Abstract
Acute ankle injuries are common problems and often lead to persistent pain. To investigate the underlying mechanism of ankle sprain pain, the response properties of spinal dorsal horn neurons were examined after ankle sprain. Acute ankle sprain was induced manually by overextending the ankle of a rat hindlimb in a direction of plantarflexion and inversion. The weight-bearing ratio (WBR) of the affected foot was used as an indicator of pain. Single unit activities of dorsal horn neurons in response to plantarflexion and inversion of the foot or ankle compression were recorded from the medial part of the deep dorsal horn, laminae IV-VI, in normal and ankle-sprained rats. One day after ankle sprain, rats showed significantly reduced WBRs on the affected foot, and this reduction was partially restored by systemic morphine. The majority of deep dorsal horn neurons responded to a single ankle stimulus modality. After ankle sprain, the mean evoked response rates were significantly increased, and afterdischarges were developed in recorded dorsal horn neurons. The ankle sprain-induced enhanced evoked responses were significantly reduced by morphine, which was reversed by naltrexone. The data indicate that movement-specific dorsal horn neuron responses were enhanced after ankle sprain in a morphine-dependent manner, thus suggesting that hyperactivity of dorsal horn neurons is an underlying mechanism of pain after ankle sprain.
Keywords: acute ankle sprain, dorsal horn neuron responses, foot movements, inflammatory pain, morphine effect
joint injuries of various causes can result in both persistent pain and locomotion disorders (Schaible et al. 2002; Schaible and Grubb 1993; Schaible and Schmidt 1988). Among joint injuries, ankle sprain is the most common musculoskeletal injury (Kannus and Renstrom 1991), and >25,000 patients require medical care for ankle sprain every day in the United States (Kannus and Renstrom 1991; Lynch and Renstrom 1999). The majority of injuries involve disruption of the lateral ligament complex (Garrick and Requa 1988) induced by excessive plantar flexion and inversion of the foot. A significant portion of patients who once had an ankle sprain suffer persistent pain and recurrent sprains (Anandacoomarasamy and Barnsley 2005; Kern-Steiner et al. 1999; Konradsen et al. 2002; Koo et al. 2002).
Despite the prevalence of ankle sprains, the detailed mechanism of ankle sprain pain has not been studied systematically. Thus, much of the understanding of ankle sprain pain relies on clinical descriptions and evidence from other joint disorders, such as joint inflammation. It is thus assumed that ankle sprain pain would be similar to the pain mechanism of inflammation. Persistent pain induced by joint inflammation has been studied extensively. Factors that initiate inflammation as well as chemicals released due to inflammation have shown to sensitize nociceptor terminals and thus lead to peripheral sensitization (McDougall and Larson 2006; Schaible et al. 2009; Stein et al. 2009). In addition, enhanced response activity of the spinal dorsal horn neurons has been shown after the administration of inflammatory agents, kaolin/carrageenan or Freud's adjuvant, into the joint capsule (Neugebauer et al. 1996; Neugebauer et al. 1995; Schaible 2004; Schaible et al. 2002; Schaible and Grubb 1993; Schaible and Schmidt 1988). Thus, both peripheral and central sensitizations may be involved in the persistent pain caused by joint inflammation.
Several animal models of musculoskeletal injury exist. These include carpal tunnel syndrome (Diao et al. 2005), muscle strain injuries (Song et al. 2004), Achilles tendon injuries (Huang et al. 2004; Messner et al. 1999), and lumbar strain by cyclic lumbar flexion (Claude et al. 2003). The most relevant animal model for the present study, however, is the rat ankle sprain pain model, which is produced by manually overextending the lateral ankle ligaments (Koo et al. 2002). This model mainly affects joint ligaments in the hindlimb with minor effects on cutaneous tissue and produces spontaneous pain and impairment of movement. Using this model of ankle sprain, the present study investigated the physiological responses of deep dorsal horn neurons that are evoked by various movements of the hind foot and compression applied to the ankle joint in normal and ankle-sprained rats.
MATERIALS AND METHODS
Experimental animals.
Adult male Sprague-Dawley rats (weight: 200∼350 g) were purchased from Harlan Sprague Dawley (Houston, TX) and used for this study. Rats in the experimental group received acute ankle sprain. Normal naïve rats were used as controls for both behavioral testing and electrophysiological recordings. Among 80 ankle-sprained rats, 32 rats were used for behavior testing and 48 rats for electrophysiological recordings. Animals were housed in plastic cages with soft bedding with free access to food and water under a 12:12-h reversed light-dark cycle (dark from 8 AM to 8 PM). All animals were acclimated for 7 days before any experiment. All experimental procedures were approved by the Institutional Animal Care and Use Committee of the University of Texas Medical Branch and were in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Ankle sprain induction.
Under gas anesthesia (3% halothane in air for induction and 1.5∼2% for maintenance), ankle sprain was induced manually as described in a previous study (Koo et al. 2002). While the leg was held, the left hind foot was overextended repeatedly in the direction of simultaneous inversion and plantarflexion for 60 times during a 1-min period with a gradual increase of bending force. The same procedures were repeated one more time. At the end, the ankle could be rotated to a position of 180° inversion (paw facing upward). Anesthesia was discontinued, and the rats recovered from anesthesia within 5–10 min and were returned to their cages.
Behavioral testing.
To estimate the level of pain in the sprained ankle, the amount of weight-bearing force (WBF) on the sprained foot was measured 1 day before and after ankle sprain. The experimental time of 1 postoperative day was chosen because our previous study (Koo et al. 2002) suggested that the level of pain after ankle sprain was maximal at 1 postoperative day, as judged by the degree of reduction in WBF. On the day of behavioral testing, each rat was weighed and then allowed to walk through a long rectangular plastic tube (10-cm width, 10-cm height, and 60-cm length) with a scale (Acculab, Pocket pro 250-B, Newton, PA) located midway down the tube that only underlay the righthand half of the tube. Thus, when the rat walked in one direction the weight on the left foot could be determined, and when the rat came down the other way, the weight placed on the right foot could be measured. The weight signals from the scale were fed into an oscilloscope, and WBFs were calculated using a data-acquisition system (CED 1401 plus with Spike 2 program, CED). To obtain the valid WBF of the foot, WBFs were measured six times, and the values were averaged for each measurement. The weight-bearing ratio (WBR) was calculated based on the following formula: WBR = (WBF of the designated foot/body weight) × 100.
Surgical preparation for in vivo electrophysiological recording.
Extracellular recordings were made from the dorsal horn neurons in 48 rats on 1 day after ankle sprain as well as in 40 normal rats. Rats were anesthetized with urethane (1.5 g/kg ip), and supplemental urethane (200 mg/kg ip) was administered when a sign of low anesthesia level was detected. The depth of anesthesia was monitored by observing the heart rate, end-tidal CO2 level, and pupil size. The rat was artificially ventilated at a rate of 80–100 breaths/min after tracheotomy, and the end-tidal expiration CO2 level was maintained at 3.5–4.5%. The animal was paralyzed with an initial bolus of pancuronium bromide (1 mg/kg iv) and maintained by continuous infusion (0.4–0.6 mg·kg−1·h−1). A laminectomy was done from T12 to L2 vertebrae to expose the L4-L6 spinal cord segments, and rats were securely fixed in a stereotaxic frame. The dura of the exposed spinal cord was then removed, and the vertebral column was held rigid by clamps. The spinal cord was continually bathed in a pool of warm mineral oil held by skin flaps. Throughout the experiment, the core body temperature was monitored by a rectal thermal probe and maintained at 36.5–37°C by a heating blanket equipped with an automatic feedback control unit. At the end of the experiment, rats were euthanized with an injection of a lethal dose of urethane (3 g/kg ip), and death was confirmed by opening the chest.
Recordings of spinal dorsal horn neurons.
Extracellular recordings of dorsal horn neuron activities were made from the ipsilateral lumbar spinal cord in normal rats as well as in ankle-sprained rats at 1 postoperative day (the same time point as the behavioral test), when sprain pain is maximal (Koo et al. 2002). Most recordings were made using carbon fiber CARBOSTAR-1 electrodes (0.4–0.8 MΩ, Kation Scientific), but, in some cases, three-barrel carbon fiber CARBOSTAR-3 electrodes (0.4–0.8 MΩ, Kation Scientific) were used to mark recording sites by the injection of methylene blue dye. A microelectrode was lowered into the cord using an electronic micromanipulator. The depth of the recording site from the dorsal surface of the cord was measured on each recording. The activity recordings were fed into an amplifier (CYBERAMP 320, Axon Instruments) and then displayed on an oscilloscope. Only single unit activity was isolated using a window discriminator (WPI) and the Spike 2 program (version 4, CED). Responses to mechanical stimuli applied to the foot were quantified by a data-acquisition system (CED 140l, CED).
Mechanical stimulation of the ankle.
The foot movements that are critical for weight bearing and affected most by ankle sprain are plantarflexion and inversion; thus, these two movements were chosen to activate sensory afferents originating from deep tissue. Ankle joint compression was also used to activate deep nociceptive sensory receptors. For the accurate measurement of ankle joint movement, a special device equipped with a potentiometer (Bourns) was designed and patched to the plantar surface of rat hindpaw. This device measured the angle of movement accurately when the foot was passively moved. To standardize the experimental procedures, the criteria of each movement were arbitrarily defined and applied in the same way for each stimulus. The resting position was defined as when the tibiotarsal-tarsometatarsal (TT-TM) angle was 90°. For plantarflexion stimulus, the foot was placed at the resting position, where the TT-TM angle was 90°, and then slowly plantarflexed to 190°, the maximum plantarflexed position. For inversion stimulus movement, the foot was rotated medially from the resting position (0° on the horizontal plane) up to 120°. For each movement stimulus, the foot was slowly moved from the starting position to the maximally moved position during the first 2 s, held at the maximally moved position for 12 s, and then moved back to the starting position during the last 1 s. The range of each movement was set to where the joint showed appreciable resistance felt at the maximum point but did not induce tissue injury.
For the compression stimulus, the pressure was applied mediolaterally to the ankle using a pair of large blunt forceps (20-cm long, contact area: 4 × 4 mm) equipped with strain gauges (Yu et al. 2002). Each compression stimulus started from 0 g intensity but quickly reached to 1,500 g within 2 s and was held at the maximum intensity for 13 s and then quickly released. The intensity of each stimulus was calibrated and fed into the data-acquisition system (CED 140l, CED) to synchronize with neuronal responses. Thus, the total compression stimulus was applied for 15 s.
Experimental design for evoked neuronal response measurement.
Neurons showing evoked responses to plantarflexion or inversion movement of the foot or compression of the ankle were subjected to study. Neurons responding to cutaneous stimulation (brushing or squeezing of the foot skin) were excluded from the study. To establish reliable responses of each neuron, neuronal responses were recorded initially two to three times by repeating the same mechanical stimulus at 10-min intervals. When the responses to each stimulus produced no more than 10% variations from the original, they were considered as stable responses. When a neuron that responded to a specific stimulus modality was identified, the spontaneous background activity was recorded for 60 s followed by the response recordings in response to a specific movement of the foot and then compression of the ankle. The response recordings were made three times with the same stimulus repeated three times, and the average of the three recordings was used as the average response to that stimulus. If spontaneous activity was present, the evoked responses were calculated by subtracting the background spontaneous activity. The recorded neurons were classified based on their responses to different mechanical stimuli: 1) plantarflexion neurons were those responding only to plantarflexion movement, 2) inversion neurons were those responding only to inversion movement, and 3) compression neurons were those responding only to compression of the ankle joint. Neurons responding to multiple stimuli were rare and not included in the data analysis. Recordings of one or two neurons were made from each animal.
Drug administration.
Morphine (2 or 5 mg/kg, morphine sulfate; Sigma, St. Louis, MO) or naltrexone (10 mg/kg, Sigma) was dissolved in saline and given intraperitoneally at a volume of 1 ml/kg. Drugs were prepared immediately before each use. Control groups received the same volume of saline. In behavioral experiments, morphine effects (2 or 5 mg/kg ip) were tested 60 min after injections (Fig. 1). In electrophysiological experiments, morphine (2 mg/kg ip) was given immediately after control recordings, and the dorsal horn neuron responses to morphine were recorded 30 min after morphine treatment. Immediately afterward, naltrexone (10 mg/kg ip) was injected, and responses were recorded again at 30 min after the naltrexone treatment (60 min after morphine injection).
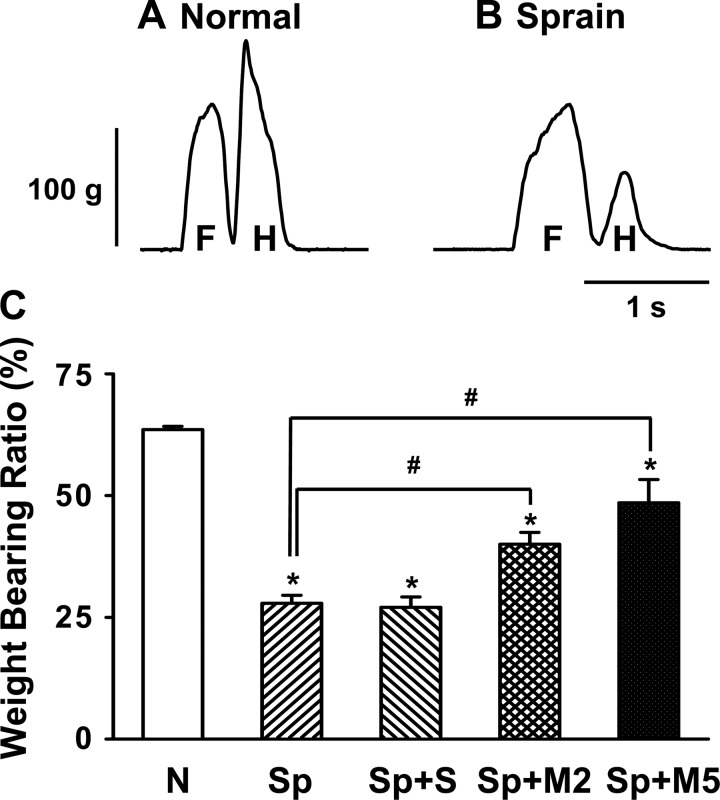
Fig. 1.
Changes in weight-bearing forces of the rat hindlimb after ankle sprain and the effect of morphine treatment. A and B: examples of weight-bearing force traces of the forelimb (F) and hindlimb (H) in a normal rat (A) and a rat 1 day after ankle sprain (B). As shown in C, the stepping force of the hindlimb is ∼64% of total body weight in normal locomotion (N). One day after ankle sprain (Sp), the weight-bearing ratio decreased to 27%. Morphine treatments [1 hr after 2 (M2) and 5 mg/kg ip (M5) of morphine] induced a recovery of the weight-bearing forces in a dose-dependent manner. Saline treatment (S) had no effect. The value of the ordinate was expressed as the percent ratio of the stepping force to body weight. Values are means ± SE; n = 10 rats in the normal group, 8 rats in the Sp group, 8 rats in the Sp + S group, 8 rats in the Sp + M2 group, and 8 rats in the Sp + M5 group. Abscissa indicate animal groups. *Value was significantly different (P < 0.05) from the normal condition; #value was significantly different (P < 0.05) from Sp condition.
Histology.
To determine the location of the recording site, methylene blue was iontophoretically injected (1∼3 μA for 3 min) using a three-barrel carbon fiber microelectrode (CARBOSTAR-3, Kation Scientific) immediately after the recordings from single neurons of 10 randomly selected rats (1 neuron/rat). The spinal cord was removed after perfusion with fixative containing 4% paraformaldehyde, cryosectioned at 30-μm thickness, mounted on slides, and then examined under a light microscope. Locations of methylene blue injection sites were plotted from 10 rats (1 site/rat, total of 10 sites; see Fig. 5A).
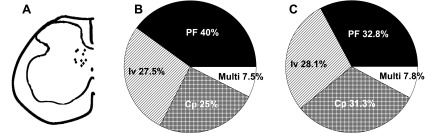
Fig. 5.
Location of recorded neurons in the spinal cord and afferent modalities of the neurons receiving inputs from the ankle. A: schematic drawing of the spinal cord showing the locations of 10 recorded neurons. Each location was marked with an injection of methylene blue after the recording session, and the marked spot was recovered by histological procedures. Marked spots are clustered in the medial deep laminae of the dorsal horn, and neurons having different afferent modalities were mixed within the same region. B and C: pie charts showing the proportion of recorded dorsal horn neurons that respond to PF, Iv, or Cp stimuli in normal rats (n = 40; B) and ankle-sprained rats (n = 64; C). The proportion of afferent modalities was about the same in normal and ankle-sprained rats. Multi, multiresponsive neurons.
Data analysis and statistics.
Statistical significance was determined using one-way ANOVA with the Duncan's multiple-comparison post hoc test for behavioral tests and an unpaired Student's t-test for the others, using Sigmastat (version 3.0, SPSS). All data are presented as means ± SE. P values of <0.05 were considered significant.
RESULTS
WBF on the sprained ankle is reduced partly due to pain.
Rats with a sprained ankle limped on the injured side, frequently tended to the sprained ankle by licking or biting, and showed an occasional irritable jump 1 day after ankle sprain. Postmortem examination showed that the manual ankle sprain manipulation produced grade I or II type of ankle sprain, which included overstretching and minor tears of lateral ankle ligaments: the calcaneofibular and anterior and posterior talofibular ligaments. In addition, several other ligaments that connect various tarsal bones, such as the calcaneocuboid, talonavicular, and talocalcanean ligaments, were also stretched. In addition, a minor hemorrhage on the extensor retinaculum and swelling of the ankle joint were found. This lateral ankle sprain without rupturing any lateral ankle ligaments is equivalent to ankle sprain grade I or II in humans (Cotler 1984).
To examine pain after sprain, stepping forces of the limbs during locomotion were analyzed before and after ankle sprain. Since the stepping forces of the forelimb were not significantly changed after ankle sprain of the hindlimb or morphine treatment (Koo et al. 2002), detailed analysis was limited to the affected hindlimb. Figure 1A shows an example of stepping force recordings of the forelimb and hindlimb in a normal rat. Normal rats step on the forelimb first followed by the hindlimb. The peak stepping force of the hindlimb was, on average, 64% of body weight. One day after ankle sprain, WBFs of the injured hindlimb decreased to 27% of body weight (Fig. 1, B and C).
To test whether the decreased weight bearing on the sprained foot is due to pain, the effect of an analgesic drug, morphine, on WBFs was examined 1 day after ankle sprain. The systemic injection of morphine (2 or 5 mg/kg) significantly improved WBFs of the affected foot (the maximum effect was observed at 1 h after morphine; Fig. 1C). This suggests that the reduction of WBF after ankle sprain is at least in part due to pain.
Dorsal horn neurons respond to deep afferent stimuli of the foot in a modality-dependent manner.
The evoked responses of the dorsal horn neurons that respond to foot movements or an ankle compression were recorded from normal rats and compared with those of ankle-sprained rats. About 70% of 40 and 64 neurons recorded from normal and ankle-sprained rats, respectively, showed a background spontaneous activity of 1 Hz or higher. However, the rates of individual units were highly variable, and no consistent and significant changes after ankle sprain were found.
The majority of dorsal horn neurons responded only to one specific foot movement, and, hence, these neurons were defined by the corresponding movement type. Thus, three types of neurons were examined: plantarflexion, inversion, and ankle compression neurons.
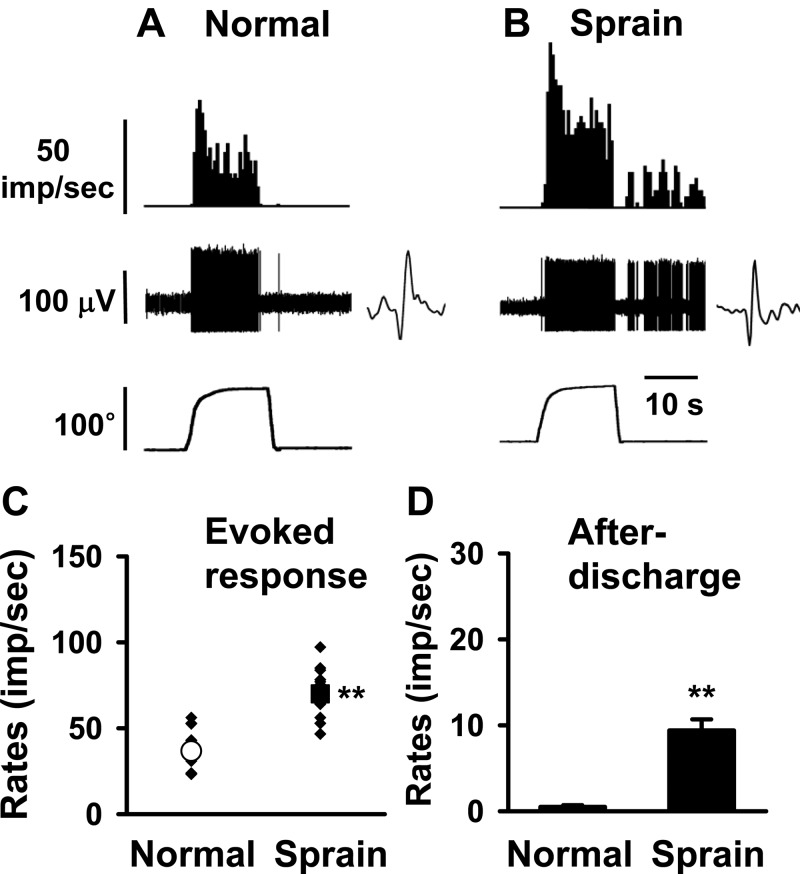
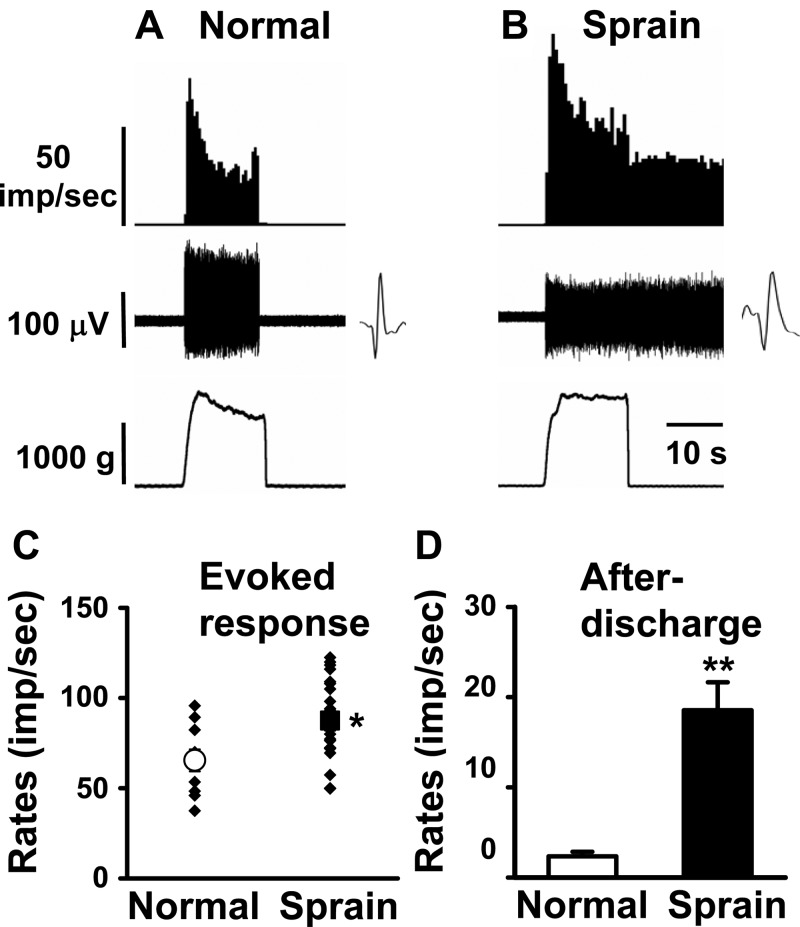
Examples of evoked responses of plantarflexion neurons in a normal rat and a rat 1 day after ankle sprain are shown in Fig. 2, A and B, respectively. In normal rats, plantarfexion neurons (n = 16) showed an increasing evoked activity during plantarflexion (from TT-TM angle 90° to 190°), and the activity peaked when the foot was maximally plantarflexed. The response thresholds to plantarflexion ranged from 96° to 135° (118.3 ± 4.6°). While the foot was held at the maximum plantarflexion position, the evoked responses gradually decreased from the peak response but still maintained significantly high levels. The response activity was quickly stopped when the foot was returned to the basal position (Fig. 2A). One day after ankle sprain, plantarflexion neurons (n = 21) showed significantly enhanced peak and mean evoked responses compared with those of normal rats. In addition, moderate levels of responses were sustained even after the foot was returned to the basal position, thus showing significant levels of afterdischarges (Fig. 2B). Group data of these changes obtained from normal and ankle-sprained rats are shown in Fig. 2C. The afterdischarges that developed after ankle sprain are shown in Fig. 2D. However, a significant change in the response thresholds of the plantarflexion neurons could not be determined.
Fig. 2.
Response characteristics of spinal dorsal horn neurons to plantarflexion (PF) of the foot in normal and ankle-sprained rats. PF was initiated from the tibiotarsal-tarsometatarsal (TT-TM) angle of 90° (baseline) and ended at 190°. The neurons responding exclusively to PF were recorded in normal (A) and sprained (B) rats. Insets show raw recordings of single spikes. C: mean evoked responses to PF. D: afterdischarges of the dorsal horn neurons recorded from normal rats (n = 16) and 1 day postankle-sprained rats (n = 21). Asterisks indicate values significantly different from the normal value (*P < 0.05; **P < 0.01).
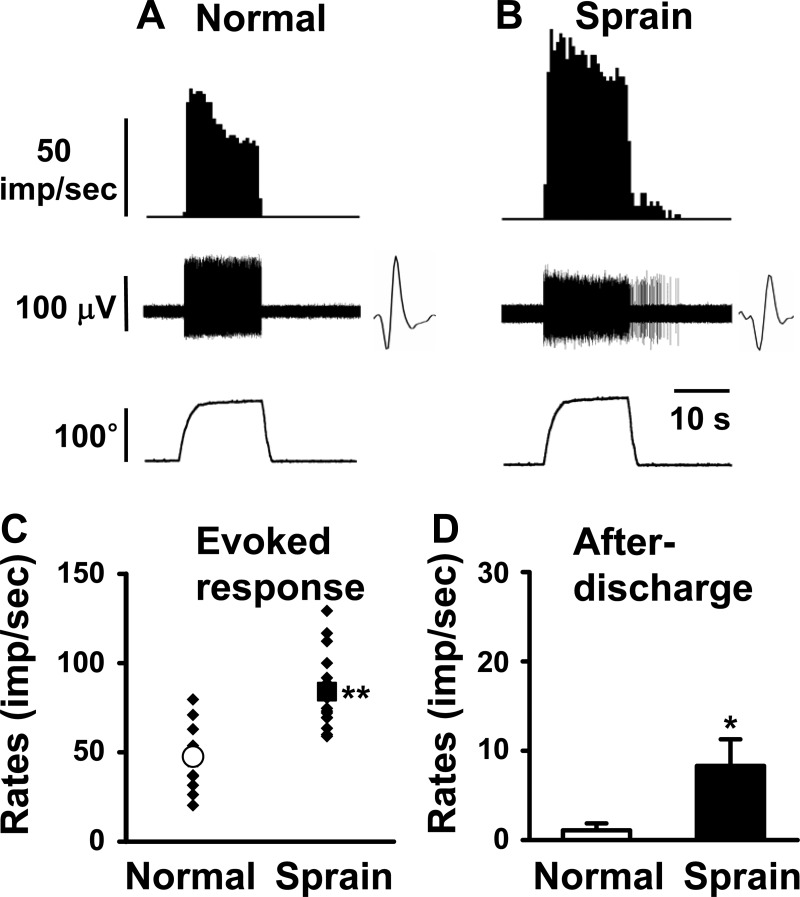
The properties of 11 inversion-specific neurons recorded from normal rats and 18 additional inversion-specific neurons from 1 day postankle-sprained rats are shown in Fig. 3. The dorsal horn neurons that responded only to inversion movement showed an average response rate of 47.6 ± 5.6 imp/s in normal control rats (Fig. 3, A and C). One day after ankle sprain, the mean response rate was increased to 84 ± 4.7 imp/s (Fig. 3, B and C). The afterdischarge rates were also increased after ankle sprain (Fig. 3D).
Fig. 3.
Response characteristics of spinal dorsal horn neurons to inversion (Iv) of the foot in normal and ankle-sprained rats. Iv was initiated at 0° on the horizontal plane and medially rotated up to 120°. The neurons responding exclusively to Iv were recorded in normal (A) and ankle-sprained (B) rats. Insets show raw recordings of single spikes. C: mean evoked responses to Iv. D: afterdischarges of the dorsal horn neurons recorded from normal rats (n = 11) and 1 day postankle-sprained rats (n = 18). Asterisks indicate values significantly different from the normal value (*P < 0.05; **P < 0.01).
For compression responsiveness, activity recordings were made from 10 dorsal horn neurons of normal rats and 20 more from ankle-sprained rats (Fig. 4). The compression intensity ranged from 0 to 1,500 g. In ankle-sprained rats, the evoked responses were significantly increased compared with those of normal rats (Fig. 4, A–C). The response activities completely ceased when the stimulation was removed in normal rats (Fig. 4, A and D), whereas neurons in ankle-sprained rats showed prominent afterdischarges (Fig. 4, B and D).
Fig. 4.
Response characteristics of spinal dorsal horn neurons to compression (Cp) of the ankle joint in normal and ankle-sprained rats. Cp was applied from the medial to lateral sides of the ankle with a pair of forceps equipped with Cp force output reading. The neurons responding exclusively to Cp were recorded in normal (A) and ankle-sprained (B) rats. Insets show raw recordings of single spikes. C: mean evoked responses to Cp. D: afterdischarges of the dorsal horn neurons recorded from normal rats (n = 10) and 1 day postankle-sprained rats (n = 20). Asterisks indicate values significantly different from the normal value (*P < 0.05; **P < 0.01).
Neurons responding to ankle stimuli are located in the medial deep dorsal horn.
The locations of 10 recording sites (4 from normal rats and 6 from ankle-sprained rats) were identified by methylene blue injection at the end of the recordings and then recovered by a histological preparation. All recorded sites (all modalities) were located in the medial part of the deep dorsal horn (laminae IV-VII; Fig. 5A). The depths of the recording sites ranged 772–1,193 μm from the spinal cord surface of the L5 to L6 spinal cord.
Among the 40 recorded cells in normal rats, plantarflexion, inversion, and compression neurons comprised 16 cells (40%), 11 cells (27.5%), and 10 cells (25%), respectively. The remaining three cells were multiresponsive neurons (Fig. 5B). In ankle-sprained rats, the proportion of each type neuron did not change, suggesting that the phenotype of dorsal horn neurons does not change after ankle sprain (n = 64; Fig. 5C).
Enhanced dorsal horn neuronal responses are sensitive to morphine.
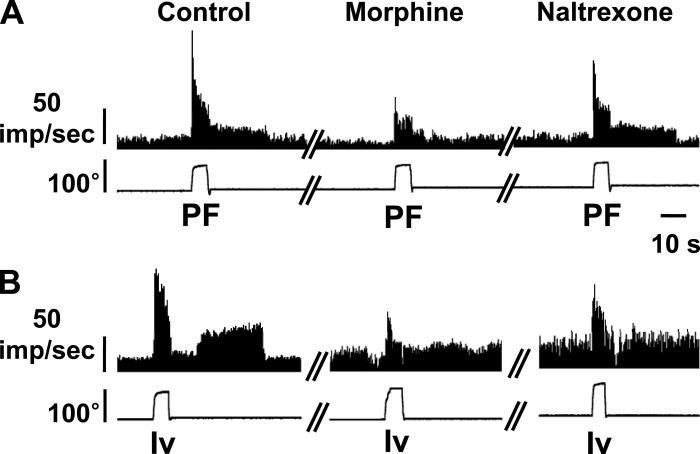
After acute ankle sprain, WBFs of the affected foot were decreased, and these reduced WBFs were partially restored with systemic morphine (Fig. 1). To see if the enhanced responses of dorsal horn neurons to ankle stimulation after sprain are sensitive to systemic morphine as well, five dorsal horn neurons (1 neuron/rat) were examined after ankle sprain. Of these five neurons, three neurons were plantarflexion neurons and two neurons were inversion neurons. Figure 6 shows an example of a planterflexion neuron and an inversion neuron to corresponding foot movement stimuli before and after morphine (2 mg/kg ip) and after naltrexone injection. The evoked responses reduced after the intraperitoneal injection of morphine (2 mg/kg) and then recovered with the injection of naltrexone (10 mg/kg ip), an opioid receptor antagonist. Not only the evoked responses but also the afterdischarges were changed by opioid ligands, particularly in the plantarflexion unit. These data suggest that an enhanced part of dorsal horn responses may be related to nociceptive inputs.
Fig. 6.
Effects of morphine and naltrexone on the responses of dorsal horn neurons recorded 1 day after ankle sprain. A: one neuron responding to PF. B: one neuron responding to Iv. Both morphine and naltrexone effects were tested 30 min after intraperitoneal injections. Two more PF neurons and one more Iv neuron were tested with similar results.
DISCUSSION
The relationship between painful behaviors and dorsal horn neuron responses to foot movements was investigated in the rat after ankle sprain. WBRs of the affected foot were greatly reduced after ankle sprain and then recovered significantly by morphine. Extracellular recordings showed that the majority of dorsal horn neurons responded exclusively to a specific foot movement and that neurons responding to multiple inputs were rare. After ankle sprain, the evoked responses increased in plantarflexion, inversion, and compression responsive neurons. In addition, afterdischarges were developed so that discharges continued for some time after the termination of these stimuli. These changes in dorsal horn neuronal responses to ankle movements likely underlie the reduced WBR after sprain of the ankle. Neurons recorded in this study were located in the medial part of the deep dorsal horn (laminae IV-VII; Fig. 5A). Their locations are consistent with previously recorded neurons receiving nociceptive afferent inputs from the ankle joint or those that respond to flexion/extension of the ankle joint (Schaible and Ebersberger 2009; Schaible and Grubb 1993; Willis and Coggeshall 2004).
Roughly 25% of all sports-related injuries involve ankle sprain, which damage the lateral ankle ligament complex (Hume and Gerrard 1998; Puffer 2001). The lateral ankle ligament complex is composed of three ligaments in humans: the anterior and posterior talofibular ligaments and the calcaneofibular ligament. In human patients, ankle sprain is categorized into three grades based on the severity of injuries: ligaments are stretched without any tear (grade I), one or more ligaments are partially torn (grade II), or one or more ligaments are completely torn (grade III). It usually happens during an unanticipated hyperplantarflexion in combination with hyperinversion of the fixed foot with external rotation of the tibia (Cotler 1984; Lynch and Renstrom 1999; Safran et al. 1999). Typically, the lateral ankle ligament injury is likely accompanied by injuries to other joint structures, such as the joint capsule, subtalar ligament (Lynch and Renstrom 1999), tibiofibular ligament (Safran et al. 1999), and muscle tissues surrounding the ankle complex (Hertel 2000). By postmortem autopsies, the extent of ligament injuries in our rat ankle sprain model was identified. The ligaments affected by sprain maneuvers include the calcaneocuboidal, talonavicular, and talocalcanean ligaments in addition to the major lateral ankle ligaments. Although the involved ligaments were elongated or partially torn in some cases and thus widened the gaps between bones (Koo et al. 2002), there was no sign of complete tear. Thus, our ankle sprain model mimics grades I or II of human ankle sprain (Cotler 1984). In addition, there was a minor hemorrhage on the extensor retinaculum and swelling of the ankle joint, thus indicating inflammation. These rats showed a significant recovery in 4 days after ankle sprain; thus, the recovery rate was also comparable to that of grade I and II ankle-sprained patients shown in clinical studies (Lynch and Renstrom 1999; Safran et al. 1999).
In this study, we grouped the dorsal horn neurons according to responding stimulus modality, such as plantarflexion, inversion, and compression of the ankle. Our results indicate that >90% of the dorsal horn neurons responding to ankle stimulation exclusively showed a specific stimulus modality and relative proportions of the populations did not change after ankle sprain. This seems to be consistent with the previous data in which 50% of primary afferent nerve fibers innervating the rat knee joint respond predominantly or exclusively in one directional knee movement (Just et al. 2000).
The most prominent changes in the responses of dorsal horn neurons after ankle sprain are a marked enhancement of the evoked responses to ankle stimulation and the development of prominent afterdischarges. The enhancement of evoked responses suggests that dorsal horn neurons are hyperactive after ankle sprain. The exact cause of such hyperactivity, however, is not clear. One possibility is that dorsal horn neurons are known to develop sensitization after receiving sustained noxious peripheral input, such as nociceptive input after sprain. Another possibility is that ankle sprain has likely caused nociceptor sensitization, and sensitized nociceptors are hyperreactive to ankle stimulation; thus, hyperresponses of dorsal horn neurons could be secondary to enhanced evoked responses of nociceptors. However, the above two possibilities are not mutually exclusive, and, in fact, it is likely that both factors contribute to that hyperactivity of dorsal horn neurons in this particular case.
In conclusion, ankle sprain injury induced the reduction of WBF on the affected foot and the enhancement of evoked activity as well as the development of afterdischarges in the dorsal horn neurons receiving afferent inputs from foot movement and ankle compression. The present study showed the neuronal basis for pain associated with ankle sprain and described some response characteristics of dorsal horn neurons receiving inputs from the sprained ankle. The partial recovery of WBF and reduction of dorsal horn responsiveness after morphine treatment suggests that these changes may be related to pain after ankle sprain.
GRANTS
This work was supported by National Institutes of Health Grants R01-AT-01474, P01-NS-11255, and R01-NS-31680. J. H Kim was supported, in part, by the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education, Science, and Technology (no. R13–2008-028-01000-0).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank Griselda Gonzales for editing the manuscript.
REFERENCES
- Anandacoomarasamy A, Barnsley L. Long term outcomes of inversion ankle injuries. Br J Sports Med 39: e14, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claude LN, Solomonow M, Zhou BH, Baratta RV, Zhu MP. Neuromuscular dysfunction elicited by cyclic lumbar flexion. Muscle Nerve 27: 348–358, 2003. [DOI] [PubMed] [Google Scholar]
- Cotler JM. Lateral ligamentous injuries of the ankle. In: Traumatic Disorders of the Ankle, edited by Hamilton WC, Gigliotti SP. New York: Springer-Verlag, 1984, p. 113–123. [Google Scholar]
- Diao E, Shao F, Liebenberg E, Rempel D, Lotz JC. Carpal tunnel pressure alters median nerve function in a dose-dependent manner: a rabbit model for carpal tunnel syndrome. J Orthop Res 23: 218–223, 2005. [DOI] [PubMed] [Google Scholar]
- Garrick JG, Requa RK. The epidemiology of foot and ankle injuries in sports. Clin Sports Med 7: 29–36, 1988. [PubMed] [Google Scholar]
- Hertel J. Functional instability following lateral ankle sprain. Sports Med 29: 361–371, 2000. [DOI] [PubMed] [Google Scholar]
- Huang TF, Perry SM, Soslowsky LJ. The effect of overuse activity on Achilles tendon in an animal model: a biomechanical study. Ann Biomed Eng 32: 336–341, 2004. [DOI] [PubMed] [Google Scholar]
- Hume PA, Gerrard DF. Effectiveness of external ankle support. Bracing and taping in rugby union. Sports Med 25: 285–312, 1998. [DOI] [PubMed] [Google Scholar]
- Just S, Pawlak M, Heppelmann B. Responses of fine primary afferent nerve fibres innervating the rat knee joint to defined torque. J Neurosci Methods 103: 157–162, 2000. [DOI] [PubMed] [Google Scholar]
- Kannus P, Renstrom P. Treatment for acute tears of the lateral ligaments of the ankle. Operation, cast, or early controlled mobilization. J Bone Joint Surg Am 73: 305–312, 1991. [PubMed] [Google Scholar]
- Kern-Steiner R, Washecheck HS, Kelsey DD. Strategy of exercise prescription using an unloading technique for functional rehabilitation of an athlete with an inversion ankle sprain. J Orthop Sports Phys Ther 29: 282–287, 1999. [DOI] [PubMed] [Google Scholar]
- Konradsen L, Bech L, Ehrenbjerg M, Nickelsen T. Seven years follow-up after ankle inversion trauma. Scand J Med Sci Sports 12: 129–135, 2002. [DOI] [PubMed] [Google Scholar]
- Koo ST, Lim KS, Chung K, Ju H, Chung JM. Electroacupuncture-induced analgesia in a rat model of ankle sprain pain is mediated by spinal α-adrenoceptors. Pain 135: 11–19, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo ST, Park YI, Lim KS, Chung K, Chung JM. Acupuncture analgesia in a new rat model of ankle sprain pain. Pain 99: 423–431, 2002. [DOI] [PubMed] [Google Scholar]
- Lynch SA, Renstrom PA. Treatment of acute lateral ankle ligament rupture in the athlete. Conservative versus surgical treatment. Sports Med 27: 61–71, 1999. [DOI] [PubMed] [Google Scholar]
- McDougall JJ, Larson SE. Nociceptin/orphanin FQ evokes knee joint pain in rats via a mast cell independent mechanism. Neurosci Lett 398: 135–138, 2006. [DOI] [PubMed] [Google Scholar]
- Messner K, Wei Y, Andersson B, Gillquist J, Rasanen T. Rat model of Achilles tendon disorder. A pilot study. Cells Tissues Organs 165: 30–39, 1999. [DOI] [PubMed] [Google Scholar]
- Neugebauer V, Rumenapp P, Schaible HG. Calcitonin gene-related peptide is involved in the spinal processing of mechanosensory input from the rat's knee joint and in the generation and maintenance of hyperexcitability of dorsal horn-neurons during development of acute inflammation. Neuroscience 71: 1095–1109, 1996. [DOI] [PubMed] [Google Scholar]
- Neugebauer V, Weiretter F, Schaible HG. Involvement of substance P and neurokinin-1 receptors in the hyperexcitability of dorsal horn neurons during development of acute arthritis in rat's knee joint. J Neurophysiol 73: 1574–1583, 1995. [DOI] [PubMed] [Google Scholar]
- Puffer JC. The sprained ankle. Clin Cornerstone 3: 38–49, 2001. [DOI] [PubMed] [Google Scholar]
- Safran MR, Benedetti RS, Bartolozzi AR, 3rd, Mandelbaum BR. Lateral ankle sprains: a comprehensive review: part 1: etiology, pathoanatomy, histopathogenesis, and diagnosis. Med Sci Sports Exerc 31: S429–S437, 1999. [DOI] [PubMed] [Google Scholar]
- Schaible H, Ebersberger A. Pain from the arthritic joint. Synaptic Plasticity Pain: 271–288, 2009. [Google Scholar]
- Schaible HG. Spinal mechanisms contributing to joint pain. Novartis Found Symp 260: 4–227, 100,–104, and 277–109, 2004. [PubMed] [Google Scholar]
- Schaible HG, Ebersberger A, Von Banchet GS. Mechanisms of pain in arthritis. Ann NY Acad Sci 966: 343–354, 2002. [DOI] [PubMed] [Google Scholar]
- Schaible HG, Grubb BD. Afferent and spinal mechanisms of joint pain. Pain 55: 5–54, 1993. [DOI] [PubMed] [Google Scholar]
- Schaible HG, Richter F, Ebersberger A, Boettger MK, Vanegas H, Natura G, Vazquez E, Segond von Banchet G. Joint pain. Exp Brain Res 196: 153–162, 2009. [DOI] [PubMed] [Google Scholar]
- Schaible HG, Schmidt RF. Time course of mechanosensitivity changes in articular afferents during a developing experimental arthritis. J Neurophysiol 60: 2180–2195, 1988. [DOI] [PubMed] [Google Scholar]
- Song H, Nakazato K, Nakajima H. Effect of increased excursion of the ankle on the severity of acute eccentric contraction-induced strain injury in the gastrocnemius: an in vivo rat study. Am J Sports Med 32: 1263–1269, 2004. [DOI] [PubMed] [Google Scholar]
- Stein C, Clark JD, Oh U, Vasko MR, Wilcox GL, Overland AC, Vanderah TW, Spencer RH. Peripheral mechanisms of pain and analgesia. Brain Res Rev 60: 90–113, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis W, Coggeshall R. Sensory Mechanisms of the Spinal Cord. New York: Kluwer Academic/Plenum, 2004. [Google Scholar]
- Yu YC, Koo ST, Kim CH, Lyu Y, Grady JJ, Chung JM. Two variables that can be used as pain indices in experimental animal models of arthritis. J Neurosci Methods 115: 107–113, 2002. [DOI] [PubMed] [Google Scholar]