, even if NMDARs and CP-AMPARs were blocked pharmacologically, suggesting the decrease reflected alterations in synaptic Glur2-containing, calcium-impermeable AMPARs. Despite the transient increase in CP-AMPAR activity that we observed, the overriding effect of mechanical injury was long-term depression of excitatory neurotransmission that would be expected to contribute to the cognitive deficits of TBI.
Keywords: traumatic brain injury, α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate receptors, N-methyl-d-aspartate, oscillations
traumatic brain injury (TBI) can produce cognitive, motor, and behavioral dysfunction including deficits in memory, attention, and executive function (Ashman et al. 2006). Deficits following TBI reflect neuronal loss and defective neurotransmission in surviving neurons, including altered connectivity and network circuitry, changes in excitability, unbalanced excitatory and inhibitory input, and abnormal synaptic function and plasticity (Cohen et al. 2007).
Most studies of neuronal electrical activity following experimental TBI have assayed synaptic function and plasticity in the hippocampus (Cohen et al. 2007). These studies reveal region-specific changes in circuit excitability and an inability to induce long-term potentiation (LTP) (D'Ambrosio et al. 1998; Miyazaki et al. 1992; Reeves et al. 1995; Sanders et al. 2000; Schwarzbach et al. 2006) and are attributed to injury-induced cell death (Golarai et al. 2001), aberrant synaptic connectivity (Golarai et al. 2001; Santhakumar et al. 2001), abnormal presynaptic function (Cole et al. 2010; Reeves et al. 2000), and changes in excitatory and inhibitory postsynaptic currents/potentials mediated by glutamate and GABAA receptors, respectively (Cohen et al. 2007; Schwarzbach et al. 2006; Witgen et al. 2005). There are fewer studies of cortical electrophysiology following TBI, hindering our understanding of how mechanical injury affects synaptic transmission in cortex.
We therefore examined the effect of in vitro mechanical injury on synaptic and network activity in cortical pyramidal neurons and focused on alterations of excitatory synaptic transmission mediated by postsynaptic glutamatergic α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate receptors (AMPARs) and N-methyl-d-aspartate (NMDARs). We previously demonstrated alterations in whole-cell NMDAR and AMPAR-mediated currents in cortical pyramidal neurons subjected to stretch-injury in vitro (Goforth et al. 1999; 2004; Zhang et al. 1996). In addition, differences in the expression of the NMDAR subunits NR1, NR2A, and NR2B, and the AMPAR subunits GluR1 and Glur2 are reported in hippocampus and cortex following in vivo TBI (Bell et al. 2009; Biegon et al. 2004; Kumar et al. 2002; Osteen et al. 2004; Schumann et al. 2008). Recent studies report a switch in AMPAR composition from Glur2-containing receptors that are mostly calcium impermeable to calcium-permeable receptors lacking Glur2 (CP-AMPARs) following mechanical injury of cortical neurons in vitro and that activation of CP-AMPARs post-injury increases neuronal cell death (Bell et al. 2009; Spaethling et al. 2008).
Despite the progress made toward understanding how TBI alters NMDAR and AMPAR expression and function, the effect of these alterations on functional cortical synaptic transmission remains unclear. Previous evidence of increased whole-cell NMDA and AMPA currents and CP-AMPAR upregulation following mechanical trauma of cortical neurons supports the hypothesis that there is enhanced glutamatergic synaptic transmission following injury (Park et al. 2008). In contrast, we demonstrate here that trauma caused prolonged depression of excitatory synaptic transmission and network activity, while only transiently altering the contribution of CP-AMPARs to synaptic transmission following in vitro TBI.
MATERIALS AND METHODS
Cortical cell culture and injury.
All animal use was in compliance with protocols approved by the University of Michigan Committee on Use and Care of Animals. Primary cultures of neurons and glia were prepared using a modified version of a standard method (Huettner and Baughman 1986). In brief, neocortices were isolated from 1 to 2-day-old Sprague-Dawley rats (Harlan, Indianapolis, IN) and tissue was minced coarsely and incubated at 34°C for 1.5 h in a solution containing 10 U/ml papain. Following incubation, the tissue was rinsed with trypsin inhibitors followed by growth media consisting of MEM supplemented with 5% calf serum, 25 mM glucose, 100 U/ml penicillin, 100 μg/l streptomycin, and 500 nM glutamine. The growth medium was first conditioned by overnight incubation in flasks containing confluent astrocytes. After being rinsed, tissue was gently triturated in 1 to 2 ml of growth media using fired-polished Pasteur pipettes of decreasing tip size. The resulting cell suspension was then plated at a density of 5 × 105 cells/25-mm well onto a confluent layer of astrocytes grown on deformable Silastic membranes that formed the bottom of 6-well plates (Flexcell International, Hillsborough, NC). Cell cultures were incubated at 37°C in 95% air-5% CO2 fed twice weekly with conditioned growth media and were utilized after 14–21 days in vitro. Mechanical injury was delivered to each well using a Cell Injury Controller II, as described (Ellis et al. 1995). To simulate mild/moderate injury, a 50-ms pulse of compressed air was used to deform the Silastic membranes of the 6-well culture plate (2.5 cm diameter) by 6.5 mm, corresponding to 38% stretch of the membrane and attached cells (Ellis et al. 1995; McKinney et al. 1996). Cells were then washed three times and growth medium was replaced with extracellular recording solution (see below). For studies that were conducted at 4 h or 2 days post-injury, cells were incubated in media at 37°C following injury. Control cells were treated identically except that no injury was delivered.
Measurement of intracellular free calcium.
Intracellular free calcium ([Ca2+]i) was assayed using the calcium indicator fura-2 AM (Invitrogen). Neuronal cultures were incubated with 5 μM fura-2 AM plus 0.01% pluronic acid for 1 h at 37°C, conditions that minimized fura-2 loading of astrocytes. Neuronal fluorescence was imaged using an Olympus BX51W1 (Olympus; Tokyo, Japan) upright microscope equipped with a Hamamatsu Orca camera (Hamamatsu, Japan) and Ludl shutter/filter wheel (Ludl, Germany). Intracellular fura-2 was sequentially excited at 340 and 380 nm, the excitation wavelengths of Ca2+-bound and Ca2+-free fura-2, respectively, and emitted fluorescence was collected at 510 nm. Images were collected using IPLab 3.6.5 software every 2 s, and the ratio of fluorescence intensity generated in response to excitation at 340 and 380 nm (F340/F380) was calculated and plotted versus time. Images were simultaneously collected from clusters of cultured cortical neurons, typically 8–15 neurons per experiment. [Ca2+]i was monitored under basal conditions (no drug treatment) and during the subsequent application of 30 μM bicuculline methiodide (BMI), 20 μM d-(-)-2-amino-5-phosphonopentanoic acid (d-APV), and 50 μM 1-naphthylacetyl spermine trihydrochloride (Naspm). KCl (30 Mm) was added at the end of each experiment to activate voltage-gated Ca2+ channels to confirm cell viability, and only those cells exhibiting an increase in [Ca2+]i in response to KCl were included in the analysis. Drugs were applied at a flow rate of ∼1 ml/min using a peristaltic pump. Oscillation parameters were measured at least 3 min following drug application to ensure complete solution exchange and to allow oscillations to reach a steady state. Neurons were defined as having basal oscillatory activity if at least three transient increases in F340/F380 greater than 0.02 relative ratio units were observed. The presence of basal oscillatory activity was obvious upon visual inspection. Relative basal [Ca2+]i levels were measured as the initial F340-to-F380 ratio. F340/F380 plots were then baseline subtracted and analyzed using a custom program implemented in MatLab software (2007b; The MathWorks, Natick, MA). Oscillation amplitude was calculated as the change in fura-2 ratio from baseline (ΔF340/F380). Recordings were made from control and injured neurons matched from the same culture at each time point post-injury, and experiments were repeated using neurons from at least three different culture preparations. All experiments were conducted at room temperature.
Measurement of spontaneous and miniature excitatory postsynaptic currents.
Spontaneous and miniature excitatory postsynaptic currents (sEPSCs and mEPSCs) were measured from individual cortical pyramidal neurons using the tight-seal whole-cell voltage clamp technique (Hamill et al. 1981) and an Axopatch 200B amplifier (Molecular Devices, Sunnyvale, CA). Pyramidal neurons were identified by their characteristic morphology. Currents were filtered at 2 kHz and digitized at 5 kHz using a Macintosh G4 computer equipped with an Instrutech ITC-16 computer interface (Instrutech, Great Neck, NY) and Pulse Control (Herrington and Bookman 1994) and Igor Pro software (Wavemetrics, Lake Oswego, OR). Patch electrodes were made from borosilicate glass capillaries (WPI, Sarasota, FL) that were pulled to a tip resistance of 3–6 MΩ using a Brown/Flaming P-97 micropipette puller (Sutter Instrument, Novato, CA) and filled with a solution containing (in mM) 135 CsAsp, 4 KCl, 2 NaCl, 10 EGTA, 0.2 CaCl2, 2 MgATP, 0.6 Na2GTP, and 10 HEPES (pH 7.2). The external recording solution contained (in mM) 130 NaCl, 4 KCl, 3 CaCl2, 2 MgCl2, 10 HEPES, 11 glucose, 0.01 glycine, 0.0005 tetrodotoxin (TTX), and 30 μM bicuculline methiodide (pH 7.3). For low magnesium experiments, extracellular MgCl2 was reduced to 0.05 mM without substitution of other ions. For sEPSC recordings, TTX was excluded from the extracellular solution and 5 mM QX314 was added to the intracellular solution to block Na2+-dependent action potentials in the cell from which sEPSC recordings were made. Drugs were applied using a SF-77 Fast-Step rapid perfusion system (Warner Instrument, Hamden, CT) as previously described (Goforth et al. 1999). Cells were continuously superfused with bath-applied standard external solution at a rate of ∼2 ml/min. Neurons selected for analysis had stable series resistances (Rs) <30 MΩ.
Data analysis and statistics.
In our cultures, sEPSCs occur in bursts that can exhibit complex waveforms. For each cell, maximal burst amplitude, charge transfer during the burst, and burst duration were averaged from at least five bursts and compared for control and injured neurons. mEPSC events were analyzed using a commercial software package (Synaptosoft; Decatur, GA). Individual events were detected using an amplitude threshold value of 5 pA and confirmed visually. mEPSC amplitude, 10–90% rise time, half-width, and charge transfer were determined in the presence and absence of drug treatments. Decay time constants (τ) were calculated from single exponential fits of averaged mEPSC traces. To construct cumulative probability distributions, 75 random events were selected from each neuron using Synaptosoft software and events from control and injured cells were respectively pooled for each experimental condition. Statistical comparisons of cumulative distributions were made with MatLab using the nonparametric Kolmogorov-Smirnov test, and significance was defined as P < 0.001. All other statistical analyses were performed using GraphPad Prism 4.0 (GraphPad Software, San Diego, CA) and statistical significance was determined using Fisher's exact test for percentages, Student's t-test for comparison of two groups, or ANOVA and Newman-Keuls multiple comparison post hoc test or the nonparametric Kruskall Wallis and Dunn's post-test for comparison of multiple groups. Statistical differences were defined as P < 0.05.
RESULTS
Mechanical injury depresses synaptically driven [Ca2+]i oscillations in cortical neurons.
As cortical neurons mature in culture, they develop spontaneous and synchronous [Ca2+]i oscillations that have been shown to reflect excitatory synaptic transmission (Murphy et al. 1992; Robinson et al. 1993). These oscillations emerge in parallel with the formation of synaptic connections (Nakanishi and Kukita 1998), and their timing coincides with bursts of action potentials (Robinson et al. 1993) and EPSPs (Murphy et al. 1992) recorded simultaneously in adjacent neurons or in the same neurons, respectively. [Ca2+]i oscillations are abolished by blocking glutamate receptors or action potentials, further indicating their mediation by excitatory synaptic transmission. We thus measured simultaneous [Ca2+]i oscillations in groups of cortical neurons to assay the effect of mechanical injury on excitatory neurotransmission within small neural networks.
[Ca2+]i was monitored using fura-2 AM immediately (within 10 min), 4 h, or 2 days following in vitro stretch-injury, and the results compared with recordings from uninjured control neurons (Fig. 1). Cortical neurons exhibited spontaneous [Ca2+]i oscillations (Fig. 1A) that required action potential firing and excitatory synaptic activity as they were abolished by TTX or by a combination of the NMDAR and AMPAR antagonists APV and CNQX (data not shown). Blockade of inhibitory GABAA receptors with BMI (30 μM) briskly increased the frequency and amplitude of the oscillations (Fig. 1A). The subsequent applications of APV and then Naspm, a selective inhibitor of CP-AMPARs, were used to assay the contribution NMDARs and CP-AMPARs, respectively, to the [Ca2+]i oscillations (Fig. 1).
Fig. 1.
Mechanical injury depressed intracellular free calcium ([Ca2+]i) oscillations in cortical neurons. A: recording of [Ca2+]i from an individual cortical neuron using fura-2. Fura ratios (F340/F380) are plotted vs. time. Spontaneous [Ca2+]i oscillations (arrow) occur before the application of bicuculline methiodide (BMI), which produced larger amplitude oscillations. Amino-5-phosphonopentanoic acid (APV) and 1-naphthylacetyl spermine trihydrochloride (Naspm) were added sequentially to inhibit N-methyl-d-aspartate (NMDARs) and calcium-permeable α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate receptors (CP-AMPARs), respectively. B: recording of [Ca2+]i from an individual neuron immediately (within 10 min) following stretch-injury, 4 h post-injury (C), and 2 days post-injury (D). Spontaneous [Ca2+]i oscillations are absent from injured neurons before BMI application.
As in other studies, basal [Ca2+]i increased soon after stretch-injury, as evidenced by increased baseline F340-to-F380 ratios in recordings that commenced within 10 min post injury (Table 1). Basal [Ca2+]i then returned to control levels at 4 h and remained so for 2 days post-injury (Table 1). Immediately post-injury, 30% (44/149) of injured neurons were incapable of producing [Ca2+]i oscillations, even when exposed to BMI. However, these neurons continued to exhibit normal responses to 30 mM KCl, indicating the loss of oscillations we observed was not due to a generalized loss of membrane integrity and/or cell death. The initial increase in basal [Ca2+]i was significantly higher in this subset of injured neurons compared with injured neurons that retained the capability to produce oscillations (Table 1). Injured neurons that did not demonstrate [Ca2+]i oscillations in response to BMI were excluded from further analysis, since oscillations were the endpoint we chose as a monitor of synaptic function.
Table 1.
| n | Initial F340-to-F380 Ratio | ||
|---|---|---|---|
| Control | 96 | 0.47 ± 0.01 | |
| Immediately post-injury | 149 | 0.89 ± 0.04 | *** |
| Control 4 h | 110 | 0.47 ± 0.01 | |
| 4 h Post-injury | 91 | 0.48 ± 0.01 | n.s. |
| Control 2 days | 92 | 0.53 ± 0.01 | |
| 2 Days post-injury | 87 | 0.54 ± 0.02 | n.s. |
| Immediate (−) no oscillations | 44 | 1.42 ± 0.08 | ***, ### |
| Immediate (+) oscillations | 105 | 0.65 ± 0.03 | *** |
Values are means ± SE. n.s., Not significant.
P < 0.001 vs. control;
P < 0.001 vs. injured (+) oscillations.
Of neurons capable of producing oscillations in response to BMI, injury decreased the percentage of cells exhibiting basal calcium oscillations before BMI addition (Fig. 1 arrow and Fig. 2A). Thus immediately post-injury, 52.1% of control neurons exhibited spontaneous basal [Ca2+]i oscillations versus 16.2% of the injured neurons. The decrease in the number of neurons exhibiting basal activity persisted at least 2 days, at which time 32.2% of injured neurons displayed basal oscillations versus 66.3% of control neurons. At 4 h post-injury, basal [Ca2+]i activity was not significantly different from controls (Fig. 2A), most likely due to an increase in the activation calcium permeability of postsynaptic glutamate receptors at this time point (see below).
Fig. 2.
Injury decreased neuronal [Ca2+]i oscillation frequency and amplitude A: percentage of neurons displaying spontaneous [Ca2+]i oscillations before disinhibition with BMI was significantly decreased immediately post-injury (n = 105 cells, 9 wells) vs. control (n = 96 cells from 9 culture wells, P < 0.001 by Fisher's exact test) and 2 days post-injury (n = 87 cells, 9 wells) vs. control (n = 92 cells, 8 wells, P < 0.01), yet was not statistically different at 4 h post-injury (n = 112 cells, 9 wells) vs. control (n = 109 cells, 9 wells, P > 0.05) B: frequency of [Ca2+]i oscillations recorded in the presence of 30 μM BMI did not differ immediately post-injury [0.042 ± 0.001 Hz for control (n = 91) vs. 0.042 ± 0.001 Hz for injured neurons (n = 105, P > 0.05)] but was significantly decreased 4 h post-injury [0.043 ± 0.002 Hz for control (n = 112) vs. 0.034 ± 0.001 Hz for injured neurons (n = 109, P < 0.01)] and at 2 days post-injury [0.033 ± 0.002 Hz for control (n = 83) vs. 0.022 ± 0.001 Hz for injured neurons (n = 78, P < 0.001)]. C: injury decreased the amplitude of [Ca2+]i oscillations recorded in the presence of 30 μM BMI immediately post-injury [0.31 ± 0.01 for control (n = 91) vs. 0.18 ± 0.01 for injured neurons (n = 105, P < 0.001)] and 2 days post-injury [0.32 ± 0.02 for control (n = 83) vs. 0.24 ± 0.02 for injured neurons (n = 78, P < 0.01)] but was not significantly different at 4 h post-injury [0.40 ± 0.02 for control (n = 112) vs. 0.41 ± 0.02 for injured neurons (n = 109, P > 0.05)]. Measurements are baseline subtracted (F340/F380) relative units.
Injury-induced suppression of basal [Ca2+]i oscillations suggests that injury alters the balance of excitatory and inhibitory drive. To further examine the effect of injury on excitatory neurotransmission only, we compared the frequency and amplitude of the [Ca2+]i oscillations after blocking inhibitory GABAA receptors with BMI, which resulted in more regular and larger amplitude [Ca2+]i oscillations and removed the contribution of fast inhibitory synaptic transmission from network activity. We detected no significant difference in the frequency of [Ca2+]i oscillations observed in the presence of BMI immediately post-injury; however, a significant reduction in oscillation frequency was apparent at 4 h and 2 days post injury (Fig. 2B). In addition, the mean amplitude of the [Ca2+]i oscillations decreased significantly immediately and 2 days post-injury (Fig. 2C). However, at 4 h post-injury, oscillation amplitude was not significantly different from controls (Fig. 2C). These data demonstrate that there is an overall depression of oscillatory activity mediated by the excitatory synaptic network that persisted for at least 2 days, with a transient mitigation of injury-induced suppression of amplitude apparent at 4 h post-injury. As discussed below, the rebound of oscillation amplitude occurred during a time when there was a transient upregulation of calcium-permeable glutamatergic receptors following injury.
Injury increases the contribution of NMDA and calcium-permeable AMPA receptors to [Ca2+]i oscillations.
Previous studies reported increased expression of calcium-permeable AMPA receptors (CP-AMPAR) (Bell et al. 2009; Spaethling et al. 2008) and enhanced NMDA receptor function (Lea et al. 2003; Zhang et al. 1996) following mechanical injury. Although the upregulation of CP-AMPARs after mechanical injury has been linked to increased cell death (Bell et al. 2009; Spaethling et al. 2008), the consequences of such upregulation for excitatory synaptic function in surviving neurons is unclear. We thus examined the sensitivity of the neuronal [Ca2+]i oscillations to the competitive NMDAR antagonist APV and to Naspm, a selective antagonist for CP-AMPARs that is a synthetic analog of Joro spider toxin (Koike et al. 1997; Takazawa et al. 1996), to determine the relative contribution of these receptors to synaptic and network activity in control versus injured neurons. BMI, APV, and Naspm were thus added sequentially during the experiments. The activity of NMDARs was assessed as the percent inhibition by APV, calculated by comparing [Ca2+]i oscillation amplitude in BMI versus BMI + APV. Likewise, the contribution of CP-AMPARS was determined by comparing oscillation amplitude in the presence of BMI + APV versus BMI + APV + Naspm. Naspm sensitivity was determined in the presence of APV to isolate injury effects on AMPARergic transmission and eliminate the contribution of NMDAR-mediated changes to oscillation amplitude. Oscillations were more sensitive to 20 μM APV immediately and 4 h post-injury, indicating an early increase in NMDAR activity, with NMDAR activation returning to control levels by 2 days post-injury (Fig. 3A). In addition, we observed an increased contribution of CP-AMPAR activation to oscillation amplitude, evidenced as a larger Naspm-blockable component, at 4 h post-injury only, with no significant differences noted either immediately or 2 days following injury (Fig. 3B). The combined increase in Ca2+ influx mediated by NMDARs and CP-AMPARs at 4 h post-injury thus appeared to compensate for the suppression we observed immediately and 2 days post-injury, since oscillation amplitude was not significantly different than controls at this time point (Fig. 3C). However, when Ca2+ influx though NMDARs and CP-AMPARs was acutely blocked with APV and Naspm, [Ca2+]i oscillation amplitude was then significantly smaller for injured cells versus control (Fig. 3C). These data suggest that the mechanisms responsible for oscillation depression, as observed immediately and 2 days post-injury, are still present at 4 h but are masked by the transient increase in NMDAR and CP-AMPAR activation. These findings indicate that although injury alters the function and/or expression of NMDAR and CP-AMPAR receptors, these alterations only transiently affect neuronal function at early time points post-injury, with the more predominant effect of injury being a reduction of the [Ca2+]i oscillations that persists for at least 2 days post-injury, the latest time point we monitored in this study.
Fig. 3.
Injury transiently increased the contribution of NMDARs and CP-AMPARs to neuronal [Ca2+]i oscillations. A: percent inhibition of [Ca2+]i oscillation amplitude by the NMDAR antagonist APV (20 μM) was significantly greater immediately post-injury [44 ± 2.0% for control (n = 79 cells from 9 wells) vs. 52.4 ± 2.0% for injured neurons (n = 87 cells from 8 wells, P < 0.05)] and 4 h post-injury [42.3 ± 1.7% for control (n = 110 cells from 9 wells) vs. 53.8 ± 1.2% for injured neurons (n = 109 cells from 9 wells, P < 0.001)] and did not differ at 2 days post-injury [33.6 ± 4.0% for control (n = 83 cells from 8 wells) vs. 32.6 ± 4.6% for injured neurons (n = 66 cells from 8 wells, P > 0.05)]. B: percent inhibition of [Ca2+]i oscillation amplitude by the selective CP-AMPAR antagonist Naspm (50 μM) was significantly greater 4 h post-injury [15.5 ± 3.4% for control (n = 95 cells from 8 wells) vs. 33.8 ± 1.7% for injured neurons (n = 92 cells from 8 wells, P < 0.001)] yet did not differ from controls immediately post-injury [12.9 ± 1.9% for control (n = 58 cells from 5 wells) vs. 6.3 ± 3.6% for injured neurons (n = 33 cells from 3 wells, P > 0.05)] or at 2 days post-injury [18.3 ± 2.8% for control (n = 44 cells from 4 wells) vs. 20.5 ± 3.5% for injured neurons (n = 33 cells from 4 wells, P > 0.05)]. All measurements were made in the presence of 30 μM BMI. C: calcium oscillation amplitude did not differ at 4 h when measured in the presence 30 μM BMI only yet was significantly decreased when measured in the presence of 30 μM BMI + 20 μM APV [0.22 ± 0.01 for control (n = 111 cells from 9 wells) vs. 0.19 ± 0.01 for injured (n = 109 cells from 9 wells; P < 0.01)]. Oscillation amplitude was also significantly reduced when recorded in the presence of BMI + APV + 50 μM Naspm [0.18 ± 0.01 for control (n = 96) vs. 0.14 ± 0.01 for injured (n = 92), P < 0.001].
Injury-induced enhancement of CP-AMPAR function is selectively prevented by inhibiting protein synthesis.
An upregulation of CP-AMPARs is observed in many neuropathologies, including ischemia (Calderone et al. 2003; Kwak and Weiss 2006; Liu and Zukin 2007), neurodegeneration (Kwak and Weiss 2006), epilepsy (Rogawski and Donevan 1999), inflammatory pain (Jones and Sorkin 2004; Vikman et al. 2008), and neurotrauma (Bell et al. 2007), but also occurs in certain forms of synaptic plasticity (Cull-Candy et al. 2006; Isaac et al. 2007), including homeostatic plasticity, the process by which neurons dynamically scale their synaptic strength to compensate for increases or decreases in overall synaptic activity (Ju et al. 2004; Sutton et al. 2006; Turrigiano 2008). The insertion of synaptic CP-AMPARs during homeostatic plasticity triggered by drug-induced mEPSC blockade requires protein synthesis (Ju et al. 2004; Sutton et al. 2006). We therefore examined whether the injury-induced upregulation of CP-AMPARs we observed at 4 h post-injury involved protein synthesis. Neuronal cultures were thus incubated with the protein synthesis inhibitor anisomycin (40 μM) starting 30 min before injury and continuing for 4 h post-injury. The relative contributions of NMDARs and CP-AMPARs were again assessed by quantifying the sensitivity of [Ca2+]i oscillation amplitudes to APV and Naspm, respectively. As shown in Fig. 4A, anisomycin prevented the increase in Naspm sensitivity that was observed at 4 h post-injury, demonstrating that protein synthesis was required for increased CP-AMPAR activity following injury. We also examined the effect of anisomycin treatment on [Ca2+]i oscillation amplitude, since we hypothesize that increased CP-AMPAR, in conjunction with increased NMDAR activity, compensates for the reduction in [Ca2+]i oscillation amplitude observed immediately and 2 days post-injury. As shown in Fig. 4B, in the presence of APV, [Ca2+]i oscillation amplitude measured from untreated injured neurons at 4 h was not significantly different from control. However, [Ca2+]i oscillation amplitude was significantly decreased in neurons where the potentiation of CP-AMPARs was prevented by anisomycin treatment. These data support the hypothesis that increased CP-AMPAR activity contributes to the rebound of [Ca2+]i oscillation amplitude that we observe 4 h post-injury. In addition, when the upregulation of CP-AMPARs was prevented by anisomycin treatment, a significantly smaller percentage of treated injured neurons exhibited basal oscillations (Fig. 4C), revealing the same hypoexcitability that we observed immediately and 2 days post-injury (Fig. 2A). Anisomycin also failed to prevent the injury-induced reductions we observed in oscillation frequency, indicating that these alterations must occur independently from enhancement of CP-AMPAR activity (Fig. 4D).
Fig. 4.
Injury-induced upregulation of CP-AMPARs was dependent on protein synthesis. A: incubation with the protein synthesis inhibitor anisomycin (40 μM) prevented upregulation of CP-AMPARs at 4 h post-injury. The percent inhibition of [Ca2+]i oscillation amplitude by Naspm (50 μM) was significantly greater at 4 h post-injury for untreated injured neurons (47.8 ± 6.4%, n = 57 cells from 5 wells) compared with untreated control neurons (7.1 ± 5.6%, n = 50 cells from 5 wells, ***P < 0.001), anisomycin-treated injured neurons (8.5 ± 4.1%, n = 55 cells from 5 wells, ###P < 0.001), or anisomycin-treated controls (10.3 ± 6.3%, n = 30 cells from 3 wells, +++P < 0.001). Percent inhibition by Naspm did not differ between anisomycin-treated injured neurons, untreated control neurons, or anisomycin-treated control neurons (P > 0.05). B: [Ca2+]i oscillation amplitude was significantly decreased for anisomycin-treated injured neurons 4 h post-injury (0.18 ± 0.02) compared with control (0.25 ± 0.02, **P < 0.01) or untreated injured neurons (0.25 ± 0.01, ##P < 0.01). Recordings were made in the presence of BMI and APV. C: percentage of anisomycin-treated injured neurons displaying basal [Ca2+]i oscillations (23.6%) was significantly smaller than untreated control neurons (50.9%, **P < 0.01), anisomycin-treated control neurons (47.4%, ##P < 0.01), and untreated injured neurons (62.8%, n = 43, +++P < 0.001). D: [Ca2+]i oscillation frequency was significantly decreased for untreated injured neurons (0.038 ± 0.002 Hz) and anisomycin-treated injured neurons (0.037 ± 0.002 Hz) compared with untreated control (0.056 ± 0.004 Hz, ***P < 0.001) and anisomycin-treated control neurons (0.062 ± 0.006 Hz, ###P < 0.001).
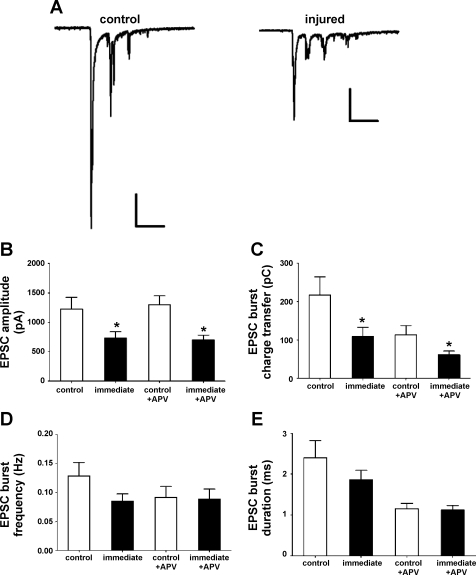
Mechanical injury diminishes the amplitude of excitatory postsynaptic currents.
Although neuronal [Ca2+]i oscillations are dependent upon synaptic activity and the activation of NMDARs and AMPARs, their generation also involves action potential firing within the neuronal network, membrane depolarization, and the activation of voltage-gated Na+ and Ca2+ channels (Wang and Gruenstein 1997). Therefore, to confirm that the injury-induced suppression of [Ca2+]i oscillations we observed reflects alterations in underlying excitatory neurotransmission, we next examined the effect of injury on spontaneous (action-potential dependent) and miniature excitatory postsynaptic currents (sEPSCs and mEPSCs). As shown in Fig. 5A, in the presence of bicuculline, sEPSCs occur as bursts of multiple synaptic events. Immediately following injury, maximal sEPSC burst amplitude (Fig. 5B) and charge transfer during the bursts of sEPSCs (Fig. 5C) were significantly reduced compared with control neurons. These reductions in amplitude and charge transfer persisted during the acute application of 20 μM APV, indicating injury primarily decreases AMPAergic synaptic transmission (Fig. 5, B and C). We observed no significant differences in sEPSC burst frequency (Fig. 5D) or sEPSC burst duration (Fig. 5E). We also did not detect any significant differences in the percent inhibition of sEPSC burst peak amplitude or charge transfer by 20 μM APV for injured neurons versus control (data not shown).
Fig. 5.
Injury reduces spontaneous excitatory postsynaptic currents (sEPSC) amplitude and charge transfer. A: examples of bursts of sEPSCs in a control and injured neuron immediately post injury. Scale bars, 200 pA, 0.5 s. B: injury reduced the maximal amplitude of sEPSC bursts immediately post-injury (732.4 ± 107.7 pA, n = 12) vs. control (1228.0 ± 196.8 pA, n = 14, *P < 0.05). The maximal amplitude of AMPAR-mediated sEPSC bursts, recorded in the presence of APV, was also reduced (701.6 ± 81.6 pA for injured neurons vs. 1302.1 ± 148.5 pA for control, P < 0.05). C: injury reduced the charge transfer during sEPSC bursts immediately post-injury in the absence of APV (109.3 ± 23.9 pC for injured vs. 217.4 ± 47.1 pC for control, P < 0.05) and in the presence of APV (61.4 ± 9.8 pC for injured vs. 114.1 ± 24.1 pC, P < 0.05). D: sEPSC burst frequency recorded from neurons immediately post-injury in either the presence or absence of APV did not differ from control neurons (P > 0.05). E: sEPSC burst duration recorded from neurons immediately post-injury in either the presence or absence of APV did not differ from control neurons (P > 0.05).
sEPSCs result from action potential-dependent activity in the synaptic network and, as such, changes in sEPSCs may reflect changes in quantal release at numerous synaptic sites in the network, presynaptic membrane excitability, and the cellular links between action potentials and the release of quanta in multiple neurons. Thus to more directly assess the function of postsynaptic glutamate receptors following injury, we also examined mEPSCs. Miniature synaptic events occur due to the spontaneous release of a single vesicle or quantum of glutamate onto a postsynaptic neuron, which in turn activates a small ensemble of postsynaptic glutamate receptors. Using whole-cell patch-clamp, mEPSC currents were recorded from individual voltage-clamped pyramidal neurons held at −65 mV in the presence of 0.5 μM TTX to block action potential-dependent synaptic currents and 30 μM BMI to block inhibitory synaptic currents mediated by GABAA receptors. The remaining mEPSCs were abolished by the coapplication of APV and CNQX, confirming their mediation by glutamate receptors (data not shown).
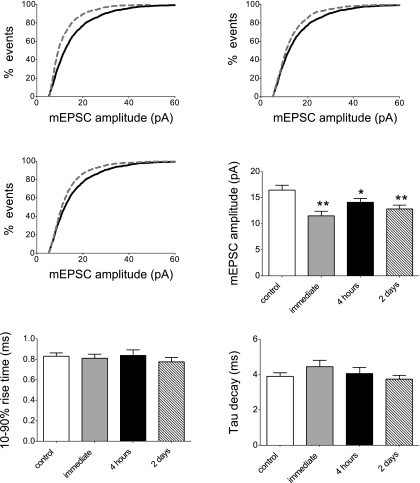
Cumulative probability distributions of mEPSC amplitude and kinetics were constructed and compared for control and injured neuronal populations immediately, 4 h, and 2 days post-injury. Paralleling the changes we observed in [Ca2+]i oscillation amplitude following injury, mEPSC amplitude was significantly decreased immediately, 4 h, and 2 days post-injury compared with controls (Fig. 6A), as denoted by a leftward shift of the cumulative probability distributions (Fig. 6B). Alterations in mEPSC amplitude also significantly decreased total charge transfer across the membrane during the mEPSC, indicating that injury results in reduced excitatory synaptic strength that persists for at least 2 days (Fig. 6C). There were no significant changes in mEPSC rise time, half-width, or mEPSC decay time constant (τ) at any time point following injury (Fig. 6D).
Fig. 6.
Injury alters miniature excitatory postsynaptic currents (mEPSC) amplitude. A: voltage-clamp recordings of mEPSCs from individual cortical pyramidal neurons. B: cumulative probability distributions of mEPSC amplitude for control neurons (1,800 events, n = 24 cells) and injured populations immediately (900 events, n = 12 cells), 4 h (1050 events, n = 14 cells), and 2 days post-injury (1,050 events, n = 14 cells). Control distributions are indicated by a solid black line, and injured distributions are indicated by the dashed gray line. Injury decreased mEPSC amplitude, as indicated by a significant leftward shift of the cumulative probability distribution immediately, 4 h, and 2 days post-injury (***P < 0.001). Inset: average mEPSC traces for cells in A (scale = 5 pA, 5 ms; control = black trace and injured = gray trace). Mean mEPSC amplitude averaged from individual cells was 17.2 ± 1.0 pA (n = 24) for control neurons, 13.1 ± 1.1 pA (n = 12) immediately, 14.4 ± 0.8 pA (n = 14) 4 h, and 13.0 ± 1.2 pA (n = 14) 2 days post injury (*P < 0.05 vs. control). C: injury significantly decreased mEPSC charge transfer, as indicated by significant leftward shifts of the cumulative distributions immediately, 4 h, and 2 days post-injury (***P < 0.001). Mean mEPSC charge transfer averaged from individual cells was 75.0 ± 7.9 fC for control neurons, 55.4 ± 9.1 fC immediately post-injury, 53.3 ± 8.0 fC 4 h post-injury, and 43.5 ± 4.7 fC 2 days post-injury. *P < 0.05 vs. control. D: injury did not alter mEPSC kinetics. Mean mEPSC 10–90% rise time was 1.32 ± 0.08 ms for control neurons, 1.41 ± 0.11 ms immediately, 1.29 ± 0.08 ms at 4 h, and 1.19 ± 0.11 ms at 2 days post-injury (P > 0.05). Mean mEPSC half-width duration was 3.9 ± 0.3 ms for control neurons, 3.7 ± 0.3 ms immediately, 3.7 ± 0.4 ms at 4 h, and 3.0 ± 0.2 ms at 2 days post-injury (P > 0.05). Mean τ decay was 4.6 ± 0.3 ms for control neurons, 5.0 ± 0.5 ms immediately, 4.8 ± 0.4 ms at 4 h, and 4.1 ± 0.2 ms at 2 days post-injury (P > 0.05).
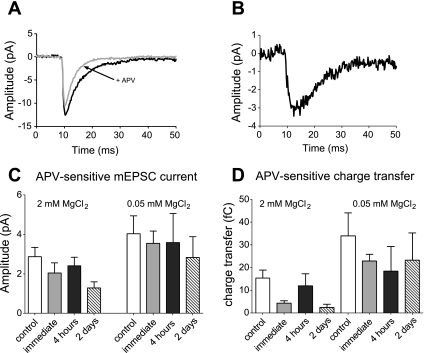
To test whether decreased mEPSC amplitude was due to a reduction in the NMDAR and/or the AMPAR components of the postsynaptic current, 20 μM APV was used to inhibit synaptic NMDARs and pharmacologically isolate the two components. mEPSCs were recorded in 2 mM extracellular MgCl2, to match the conditions of [Ca2+]i oscillation recordings, or 0.05 mM extracellular MgCl2, which reduces Mg2+-blockade of NMDARs, thus allowing a more thorough examination of the activity of NMDARs at the synapse. As shown in Fig. 7A, mEPSCs contained an APV-sensitive component mediated by NMDARs. To quantify the NMDAergic component of the mEPSC, the average waveform of mEPSCs recorded in the presence of APV was subtracted from the average of mEPSCs in the absence of APV in the same cell, producing an APV-sensitive, NMDAergic difference current (Fig. 7B). The amplitude of APV-sensitive difference currents measured in either 2 mM or 0.05 mM MgCl2 did not differ significantly between control and injured neurons at any time point post-injury (Fig. 7C). We also compared the amount of mEPSC charge transfer inhibited by APV for control and injured neurons. As shown in Fig. 7D, although there appeared to be a trend toward a decrease in the amount of mEPSC charge transfer mediated by NMDARs following injury, these changes were not statistically significant (Fig 7D).
Fig. 7.
Injury does not alter synaptic NMDAR function. A: average mEPSC waveform recorded from an individual control neuron, in 0.05 MgCl2, in the presence (black trace) and absence (gray trace) of APV. B: APV-sensitive difference current, calculated as the average mEPSC-no APV minus average mEPSC + APV. C: mean APV-sensitive mEPSC currents did not differ for control and injured neurons either in 2 mM MgCl2 (control, n = 22; immediate, n = 10; 4 h, n = 11; 2 days, n = 10, P > 0.05) or 0.05 mM MgCl2 (control, n = 15; immediate, n = 8; 4 h, n = 7; 2 days, n = 6). D: APV-sensitive difference in mEPSC charge transfer did not differ significantly for injured neurons vs. control in either 2 mM MgCl2 or 0.05 mM MgCl2.
To confirm that reduced mEPSC amplitude was in fact due to a decrease in synaptic AMPAR activity, we compared mEPSCs recorded in the presence of APV for control and injured neurons. As shown in Fig. 8, A-D, injury significantly reduced the amplitude of AMPA-mediated mEPSCs immediately, 4 h, and 2 days post-injury. We observed no significant change in the 10–90% rise time or τ of decay of AMPARergic mEPSCs following injury (Fig. 8, E and F). These data taken together therefore indicate that injury-induced suppression of mEPSCs is primarily due to reduced AMPAR but not NMDAR activity.
Fig. 8.
Injury reduces the AMPAergic component of mEPSCs. A-C: cumulative probability distributions of mEPSC amplitude recorded in the presence of 20 μM APV for control neurons (n = 25 cells) and injured populations immediately (n = 11 cells), 4 h (n = 19 cells), and 2 days post-injury (n = 22 cells). Control distributions are indicated by a solid black line, and injured distributions are indicated by the dashed gray line. Injury decreased mEPSC amplitude, as indicated by a significant leftward shift of the cumulative probability distribution immediately, 4 h, and 2 days post-injury (***P < 0.001). D: mean mEPSC amplitude averaged from individual cells was 16.5 ± 0.9 pA for control neurons, 11.5 ± 0.9 pA immediately, 14.1 ± 0.7 pA 4 h, and 12.8 ± 0.8 pA 2 days post injury (*P < 0.05; **P < 0.01 vs. control). E: injury did not alter the kinetics of the AMPAergic component of the mEPSC. Mean mEPSC 10–90% rise time in the presence of APV was 0.83 ± 0.03 ms for control, 0.81 ± 0.04 ms immediately, 0.84 ± 0.06 ms at 4 h, and 0.78 ± 0.04 ms at 2 days post-injury (P > 0.05). F: Mean τ decay of mEPSCs recorded in the presence of APV was 3.9 ± 0.2 ms for control neurons, 4.4 ± 0.4 ms immediately, 4.1 ± 0.4 ms at 4 h, and 3.7 ± 0.2 ms at 2 days post-injury (P > 0.05).
To determine whether injury increased the contribution of postsynaptic CP-AMPARs, as observed for [Ca2+]i oscillations, we examined the effect of 50 μM Naspm on mEPSC amplitude. We observed a transient increase in the contribution of CP-AMPARs to mEPSC amplitude 4 h post-injury, as evidenced by changes in the sensitivity of mEPSC amplitude to Naspm (Fig. 9). mEPSCs recorded from uninjured control neurons contained a small CP-AMPAR-mediated component, since the application of Naspm significantly reduced mEPSC amplitude in controls (Fig. 9, A and E). Interestingly, immediately post-injury, mEPSC amplitude appeared insensitive to Naspm (Fig. 9, B and E), suggesting an early loss of CP-AMPAR activity at the synapse. Naspm sensitivity was also observed for mEPSCs recorded from neurons 4 h and 2 days post-injury (Fig. 9, C–E). As we observed for [Ca2+]i oscillations, the sensitivity of mEPSC amplitude to Naspm was significantly increased at 4 h post-injury compared with control, indicating enhanced synaptic CP-AMPAR activity at this time point only (Fig. 9F).
Fig. 9.
Injury increases postsynaptic CP-AMPAR function. A–D: cumulative probability distributions of mEPSC amplitude recorded in the presence (dashed gray line) and absence (solid black line) of 50 μM Naspm (+BMI and APV). Insets: average mEPSC in the presence (black) and absence (gray) of Naspm from individual neurons. Naspm significantly decreased mEPSC amplitude (noted as a leftward shift of the cumulative distribution) for control neurons, 4 h post-injury, and 2 days post-injury (***P < 0.001). E: Naspm decreased mean mEPSC amplitude in control neurons (n = 21, P < 0.05), 4 h post-injury (n = 12, P < 0.01), and 2 days post-injury (n = 19, P < 0.05) but not immediately post-injury (n = 10, P > 0.05). F: percent inhibition of mEPSC amplitude by Naspm was significantly increased at 4 h post-injury when compared with control (22.1 ± 3.7% vs. 8.9 ± 4.1%; P < 0.05) but not immediately (0.01 ± 4.5%, P > 0.05) or 2 days post-injury (6.0 ± 3.5%, P > 0.05).
When NMDARs and CP-AMPARs were both acutely blocked after injury by the coapplication of APV and Naspm, thereby isolating postsynaptic Glur2-containing AMPARs, we observed an overall reduction in mEPSC amplitude at all time points post-injury (Fig. 10). These findings demonstrate that despite the transient modulation of CP-AMPAR synaptic activity we observed with injury, alterations of Glur2-containing AMPARs mediate the persistent depression in the excitatory currents we observed.
Fig. 10.
Injury alters Glur2-containing AMPAR function. A: cumulative probability distributions of mEPSC amplitude recorded during blockade of NMDAR and CP-AMPAR with 20 μM APV + 50 μM Naspm show a significant decrease in mEPSC amplitude at all time points post-injury (noted by a leftward shift of the cumulative distribution; ***P < 0.001). Control distributions are indicated by the solid black line, and injured distributions are indicated by the dashed gray line. B: mean mEPSC amplitude recorded in APV + Naspm averaged from individual neurons (*P < 0.05 vs. control).
DISCUSSION
Following mechanical injury, cortical neurons exhibited abnormal excitatory synaptic transmission and network activity due, in part, to alterations in postsynaptic AMPARs. Although injury transiently increased synaptic CP-AMPAR activity at 4 h post-injury, the predominant effect we observed was the depression of mEPSCs and synaptically driven neuronal [Ca2+]i oscillations that occurred immediately post-injury and persisted at least 2 days. This depression most likely reflected decreased function and/or expression of synaptic AMPARs containing Glur2.
[Ca2+]i oscillation amplitude decreased immediately post-injury, recovered by 4 h, but was again decreased at 2 days post-injury. Changes in [Ca2+]i oscillation amplitude may reflect calcium influx through glutamate receptors but could also occur via alterations in action potential firing, membrane depolarization, and the activation of voltage-gated calcium channels that indirectly cause a rise in [Ca2+]i (Wang and Gruenstein 1997). The rebound of [Ca2+]i oscillation amplitude seen at 4 h was likely due to an increase in NMDAR and CP-AMPAR activity. Although NMDAR activity was also increased immediately post-injury, without the additional potentiation of CP-AMPARs, this enhancement was not sufficient to compensate for the decrease in [Ca2+]i oscillation amplitude.
The suppression of [Ca2+]i oscillation amplitude was paralleled by a reduction in mEPSC amplitude immediately and 2 days post-injury. Decreased mEPSC amplitude persisted when NMDARs and CP-AMPARs were acutely inhibited, indicating that synaptic depression resulted from alterations in synaptic Glur2-containing AMPARs that were insensitive to these agents.
As for [Ca2+]i oscillation amplitude, we also observed an increase in CP-AMPAR activation during mEPSCs recorded 4 h post-injury. Although CP-AMPARs have larger channel conductances compared with Glur2-containing AMPARs (Li et al. 2003; Swanson et al. 1997), in our hands, this did not result in larger mEPSCs. In fact, synaptic charge transfer actually decreased, thereby reducing the strength of the excitatory synapses. Thus it appears that the contribution of CP-AMPARs to [Ca2+]i oscillation amplitude occurs via calcium influx through these channels rather than indirectly through increased membrane depolarization. We also found no significant change in the kinetics of AMPARergic mEPSCs, despite the fact that CP-AMPARS exhibit more rapid kinetics (Grosskreutz et al. 2003; Isaac et al. 2007; Oh and Derkach 2005) and their synaptic incorporation can shorten mEPSC decay time (Guire et al. 2008). This may reflect a more modest change in the composition of synaptic AMPARs in our system following injury. Thus, although the Naspm sensitivity of mEPSCs is significantly greater 4 h post-injury versus control, the increased activity of synaptic CP-AMPARs at this time point does not substantially increase mEPSC amplitude (Figs. 6 and 8) and the mEPSC waveform may still be governed primarily by GluR-containing AMPARs.
[Ca2+]i oscillations exhibited enhanced sensitivity to APV immediately and 4 h post-injury, indicating an increase in NMDAR contribution to the oscillations at these time points. This is consistent with our previous observation of increased NMDA-elicited calcium influx due to reduced Mg2+ blockade of the NMDARs immediately post-injury (Zhang et al. 1996). However, we did not observe a corresponding increase in the NMDAR-mediated component of the mEPSC or sEPSC bursts at any time point post-injury, suggesting that increased NMDAR activity was unlikely to reflect changes in postsynaptic NMDAR function. Thus, enhanced NMDAR activity, as measured via [Ca2+]i oscillations, may reflect the activation of extrasynaptic or presynaptic NMDARs that would not be expected to contribute to the mEPSC current but would be evident when measuring network activity. The effect of injury on different populations of NMDARs clearly requires additional studies.
In addition to depressed oscillation amplitude, we found that fewer injured neurons exhibited spontaneous [Ca2+]i oscillations under basal conditions and oscillation frequency was reduced at 4 h and 2 days post-injury. The lower incidence of basal [Ca2+]i oscillations in injured neurons could reflect not only decreased excitatory postsynaptic function but enhanced inhibitory GABAergic function or a loss of excitatory neurons from the network. Hippocampal inhibitory postsynaptic currents are known to be altered by fluid percussion injury (Witgen et al. 2005), and GABA-activated currents from cortical neurons are increased by in vitro stretch-injury in our model (Kao et al. 2004). Neuronal death is unlikely here, since the mild injury we used is not associated with a significant degree of neuronal death at these time points (McKinney et al. 1996). Furthermore, alteration of mEPSCs cannot be explained by neuronal death, since these events directly reflect the functioning of individual synapses. Although we observed a trend toward decreased mEPSC frequency post-injury (data not shown), which may reflect decreased presynaptic function after TBI, these changes were not significant. Further studies will thus be necessary to explore the contribution of presynaptic changes to overall changes in excitatory transmission.
Our finding of depressed excitatory postsynaptic function in cortical neurons following mechanical injury qualitatively agrees with reports of reduced field excitatory post-synaptic potentials (fEPSPs) and population spike amplitude in hippocampal CA1 neurons 1 to 48 hours following FPI (Cohen et al. 2007; Miyazaki et al. 1992; Reeves et al. 2000). Concomitant decreases in NMDA-mediated postsynaptic potentials and glutamate-activated NMDAR and AMPAR currents also suggest there are alterations in postsynaptic glutamate receptor function after FPI injury (Schwarzbach et al. 2006). Although our study focuses on mild/moderate injury, it is possible that differences in injury mechanism and/or severity produce differential synaptic changes. Thus, models where posttraumatic epilepsy is observed following cortical deafferentation, such as the partially isolated (undercut) cortex model, produce hyperexcitability and epileptiform activity (Avramescu and Timofeev 2008; Hoffman et al. 1994; Li and Prince 2002; Prince and Tseng 1993; Salin et al. 1995; Topolnik et al. 2003). Li and Prince (2002) report increased sEPSC and mEPSC frequency and amplitude in layer V pyramidal neurons in slices from chronically injured neocortex (Li and Prince 2002). Similarly, deafferentation due to the removal of superficial cortical layers in an in vitro neocortical slice preparation results in increased sEPSC amplitude and frequency in layer V pyramidal neurons 2–8 h after injury (Yang et al. 2007). Changes in excitatory synaptic function occur in the context of complex pathophysiology that includes changes in intrinsic membrane properties (Avramescu and Timofeev 2008; Prince and Tseng 1993), axonal sprouting (Salin et al. 1995), increased excitatory synaptic connectivity (Avramescu and Timofeev 2008; Jin et al. 2006), and decreased inhibitory synaptic transmission (Li and Prince 2002; Yang et al. 2007). Thus, although the findings of these studies would appear at face value to contradict our study, these other cortical models correspond to more focal and severe penetrating brain injuries, whereas our model directly assessed the effect of mild, non-lethal injury, such as that encountered in closed head concussion. This suggests that the cortex may respond quite differently depending on type and severity of the brain injury.
Other reports of injury-induced alterations of postsynaptic NMDARs and AMPARs include enhanced NMDAR and AMPA-mediated whole-cell currents immediately following stretch-injury in vitro (Cohen et al. 2007; Goforth et al. 1999; Lea et al. 2002; Zhang et al. 1996); and short-lived hyperactivation of hippocampal NMDARs following in vivo TBI (Biegon et al. 2004). Early enhancement of NMDAR activity is followed by a more sustained depression of activity in the cortex and the hippocampus, which lasted hours to days following injury (Biegon et al. 2004). Kumar et al. (2002) report decreased expression of NR1, NR2A, and NR2B subunits in the hippocampus 6 to 12 h following controlled cortical impact (CCI), and decreased NR2A and NR2B expression is observed in the cortex 1 to 4 days following FPI (Osteen et al. 2004). A biphasic effect of injury on NMDAR function is supported by the evidence for a short (<1 h) therapeutic time window for applying NMDAR antagonists following trauma and improvement in neurological deficits observed with more delayed treatment with NMDA or NMDAR agonists following TBI in vivo (Biegon et al. 2004; Yaka et al. 2007). We found there was early transient hyperactivation, but not long-term suppression, of NMDAR activity within the neuronal network. We also observed injury-induced alterations in postsynaptic AMPAR function that contributed to reduced excitatory synaptic strength. Synaptic strength is governed in large part by the trafficking of synaptic AMPARs under both physiological and pathological conditions (Isaac et al. 2007; Malinow and Malenka 2002; Santos et al. 2009). Although TBI activates numerous calcium-dependent kinases [CaMKII, PKC (Atkins et al. 2006; Yang et al. 1993; Zhang et al. 1996)], phosphatases [calcineurin; (Kurz et al. 2005)], and proteases [calpain; (Kampfl et al. 1997)], which can modify AMPAR trafficking and function, it is unclear how the convergence of these processes affects synaptic function.
In contrast with our observation of decreased synaptic AMPAR function, our laboratory previously described an increase in steady-state whole-cell AMPAR-mediated currents due to the slower activation and desensitization of AMPARs in response to exogenous agonist (Goforth et al. 1999; 2004). However, our previous study of whole-cell currents likely included the contribution of both synaptic and extrasynaptic receptors, whereas the present study included a specific examination of synaptic AMPAR function, which may not reveal the altered receptor kinetics such as those observed during prolonged agonist exposure. Our observed changes in synaptic AMPAR function are consistent with a report of decreased GluR1 expression in the cortex 15 min after closed head injury (Schumann et al. 2008) as well as reports demonstrating increased CP-AMPAR expression in cortical and cerebellar neurons following in vitro stretch-injury (Bell et al. 2007; Bell et al. 2009; Spaethling et al. 2008) and in CA1 hippocampal neurons following FPI (Bell et al. 2009). As in the present study, Spaethling et al. (2008) found increased Ca2+ influx and ionic current through CP-AMPARs at 4 h post injury in cortical neurons. Interestingly, these authors also reported decreased AMPA-evoked [Ca2+]i responses at time points earlier than 4 h post-injury, consistent with our finding that the contribution of CP-AMPARs to mEPSC amplitude decreased immediately following injury. An increase in postsynaptic CP-AMPAR expression was also reported 1 h following a combined insult of stretch-injury plus exogenous NMDA in cortical neurons (Bell et al. 2009). In this study, upregulation of CP-AMPARs required the activation of NR2B-containing NMDARs, phosphorylation of Glur2 Ser880 by PKC, and PICK1-mediated endocytosis of Glur2-containing receptors (Bell et al. 2009). However, in contrast with our findings, Glur2 endocytosis paired with the insertion of CP-AMPARs increased mEPSC amplitude in this model. The differences between the former study and our study are likely due to their use of a milder degree of stretch-injury paired with the coincident application of exogenous NMDA, which may alter the time course of cellular signaling events, and/or activate additional NMDA-dependent signaling pathways targeting postsynaptic glutamate receptor expression and function differently (Cull-Candy et al. 2006; Cull-Candy and Leszkiewicz 2004; Isaac et al. 2007). It is possible that PICK1-dependent endocytosis of Glur2-containing receptors contributes to the decreased excitatory synaptic transmission that we observed following injury, since this mechanism is linked not only to LTP, but long-term depression (LTD) in cerebellar and hippocampal neurons (Chung et al. 2003; Seidenman et al. 2003; Terashima et al. 2008). Both Spaethling et al. (2008) and Bell et al. (2009) provide evidence that the prevention of CP-AMPAR upregulation imparts significant protection against secondary excitotoxic insult and delayed neuronal death 20–24 h following injury. However, neither study examined CP-AMPAR expression nor synaptic function at time points longer than 6 h post-injury, as addressed in the present study.
The insertion of synaptic CP-AMPARs plays a role in mediating diverse forms of physiological synaptic plasticity (Cull-Candy et al. 2006; Isaac et al. 2007). In cortical cultures, a 3-h suppression of neuronal activity and mEPSC blockade results in the insertion of calcium-permeable Glur1 homomers in the synapse (Sutton et al. 2006). This process, a form of homeostatic plasticity, depends upon local dendritic protein synthesis and trafficking of Glur1 subunits and is prevented by protein synthesis inhibitors (Sutton et al. 2006). The upregulation of CP-AMPARs is transient, since the Glur1 homomers are then replaced by Glur 2-containing AMPARs over a 24-h period (Sutton et al. 2006). Likewise, the increase in CP-AMPAR activity that we observed depended upon protein synthesis and may reflect an attempt by the neurons to restore synaptic strength following an initial depression due to injury. Future studies are needed to confirm whether injury increases the synthesis and trafficking of Glur1 homomers. The short-lived nature of CP-AMPAR enhancement coupled with the persistent depression of mEPSCs following injury suggests that neurons are unable to sustain the transient increase in AMPARs and may lack the ability to replenish synaptic Glur2-containing receptors or that extant Glur2-containing synaptic AMPARs are chronically altered by injury.
To our
knowledge, this is the first report of decreased AMPAR-mediated excitatory synaptic transmission and neuronal [Ca2+]i oscillations in cortex following mechanical injury. Because glutamatergic synaptic transmission is essential for proper brain function, it will be a challenge to identify new therapeutic approaches to TBI to mitigate neuronal death and dysfunction while not hindering normal synaptic function and plasticity. Although pharmacological inhibition of NMDARs and CP-AMPARs appears to be an attractive means for preventing neuronal death after TBI, their blockade may not ameliorate cognitive deficits due to the more persistent suppression of cortical excitatory synaptic transmission we describe here. Elucidation of both mechanisms would thus benefit the development of potential new therapies for TBI.
GRANTS
This work was supported by RO1 NS49519 from the National Institutes of Health (to L. Satin).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Khaled Houamed and Mary Clark for assistance with the cortical cell culturing.
REFERENCES
- Ashman TA, Gordon WA, Cantor JB, Hibbard MR. Neurobehavioral consequences of traumatic brain injury. Mt Sinai J Med 73: 999–1005, 2006 [PubMed] [Google Scholar]
- Atkins CM, Chen S, Alonso OF, Dietrich WD, Hu BR. Activation of calcium/calmodulin-dependent protein kinases after traumatic brain injury. J Cereb Blood Flow Metab 26: 1507–1518, 2006 [DOI] [PubMed] [Google Scholar]
- Avramescu S, Timofeev I. Synaptic strength modulation after cortical trauma: a role in epileptogenesis. J Neurosci 28: 6760–6772, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell JD, Ai J, Chen Y, Baker AJ. Mild in vitro trauma induces rapid Glur2 endocytosis, robustly augments calcium permeability and enhances susceptibility to secondary excitotoxic insult in cultured Purkinje cells. Brain 130: 2528–2542, 2007 [DOI] [PubMed] [Google Scholar]
- Bell JD, Park E, Ai J, Baker AJ. PICK1-mediated GluR2 endocytosis contributes to cellular injury after neuronal trauma. Cell Death Differ 16: 1665–1680, 2009 [DOI] [PubMed] [Google Scholar]
- Biegon A, Fry PA, Paden CM, Alexandrovich A, Tsenter J, Shohami E. Dynamic changes in N-methyl-D-aspartate receptors after closed head injury in mice: implications for treatment of neurological and cognitive deficits. Proc Natl Acad Sci USA 101: 5117–5122, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderone A, Jover T, Noh KM, Tanaka H, Yokota H, Lin Y, Grooms SY, Regis R, Bennett MV, Zukin RS. Ischemic insults derepress the gene silencer REST in neurons destined to die. J Neurosci 23: 2112–2121, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung HJ, Steinberg JP, Huganir RL, Linden DJ. Requirement of AMPA receptor GluR2 phosphorylation for cerebellar long-term depression. Science 300: 1751–1755, 2003 [DOI] [PubMed] [Google Scholar]
- Cohen AS, Pfister BJ, Schwarzbach E, Grady MS, Goforth PB, Satin LS. Injury-induced alterations in CNS electrophysiology. Prog Brain Res 161: 143–169, 2007 [DOI] [PubMed] [Google Scholar]
- Cole JT, Mitala CM, Kundu S, Verma A, Elkind JA, Nissim I, Cohen AS. Dietary branched chain amino acids ameliorate injury-induced cognitive impairment. Proc Natl Acad Sci USA 107: 366–371, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy S, Kelly L, Farrant M. Regulation of Ca2+-permeable AMPA receptors: synaptic plasticity and beyond. Curr Opin Neurobiol 16: 288–297, 2006 [DOI] [PubMed] [Google Scholar]
- Cull-Candy SG, Leszkiewicz DN. Role of distinct NMDA receptor subtypes at central synapses. Sci STKE 2004 (255): re16, 2004 [DOI] [PubMed] [Google Scholar]
- D'Ambrosio R, Maris DO, Grady MS, Winn HR, Janigro D. Selective loss of hippocampal long-term potentiation, but not depression, following fluid percussion injury. Brain Res 786: 64–79, 1998 [DOI] [PubMed] [Google Scholar]
- Ellis EF, McKinney JS, Willoughby KA, Liang S, Povlishock JT. A new model for rapid stretch-induced injury of cells in culture: characterization of the model using astrocytes. J Neurotrauma 12: 325–339, 1995 [DOI] [PubMed] [Google Scholar]
- Goforth PB, Ellis EF, Satin LS. Enhancement of AMPA-mediated current after traumatic injury in cortical neurons. J Neurosci 19: 7367–7374, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goforth PB, Ellis EF, Satin LS. Mechanical injury modulates AMPA receptor kinetics via an NMDA receptor-dependent pathway. J Neurotrauma 21: 719–732, 2004 [DOI] [PubMed] [Google Scholar]
- Golarai G, Greenwood AC, Feeney DM, Connor JA. Physiological and structural evidence for hippocampal involvement in persistent seizure susceptibility after traumatic brain injury. J Neurosci 21: 8523–8537, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosskreutz J, Zoerner A, Schlesinger F, Krampfl K, Dengler R, Bufler J. Kinetic properties of human AMPA-type glutamate receptors expressed in HEK293 cells. Eur J Neurosci 17: 1173–1178, 2003 [DOI] [PubMed] [Google Scholar]
- Guire ES, Oh MC, Soderling TR, Derkach VA. Recruitment of calcium-permeable AMPA receptors during synaptic potentiation is regulated by CaM-kinase I. J Neurosci 28: 6000–6009, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch 391: 85–100, 1981 [DOI] [PubMed] [Google Scholar]
- Herrington J, Bookman RJ. Pulse control v4.3: Igor XOP's for patch clamp data acquisition. Miami, FL: Univ of Miami Press, 1994 [Google Scholar]
- Hoffman SN, Salin PA, Prince DA. Chronic neocortical epileptogenesis in vitro. J Neurophysiol 71: 1762–1773, 1994 [DOI] [PubMed] [Google Scholar]
- Huettner JE, Baughman RW. Primary culture of identified neurons from the visual cortex of postnatal rats. J Neurosci 6: 3044–3060, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac JT, Ashby M, McBain CJ. The role of the GluR2 subunit in AMPA receptor function and synaptic plasticity. Neuron 54: 859–871, 2007 [DOI] [PubMed] [Google Scholar]
- Jin X, Prince DA, Huguenard JR. Enhanced excitatory synaptic connectivity in layer v pyramidal neurons of chronically injured epileptogenic neocortex in rats. J Neurosci 26: 4891–4900, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones TL, Sorkin LS. Calcium-permeable alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid/kainate receptors mediate development, but not maintenance, of secondary allodynia evoked by first-degree burn in the rat. J Pharmacol Exp Ther 310: 223–229, 2004 [DOI] [PubMed] [Google Scholar]
- Ju W, Morishita W, Tsui J, Gaietta G, Deerinck TJ, Adams SR, Garner CC, Tsien RY, Ellisman MH, Malenka RC. Activity-dependent regulation of dendritic synthesis and trafficking of AMPA receptors. Nat Neurosci 7: 244–253, 2004 [DOI] [PubMed] [Google Scholar]
- Kampfl A, Posmantur RM, Zhao X, Schmutzhard E, Clifton GL, Hayes RL. Mechanisms of calpain proteolysis following traumatic brain injury: implications for pathology and therapy: implications for pathology and therapy: a review and update. J Neurotrauma 14: 121–134, 1997 [DOI] [PubMed] [Google Scholar]
- Kao CQ, Goforth PB, Ellis EF, Satin LS. Potentiation of GABAA currents after mechanical injury of cortical neurons. J Neurotrauma 21: 259–270, 2004 [DOI] [PubMed] [Google Scholar]
- Koike M, Iino M, Ozawa S. Blocking effect of 1-naphthyl acetyl spermine on Ca2+-permeable AMPA receptors in cultured rat hippocampal neurons. Neurosci Res 29: 27–36, 1997 [DOI] [PubMed] [Google Scholar]
- Kumar A, Zou L, Yuan X, Long Y, Yang K. N-methyl-D-aspartate receptors: transient loss of NR1/NR2A/NR2B subunits after traumatic brain injury in a rodent model. J Neurosci Res 67: 781–786, 2002 [DOI] [PubMed] [Google Scholar]
- Kurz JE, Hamm RJ, Singleton RH, Povlishock JT, Churn SB. A persistent change in subcellular distribution of calcineurin following fluid percussion injury in the rat. Brain Res 1048: 153–160, 2005 [DOI] [PubMed] [Google Scholar]
- Kwak S, Weiss JH. Calcium-permeable AMPA channels in neurodegenerative disease and ischemia. Curr Opin Neurobiol 16: 281–287, 2006 [DOI] [PubMed] [Google Scholar]
- Lea PM, Custer SJ, Vicini S, Faden AI. Neuronal and glial mGluR5 modulation prevents stretch-induced enhancement of NMDA receptor current. Pharmacol Biochem Behav 73: 287–298, 2002 [DOI] [PubMed] [Google Scholar]
- Lea PM, 4th, Custer SJ, Stoica BA, Faden AI. Modulation of stretch-induced enhancement of neuronal NMDA receptor current by mGluR1 depends upon presence of glia. J Neurotrauma 20: 1233–1249, 2003 [DOI] [PubMed] [Google Scholar]
- Li G, Pei W, Niu L. Channel-opening kinetics of GluR2Q(flip) AMPA receptor: a laser-pulse photolysis study. Biochemistry 42: 12358–12366, 2003 [DOI] [PubMed] [Google Scholar]
- Li H, Prince DA. Synaptic activity in chronically injured, epileptogenic sensory-motor neocortex. J Neurophysiol 88: 2–12, 2002 [DOI] [PubMed] [Google Scholar]
- Liu SJ, Zukin RS. Ca2+-permeable AMPA receptors in synaptic plasticity and neuronal death. Trends Neurosci 30: 126–134, 2007 [DOI] [PubMed] [Google Scholar]
- Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci 25: 103–126, 2002 [DOI] [PubMed] [Google Scholar]
- McKinney JS, Willoughby KA, Liang S, Ellis EF. Stretch-induced injury of cultured neuronal, glial, and endothelial cells. Effect of polyethylene glycol-conjugated superoxide dismutase. Stroke 27: 934–940, 1996 [DOI] [PubMed] [Google Scholar]
- Miyazaki S, Katayama Y, Lyeth BG, Jenkins LW, DeWitt DS, Goldberg SJ, Newlon PG, Hayes RL. Enduring suppression of hippocampal long-term potentiation following traumatic brain injury in rat. Brain Res 585: 335–339, 1992 [DOI] [PubMed] [Google Scholar]
- Murphy TH, Blatter LA, Wier WG, Baraban JM. Spontaneous synchronous synaptic calcium transients in cultured cortical neurons. J Neurosci 12: 4834–4845, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi K, Kukita F. Functional synapses in synchronized bursting of neocortical neurons in culture. Brain Res 795: 137–146, 1998 [DOI] [PubMed] [Google Scholar]
- Oh MC, Derkach VA. Dominant role of the GluR2 subunit in regulation of AMPA receptors by CaMKII. Nat Neurosci 8: 853–854, 2005 [DOI] [PubMed] [Google Scholar]
- Osteen CL, Giza CC, Hovda DA. Injury-induced alterations in N-methyl-D-aspartate receptor subunit composition contribute to prolonged 45calcium accumulation following lateral fluid percussion. Neuroscience 128: 305–322, 2004 [DOI] [PubMed] [Google Scholar]
- Park E, Bell JD, Baker AJ. Traumatic brain injury: can the consequences be stopped? CMAJ 178: 1163–1170, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince DA, Tseng GF. Epileptogenesis in chronically injured cortex: in vitro studies. J Neurophysiol 69: 1276–1291, 1993 [DOI] [PubMed] [Google Scholar]
- Reeves TM, Kao CQ, Phillips LL, Bullock MR, Povlishock JT. Presynaptic excitability changes following traumatic brain injury in the rat. J Neurosci Res 60: 370–379, 2000 [DOI] [PubMed] [Google Scholar]
- Reeves TM, Lyeth BG, Povlishock JT. Long-term potentiation deficits and excitability changes following traumatic brain injury. Exp Brain Res 106: 248–256, 1995 [DOI] [PubMed] [Google Scholar]
- Robinson HP, Kawahara M, Jimbo Y, Torimitsu K, Kuroda Y, Kawana A. Periodic synchronized bursting and intracellular calcium transients elicited by low magnesium in cultured cortical neurons. J Neurophysiol 70: 1606–1616, 1993 [DOI] [PubMed] [Google Scholar]
- Rogawski MA, Donevan SD. AMPA receptors in epilepsy and as targets for antiepileptic drugs. Adv Neurol 79: 947–963, 1999 [PubMed] [Google Scholar]
- Salin P, Tseng GF, Hoffman S, Parada I, Prince DA. Axonal sprouting in layer V pyramidal neurons of chronically injured cerebral cortex. J Neurosci 15: 8234–8245, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders MJ, Sick TJ, Perez-Pinzon MA, Dietrich WD, Green EJ. Chronic failure in the maintenance of long-term potentiation following fluid percussion injury in the rat. Brain Res 861: 69–76, 2000 [DOI] [PubMed] [Google Scholar]
- Santhakumar V, Ratzliff AD, Jeng J, Toth Z, Soltesz I. Long-term hyperexcitability in the hippocampus after experimental head trauma. Ann Neurol 50: 708–717, 2001 [DOI] [PubMed] [Google Scholar]
- Santos SD, Carvalho AL, Caldeira MV, Duarte CB. Regulation of AMPA receptors and synaptic plasticity. Neuroscience 158: 105–125, 2009 [DOI] [PubMed] [Google Scholar]
- Schumann J, Alexandrovich GA, Biegon A, Yaka R. Inhibition of NR2B phosphorylation restores alterations in NMDA receptor expression and improves functional recovery following traumatic brain injury in mice. J Neurotrauma 25: 945–957, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzbach E, Bonislawski DP, Xiong G, Cohen AS. Mechanisms underlying the inability to induce area CA1 LTP in the mouse after traumatic brain injury. Hippocampus 16: 541–550, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidenman KJ, Steinberg JP, Huganir R, Malinow R. Glutamate receptor subunit 2 Serine 880 phosphorylation modulates synaptic transmission and mediates plasticity in CA1 pyramidal cells. J Neurosci 23: 9220–9228, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaethling JM, Klein DM, Singh P, Meaney DF. Calcium-permeable AMPA receptors appear in cortical neurons after traumatic mechanical injury and contribute to neuronal fate. J Neurotrauma 25: 1207–1216, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton MA, Ito HT, Cressy P, Kempf C, Woo JC, Schuman EM. Miniature neurotransmission stabilizes synaptic function via tonic suppression of local dendritic protein synthesis. Cell 125: 785–799, 2006 [DOI] [PubMed] [Google Scholar]
- Swanson GT, Kamboj SK, Cull-Candy SG. Single-channel properties of recombinant AMPA receptors depend on RNA editing, splice variation, and subunit composition. J Neurosci 17: 58–69, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takazawa A, Yamazaki O, Kanai H, Ishida N, Kato N, Yamauchi T. Potent and long-lasting anticonvulsant effects of 1-naphthylacetyl spermine, an analogue of Joro spider toxin, against amygdaloid kindled seizures in rats. Brain Res 706: 173–176, 1996 [DOI] [PubMed] [Google Scholar]
- Terashima A, Pelkey KA, Rah JC, Suh YH, Roche KW, Collingridge GL, McBain CJ, Isaac JT. An essential role for PICK1 in NMDA receptor-dependent bidirectional synaptic plasticity. Neuron 57: 872–882, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topolnik L, Steriade M, Timofeev I. Hyperexcitability of intact neurons underlies acute development of trauma-related electrographic seizures in cats in vivo. Eur J Neurosci 18: 486–496, 2003 [DOI] [PubMed] [Google Scholar]
- Turrigiano GG. The self-tuning neuron: synaptic scaling of excitatory synapses. Cell 135: 422–435, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vikman KS, Rycroft BK, Christie MJ. Switch to Ca2+-permeable AMPA and reduced NR2B NMDA receptor-mediated neurotransmission at dorsal horn nociceptive synapses during inflammatory pain in the rat. J Physiol 586: 515–527, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Gruenstein EI. Mechanism of synchronized Ca2+ oscillations in cortical neurons. Brain Res 767: 239–249, 1997 [DOI] [PubMed] [Google Scholar]
- Witgen BM, Lifshitz J, Smith ML, Schwarzbach E, Liang SL, Grady MS, Cohen AS. Regional hippocampal alteration associated with cognitive deficit following experimental brain injury: a systems, network and cellular evaluation. Neuroscience 133: 1–15, 2005 [DOI] [PubMed] [Google Scholar]
- Yaka R, Biegon A, Grigoriadis N, Simeonidou C, Grigoriadis S, Alexandrovich AG, Matzner H, Schumann J, Trembovler V, Tsenter J, Shohami E. D-cycloserine improves functional recovery and reinstates long-term potentiation (LTP) in a mouse model of closed head injury. Faseb J 21: 2033–2041, 2007 [DOI] [PubMed] [Google Scholar]
- Yang K, Taft WC, Dixon CE, Todaro CA, Yu RK, Hayes RL. Alterations of protein kinase C in rat hippocampus following traumatic brain injury. J Neurotrauma 10: 287–295, 1993 [DOI] [PubMed] [Google Scholar]
- Yang L, Benardo LS, Valsamis H, Ling DS. Acute injury to superficial cortex leads to a decrease in synaptic inhibition and increase in excitation in neocortical layer V pyramidal cells. J Neurophysiol 97: 178–187, 2007 [DOI] [PubMed] [Google Scholar]
- Zhang L, Rzigalinski BA, Ellis EF, Satin LS. Reduction of voltage-dependent Mg2+ blockade of NMDA current in mechanically injured neurons. Science 274: 1921–1923, 1996 [DOI] [PubMed] [Google Scholar]