Abstract
Circadian oscillations in the suprachiasmatic nucleus (SCN) depend on transcriptional repression by Period (PER)1 and PER2 proteins within single cells and on vasoactive intestinal polypeptide (VIP) signaling between cells. Because VIP is released by SCN neurons in a circadian pattern, and, after photic stimulation, it has been suggested to play a role in the synchronization to environmental light cycles. It is not known, however, if or how VIP entrains circadian gene expression or behavior. Here, we tested candidate signaling pathways required for VIP-mediated entrainment of SCN rhythms. We found that single applications of VIP reset PER2 rhythms in a time- and dose-dependent manner that differed from light. Unlike VIP-mediated signaling in other cell types, simultaneous antagonism of adenylate cyclase and phospholipase C activities was required to block the VIP-induced phase shifts of SCN rhythms. Consistent with this, VIP rapidly increased intracellular cAMP in most SCN neurons. Critically, daily VIP treatment entrained PER2 rhythms to a predicted phase angle within several days, depending on the concentration of VIP and the interval between VIP applications. We conclude that VIP entrains circadian timing among SCN neurons through rapid and parallel changes in adenylate cyclase and phospholipase C activities.
Keywords: phase response curve, pacemaker, Period gene, luciferase, Förster resonance energy transfer
coordinated rhythms across populations of neurons are believed critical to many behavioral and cognitive functions (Buzsáki 2006). The mechanisms that synchronize the periods of neural oscillators can include gap junctions that produce in-phase rhythms (Mancilla et al. 2007; Schneider et al. 2006), reciprocal inhibition producing either in-phase or anti-phase cycling (Wang and Rinzel 1992), and fast, weighted, excitatory synapses producing a range of phase relationships (Smarandache et al. 2009). Daily, or circadian, rhythms in behavior and physiology, however, depend on the neuropeptide VIP. The mechanisms by which VIP synchronizes circadian rhythms among cells are unknown.
The daily resetting of circadian timing establishes a stable phase relationship (i.e., the phase angle of entrainment) between behavioral and physiological rhythms and environmental cues. VIP is well positioned to reset circadian oscillators in the brain to each other and to exogenous timing cues. Vip and its receptors, Vipr1 and Vipr2, are expressed in the central and peripheral nervous systems (Chaudhury et al. 2008; Dietl et al. 1990; Mohney and Zigmond 1998), including in the suprachiasmatic nucleus (SCN), a master circadian pacemaker (Cagampang et al. 1998; Shinohara et al. 1999). VIP applied to SCN explants in the late subjective night induces the transcription of Period (Per)1 and Per2, two genes implicated in rhythm generation and entrainment (Nielsen et al. 2002). VIP can shift the daily rhythms in locomotion (Piggins et al. 1995) and in electrical discharge (Reed et al. 2001) and vasopressin release (Watanabe et al. 2000) of SCN explants. These actions of VIP in the SCN have been shown to depend on the activities of phospholipase C (PLC) (Nielsen et al. 2002), adenylate cyclase (AC), or protein kinase A (PKA) (Meyer-Spasche and Piggins 2004), but the signaling underlying entrainment by VIP has not been studied.
A phase-response curve (PRC) plots the steady-state shift in a rhythm as a function of the time of stimulation. A PRC can be used to predict features of entrainment, including the phase angle of entrainment, the range of periods to which the oscillator can entrain, and how long it will take to entrain (Pittendrigh 1960). Importantly, existing PRCs have not been tested for their ability to predict these features of SCN entrainment. This study aimed to generate a PRC to VIP that would predict features of entrainment and could be used to test the underlying molecular mechanisms and kinetics. We combined pharmacology with recordings of bioluminescence from a reporter of PER2 levels and of Förster resonance energy transfer (FRET) from a reporter of cAMP levels. We found that VIP directly entrains the PER2 rhythms of SCN neurons through rapid, parallel changes in AC and PLC signaling.
MATERIALS AND METHODS
Animals.
PER2::LUCIFERASE (PER2::LUC) knockin mice (Yoo et al. 2004; founders generously provided by J. S. Takahashi, Univ. of Texas Southwestern Medical Center, Dallas, TX) were housed in a 12:12-h light-dark cycle and bred as homozygous pairs in the Danforth Animal Facility of Washington University. All procedures were approved by the Animal Care and Use Committee of Washington University or Oregon Health Sciences University and followed National Institutes of Health guidelines.
Drugs.
VIP was purchased from Bachem (King of Prussia, PA) or Tocris (Ellisville, MO). MDL-12,330a (MDL), 9-(tetrahydro-2-furyl)-adenine (THFA), 1-(6-{[(17b)-3-methoxyestra-1,3,5(10)-trien-17-yl]amino}hexyl)-1H-pyrrole-2,5-dione (U-73122), forskolin, and 3-isobutyl-1-methylxanthine (IBMX) were purchased from Sigma (St. Louis, MO). (7R)-4-hydroxy-7-methoxy-N,N,N-trimethyl-3,5,9-trioxa-4-phosphaheptacosan-1-aminium-4-oxide (edelfosine) was from Tocris. Drugs were dissolved in DMSO or deionized water as stock solutions, stored at −20°C, and diluted with culture medium so that the final DMSO concentration was below 0.4% of the total volume. Culture media consisted of DMEM (Sigma) supplemented with 2% B27 (Invitrogen, Carlsbad, CA), 10 mM HEPES (Sigma), and 2.2 mg/ml NaHCO3 (Invitrogen). VIP was dissolved in culture medium, and vehicle controls consisted of an equal volume of culture medium.
Bioluminescence recording.
We recorded bioluminescence rhythms from 300-μm coronal SCN slices from PER2::LUC mice (age: 8–20 days) using a photomultiplier tube (model HC135-11, Hamamatsu, Shizuoka, Japan) as previously described (Abe et al. 2002). SCN explants were cultured on 0.4-mm membrane inserts (Millipore, Billerica, MA) in sealed 35-mm culture dishes (BD Biosciences, San Jose, CA) with 1 ml prewarmed air-buffered medium supplemented with 10% newborn calf serum (Invitrogen) and 100 μM beetle luciferin (Promega, Madison, WI) as a final concentration at 34°C. Bioluminescence counts were integrated and stored at 1-min intervals for up to 15 days of recording.
During drug applications, 500 μl of the culture medium were mixed with VIP, antagonists, or vehicle (culture medium, 5–50 μl) and added back to the culture dish. For the phase shift experiments, circadian times were calculated in hours from the peak of the PER2::LUC rhythm (CT12) (Yoo et al. 2004). To minimize artifacts, treatments were added without subsequent removal.
To measure the phase shift, bioluminescence data were detrended by subtracting a 24-h running average (Abe et al. 2002), and the daily peak of expression was determined using an acrophase fitting function with Clocklab software (Actimetrics, Wilmette, IL). Phase shifts were measured as the time difference between linear regressions of the acrophases on the days before a treatment and the 4–5 days after treatment. In some cases, the shift was measured after one to three cycles of transient shifts. The period of the PER2::LUC rhythm was measured as the average time between acrophases from at least 4 days of recording. The induction of PER2 expression was measured by averaging the raw bioluminescence signal of the cycle with the VIP or vehicle treatment. All statistics were performed with Origin 7.0 software (Origin, Northampton, MA).
ELISA.
We measured VIP concentrations to determine the profile of the neuropeptide from eight mouse SCN explants treated with either 1 μM VIP (n = 4) or vehicle (n = 4). Medium (40 μl) was collected from each culture at 0, 10, 30, 60, and 120 min and 24 h after treatment, immediately frozen at −35°C, and stored at −80°C. A competitive ELISA was performed according to the manufacturer's protocol (Peninsula Laboratories, San Carlos, CA). Absorbance was read at 450 nm with a microplate spectrophotometer (Molecular Devices, Menlo Park, CA). A standard curve was generated with serially diluted standards ranging from 0 to 10 ng/ml and an IC50 of 0.24 ng/ml.
cAMP measurement.
SCN cultures were prepared from neonatal Sprague-Dawley rats and transfected with a cAMP reporter using the biolistic method as previously described (Ikeda et al. 2003). Briefly, neonatal rat pups (3–7 days old) were decapitated, the brains were removed, and 200- to 300-μm-thick coronal slices were cut with a vibrating blade microtome (Camden Instruments, Lafayette, IN). Slices were placed on Millicell-CM membranes (30-mm diameter, 0.4 μm, Millipore) and maintained in an incubator at 37°C with 5% CO2. Organotypic cultures were grown in culture media consisting of DMEM-High without l-glutamine and with sodium pyruvate (Hyclone, Thermo Scientific, Waltham, MA), 2% B27 supplement (GIBCO, Carlsbad, CA), 10 mM HEPES (GIBCO), and 1× GlutaMax (GIBCO).
cAMP activity was measured using a fusion protein consisting of cyan fluorescent protein (CFP), truncated Epac1 expressing a cAMP-binding site, and yellow fluorescent protein (YFP) (DiPilato et al. 2004; Dunn et al. 2006). The cDNA for ICUE2 was kindly provided by Dr. Jin Zhang and Dr. Marla B. Feller (DiPilato et al. 2004; Dunn et al. 2006; Violin et al. 2008). A Helios Gene Gun (Bio-Rad Laboratories) was used according to the manufacturer's instructions to transfect the ICUE2 cDNA, driven by a cytomegalovirus promoter, into 2- to 20-day-old cultures. Individual neurons were imaged between 2 and 7 days after transfection. Slice cultures were transferred to a recording chamber (35°C) with a laminar flow (6–8 ml/min) of ACSF solution consisting of (in mM) 124 NaCl, 2.5 KCl, 1.2 NaH2PO4, 1.2 MgCl2, 2.4 CaCl2, 10 glucose, and 24 NaHCO3, adjusted to 300 mosM and bubbled with 5% CO2 and 95% O2. The recording chamber was located on the stage of an inverted microscope (Nikon TE2000E, Toyko, Japan), illuminated using a xenon arc lamp, and passed through a 436/20-nm filter (Chroma, Technical Corporation, Bellows Fall, VT) within a Lambda 10-3 filterwheel (Sutter Instruments, Novata, CA) and with light reflected by a 455dcxru dichroic filter (Chroma, Technical Corporation). Images were visualized using an ORCA ER charge-coupled device camera (Hamamatsu Photonics) after being passed through a Dual-View beam splitter at 505dcxr (Optical Insights), with 535/40- and 480/30-nm emission filters. Data acquisition was controlled by Metafluor software (Molecular Devices, Sunnyvale, CA) with binning and light exposure optimized to minimize photobleaching. The FRET ratio, fluorescence at 535/480 nm after background subtraction at each wavelength, was normalized using the ratio before the application of VIP. Neurons were identified by morphological appearance. At the end of each experiment, neurons were treated with forskolin (20 μM) and IBMX (75 μM). Cells that did not respond were excluded.
Statistics.
Unless otherwise specified, comparison between two different groups with one variable was performed using one-way ANOVA with a Scheffé post hoc test and comparison between two different groups with two variables using two-way ANOVA with a Tukey post hoc test. Values were considered significant if P < 0.05. All statistics and curve fits were performed with Origin 7.0 (Origin) or Excel 11.5 (Microsoft, Redmond, WA) except Rayleigh statistics (Oriana, Kovach Computing Services, UK).
RESULTS
VIP shifts of PER2::LUC rhythms depend on phase and dose.
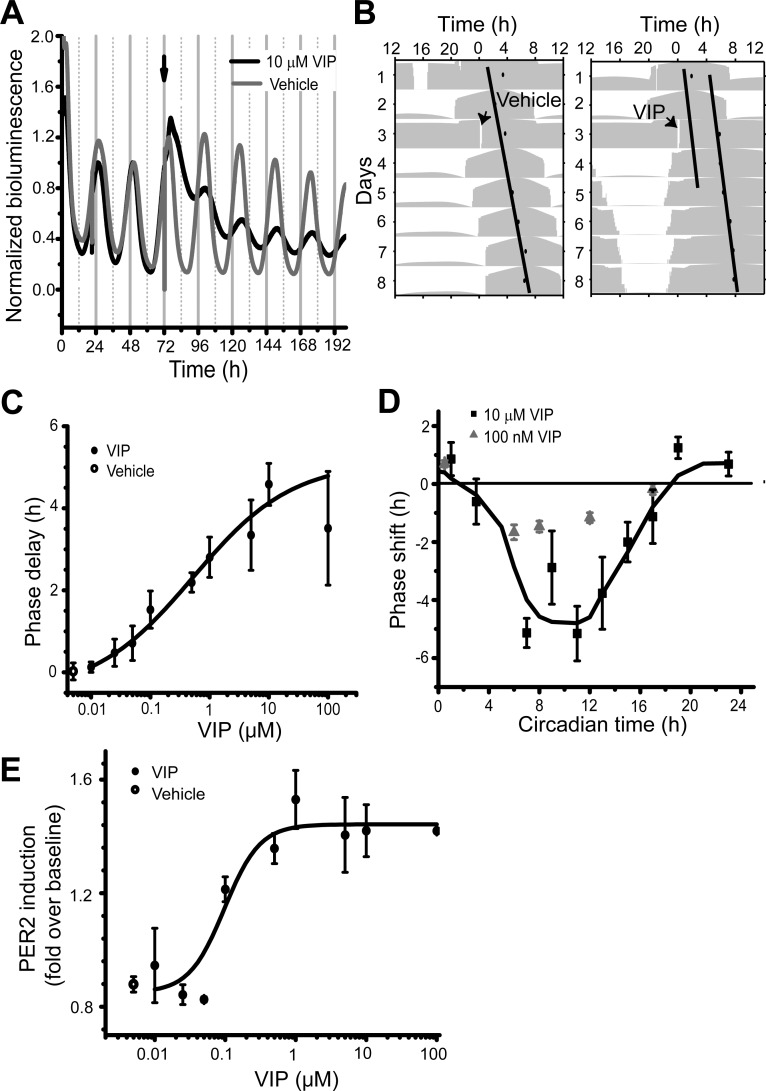
We monitored the effects of 10 nM–100 μM VIP on PER2-driven LUC activity in SCN explants. The half-life of VIP applied to SCN cultures was ∼2 h based on ELISA measurements (Supplemental Material, Supplemental Fig. S1).1 VIP application near the peak of PER2 expression (CT12) reduced the subsequent amplitude and delayed the peak of subsequent cycles compared with vehicle-treated cultures (Fig. 1A). This study focused on the phase-shifting effects of VIP, leaving cause and relevance of the amplitude effects for a subsequent analysis. The steady-state phase shift after 4 days (Fig. 1B) was measured as a function of VIP concentration (Fig. 1C). When applied at CT12, VIP induced a dose-dependent delay in the peak of PER2 expression, with a threshold of ∼100 nM (P < 0.05 compared with vehicle treatment, one-way ANOVA, Scheffé post hoc test, F1,15 = 11.15), an EC50 near 500 nM, and saturation of ∼10 μM. VIP also induced a transient increase in PER2 expression with a similar dose dependence (Fig. 1E). The threshold for VIP-mediated PER2 induction was ∼100 nM, similar to the threshold for phase shifting (P < 0.05 compared with vehicle treatment, one-way ANOVA, Scheffé post hoc test, F1,6 = 7.85; EC50 near 100 nM, and saturation of ∼5 μM). Thus, a 10-fold increase in VIP concentration approximately doubled the steady-state phase delay of SCN rhythmicity (r2 = 0.98, n = 30 SCN explants).
Fig. 1.
VIP phase shifts Period (PER)2::LUCIFERASE (LUC) rhythms in the suprachiasmatic nucleus (SCN). A: representative PER2::LUC traces with 10 μM VIP (black trace) or vehicle (gray trace) treatment at CT12 (arrow). Each PER2::LUC rhythm was normalized to the peak before treatment. B: single-plotted actograms of the bioluminescence traces from A. The acrophase (black circle) of PER2::LUC expression was the daily peak of a sine function fit to each cycle's bioluminescence trace (Herzog et al. 2004). Phase shifts were measured as the time difference between linear fits to the acrophases before and after the treatment. C: dose-dependent phase delays induced by VIP applied at CT12. Above 100 nM VIP and below 10 μM VIP, the delay of the PER2::LUC rhythm increased linearly with logarithmic increases in the VIP concentration. Data were fitted with a logistic function (black line). D: phase-response curves (PRCs) for 10 μM or 100 nM VIP (n = 63 and n = 33, respectively) as a function of circadian time of VIP application. Phase delays and advances are shown as negative and positive values, respectively. The PRC for 10 μM VIP was fitted with a fast Fourier transform and adjacent-point average (line). Note that the PRC is dominated by delays and unlike the PRC to light. E: concentration-response curve for the transient induction of PER2 by VIP. Data were fitted with a logistic function (black line).
To measure whether VIP has phase-dependent effects on SCN rhythms, either 100 nM or 10 μM VIP was applied at various circadian time points. The resulting VIP PRCs had a large delay zone from early subjective day to early subjective night (CT3–18) and a small advance zone from late subjective night to early subjective day (CT19–1) with significant differences between the times of advances and delays (P < 0.01, F1,115 = 7.11, n = 63, two-way ANOVA, Tukey post hoc test; Fig. 1D). VIP (10 μM) treatments at CT11–12 induced a phase delay that was similar in magnitude at 1, 2, and 3 days after treatment (P = 0.77, one-way ANOVA, F1,18 = 0.09). In contrast, the same VIP treatment at CT19–23 induced advances that were larger when measured the day after treatment than on subsequent days (P = 0.02, one-way ANOVA, F1,16 = 6.17; Supplemental Fig. 2). VIP (10 μM) induced larger delays than 100 nM VIP at most of the time points tested except the early subjective day. Vehicle treatment induced no or little shift (P < 0.05, two-way ANOVA, Tukey post hoc test, compared with 10 μM VIP, n = 41). Therefore, adjustment of daily PER2 rhythms depended on the time of administration and concentration of VIP.
Blockade of both AC and PLC activities is required to suppress VIP-induced phase shifts.
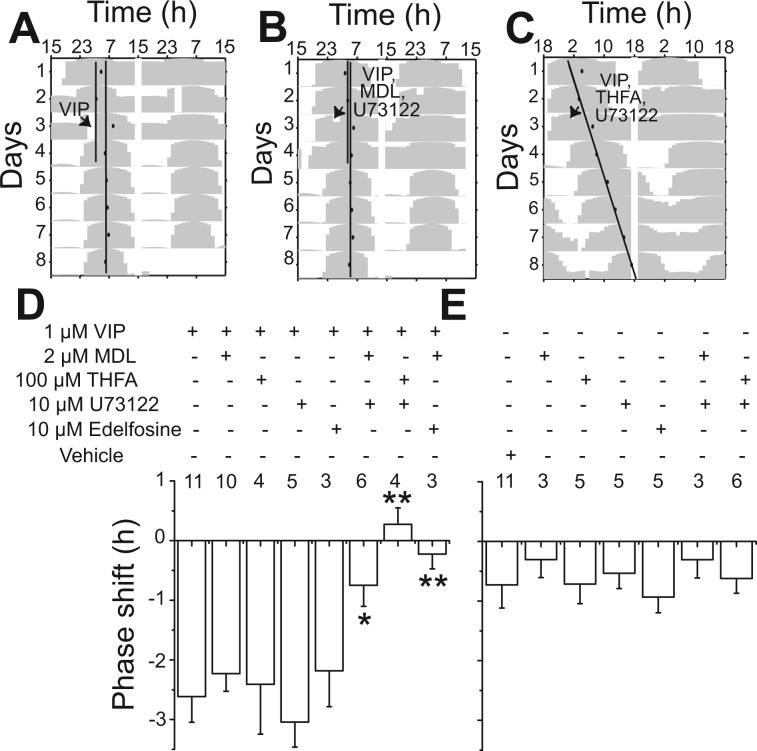
Previous findings have indicated that in the SCN, VIP may signal through cAMP- or Ca2+-mediated pathways. We examined the effects of two inhibitors of AC and two inhibitors of PLC on VIP-induced phase shifts. At the time of VIP administration, we included MDL, an irreversible, competitive inhibitor of AC (Lippe and Ardizzone 1991), THFA, a noncompetitive inhibitor of AC (O'Neill et al. 2008), U-73122, an inhibitor of PLC (Smith et al. 1990), or edelfosine, a specific inhibitor of PLC (Powis et al. 1992). VIP-induced phase shifts of the PER2::LUC rhythms at CT12 were significantly reduced by the combined application of MDL (2 μM) or THFA (100 μM) with U-73122 (10 μM) or edelfosine (10 μM) 1 h before VIP application (P < 0.05, F1,15 = 8.47 for MDL + U-73122; P < 0.01, F1,12 = 10.6 for THFA + U-73122; P < 0.01, F1,15 = 15.89 for MDL + edelfosine, one-way ANOVA, Scheffé post hoc test; Fig. 2, A–D). None of the inhibitors alone reduced the VIP-induced phase shift compared with VIP (P > 0.05; Fig. 2D) or induced a significant shift compared with vehicle (P > 0.05, one-way ANOVA, Scheffé post hoc test; Fig. 2E). The inhibitors alone or in combination also did not reduce the amplitude of PER2 rhythms compared with vehicle. These results indicate that VIP signals in parallel through AC and PLC to phase shift SCN rhythms.
Fig. 2.
Blockade of cAMP and phospholipase C (PLC) signaling was required to reduce VIP-induced phase shifts. A–C: representative actograms of PER2::LUC rhythms with treatment of 1 μM VIP alone (A), VIP with 2 μM MDL-12,300a [MDL; inhibitor of adenylate cyclase (AC)] + 10 μM U-73122 (inhibitor of PLC) (B), and VIP with 100 μM 9-(tetrahydro-2furyl)-adenosine (THFA; inhibitor of AC) + 10 μM U-73122 (C), respectively. The inhibitor cocktails were applied 1 h before the VIP application at CT12. D: the phase shift (means ± SE) of PER2::LUC rhythms by VIP applied at CT12 was significantly attenuated by the combination of AC and PLC inhibitors (*P < 0.05 and **P < 0.01, one-way ANOVA with Scheffé post hoc test). E: in the absence of VIP, the inhibitors induced little to no phase shifts (n = 25, compared with vehicle-treated cultures, P > 0.05). The number of cultures recorded is shown for each treatment.
VIP elevates cAMP in most SCN neurons.
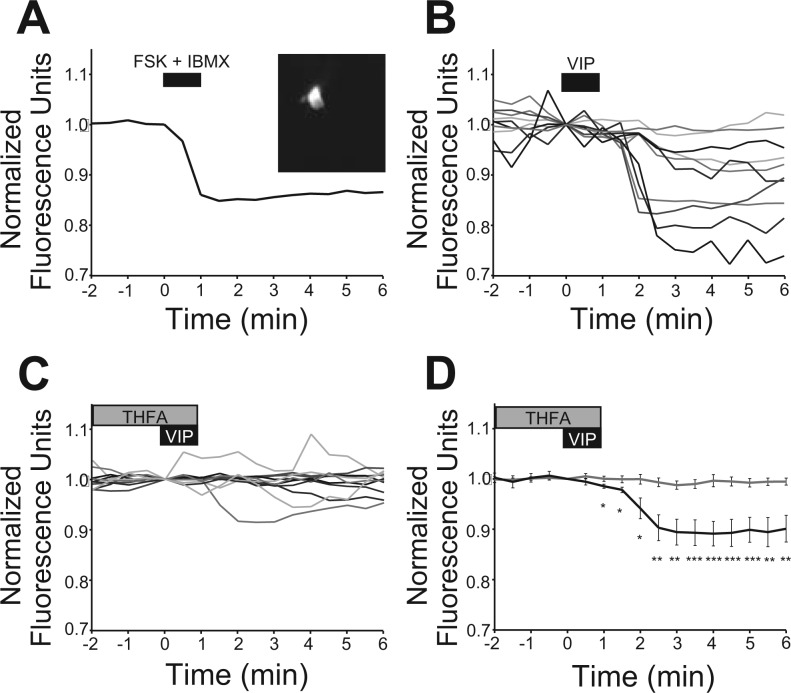
Previous reports have indicated that VIP induces the accumulation of cAMP in the SCN independent of circadian time (Rea 1990; Vanecek and Watanabe 1998). To measure the time course of this response in individual SCN neurons and to validate the efficacy of antagonists of AC activity, the cAMP reporter ICUE2 was transfected into individual SCN neurons (Fig. 3A). ICUE2 and other Epac-based FRET reporters detect increases in cAMP as a decrease in the YFP-to-CFP ratio reliably in the fly brain (Shafer et al. 2008), cultured retina (Dunn et al. 2006), hippocampus (Nikolaev et al. 2004), dorsal root ganglion (Murray et al. 2009), and mammalian cell lines (DiPilato et al. 2004; Nikolaev et al. 2004; Ponsioen et al. 2004). To confirm the reliability of ICUE2 in the SCN, we applied a combination of forskolin (20 μM), an activator of most ACs, and IBMX (75 μM), an inhibitor of cAMP phosphodiesterase, to ICUE2-expressing SCN neurons at the end of each experiment. This treatment reduced the normalized FRET ratio (Fig. 3A) within 4 min by 0.14 ± 0.02 ratio units in 21 neurons from 10 slice cultures. Application of VIP (1 μM) for 1 min reduced the FRET ratio in 10 SCN neurons in 4 slice cultures, consistent with a second messenger process (Fig. 3, B and D). The duration of the FRET response varied with some neurons returning to near baseline within 10 min while others persisted longer than 20 min or as long as we recorded. Addition of THFA (100 μM) to the bath 2 min before and during VIP application significantly reduced the response of SCN neurons (measured 3 min after VIP, t13 = 3.88, P ≤ 0.005, unpaired two-tailed t-test, n = 11, 6 slice cultures; Fig. 3, C and D), suggesting that intracellular VIP signaling acts, at least in part, through cAMP signaling.
Fig. 3.
VIP increased cAMP in SCN neurons. A: a representative SCN neuron expressing the ICUE2 reporter (inset image) showed a reduced Förster resonance energy transfer (FRET) ratio (535/480 nm) after treatment with forskolin (FSK; 20 μM) and 3-isobutyl-1-methylxanthine (IBMX; 75 μM), indicative of increased intracellular cAMP. Data were normalized to the ratio at the initiation of treatment. B: treatment with VIP (1 μM) reduced the FRET ratio in SCN neurons. Each line represents a different neuron. C: pretreatment (2 min) with THFA (100 μM) attenuated the VIP (1 μM)-induced reduction in the FRET ratio. D: plot of mean ± SE responses to VIP (black, n = 10; see B) and THFA + VIP (gray, n = 11; see C) showing that THFA treatment significantly reduced cAMP induction by VIP (unpaired two-tailed t-test, *P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.005).
VIP entrains PER2::LUC rhythms to a predicted phase angle.
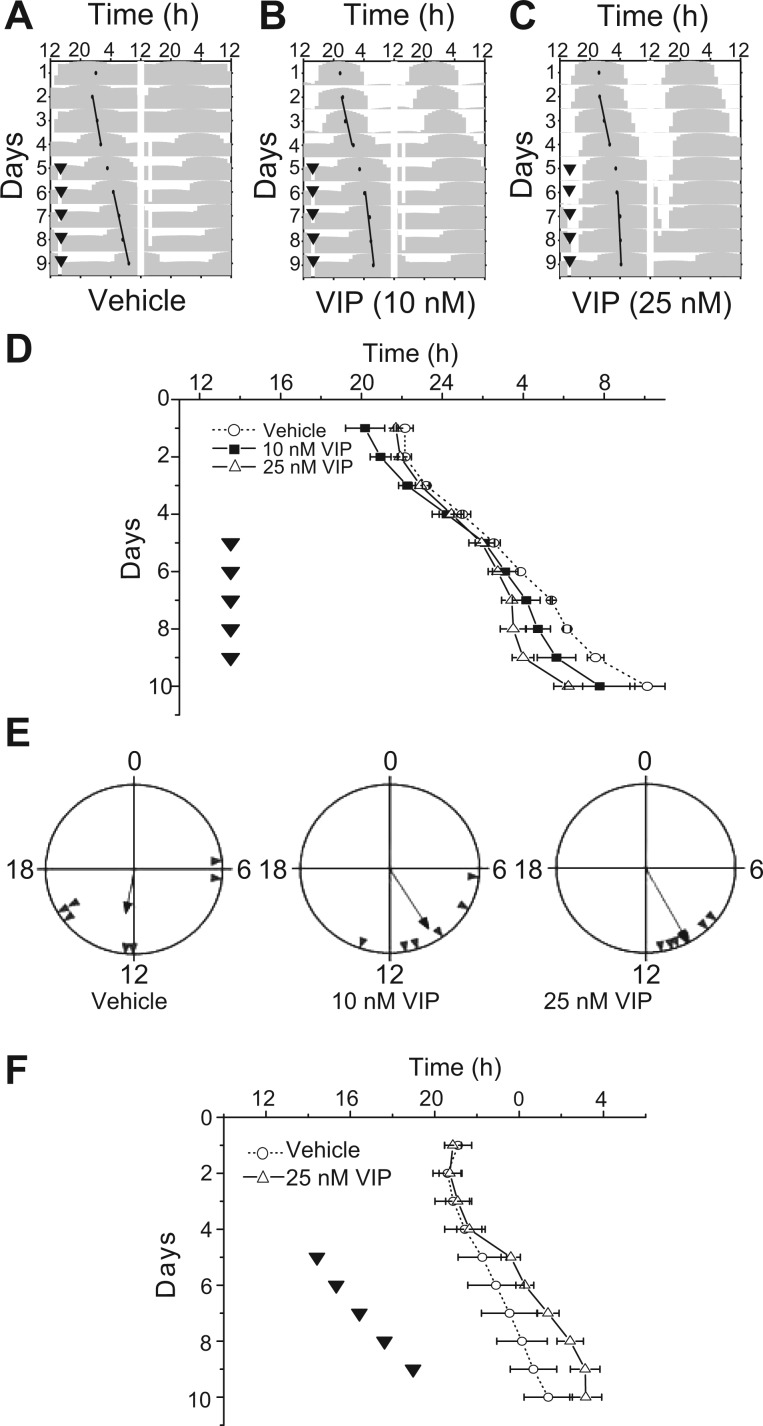
We investigated if and how the SCN circadian pacemaker might entrain to daily VIP stimulation. Our VIP PRC predicted that daily VIP treatment should fall around CT2 to entrain the SCN, ∼10 h before the peak of PER2::LUC bioluminescence (CT12). VIP (10 or 25 nM) or vehicle was applied to SCN cultures for 5 consecutive days starting on the fifth day of the bioluminescence recording. The period of SCN cultures was not altered by the vehicle treatment (before: 24.5 ± 0.3 h vs. during: 24.5 ± 0.2 h, n = 7 cultures, P = 0.97, one-way ANOVA; Fig. 4, A and D). In contrast, the circadian period was shortened by daily 25 nM VIP, with the bioluminescence peak occurring 10.1 ± 0.4 h after VIP on the last day of application (period before: 24.7 ± 0.2 h vs. days 2–5 of the treatment: 24.4 ± 0.2 h, n = 7 cultures; Fig. 4, C and D). Notably, the phases of cultures treated with 25 nM VIP were more synchronized (Rayleigh test, P < 0.05, r = 0.97) than those of vehicle-treated cultures (P > 0.1, r = 0.52; Fig. 4E). A lower dose of VIP (10 nM) had a smaller effect on the period (before: 25.0 ± 0.3 h vs. during: 24.7 ± 0.2 h) and a less reliable effect on the phase angle of entrainment (10.0 ± 1.1 h, n = 6 cultures; Fig. 4, B and D). Application of 25 nM VIP on a 25-h cycle also entrained the period of SCN cultures (24.4 ± 0.1 h before and 25.0 ± 0.2 h during the application, n = 4 cultures) to the predicted phase so that the VIP application fell 7.6 ± 0.7 h before the peak of bioluminescence (Fig. 4F). In contrast, vehicle treatments every 25-h failed to change the period (24.4 ± 0.2 h before vs. 24.6 ± 0.1 h during, n = 2 cultures). Therefore, daily VIP applications entrained PER2::LUC rhythms to the predicted phase angles.
Fig. 4.
VIP entrained PER2::LUC rhythms. A–C: representative actograms of SCN PER2::LUC rhythms during 5 consecutive days of treatment (▾) with either vehicle (A), 10 nM VIP (B), or 25 nM VIP (C). Note that the daily peak of PER2::LUC rhythms shifted to follow the daily VIP pulse by ∼10 h and free ran from that phase after the last VIP treatment. D: the daily peak of PER2::LUC rhythms (means ± SE) of SCN cultures treated with VIP or vehicle indicated that VIP entrained circadian rhythms in the cultured SCN (vehicle, n = 2; 10 nM VIP, n = 3; and 25 nM VIP, n = 3). Similar results were found in two additional replications of this experiment. E: representative Rayleigh plots showing the phase angle of PER2::LUC expression (gray triangles) from cultures treated with vehicle (n = 7), 10 nM VIP (n = 6), or 25 nM VIP (n =7) on the fifth day of treatment. The time between the peak phase of the SCN cultures and the time of treatment is the phase angle of entrainment. Note that cultures treated with VIP had similar times of peak PER2::LUC bioluminescence, whereas vehicle-treated cultures did not tend to peak at similar times. F: the daily peak of PER2::LUC rhythms (means ± SE) over 10 days showed that SCN cultures (▵) synchronized their phase and period to 25 nM VIP applied every 25 h (▾) for 5 consecutive days and free ran (○) when treated with vehicle.
DISCUSSION
The mechanisms by which circadian rhythms synchronize to daily timing cues have been formally described as a result of rapid changes in either phase or period of the endogenous oscillator (Comas et al. 2006). Rapid phase adjustment, or nonparametric entrainment, has been shown in the eclosion rhythm of flies (Zimmerman et al. 1968), locomotor activity of nocturnal rodents (Pittendrigh and Daan 1976), and in vitro oscillation of cyanobacterial genes (Yoshida et al. 2009) and predicted for the SCN (Best et al. 1999). Our results implicate VIP in rapid phase adjustment of the SCN on a daily basis. Single pulses of VIP shifted the phase, rather than the period, of the SCN (Supplemental Fig. S3) and repeated pulses entrained SCN rhythms. Importantly, VIP doses near the threshold for phase shifts, when applied daily, entrained circadian rhythms of PER2. VIP similarly entrains circadian rhythms in cortical astrocytes (Marpegan et al. 2009). Thus, we postulate that VIP shifts the oscillations of SCN cells through rapid changes in their clock gene expression. In the SCN, VIP release is both circadian (Shinohara et al. 1995) and increased by light (Shinohara et al. 1995; Shinohara et al. 1993). Based on the PRCs measured here, we make two predictions: 1) increases in VIP release due to light during the day delay SCN rhythms; and 2) in the absence of light, circadian release of VIP peaking around midday delays free-running SCN rhythms. These predictions are consistent with the advanced phase angle of entrainment in a light cycle and shortened free-running period in constant darkness reported in VIP-deficient mice (Colwell et al. 2003).
The PRC to VIP described here is the first based on shifts in PER2 expression in the SCN and differs in shape and amplitude from a PRC to light and also differs in some respects from published responses to VIP. The PRCs for VIP-induced shifts in vasopressin and multiunit firing rate rhythms were previously described as light like with advances three to eight times larger than the shifts reported here (Reed et al. 2001; Watanabe et al. 2000). We found that advances were larger when measured on the day after VIP treatment (as was done in previous studies) compared with the steady-state shift (Supplemental Fig. S2). This illustrates that the isolated SCN can exhibit large, transient adjustments in phase similar to what has been described for behavioral shifts to light during the late night. Importantly, PRCs based on the first day or two after a treatment can differ substantially from the steady-state PRC. By measuring the steady-state PRC to VIP applied at many different circadian times from long-term recordings of SCN, we conclude the VIP PRC differs from the effects of light in vivo or glutamate in vitro on SCN rhythms. The VIP PRC is dominated by a large delay zone. Although this could be unique to PER2, it is consistent with the period-lengthening effects of chronic VIP infusion on locomotor activity (Pantazopoulos et al. 2010).
Interestingly, the PRC to VIP in the SCN shared a similar shape and amplitude as the PRC to the neuropeptide pigment dispersing peptide (PDF) in the cockroach (Petri and Stengl 1997). PDF in flies appears to play roles similar to mammalian VIP in entrainment and synchrony among circadian oscillators (Lin et al. 2004). Thus, the steady-state PRC with a large delay zone and low-amplitude, narrow advance zone may have features that facilitate coordinated rhythmicity in populations of circadian cells.
The rate of entrainment and the phase angle of entrainment both depended on the concentration of VIP and whether VIP was applied on a 24-h cycle or a 25-h cycle. Thus, aging, light intensity, and other events that change VIP levels or time of release would be expected to impact circadian behaviors. This is consistent with, for example, evidence that age-related changes in VIP timing and levels are intimately associated with menopause in rats (Gerhold et al. 2005; Krajnak et al. 1998). It is clear, however, that VIP is not the sole entraining agent of the SCN since, for example, mice lacking VIP or its receptor can still entrain to light cycles. Photic entrainment likely depends on neuropeptides and transmitters including VIP (Piggins et al. 1995; Reed et al. 2001; Watanabe et al. 2000), pituitary adenylate cyclase-activating polypeptide (Hannibal et al. 1997; Harrington et al. 1999), gastrin-releasing peptide (Albers et al. 1995; Kallingal and Mintz 2006; McArthur et al. 2000; Piggins et al. 1995), glutamate (Asai et al. 2001; Ding et al. 1994; Meijer et al. 1988), and nitric oxide (Ding et al. 1994) and intracellular signals including cAMP (Prosser and Gillette 1989) and cGMP (Liu et al. 1997; Prosser et al. 1989).
Although the exact sites of action for VIP-induced entrainment in the SCN are unknown, we found that VIP increased intracellular cAMP in individual SCN neurons (Fig. 3B). The VPAC2 receptor, encoded by the Vipr2 gene, is presumed to be the primary mediator of VIP activity since the loss of the receptor produces a phenotype similar to loss of VIP (Colwell et al. 2003; Harmar et al. 2002). Consistent with our findings, Vipr2 mRNA (Cagampang et al. 1998; Shinohara et al. 1999) and a transgenic reporter using the human Vipr2 promoter (Kallo et al. 2004) have been shown to be widely expressed in the SCN.
We found convergence of VIP signaling to both AC and PLC signaling pathways in the SCN. Here, low doses of antagonists against the two pathways suppressed VIP-induced shifts, whereas blockade of a single pathway had little effect on shifts. This implicates Gs in VIP-mediated responses and also raises the possibility for the involvement of Gq or Gi/o activities (Aton et al. 2006; Gillette and Mitchell 2002; Hains et al. 2004; Stewart et al. 2007; Trimble et al. 1987; Van Rampelbergh et al. 1997). These results are consistent with previous observations that signals like glutamate can shift SCN rhythms while raising cAMP levels at times when cAMP alone does not shift SCN rhythms (Prosser and Gillette 1989; Tischkau et al. 2000) and that the blockade of cAMP production does not prevent these shifts (Tischkau et al. 2000). We postulate that VIP requires concurrent increases in AC and PLC activities to shift SCN circadian timing. It remains an interesting possibility that changes in the contributions of AC and PLC pathways after VIP stimulation shape the response to VIP at different circadian times (Jenkins et al. 2007; O'Neill et al. 2008; Obrietan et al. 1999).
Previous reports have shown that prolonged blockade of cAMP production or Ca2+ in the rodent SCN or snail retina stop the progression of circadian rhythms (Khalsa et al. 1993; Lundkvist et al. 2005; O'Neill et al. 2008; Ralph et al. 1992). Here, the low concentrations of antagonists that blocked VIP-induced phase shifts had little effect on PER2 rhythms over 5–6 days. We conclude that the mechanisms mediating shifts to VIP are more sensitive to acute changes in cAMP and Ca2+ levels than the mechanisms involved in rhythm generation.
Taken together, the findings presented here implicate VIP in the synchronization of SCN neurons to each other and environmental cycles via increases in AC and PLC signaling to rapidly shift clock gene rhythms.
GRANTS
This work was supported by Imaging Science Pathway Fellowship T90 DA022871 and National Institutes of Health Grants MH-63107 (to E. D. Herzog) and NS-036607 (to C. N. Allen).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Tatiana Simon for technical support and Luciano Marpegan and other Herzog laboratory members for helpful discussions. Karin Mullendorff and Michelle Sorensen provided invaluable assistance in the preparation of the organotypic cultures and ICUE2 expression. The authors are grateful to J. Diani, T. Keadle, and D. Piatchek of the Washington University Danforth Campus animal facility for animal care.
Footnotes
Supplemental Material for this article is available at the Journal of Neurophysiology website.
REFERENCES
- Abe M, Herzog ED, Yamazaki S, Straume M, Tei H, Sakaki Y, Menaker M, Block GD. Circadian rhythms in isolated brain regions. J Neurosci 22: 350–356, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albers HE, Gillespie CF, Babagbemi TO, Huhman KL. Analysis of the phase shifting effects of gastrin releasing peptide when microinjected into the suprachiasmatic region. Neurosci Lett 191: 63–66, 1995. [DOI] [PubMed] [Google Scholar]
- Asai M, Yamaguchi S, Isejima H, Jonouchi M, Moriya T, Shibata S, Kobayashi M, Okamura H. Visualization of mPer1 transcription in vitro: NMDA induces a rapid phase shift of mPer1 gene in cultured SCN. Curr Biol 11: 1524–1527, 2001. [DOI] [PubMed] [Google Scholar]
- Aton SJ, Huettner JE, Straume M, Herzog ED. GABA and Gi/o differentially control circadian rhythms and synchrony in clock neurons. Proc Natl Acad Sci USA 103: 19188–19193, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best JD, Maywood ES, Smith KL, Hastings MH. Rapid resetting of the mammalian circadian clock. J Neurosci 19: 828–835, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G. Rhythms of the Brain. Oxford: Oxford Univer. Press, 2006, p. xiv. [Google Scholar]
- Cagampang FR, Sheward WJ, Harmar AJ, Piggins HD, Coen CW. Circadian changes in the expression of vasoactive intestinal peptide 2 receptor mRNA in the rat suprachiasmatic nuclei. Brain Res Mol Brain Res 54: 108–112, 1998. [DOI] [PubMed] [Google Scholar]
- Chaudhury D, Loh DH, Dragich JM, Hagopian A, Colwell CS. Select cognitive deficits in vasoactive intestinal peptide deficient mice. BMC Neurosci 9: 63, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colwell CS, Michel S, Itri J, Rodriguez W, Tam J, Lelievre V, Hu Z, Liu X, Waschek JA. Disrupted circadian rhythms in VIP and PHI deficient mice. Am J Physiol Regul Integr Comp Physiol 285: R939–R949, 2003. [DOI] [PubMed] [Google Scholar]
- Comas M, Beersma DG, Spoelstra K, Daan S. Phase and period responses of the circadian system of mice (Mus musculus) to light stimuli of different duration. J Biol Rhythms 21: 362–372, 2006. [DOI] [PubMed] [Google Scholar]
- Dietl MM, Hof PR, Martin JL, Magistretti PJ, Palacios JM. Autoradiographic analysis of the distribution of vasoactive intestinal peptide binding sites in the vertebrate central nervous system: a phylogenetic study. Brain Res 520: 14–26, 1990. [DOI] [PubMed] [Google Scholar]
- Ding JM, Chen D, Weber ET, Faiman LE, Rea MA, Gillette MU. Resetting the biological clock: mediation of nocturnal circadian shifts by glutamate and NO. Science 266: 1713–1717, 1994. [DOI] [PubMed] [Google Scholar]
- DiPilato LM, Cheng X, Zhang J. Fluorescent indicators of cAMP and Epac activation reveal differential dynamics of cAMP signaling within discrete subcellular compartments. Proc Natl Acad Sci USA 101: 16513–16518, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn TA, Wang CT, Colicos MA, Zaccolo M, DiPilato LM, Zhang J, Tsien RY, Feller MB. Imaging of cAMP levels and protein kinase A activity reveals that retinal waves drive oscillations in second-messenger cascades. J Neurosci 26: 12807–12815, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhold LM, Rosewell KL, Wise PM. Suppression of vasoactive intestinal polypeptide in the suprachiasmatic nucleus leads to aging-like alterations in cAMP rhythms and activation of gonadotropin-releasing hormone neurons. J Neurosci 25: 62–67, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillette MU, Mitchell JW. Signaling in the suprachiasmatic nucleus: selectively responsive and integrative. Cell Tissue Res 309: 99–107, 2002. [DOI] [PubMed] [Google Scholar]
- Hains MD, Siderovski DP, Harden TK. Application of RGS box proteins to evaluate G-protein selectivity in receptor-promoted signaling. Methods Enzymol 389: 71–88, 2004. [DOI] [PubMed] [Google Scholar]
- Hannibal J, Ding JM, Chen D, Fahrenkrug J, Larsen PJ, Gillette MU, Mikkelsen JD. Pituitary adenylate cylcase-activating peptide (PACAP) in the retinohypothalamic tract: a potential daytime regulator of the biological clock. J Neurosci 17: 2637–2644, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmar AJ, Marston HM, Shen S, Spratt C, West KM, Sheward WJ, Morrison CF, Dorin JR, Piggins HD, Reubi JC, Kelly JS, Maywood ES, Hastings MH. The VPAC2 receptor is essential for circadian function in the mouse suprachiasmatic nuclei. Cell 109: 497–508, 2002. [DOI] [PubMed] [Google Scholar]
- Harrington ME, Hoque S, Hall A, Golombek D, Biello S. Pituitary adenylate cyclase activating peptide phase shifts circadian rhythms in a manner similar to light. J Neurosci 19: 6637–6642, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog ED, Aton SJ, Numano R, Sakaki Y, Tei H. Temporal precision in the mammalian circadian system: a reliable clock from less reliable neurons. J Biol Rhythms 19: 35–46, 2004. [DOI] [PubMed] [Google Scholar]
- Ikeda M, Sugiyama T, Wallace CS, Gompf HS, Yoshioka T, Miyawaki A, Allen CN. Circadian dynamics of cytosolic and nuclear Ca2+ in single suprachiasmatic nucleus neurons. Neuron 38: 253–263, 2003. [DOI] [PubMed] [Google Scholar]
- Jenkins TC, Andrews JB, Meyer-Bernstein EL. Daily oscillation of phospholipase C β4 in the mouse suprachiasmatic nucleus. Brain Res 1178: 83–91, 2007. [DOI] [PubMed] [Google Scholar]
- Kallingal GJ, Mintz EM. Glutamatergic activity modulates the phase-shifting effects of gastrin-releasing peptide and light. Eur J Neurosci 24: 2853–2858, 2006. [DOI] [PubMed] [Google Scholar]
- Kallo II, Kalamatianos T, Wiltshire N, Shen S, Sheward WJ, Harmar AJ, Coen CW. Transgenic approach reveals expression of the VPAC receptor in phenotypically defined neurons in the mouse suprachiasmatic nucleus and in its efferent target sites. Eur J Neurosci 19: 2201–2211, 2004. [DOI] [PubMed] [Google Scholar]
- Khalsa SBS, Ralph MR, Block GD. The role of extracellular calcium in generating and in phase–shifting the Bulla ocular circadian rhythm. J Biol Rhythms 8: 125–139, 1993. [DOI] [PubMed] [Google Scholar]
- Krajnak K, Kashon ML, Rosewell KL, Wise PM. Aging alters the rhythmic expression of vasoactive intestinal polypeptide mRNA but not arginine vasopressin mRNA in the suprachiasmatic nuclei of female rats. J Neurosci 18: 4767–4774, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Stormo GD, Taghert PH. The neuropeptide pigment-dispersing factor coordinates pacemaker interactions in the Drosophila circadian system. J Neurosci 24: 7951–7957, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippe C, Ardizzone C. Actions of vasopressin and isoprenaline on the ionic transport across the isolated frog skin in the presence and the absence of adenyl cyclase inhibitors MDL12330A and SQ22536. Comp Biochem Physiol C 99: 209–211, 1991. [DOI] [PubMed] [Google Scholar]
- Liu C, Ding JM, Faiman LE, Gillette MU. Coupling of muscarinic cholinergic receptors and cGMP in nocturnal regulation of the suprachiasmatic circadian clock. J Neurosci 17: 659–666, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundkvist GB, Kwak Y, Davis EK, Tei H, Block GD. A calcium flux is required for circadian rhythm generation in mammalian pacemaker neurons. J Neurosci 25: 7682–7686, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancilla JG, Lewis TJ, Pinto DJ, Rinzel J, Connors BW. Synchronization of electrically coupled pairs of inhibitory interneurons in neocortex. J Neurosci 27: 2058–2073, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marpegan L, Krall TJ, Herzog ED. Vasoactive intestinal polypeptide entrains circadian rhythms in astrocytes. J Biol Rhythms 24: 135–143, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArthur AJ, Coogan AN, Ajpru S, Sugden D, Biello SM, Piggins HD. Gastrin-releasing peptide phase-shifts suprachiasmatic nuclei neuronal rhythms in vitro. J Neurosci 20: 5496–5502, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer JH, Van der Zee EA, Dietz M. Glutamate phase shifts circadian activity rhythms in hamsters. Neurosci Lett 86: 177–183, 1988. [DOI] [PubMed] [Google Scholar]
- Meyer-Spasche A, Piggins HD. Vasoactive intestinal polypeptide phase-advances the rat suprachiasmatic nuclei circadian pacemaker in vitro via protein kinase A and mitogen-activated protein kinase. Neurosci Lett 358: 91–94, 2004. [DOI] [PubMed] [Google Scholar]
- Mohney RP, Zigmond RE. Vasoactive intestinal peptide enhances its own expression in sympathetic neurons after injury. J Neurosci 18: 5285–5293, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray AJ, Tucker SJ, Shewan DA. cAMP-dependent axon guidance is distinctly regulated by Epac and protein kinase A. J Neurosci 29: 15434–15444, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen HS, Hannibal J, Fahrenkrug J. Vasoactive intestinal polypeptide induces per1 and per2 gene expression in the rat suprachiasmatic nucleus late at night. Eur J Neurosci 15: 570–574, 2002. [DOI] [PubMed] [Google Scholar]
- Nikolaev VO, Bunemann M, Hein L, Hannawacker A, Lohse MJ. Novel single chain cAMP sensors for receptor-induced signal propagation. J Biol Chem 279: 37215–37218, 2004. [DOI] [PubMed] [Google Scholar]
- O'Neill JS, Maywood ES, Chesham JE, Takahashi JS, Hastings MH. cAMP-dependent signaling as a core component of the mammalian circadian pacemaker. Science 320: 949–953, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obrietan K, Impey S, Smith D, Athos J, Storm DR. Circadian regulation of cAMP response element-mediated gene expression in the suprachiasmatic nuclei. J Biol Chem 274: 17748–17756, 1999. [DOI] [PubMed] [Google Scholar]
- Pantazopoulos H, Dolatshad H, Davis FC. Chronic stimulation of the hypothalamic vasoactive intestinal peptide receptor lengthens circadian period in mice and hamsters. Am J Physiol Regul Integr Comp Physiol 299: R379–R385, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petri B, Stengl M. Pigment-dispersing hormone shifts the phase of the circadian pacemaker of the cockroach Leucophaea maderae. J Neurosci 17: 4087–4093, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piggins HD, Antle MC, Rusak B. Neuropeptides phase shift the mammalian circadian pacemaker. J Neurosci 15: 5612–5622, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittendrigh CS. Circadian rhythms and the circadian organization of living systems. Cold Spring Harbor Symp Quant Biol 25: 159–182, 1960. [DOI] [PubMed] [Google Scholar]
- Pittendrigh CS, Daan S. A functional analysis of circadian pacemakers in rodents: IV. Entrainment: pacemaker as clock. J Comp Physiol [A] 106: 291–331, 1976. [Google Scholar]
- Ponsioen B, Zhao J, Riedl J, Zwartkruis F, van der Krogt G, Zaccolo M, Moolenaar WH, Bos JL, Jalink K. Detecting cAMP-induced Epac activation by fluorescence resonance energy transfer: Epac as a novel cAMP indicator. EMBO Rep 5: 1176–1180, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powis G, Seewald MJ, Gratas C, Melder D, Riebow J, Modest EJ. Selective inhibition of phosphatidylinositol phospholipase C by cytotoxic ether lipid analogues. Cancer Res 52: 2835–2840, 1992. [PubMed] [Google Scholar]
- Prosser RA, Gillette MU. The mammalian circadian clock in the suprachiasmatic nuclei is reset in vitro by cAMP. J Neurosci 9: 1073–1081, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser RA, McArthur AJ, Gillette MU. cGMP induces phase shifts of a mammalian circadian pacemaker at night, in antiphase to cAMP effects. Proc Natl Acad Sci USA 86: 6812–6815, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph MR, Khalsa SBS, Block GD. Cyclic nucleotides and circadian rhythm generation in Bulla gouldiana. Comp Biochem Physiol 105: 2289–2296, 1992. [Google Scholar]
- Rea MA. VIP-stimulated cyclic AMP accumulation in the suprachiasmatic hypothalamus. Brain Res Bull 25: 843–847, 1990. [DOI] [PubMed] [Google Scholar]
- Reed HE, Meyer-Spasche A, Cutler DJ, Coen CW, Piggins HD. Vasoactive intestinal polypeptide (VIP) phase-shifts the rat suprachiasmatic nucleus clock in vitro. Eur J Neurosci 13: 839–843, 2001. [DOI] [PubMed] [Google Scholar]
- Schneider AR, Lewis TJ, Rinzel J. Effects of correlated input and electrical coupling on synchrony in fast-spiking cell networks. Neurocomputing 69: 1125–1129, 2006. [Google Scholar]
- Shafer OT, Kim DJ, Dunbar-Yaffe R, Nikolaev VO, Lohse MJ, Taghert PH. Widespread receptivity to neuropeptide PDF throughout the neuronal circadian clock network of Drosophila revealed by real-time cyclic AMP imaging. Neuron 58: 223–237, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara K, Funabashi T, Kimura F. Temporal profiles of vasoactive intestinal polypeptide precursor mRNA and its receptor mRNA in the rat suprachiasmatic nucleus. Brain Res Mol Brain Res 63: 262–267, 1999. [DOI] [PubMed] [Google Scholar]
- Shinohara K, Honma S, Katsuno Y, Abe H, Honma KI. Two distinct oscillators in the rat suprachiasmatic nucleus in vitro. Proc Natl Acad Sci USA 92: 7396–7400, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara K, Tominaga K, Isobe Y, Inouye ST. Photic regulation of peptides located in the ventrolateral subdivision of the suprachiasmatic nucleus of the rat: daily variations of vasoactive intestinal polypeptide, gastrin- releasing peptide, and neuropeptide Y. J Neurosci 13: 793–800, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smarandache C, Hall WM, Mulloney B. Coordination of rhythmic motor activity by gradients of synaptic strength in a neural circuit that couples modular neural oscillators. J Neurosci 29: 9351–9360, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJ, Sam LM, Justen JM, Bundy GL, Bala GA, Bleasdale JE. Receptor-coupled signal transduction in human polymorphonuclear neutrophils: effects of a novel inhibitor of phospholipase C-dependent processes on cell responsiveness. J Pharmacol Exp Ther 253: 688–697, 1990. [PubMed] [Google Scholar]
- Stewart AJ, Morgan K, Farquharson C, Millar RP. Phospholipase C-eta enzymes as putative protein kinase C and Ca2+ signalling components in neuronal and neuroendocrine tissues. Neuroendocrinology 86: 243–248, 2007. [DOI] [PubMed] [Google Scholar]
- Tischkau SA, Gallman EA, Buchanan GF, Gillette MU. Differential cAMP gating of glutamatergic signaling regulates long-term state changes in the suprachiasmatic circadian clock. J Neurosci 20: 7830–7837, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimble ER, Bruzzone R, Biden TJ, Meehan CJ, Andreu D, Merrifield RB. Secretin stimulates cyclic AMP and inositol trisphosphate production in rat pancreatic acinar tissue by two fully independent mechanisms. Proc Natl Acad Sci USA 84: 3146–3150, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Rampelbergh J, Poloczek P, Francoys I, Delporte C, Winand J, Robberecht P, Waelbroeck M. The pituitary adenylate cyclase activating polypeptide (PACAP I) and VIP (PACAP II VIP1) receptors stimulate inositol phosphate synthesis in transfected CHO cells through interaction with different G proteins. Biochim Biophys Acta 1357: 249–255, 1997. [DOI] [PubMed] [Google Scholar]
- Vanecek J, Watanabe K. Melatonin inhibits the increase of cyclic AMP in rat suprachiasmatic neurons induced by vasoactive intestinal peptide. Neurosci Lett 252: 21–24, 1998. [DOI] [PubMed] [Google Scholar]
- Violin JD, DiPilato LM, Yildirim N, Elston TC, Zhang J, Lefkowitz RJ. β2-Adrenergic receptor signaling and desensitization elucidated by quantitative modeling of real time cAMP dynamics. J Biol Chem 283: 2949–2961, 2008. [DOI] [PubMed] [Google Scholar]
- Wang XJ, Rinzel J. Alternating and synchronous rhythms in reciprocally inhibitory model neurons. Neural Comp 4: 84–97, 1992. [Google Scholar]
- Watanabe K, Vanecek J, Yamaoka S. In vitro entrainment of the circadian rhythm of vasopressin-releasing cells in suprachiasmatic nucleus by vasoactive intestinal polypeptide. Brain Res 877: 361–366, 2000. [DOI] [PubMed] [Google Scholar]
- Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong HK, Oh WJ, Yoo OJ, Menaker M, Takahashi JS. Period2::luciferase real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci USA 12: 1–8, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Murayama Y, Ito H, Kageyama H, Kondo T. Nonparametric entrainment of the in vitro circadian phosphorylation rhythm of cyanobacterial KaiC by temperature cycle. Proc Natl Acad Sci USA 106: 1648–1653, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman WF, Pittendrigh CS, Pavlidis T. Temperature compensation of the circadian oscillation in Drosophila pseudoobscura and its entrainment by temperature cycles. J Insect Physiol 14: 669–684, 1968. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.