Abstract
Integrin-mediated adhesion is a critical regulator of cell migration. Here we demonstrate that integrin-mediated adhesion to high fibronectin concentrations induces a stop signal for cell migration by inhibiting cell polarization and protrusion. On fibronectin, the stop signal is generated through α5β1 integrin-mediated signaling to the Rho family of GTPases. Specifically, Cdc42 and Rac1 activation exhibits a biphasic dependence on fibronectin concentration that parallels optimum cell polarization and protrusion. In contrast, RhoA activity increases with increasing substratum concentration. We find that cross talk between Cdc42 and Rac1 is required for substratum-stimulated protrusion, whereas RhoA activity is inhibitory. We also show that Cdc42 activity is inhibited by Rac1 activation, suggesting that Rac1 activity may down-regulate Cdc42 activity and promote the formation of stabilized rather than transient protrusion. Furthermore, expression of RhoA down-regulates Cdc42 and Rac1 activity, providing a mechanism whereby RhoA may inhibit cell polarization and protrusion. These findings implicate adhesion-dependent signaling as a mechanism to stop cell migration by regulating cell polarity and protrusion via the Rho family of GTPases.
INTRODUCTION
Cell migration plays a central role in both normal and pathological processes, including embryonic development, wound healing, inflammation, and tumor metastasis (Trinkaus, 1984). Integrin-mediated adhesion to the extracellular matrix is a critical regulator of cell migration speed for many cell types, including fibroblasts and carcinomas. These cell types exhibit a biphasic relationship between cell migration speed and substratum concentration (Goodman et al., 1989; DiMilla et al., 1993; Huttenlocher et al., 1996; Ho et al., 1997; Palecek et al., 1997), with maximum migration rates at intermediate adhesiveness where cells can both efficiently form adhesions at the cell front and release adhesive contacts at the cell rear (DiMilla et al., 1991; Duband et al., 1991; Huttenlocher et al., 1995). Previous studies have implicated reduced adhesive release at the cell's rear as an important mechanism for inhibited migration under conditions of high cell substratum adhesiveness (Marks et al., 1991; Hendey et al., 1992; Jay et al., 1995; Huttenlocher et al., 1997; Cox and Huttenlocher, 1998; Palecek et al., 1998).
Integrins are a family of heterodimeric cell surface adhesion receptors that bind to specific extracellular matrix components and cluster in the membrane to form organized adhesive contacts called focal complexes or focal adhesions (Hynes, 1992). To migrate, cells must coordinately assemble and disassemble integrin-containing adhesive complexes. Integrin-containing adhesive complexes regulate cell migration by performing both an adhesive function, linking the extracellular matrix to the actin cytoskeleton, and a signal transduction function by regulating molecules important for cell motility (Clark and Brugge, 1995; Huttenlocher et al., 1995; Ilic et al., 1995; Cary et al., 1996, 1998; Lauffenburger and Horwitz, 1996; Anand-Apte et al., 1997; Klemke et al., 1997; Cox and Huttenlocher, 1998; Klemke et al., 1998; Palecek et al., 1999). Recent studies have demonstrated that integrin-mediated adhesion to the extracellular matrix regulates the activity of the Rho family of small GTP-binding proteins (Barry et al., 1997; Clark et al., 1998; Price et al., 1998; Ren et al., 1999; del Pozo et al., 2000). Rho family members, which include Cdc42, Rac1, and RhoA, cycle between an active GTP-bound state and an inactive GDP-bound state. Members of this family play an important role in regulating cell migration (Allen et al., 1998; del Pozo et al., 1999; Nobes and Hall, 1999; Ridley et al., 1999; Banyard et al., 2000). Cdc42 stimulates the formation of filopodia and regulates cell polarity (Kozma et al., 1995; Nobes and Hall, 1995; Allen et al., 1998; Nobes and Hall, 1999), whereas Rac1 induces lamellipodia (Nobes and Hall, 1995). Cdc42 and Rac1 signal to form new focal complexes at the leading edge of the cell that promote membrane ruffling and the formation of lamellipodia (Hotchin and Hall, 1995; Kozma et al., 1995; Nobes and Hall, 1995; Rottner et al., 1999). RhoA stimulates the formation of stress fibers (Ridley and Hall, 1992) and is required for the maturation of adhesive contacts into focal adhesions (Ridley and Hall, 1992; Hotchin and Hall, 1995; Rottner et al., 1999).
Here we demonstrate that integrin-mediated adhesion regulates cell polarization and protrusion. Specifically, we find that high substratum concentrations induce a “stop” signal that inhibits cell polarization and protrusion. In contrast to previous studies that have implicated adhesive release as rate limiting to migration speed at high substratum concentrations, our studies implicate a novel mechanism, i.e., an adhesion-dependent stop signal that regulates cell polarization and protrusion. We find that the stop signal mediated by high fibronectin concentrations is transduced through the Rho family of proteins. We show that a biphasic relationship exists between fibronectin-coating concentration and Cdc42 and Rac1 activation that parallels optimum polarization and protrusion. In contrast, RhoA activity increases and plateaus, remaining elevated at high substratum concentrations. We also find that RhoA activity can inhibit substratum-stimulated protrusion by down-regulating Cdc42 and Rac1 activity. Further evidence for the importance of cross talk between the Rho family of GTPases is provided by our observation that Rac1 can directly down-regulate Cdc42 activity. Together, the findings implicate adhesion-dependent signaling as a mechanism to “stop” cell migration, through regulating cell polarization and protrusion via the Rho family of GTPases.
MATERIALS AND METHODS
Vectors
The pEXVmyc-w.t.RhoA, pRK5myc-L63RhoA, pEXVmyc-N17Cdc42, and pEXVmyc-N17Rac1, and pRK5myc-L61Rac1 were provided by Alan Hall (University College London, London, United Kingdom). Inserts were removed from the pEXVmyc vectors via EcoRI digestion and cloned into the pIRES-EGFP vector (Clontech, Palo Alto, CA). The insert was removed from the pRK5myc-L61Rac1 vector via BamHI and EcoRI digestion and cloned into the pIRES-EGFP vector. CMV5-N19RhoA was provided by Martin Schwartz (The Scripps Research Institute, La Jolla, CA). The pLEX A GST-PBD and pGEX 2T GST-RBD vectors used in the Cdc42, Rac1, and RhoA activity assays were obtained from Keith Burridge (University of North Carolina, Chapel Hill, NC). pcDNA3.1 and pcDNA3.1hygro-lacZ were purchased from Invitrogen (Carlsbad, CA).
Antibodies and Reagents
Fibronectin was purified from human plasma by affinity chromatography as previously described (Ruoslahti et al., 1982). Fibrinogen and anti-vinculin antibody were purchased from Sigma Chemical (St. Louis, MO). Rhodamine-labeled phalloidin was obtained from Molecular Probes (Eugene, OR). Anti-Cdc42 and anti-Rac1 antibody were obtained from Transduction Laboratories (San Diego, CA). Anti-RhoA antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Goat anti-mouse antibody conjugated to horseradish peroxidase and sheep anti-mouse antibody conjugated to fluorescein were obtained from Jackson ImmunoResearch (West Grove, PA).
Cell Culture and Transfection
Neutrophils were purified from human blood using Polymorphprep solution (Nycomed, Oslo, Norway) according to the manufacturer's instructions. CHO-K1 cells (Chinese hamster ovary fibroblast-like cells) were obtained from the American Type Culture Collection (Rockville, MD). The cells were grown at 37°C and 10% CO2 in complete growth media: sodium bicarbonate-buffered Dulbecco's Modified Eagle's Medium (DME) with 10% fetal bovine serum (FBS), 2 mM glutamine, 1 mM sodium pyruvate, 1% nonessential amino acids, 100 U/ml penicillin, and 100 μg/ml streptomycin (Sigma Chemical).
CHO cells were transiently transfected using 4 μg of DNA and 15 μl of Lipofectamine (Life Technologies, Gaithersburg, MD) according to the manufacturer's instructions with the following exception: after adding the DNA:lipid complexes to the cells, 2 ml of complete growth media was added and cells were incubated for 12 h before washing. For cotransfections, a ratio of 1:8 (pIRES-N17Rac:pEXV-N17Cdc42 or pcDNA3.1) was used. In cotransfection experiments, only green flourescence protein (GFP)-positive cells were analyzed. GFP-positive cells should express N17Rac because the IRES-EGFP vector (Clontech) contains an internal ribosomal entry site that permits the gene of interest (N17Rac) and the EGFP gene to be translated from a single bicistronic mRNA. Cells were used in experiments 48–60-h posttransfection. Before use in serum-free experiments, cells were plated for 24 h and then incubated for 12–18 h in growth media with reduced (1%) FBS. To plate cells for experiments, cells were removed with 0.02% EDTA in phosphate-buffered saline, washed with phosphate-buffered saline, washed with 1× Puck's Saline G (to deplete growth factors), and washed 2× with DME+ (growth media without FBS) supplemented with 0.2% bovine serum albumin (BSA). The cells were plated in DME+ with 0.2% BSA onto fibronectin-coated nontissue culture plastic plates blocked with 2% BSA. Cells were seeded onto 35-mm plates at 1.5 × 105/plate for time-lapse videomicroscopy experiments. For use in experiments with the growth factor-supplemented media, CCMI (Hyclone Laboratories, Logan, UT) cells were plated for 24–36 h before use. For all experiments, “low” substratum concentration denotes 1–2 μg/ml fibronectin, “intermediate” denotes 3–10 μg/ml, “high” denotes 20–40 μg/ml, and “very high” denotes 50–100 μg/ml fibronectin.
Time-Lapse Videomicroscopy.
Neutrophils were plated in CCM1 onto 35-mm dishes coated with fibrinogen or fibronectin. Five minutes after plating, 1 × 10−8 M formyl-methionyl-leucyl-phenylalanine (fMLP, Sigma Chemical) was added to the dish to activate the neutrophils. After a 5-min incubation with the fMLP, plates were placed on a heated microscope stage and supplied 10% CO2. Images were collected 5–10 min after cells were placed on the stage using ISee analytical software (Inovision, Raleigh, NC).
CHO-K1 cells were plated as described above and incubated for 1 h at 37°C in 10% CO2. The plated cells were either placed directly onto a heated microscope stage with 10% CO2, or the system was closed and placed onto a heated stage. To close the system, the plate was filled completely with 10% CO2-saturated media (DME+ with 0.2% BSA, or CCMI) and sealed with vacuum grease and a 40-mm glass coverslip (Bioptechs, Butler, PA). A constant pH of 7.0–7.4 and temperature of 37°C was maintained during taping. Cells were allowed to equilibrate on the microscope stage for 1 h before images were captured for analysis.
Transient Membrane Protrusion Analysis
Transient membrane protrusion analysis was performed using a 20× objective on an Olympus IX-70 inverted microscope (Olympus America, Melville, NY). Images were captured at 15-s intervals for 5 min using an LG3 frame grabber (Scion, Frederick, MD) and three or four different fields were analyzed per condition per experiment using Scion Image software. Scion Image is a variation of NIH Image, which was developed at the U.S. National Institutes of Health and is available at http://rsb.info.nih.gov/nih-image/. A macro for transient membrane protrusion was written for Scion Image based on a previously described program (Araki et al., 1996). Subsequent images in the stack were overlaid with each other and a difference image was created for each by subtracting the gray level values (0–255, white-black) for each pixel in the image, multiplying by 0.5 and adding 128. A 5% threshold was applied to the difference image so that any pixel undergoing more than a 5% change in gray level value (falling in the range of 0–113 or 143–255) was scored as positive. This threshold was chosen because it detects membrane activity at the cell edge while minimizing the detection of intracellular vesicle and organelle movement. A binary image was then created in which positive pixels were displayed as white against a black background. The white pixels in a selected area encompassing a cell were then scored. The analysis was performed for every cell that did not divide or come into contact with other cells during the experiment. For each experiment, the average number of pixels exceeding the 5% threshold per 15-s interval was determined for each condition. Then the average (transient protrusion index) and SD for three experiments were calculated. For each condition, 18–50 cells were quantified in total from three to four separate experiments.
Stabilized Membrane Protrusion Analysis
Stabilized membrane protrusion analysis was performed using a 20× objective on an Olympus IX-70 inverted microscope (Olympus America). Images were captured at 5-min intervals for 1 h and four fields were examined on each plate using an automated stage (Ludl Electronic Products, Hawthorne, NY). Cells in each pair of sequential images were traced and overlaid. ISee analytical imaging software (Inovision) was used to calculate the protrusion area (μm2) and total cell area (μm2) for each set of overlaid images. Contacting or dividing cells were omitted. For each experiment, the average area of protrusion per 5-min interval was determined and normalized to the average cell area for the condition. Then the average normalized protrusion area and SD were determined for three experiments. For each condition, 15–30 cells were quantified in total from three to four separate experiments.
Immunofluorescence
Cells were prepared for use in immunofluorescent cell staining experiments as described for time-lapse videomicroscopy. Glass coverslips were acid washed, silanated, conjugated to fibronectin, and blocked with 2% BSA using previously described methods (Crowley and Horwitz, 1995). Cells were plated at a density of 2 × 104/coverslip in DME+ with 0.2% BSA. After a 3-h incubation at 37°C, 10% CO2, cells were fixed and stained for vinculin and actin as described previously (Huttenlocher et al., 1996).
Cdc42, Rac1, and RhoA Activity Assays
All activity assays were performed with serum-starved CHO-K1 cells cultured as described above. For activity assays, cells were transfected as described above except cells were initially incubated for 2 h after addition of the lipid:DNA complexes in DME+ to boost transfection efficiency. After 2 h, the cells were supplemented with 10% FBS and the transfection was completed as described. Transfection efficiency was typically 80–90%, as determined by β-galactosidase staining of control cells transfected with pcDNA3.1hygro-lacZ. Serum-starved cells were plated onto 10-cm nontissue culture plates coated with fibronectin and blocked with BSA (as described above) at a concentration of 1.5 × 106 cells/plate. Cells were plated in serum-free conditions as described above. After a 3-h incubation, lysates were collected and activity assays were performed as described (Bagrodia et al., 1998). To assay the activity of Cdc42 and Rac1, cell lysate was affinity precipitated with a PAK binding domain-GST fusion protein that only binds to the active, GTP-bound form of Rac1. The total cell lysates and the affinity-precipitated products were run on an SDS-PAGE gel, transferred to nitrocellulose, and blotted for Cdc42 or Rac1. Staining was detected using enhanced chemiluminescence and densitometry was performed using Scion Image (Scion, Frederick, MD).
The RhoA assay was performed as described (Ren et al., 1999). This assay is similar to the Rac1 activity assay, only a Rhotekin binding domain-GST fusion protein, which binds to the GTP-bound form of RhoA, was used for the affinity precipitation. The total cell lysates and the affinity-precipitated products were run on an SDS-PAGE gel, transferred to nitrocellulose, and blotted for RhoA. Staining was detected using enhanced chemiluminescence.
RESULTS
Substratum Concentration Regulates Cell Polarization and Membrane Protrusion
The migration speed of CHO-K1 cells displays a biphasic dependence on substratum concentration, with maximum migration at intermediate substratum concentrations (Huttenlocher et al., 1996; Palecek et al., 1997). CHO cell migration on fibronectin is dependent on the α5β1 integrin because the function-perturbing antibody, PB1, blocks migration on fibronectin (Huttenlocher et al., 1996). To determine a mechanism for reduced migration rates of cells plated on high concentrations of substratum, we examined cell morphology using time-lapse videomicroscopy. We find that CHO cells and neutrophils show a substrate concentration-dependent effect on cell polarization and protrusion (Figure 1).
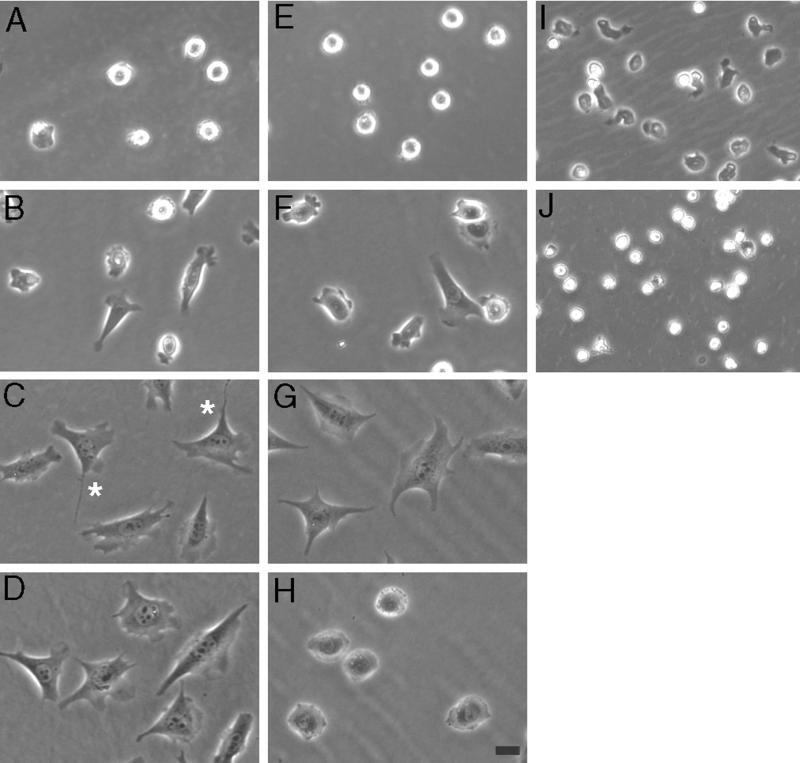
Figure 1.
Substratum concentration regulates the polarity of CHO cells (A–H) and neutrophils (I–J). Phase pictures (20×) of CHO cells plated on low (A and E), intermediate (B and F), high (C and G), and very high (D and H) concentrations of fibronectin. Cells were plated in CCMI (A–D) or serum-starved and plated in growth factor-free media (E–H, video sequences for these experiments are available online). Cells do not spread well and fail to polarize on low concentrations of fibronectin. Cells polarize well on intermediate concentrations, less on high concentrations, and only minimally on very high fibronectin concentrations. Note the reduction in cell spreading exhibited by serum-starved cells plated onto very high concentrations of fibronectin (H). Asterisks indicate cells on a high fibronectin concentration in CCMI, which exhibit inhibited tail release. Phase pictures (20×) of neutrophils plated on intermediate (I) and very high (J) concentrations of fibrinogen. Polarization and cell spreading are inhibited on very high concentrations of fibrinogen. Bar, 20 μm. Video sequences show serum-starved CHO cells plated on intermediate (Figure 1F.mov), high (Figure 1G.mov), or very high (Figure 1H.mov) concentrations of fibronectin. Video material is available at www.molbiolcell.org.
We examined the morphology of CHO cells plated onto a range of fibronectin concentrations in the presence and absence of exogenous growth factors. In the presence of the growth factor-supplemented media CCM1, CHO cells demonstrate optimum polarization at intermediate substratum concentrations that support maximum migration (Figure 1B). At low substratum concentrations, cells do not spread, polarize, or protrude (Figure 1A). At high ligand concentrations in the presence of growth factors, cells spread maximally but display a less polarized morphology with less protrusive activity (Figure 1C). Some cells plated on high fibronectin concentrations polarize, and migration of these cells is limited by rear detachment rate (Figure 1C, asterisks). However, cells plated on very high substratum concentrations do not polarize and exhibit reduced protrusive activity (Figure 1D). These findings suggest that fibronectin concentration regulates membrane protrusion and polarization and contributes to the inhibition of migration on high substratum concentrations.
To examine how fibronectin-coating concentration regulates cell morphology and polarization in cells independent of exogenous growth factors, we examined serum-starved CHO cells plated on different concentrations of fibronectin in a growth factor-free media. In the absence of exogenous growth factors, CHO cells display substratum-stimulated membrane-protrusive activity and polarization at an intermediate fibronectin-coating concentration, suggesting that fibronectin alone is sufficient to stimulate membrane protrusion without exogenous growth factors (Figure 1F, video). However, the effects are less robust than those seen in the presence of growth factors and, in contrast to cells plated in CCM1, the cells generally do not translocate. Fibronectin-coating concentration also regulates cell morphology and protrusion under growth factor-free conditions, where the effects of high fibronectin concentration on cell morphology are more dramatic. On high fibronectin concentrations, CHO cells are spread (average cell area = 4339 ± 360 μm2) but exhibit no polarization or protrusion (Figure 1G, video). In a manner analogous to neutrophils, CHO cells plated at very high fibronectin-coating concentrations are less spread (average cell area = 3271 ± 106 μm2), do not polarize, and exhibit only minimal membrane protrusion (Figure 1H, video). Together, these findings suggest that adhesion-dependent signaling pathways regulate cell polarization and protrusion in a substratum concentration-dependent manner.
Interestingly, we find that neutrophils, which are less adhesive and migrate more rapidly than fibroblasts, also exhibit reduced cell polarity and spreading at high substratum concentrations. Neutrophils were stimulated with the chemoattractant fMLP, plated on different concentrations of fibrinogen or fibronectin, and observed using time-lapse videomicroscopy. At very high fibrinogen or fibronectin-coating concentrations neutrophils do not polarize and are less spread than cells plated on intermediate concentrations of ligand (Figure 1J). Intermediate concentrations of ligand promote maximum polarization with visible membrane protrusion (Figure 1I). Together, these findings suggest that high ligand-coating concentrations induce a stop signal that inhibits the polarization and migration of many cell types, including fibroblasts and neutrophils. This is in contrast to previous studies that have implicated release of attachments at the cell rear as the rate-limiting step of cell migration on high substratum concentrations.
CHO Cells Exhibit Reduced Transient and Stabilized Membrane Protrusion on High Coating Concentrations of Fibronectin
To quantify the effects of fibronectin-coating concentration on membrane protrusive activity in CHO cells, two assays of membrane protrusion were used. The assays were designed to quantify the two prominent types of membrane activity typically observed in adherent CHO cells by time-lapse videomicroscopy. The first type of activity analyzed is transient membrane protrusion, which occurs predominantly at the cell periphery. Quantification of transient membrane protrusion is performed using an assay that calculates the changes in pixel intensity that occur at the cell periphery over short 15-s time intervals. In general, migratory cells display transient membrane protrusion, although this activity does not mean that the cell will necessarily undergo changes in net cell area or translocate. However, cells that are nonmigratory are generally quiescent with an absence of transient membrane protrusion (Huttenlocher et al., 1998). The second type of activity quantified is stabilized membrane protrusion, which measures the stabilized protrusion area over longer, 5-min time intervals. In general, cells that display stabilized protrusive activity will migrate and translocate. Using these two assays we examined the relationship between substratum concentration and membrane protrusive activity in CHO cells. Examples of cells used in the transient and stabilized membrane protrusion analysis are shown in Figures 2A and 3A, respectively. We find that fibronectin stimulates membrane protrusion in the absence of exogenous growth factors in serum-starved cells, suggesting that fibronectin alone is sufficient to stimulate membrane protrusion (Figures 2B and 3B). However, this membrane activity is substratum concentration-dependent with cells exhibiting maximum transient and stabilized protrusion at an intermediate substratum concentration. There is a striking reduction in membrane protrusion at high substratum concentrations where the cells are quiescent. This shutdown in membrane activity was not dependent on a more spread morphology because cells on a very high substratum concentration are often “rounded” but still display an absence of transient and stabilized protrusion. In the presence of growth factors, a similar trend is observed with cells exhibiting optimal membrane activity at intermediate fibronectin-coating concentrations and reduced membrane activity at higher concentrations of fibronectin (Figures 2B and 3B); this correlates with findings from a recent study of stabilized membrane protrusion in EGF-stimulated cells (Maheshwari et al., 1999). To summarize, we find that substratum concentration regulates the membrane protrusion and cell morphology of CHO-K1 cells. These findings suggest that substratum concentration regulates intracellular signaling pathways important for membrane protrusion and polarization.
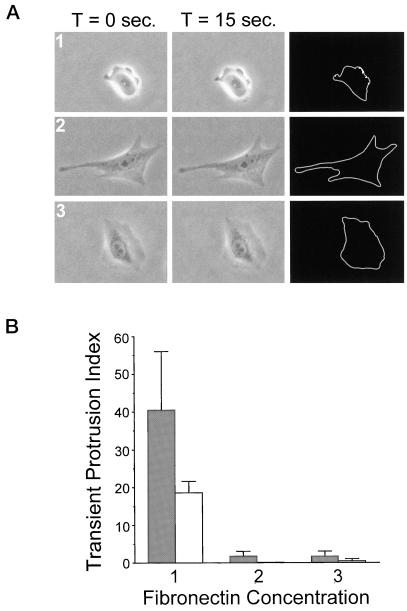
Figure 2.
Substratum concentration regulates transient membrane protrusion in CHO cells. Quantitative analysis was performed to assay transient membrane protrusion in CHO cells. Cells were plated onto intermediate (1), high (2), and very high (3) concentrations of fibronectin for 2–4 h before analysis. (A) Examples of serum-starved cells used in transient membrane protrusion analysis (performed as described in MATERIALS AND METHODS). Images were taken at 15-s intervals for 5 min. Subsequent images were overlaid and pixels undergoing a change in gray level value of 5% or more were scored as positive (white pixels on black background in far right column). This was done for all images in the stack and results were quantified as described in MATERIALS AND METHODS. (B) Summarized results from three experiments using transient membrane protrusion analysis. Gray bars represent data from cells plated in CCMI and white bars represent data from serum-starved cells plated in serum-free media. The transient protrusion index is the average number of pixels per cell undergoing a 5% or more change in gray level value per 15-s interval. Data are the average of three experiments and error bars represent the SD. Transient membrane protrusion is maximal at an intermediate fibronectin concentration and reduced on higher concentrations.
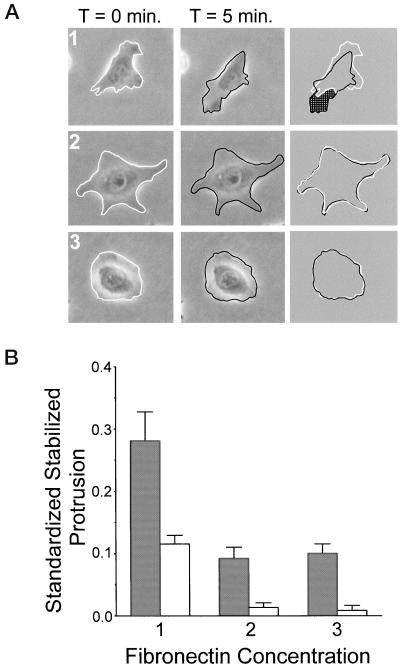
Figure 3.
Substratum concentration regulates stabilized membrane protrusion in CHO cells. Quantitative analysis was performed to assay stabilized membrane protrusion in CHO cells. Cells were plated onto intermediate (1), high (2), and very high (3) concentrations of fibronectin for 2–4 h before analysis. (A) Examples of serum-starved cells used in stabilized membrane protrusion analysis (performed as described in MATERIALS AND METHODS). Images were taken at 5-min intervals for 1 h. Subsequent images were overlaid and the area of protrusion (shaded in far right column) was calculated and normalized to the total cell area. This was done for all images in the stack and results were quantified as described in MATERIALS AND METHODS. (B) Summarized results from three experiments using stabilized membrane protrusion analysis. Gray bars represent data from cells plated in CCMI and white bars represent data from serum-starved cells plated in serum-free media. Standardized stabilized protrusion is the average area of protrusion normalized to the total cell area per cell per 5-min interval. Data are the average of three experiments and error bars represent the SD. Stabilized membrane protrusion is maximal at an intermediate fibronectin concentration and reduced on higher concentrations.
Fibronectin Concentration Regulates Focal Adhesion Morphology
To establish a role for adhesive signaling in the substratum-dependent effects on cell polarization and membrane activity, serum-starved CHO cells were plated on different concentrations of fibronectin and focal adhesion morphology was examined. Serum-starved CHO cells exhibit striking differences in focal adhesion and actin organization when adherent for 3 h to coverslips coated with different concentrations of fibronectin (Figure 4). Cells plated on intermediate concentrations of fibronectin are less spread and display visible membrane protrusion. These cells have punctate adhesive complexes located around the cell periphery and a poorly organized actin cytoskeleton (Figure 4, A and D). Cells plated on high fibronectin concentrations are more spread and have a polygonal morphology. These cells have more prominent focal adhesions with peripheral stress fibers (Figure 4, B and E). In contrast, cells plated on very high concentrations of fibronectin are less spread and less polarized. These cells display stronger focal adhesions throughout the cell with more centrally distributed stress fibers (Figure 4, C and F). These results demonstrate that cells adhering to coverslips coated with different concentrations of fibronectin form integrin-containing adhesive complexes that differ in size, number, and location. The small punctate focal complexes that form at intermediate fibronectin-coating concentrations are similar to focal complexes mediated by Cdc42 and Rac1 (Nobes and Hall, 1995). In contrast, the more prominent, centrally located complexes with central stress fibers that form at very high fibronectin-coating concentration are similar to the focal adhesions mediated by the small GTPase, RhoA (Ridley and Hall, 1992). These findings suggest that different fibronectin-coating concentrations may regulate cell morphology and migration by modulating intracellular signaling via the small GTPases Cdc42, Rac1, and RhoA.
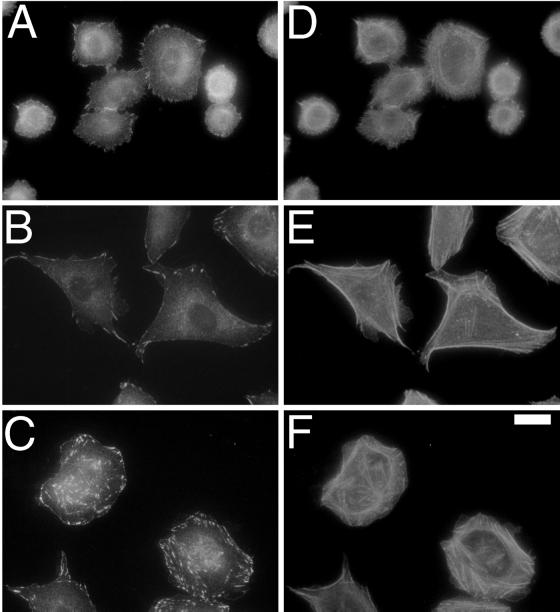
Figure 4.
Substratum concentration regulates focal adhesion morphology in serum-starved CHO cells. Cells (60×) were plated for 3 h on silanated glass coverslips coated with intermediate (A and D), high (B and E), and very high (C and F) fibronectin concentrations and stained for vinculin (A–C) and actin (D–F). On higher substratum concentrations, cells form stronger, more centrally located adhesions and stress fibers. Bar, 20 μm.
Cdc42 and Rac1 Activity Are Maximal at an Intermediate Fibronectin Concentration, whereas RhoA Activity Increases with Increasing Fibronectin Concentration
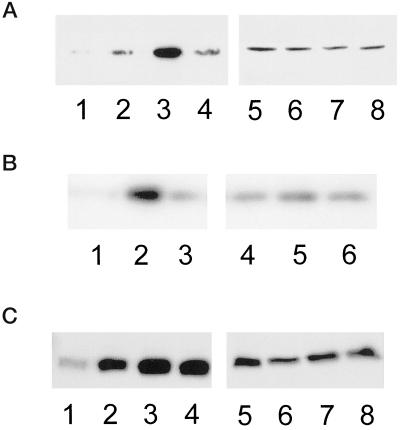
To determine whether fibronectin concentration regulates Cdc42, Rac1, and RhoA activity, activity assays were performed using serum-starved CHO cells plated on different concentrations of fibronectin. Interestingly, we find that Cdc42, Rac1, and RhoA activity is differentially regulated by fibronectin concentration. The activity levels of Cdc42 and Rac1 exhibit a biphasic pattern with respect to fibronectin-coating concentration, with optimal activity occurring at an intermediate substratum concentration (Figure 5, A and B). Cdc42 and Rac1 activity levels are reduced at both higher and lower fibronectin-coating concentrations. This is the first study that demonstrates that the activity of signaling molecules critical for cell movement exhibits a biphasic dependence on cell substratum adhesion. The activity of Cdc42 and Rac1 parallels the biphasic dependence of cell speed with substratum concentration, implicating reduced adhesion-dependent signaling via Cdc42 and Rac1 as a mechanism that contributes to reduced migration rates under conditions of high cell-substratum adhesion. In contrast to Cdc42 and Rac1 activity, RhoA activity increases and then plateaus, remaining elevated at high substratum concentrations (Figure 5C). Therefore, Cdc42 and Rac1 activity is maximal at intermediate substratum concentrations that support membrane polarization and protrusion, whereas RhoA activity remains elevated at higher substratum concentrations.
Figure 5.
Cdc42 and Rac1 activation exhibit a biphasic dependence on substrate concentration, whereas RhoA activation increases with increasing substratum concentration. (A) Representative blot showing that Cdc42 activation is optimal at an intermediate fibronectin concentration. Serum-starved CHO cells were kept in suspension (1 and 5) or plated onto low (2 and 6), intermediate (3 and 7), or very high (4 and 8) concentrations of fibronectin for 3 h and activity assays were performed as described in MATERIALS AND METHODS. Affinity-precipitated Cdc42-GTP was run in lanes 1–4 and total cell lysate was run in lanes 5–8. (B) Representative blot showing that Rac1 activation is optimal at an intermediate fibronectin concentration. Serum-starved CHO cells were plated onto low (1 and 4), intermediate (2 and 5), or very high (3 and 6) concentrations of fibronectin for 3 h and activity assays were performed as described in MATERIALS AND METHODS. Affinity-precipitated Rac1-GTP was run in lanes 1–3 and total cell lysate was run in lanes 4–6. (C) Representative blot showing that RhoA activation increases with increasing fibronectin concentration. Serum-starved CHO cells were kept in suspension (1 and 5) or plated onto low (2 and 6), intermediate (3 and 7), or very high (4 and 8) concentrations of fibronectin for 3 h and activity assays were performed as described in MATERIALS AND METHODS. Affinity-precipitated RhoA-GTP was run in lanes 1–4 and the total cell lysate was run in lanes 5–8.
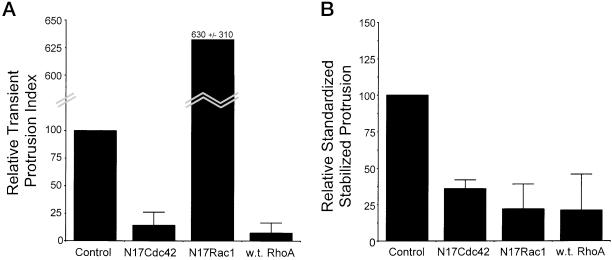
Substratum-stimulated Membrane Protrusion Requires the Activity of Cdc42 and Rac1 but Is Inhibited by RhoA
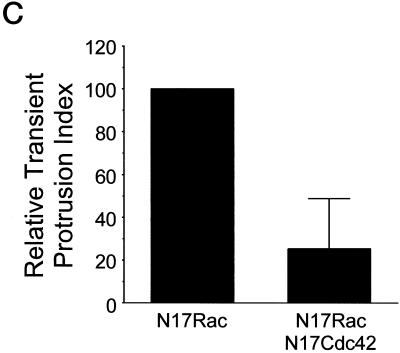
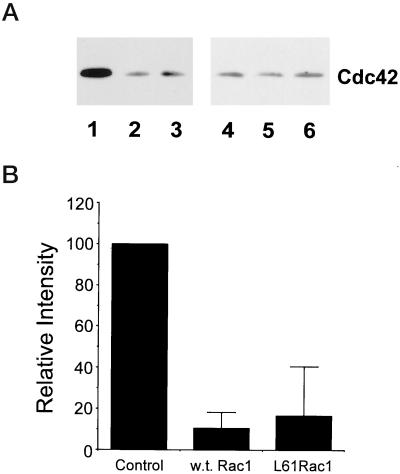
To investigate whether adhesion-dependent activation of Cdc42 and Rac1 is required for fibronectin-stimulated membrane protrusion, cells were transiently transfected with vectors that coexpress GFP and either dominant negative Cdc42 or Rac1. Expression of the proteins was determined by identifying GFP-positive cells. Membrane protrusive activity was examined in transfected cells plated onto an intermediate concentration of fibronectin using the two membrane protrusion assays described above. Expression of N17Cdc42 blocked both transient and stabilized membrane protrusion, suggesting that Cdc42 is required for fibronectin-stimulated membrane activity (Figure 6, A and B). Interestingly, expression of N17Rac1 inhibited stabilized membrane protrusion, but promoted rather than inhibited transient membrane activity. To determine whether the transient membrane protrusion stimulated by N17Rac1 is dependent on Cdc42, cells were cotransfected with pIRES-EGFP-N17Rac1 and pEXV-mycN17Cdc42 at a 1:8 ratio and transient protrusive activity was assayed in GFP-positive cells. Expression of N17Cdc42 blocks the transient protrusive activity stimulated by N17Rac1 (Figure 6C). Since inhibiting Rac1 leads to an up-regulation of Cdc42-mediated transient protrusive activity, the findings suggests that Rac1 may down-regulate Cdc42 activity. To test this possibility, cells were transfected with wild-type Rac1 or L61Rac (constitutively active) and Cdc42 activity assays were performed. Both wild-type and L61Rac down-regulate Cdc42 activity (Figure 7, A and B). These findings suggest that Rac1 inhibits Cdc42-mediated transient protrusive activity by down-regulating Cdc42 activity. Rac1-mediated inhibition of transient nondirectional membrane ruffling may be an important mechanism for the generation of directional stabilized protrusions. Collectively, these results demonstrate that Cdc42 and Rac1 activity is necessary for substratum-stimulated membrane activity and cross talk between Cdc42 and Rac1 is important for the formation of membrane protrusion.
Figure 6.
Cross talk between Cdc42 and Rac1 is required for substratum-stimulated membrane activity, whereas RhoA activity is inhibitory. (A and B) CHO cells were transfected with control vector, or vectors expressing N17Cdc42 (dominant negative Cdc42), N17Rac1 (dominant negative Rac1), or wild-type RhoA. Analysis was performed using serum-starved cells 2–4 h after plating on an intermediate concentration of fibronectin. (A) Summarized results from three experiments using transient membrane protrusion analysis. The transient protrusion index was determined for the control and standardized to 100. The transient protrusion index for other conditions was determined as a percentage of control with the error bars representing the SD. (B) Histogram showing results from three experiments using stabilized membrane protrusion analysis. The standardized stabilized protrusion was determined for controls and standardized to 100. The relative standardized stabilized protrusion for other conditions was determined as a percentage of control with the error bars representing the SD. (C) Dominant negative Cdc42 inhibits the transient membrane activity stimulated by dominant negative Rac1. Cells were cotransfected in a 1:8 ratio with pIRES-EGFP-N17Rac and either pcDNA3.1 (control) or pEXV-N17Cdc42. Cotransfected cells were identified through fluorescence and transient membrane activity was quantified as described in MATERIALS AND METHODS. The transient protrusion index was determined for the control and standardized to 100. The transient protrusion index for cells cotransfected with N17Cdc42 was determined as a percentage of control with the error bars representing the SD.
Figure 7.
Rac1 down-regulates Cdc42 activity. (A) Representative blot showing that Cdc42 activity is inhibited in cells expressing wild-type and L61Rac1. Cells were transiently transfected with control vector (1 and 4), wild-type Rac1 (2 and 5), or L61Rac1 (3 and 6) (constitutively active), and Cdc42 activity assays were performed as described in MATERIALS AND METHODS. Affinity-precipitated Cdc42-6TP was run in lanes 1–3, and total cell lysate was run in lanes 4–6. (B) Results from densitometry analysis of three experiments. The signal obtained for cells transfected with control vector was assigned a relative intensity of 100. For other samples, the intensity of signal was determined as a percentage of control. Error bars represent the SD.
In contrast to Cdc42 and Rac1, we find that the expression of wild-type RhoA inhibits substratum-stimulated transient and stabilized membrane protrusion (Figure 6, A and B). In addition, the expression of RhoA generally produces a more spread, less polarized morphology at an intermediate substratum concentration (RhoA-transfected cells have an average cell area of 4500 ± 1000 μm2 versus control cells, which have an average area of 1760 ± 320 μm2). However, on high fibronectin concentrations (at which cells are typically well spread) expression of RhoA promotes a less spread, less polarized morphology with inhibited membrane protrusive activity, typical of cells normally plated on very high fibronectin concentrations (our unpublished results). These results demonstrate that RhoA activity, in contrast to Cdc42 and Rac1, inhibits substratum-stimulated polarization and protrusion and promotes cell spreading on intermediate fibronectin concentrations. Therefore, RhoA expression promotes phenotypic traits associated with adhesion to higher substratum concentrations.
To determine a mechanism for the inhibition of polarization and protrusion induced by RhoA, the effects of RhoA expression on Cdc42 and Rac1 activity were examined. Activity assays were performed on an intermediate fibronectin-coating concentration with cells transiently transfected with control vector, wild-type RhoA, or L63RhoA (constitutively active). We find that expression of RhoA and L63RhoA down-regulates Cdc42 and Rac1 activity (Figure 8). This suggests that RhoA may inhibit polarization and protrusion through down-regulating Cdc42 and Rac1 activity. To determine whether RhoA is required for the inhibition of membrane polarization and protrusion observed at high ligand density, cells were transfected with dominant negative RhoA and observed on a high fibronectin-coating concentration through time-lapse videomicroscopy. We find that dominant negative RhoA did not increase the polarization or protrusive activity of cells on high fibronectin-coating concentrations (our unpublished results). This suggests that RhoA-independent mechanisms may contribute to reduced Cdc42 and Rac1 activity, and the inhibition of polarization and protrusion that occurs on high fibronectin concentrations. Together, the findings suggest that cross talk between Cdc42, Rac1, and RhoA plays a central role in the adhesion-dependent regulation of cell polarization and protrusion.
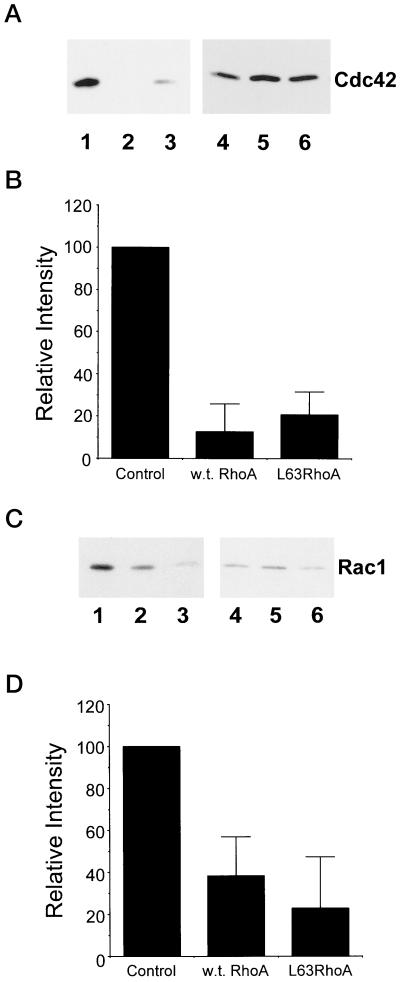
Figure 8.
RhoA down-regulates Cdc42 and Rac1 activity. Cells were transiently transfected with either control vector, wild-type RhoA, or L63RhoA (constitutively active), and Cdc42/Rac1 activity assays were performed as described in MATERIALS AND METHODS. (A and C) Representative blots showing that Cdc42 (A) and Rac1 (C) activity are reduced in CHO cells that express wild-type and L63RhoA. Cells were transfected with control vector (1 and 4), wild-type RhoA (2 and 5), or L63RhoA (3 and 6) and plated onto an intermediate fibronectin-coating concentration. Affinity-precipitated Cdc42-GTP or Rac1-GTP was run in lanes 1–3 and total cell lysate was run in lanes 4–6. (B and D) Results from densitometry analysis of three experiments from the Cdc42 (B) and Rac (D) activity assays. The signal obtained for cells transfected with control vector was assigned a relative intensity of 100. For other samples, the intensity of signal was determined as a percentage of control. Error bars represent the SD.
DISCUSSION
The migration rates of many cell types, including fibroblasts and carcinomas, exhibit a biphasic dependence on cell substratum adhesiveness, with optimal speeds occurring at intermediate levels of adhesiveness (Goodman et al., 1989; DiMilla et al., 1993; Huttenlocher et al., 1996; Ho et al., 1997; Palecek et al., 1997). We have also demonstrated that neutrophils, which move more rapidly than fibroblasts, exhibit a biphasic relationship between cell speed and substratum concentration with maximum migration rates occurring at intermediate substratum concentrations (Huttenlocher, unpublished data). The biphasic dependence of cell migration speed on cell substratum adhesiveness, therefore, appears to be an important regulatory mechanism that governs the migration of both slow-moving cell types, such as fibroblasts, as well as the more rapid, gliding movements of leukocytes.
Previous studies have implicated cell detachment as the rate-limiting step to cell migration speed under conditions of high cell substratum adhesiveness (Lauffenberger and Horwitz, 1996; Cox and Huttenlocher, 1998; Palecek et al., 1999). In addition, we and others have demonstrated that mechanisms that reduce cell detachment rate are inhibitory to cell migration speed, supporting the importance of cell detachment as a mechanism to modulate cell migration rate (Marks et al., 1991; Hendey et al., 1992; Jay et al., 1995; Huttenlocher et al., 1997; Cox and Huttenlocher, 1998; Palecek et al., 1998). However, we now provide evidence that cell substratum adhesiveness also regulates cell polarization and protrusion. Specifically, we find that high substratum concentrations induce a stop signal that inhibits cell polarization and protrusion.
The substratum concentration dependence of cell polarization and protrusion suggests that integrin-mediated adhesive signaling pathways may be involved in regulating adhesion-dependent membrane protrusive activity. Specifically, the substratum concentration-dependent effects on the organization of the actin cytoskeleton and focal adhesions implicate adhesive signaling via the Rho family of GTPases. Recent studies have demonstrated that integrin-mediated adhesion regulates the activities of Cdc42, Rac1, and RhoA and these activities are required for normal cell spreading (Barry et al., 1997; Clark et al., 1998; Price et al., 1998; Ren et al., 1999; del Pozo et al., 2000). Here, we provide evidence that α5β1 integrin-mediated adhesion to fibronectin differentially regulates the activities of Cdc42, Rac1, and RhoA. In serum-starved CHO cells, Cdc42 and Rac1 activity demonstrate a biphasic dependence on fibronectin concentration, with maximum activity occurring at intermediate substratum concentrations that support membrane protrusion and polarization. This is the first study to demonstrate a clear biphasic dependence of an adhesion-regulated signaling pathway on substratum concentration. A recent study demonstrated that the activity of FAK and ERK2, unlike Cdc42 and Rac1, increases monotonically with ligand concentration (Asthagiri et al., 1999). Like FAK and ERK2 activity, we find that RhoA activity also increases with increasing fibronectin concentration but plateaus at an intermediate ligand concentration. Therefore, Cdc42 and Rac1 are optimally active at substratum concentrations that stimulate polarization and protrusion, whereas RhoA continues to be active at higher substratum concentrations where polarization and protrusion are inhibited. Other recently published reports have demonstrated that the balance between Cdc42, Rac1, and RhoA activity is a critical determinant of the cell phenotype during cell migration, invasion, and neurite outgrowth (van Leeuwen et al., 1997; Sander et al., 1999; Banyard et al., 2000; Zondag et al., 2000).
To test whether substratum-stimulated membrane protrusion requires the activity of Cdc42 and Rac1 we expressed dominant negative forms of the proteins to inhibit their activity. We find that Cdc42 is required for transient and stabilized substratum-stimulated membrane activity, whereas Rac1 activity is required for stabilized protrusion but not transient activity. These findings are in agreement with reports in the literature that suggest that Cdc42 acts upstream of Rac1 to stimulate transient protrusive activity (Nobes and Hall, 1995). Interestingly, inhibiting Rac1 activity stimulates transient membrane protrusion, and this effect is mediated by Cdc42 activity. Furthermore, expression of wild-type and constitutive active Rac1 down-regulates Cdc42 activity. These findings implicate a novel mechanism in which Rac1 activity may feedback to inhibit the transient membrane protrusion stimulated by Cdc42. Transient Cdc42-mediated protrusive activity, including filopodia, is important for the sensing of chemotactic gradients and the establishment of cell polarity (Allen et al., 1998; Nobes and Hall, 1999; Ridley et al., 1999). CHO cells do not form classic filopodia but rather exhibit transient protrusive activity that, like filopodia, may perform a gradient-sensing function required for the establishment of cell polarity and the subsequent generation of lamellipodia and stabilized protrusion. Although the sensing function provided by transient protrusion is important to determine the location of stabilized protrusion formation, our findings suggest that the generation of stabilized protrusions by Rac1 may require localized down-regulation of Cdc42-stimulated transient protrusive activity.
In contrast to Cdc42 and Rac1, we provide evidence that RhoA activity inhibits substratum-stimulated transient and stabilized protrusion. This is in agreement with a recent report demonstrating that RhoA inhibition promotes Rac1-mediated membrane protrusion and focal complex formation (Rottner et al., 1999). Although a recent study has shown that Cdc42 and Rac1 directly down-regulate RhoA activity, there is no direct evidence in that system that RhoA regulates Cdc42 or Rac1 activity (Sander et al., 1999). However, our findings directly demonstrate that increased RhoA activity inhibits cell protrusion and polarization under adhesive conditions that are normally supportive. Furthermore, in CHO cells we find that wild-type and constitutively active RhoA down-regulate Cdc42 and Rac1 activity. Therefore, RhoA may inhibit polarization and protrusion through down-regulating Cdc42 and Rac1 activity. The inhibition of polarization and protrusion observed on high substratum concentrations, however, could not be reversed by expression of dominant negative RhoA, suggesting that RhoA-independent mechanisms may contribute to the reduced activity levels of Cdc42 and Rac1 on high fibronectin-coating concentrations.
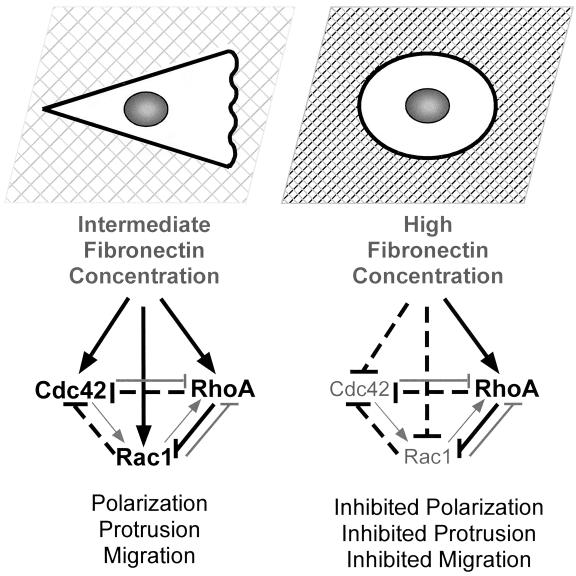
Together, these findings support a hypothetical model (Figure 9) in which cell migration rate is optimal at intermediate substratum concentrations at which Cdc42, Rac1, and RhoA are active. Cdc42 and Rac1 may stimulate cell migration by promoting protrusion, polarization, and the formation of focal complexes. RhoA may contribute to migration by stimulating contractility and promoting more stabilized adhesive contacts that are necessary to generate the traction required for cell movement. Integrin-mediated signaling on high fibronectin concentrations inhibits Cdc42 and Rac1 activity and promotes RhoA activity. Inhibited Cdc42 and Rac1 activity in combination with high RhoA activity results in a loss of cell polarity, lack of protrusion, and inhibited migration. Furthermore, cross talk between the GTPases plays a central role in regulating adhesion-dependent cell polarization and protrusion (see model in Figure 9).
Figure 9.
Schematic to demonstrate how integrin-mediated signaling on different substratum concentrations regulates cell polarity and protrusion via the Rho family GTPases. This model also highlights proposed cross talk interactions that may occur between Cdc42, Rac1, and RhoA. On intermediate fibronectin concentrations integrin-mediated signaling activates Cdc42, Rac1, and RhoA, resulting in cell polarization, protrusion, and migration. On higher fibronectin concentrations, integrin-mediated signaling inhibits Cdc42 and Rac1 activity but stimulates RhoA activity. This results in loss of cell polarity, inhibited protrusion, and inhibited migration. On all substratum concentrations, cross talk between the Rho family of GTPases is likely to influence protrusive activity. Solid black lines indicate interactions that have been documented both here and in other recent articles. Specifically, integrin-mediated signaling has been shown to activate Cdc42, Rac1, and RhoA (Barry et al., 1997; Clark et al., 1998; Price et al., 1998; Ren et al., 1999; del Pozo et al., 2000); and an antagonistic relationship has been proposed between RhoA and Rac1 (Rottner et al., 1999). Dotted black lines indicate new interactions that have been documented in this article. Specifically, we find that Rac1 and RhoA can inhibit Cdc42 activity. Gray lines indicate interactions that have been proposed in previous publications. For example, several recent studies have demonstrated that Cdc42 and Rac1 can inhibit RhoA activity (van Leeuwen et al., 1997; Sander et al., 1999; Zondag et al., 2000).
Substratum-mediated regulation of cell polarization and protrusion is likely to play a critical role in vivo, where cells are exposed to different extracellular environments with variable concentrations of extracellular matrix components and growth factors. In general, extracellular matrix concentrations in vivo are not supportive for cell motility. Our findings support a model in which high ligand concentration may normally induce a stop signal for membrane protrusion and cell polarization. There is evidence that many cell types, including tumor cells (Rooprai et al., 1998), epithelial cells (Legrand et al., 1999), and osteoclasts (Sato et al., 1997), stimulate migration by degrading extracellular matrix components at the leading edge through the secretion of matrix metalloproteinases. Secretion of matrix metalloproteinases also occurs at the edges of closing wounds (Bullard et al., 1999; Legrand et al., 1999; Lu et al., 1999) and at the advancing edge of tumors during metastasis (Airola et al., 1997). Selective degradation of the extracellular matrix may reduce the concentration of ligands to levels that stimulate Cdc42 and Rac1 activity, thereby promoting membrane protrusion and directional cell movement. A second example of an important mechanism that likely operates in vivo to inhibit membrane-protrusive activity is contact-mediated inhibition of cell migration. Our previous studies have demonstrated that α5 integrin and cadherin adhesive signaling pathways cross talk to regulate contact-mediated inhibition of membrane-protrusive activity and cell migration (Huttenlocher et al., 1998). Therefore, α5 integrin can mediate a shutdown of motile activity during cell-cell contact and in cases of high cell substratum adhesiveness.
In summary, our findings demonstrate that high substratum concentration can induce a stop signal for cell migration by inhibiting cell polarity and protrusion through integrin-mediated signaling pathways via the Rho family of GTPases. This phenomenon is likely to be an important regulatory mechanism in vivo during both normal and pathological cell migrations.
Supplementary Material
ACKNOWLEDGMENTS
We thank Amit Bhatt, Patricia Keely, and Keith Burridge for useful discussions, and Shannon Smith for excellent technical assistance. This work was supported by the National Cancer Institute (R01 CA85862-01) and by a research grant from the Arthritis Foundation. S.S. was supported by National Institutes of Health Grant GM-29860 (to K. Burridge).
Abbreviations used:
- fMLP
formyl-methionyl-leucyl-phenylalanine
Footnotes
Online version of this article contains video material for Figure 1. The online version is available at www.molbiolcell.org.
REFERENCES
- Airola K, Johansson N, Kariniemi AL, Kahari VM, Saarialho-Kere UK. Human collagenase-3 is expressed in malignant squamous epithelium of the skin. J Invest Dermatol. 1997;109:225–231. doi: 10.1111/1523-1747.ep12319441. [DOI] [PubMed] [Google Scholar]
- Allen WE, Zicha D, Ridley AJ, Jones GE. A role for Cdc42 in macrophage chemotaxis. J Cell Biol. 1998;141:1147–1157. doi: 10.1083/jcb.141.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand-Apte B, Zetter BR, Viswanathan A, Qiu RG, Chen J, Ruggieri R, Symons M. Platelet-derived growth factor and fibronectin-stimulated migration are differentially regulated by the Rac and extracellular signal-regulated kinase pathways. J Biol Chem. 1997;272:30688–30692. doi: 10.1074/jbc.272.49.30688. [DOI] [PubMed] [Google Scholar]
- Araki N, Johnson MT, Swanson JA. A role for phosphoinositide 3-kinase in the completion of macropinocytosis and phagocytosis by macrophages. J Cell Biol. 1996;135:1249–1260. doi: 10.1083/jcb.135.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asthagiri AR, Nelson CM, Horwitz AF, Lauffenburger DA. Quantitative relationship among integrin-ligand binding, adhesion, and signaling via focal adhesion kinase and extracellular signal-regulated kinase 2. J Biol Chem. 1999;274:27119–27127. doi: 10.1074/jbc.274.38.27119. [DOI] [PubMed] [Google Scholar]
- Bagrodia S, Taylor SJ, Jordon KA, Van Aelst L, Cerione RA. A novel regulator of p21-activated kinases. J Biol Chem. 1998;273:23633–23636. doi: 10.1074/jbc.273.37.23633. [DOI] [PubMed] [Google Scholar]
- Banyard J, Anand-Apte B, Symons M, Zetter BR. Motility and invasion are differentially modulated by Rho family GTPases. Oncogene. 2000;19:580–591. doi: 10.1038/sj.onc.1203338. [DOI] [PubMed] [Google Scholar]
- Barry ST, Flinn HM, Humphries MJ, Critchley DR, Ridley AJ. Requirement for Rho in integrin signaling. Cell Adhes Commun. 1997;4:387–398. doi: 10.3109/15419069709004456. [DOI] [PubMed] [Google Scholar]
- Bullard KM, Lund L, Mudgett JS, Mellin TN, Hunt TK, Murphy B, Ronan J, Werb Z, M.J. Banda MJ. Impaired wound contraction in stromelysin-1-deficient mice. Ann Surg. 1999;230:260–265. doi: 10.1097/00000658-199908000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cary LA, Chang JF, Guan JL. Stimulation of cell migration by overexpression of focal adhesion kinase and its association with Src and Fyn. J Cell Sci. 1996;109:1787–1794. doi: 10.1242/jcs.109.7.1787. [DOI] [PubMed] [Google Scholar]
- Cary LA, Han DC, Polte TR, Hanks SK, Guan JL. Identification of p130Cas as a mediator of focal adhesion kinase-promoted cell migration. J Cell Biol. 1998;140:211–221. doi: 10.1083/jcb.140.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark EA, Brugge JS. Integrins and signal transduction pathways: the road taken. Science. 1995;268:233–239. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- Clark EA, King WG, Brugge JS, Symons M, Hynes RO. Integrin-mediated signals regulated by members of the Rho family of GTPases. J Cell Biol. 1998;142:573–586. doi: 10.1083/jcb.142.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox EA, Huttenlocher A. Regulation of integrin-mediated adhesion during cell migration. Microsc Res Tech. 1998;43:412–419. doi: 10.1002/(SICI)1097-0029(19981201)43:5<412::AID-JEMT7>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Crowley E, Horwitz AF. Tyrosine phosphorylation and cytoskeletal tension regulate the release of fibroblast adhesions. J Cell Biol. 1995;131:525–537. doi: 10.1083/jcb.131.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Pozo MA, Price LS, Alderson NB, Ren XD, Schwartz MA. Adhesion to the extracellular matrix regulates the coupling of the small GTPase Rac to its effector PAK. EMBO J. 2000;19:2008–2014. doi: 10.1093/emboj/19.9.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Pozo MA, Vicente-Manzanares M, Tejedor R, Serrador JM, Sanchez-Madrid F. Rho GTPases control migration and polarization of adhesion molecules and cytoskeletal ERM components in T lymphocytes. Eur J Immunol. 1999;29:3609–3620. doi: 10.1002/(SICI)1521-4141(199911)29:11<3609::AID-IMMU3609>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- DiMilla PA, Barbee K, Lauffenburger DA. Mathematical model for the effects of adhesion and mechanics on cell migration speed. Biophys J. 1991;60:15–37. doi: 10.1016/S0006-3495(91)82027-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMilla PA, Stone JA, Quinn JA, Albeda SM, Lauffenburger DA. Maximal migration of human smooth muscle cells on fibronectin and type IV collagen occurs at an intermediate attachment strength. J Cell Biol. 1993;122:729–737. doi: 10.1083/jcb.122.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duband JL, Dufour S, Yamada SS, Yamada KM, Thiery JP. Neural crest cell locomotion induced by antibodies to beta 1 integrins. A tool for studying the roles of substratum molecular avidity and density in migration. J Cell Sci. 1991;98:517–532. doi: 10.1242/jcs.98.4.517. [DOI] [PubMed] [Google Scholar]
- Goodman SL, Risse G, von der Mark K. The E8 subfragment of laminin promotes locomotion of myoblasts over extracellular matrix. J Cell Biol. 1989;109:799–809. doi: 10.1083/jcb.109.2.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendey B, Klee CB, F.R. Maxfield FR. Inhibition of neutrophil chemokinesis on vitronectin by inhibitors of calcineurin. Science. 1992;258:296–299. doi: 10.1126/science.1384129. [DOI] [PubMed] [Google Scholar]
- Ho WC, Heinemann C, Hangan D, Uniyal S, Morris VL, B.M. Chan BM. Modulation of in vivo migratory function of alpha 2 beta 1 integrin in mouse liver. Mol BiolCell. 1997;8:1863–1875. doi: 10.1091/mbc.8.10.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotchin NA, Hall A. The assembly of integrin adhesion complexes requires both extracellular matrix and intracellular Rho/Rac GTPases. J Cell Biol. 1995;131:1857–1865. doi: 10.1083/jcb.131.6.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher A, Sandborg RR, Horwitz AF. Adhesion in cell migration. Curr Opin Cell Biol. 1995;7:697–706. doi: 10.1016/0955-0674(95)80112-x. [DOI] [PubMed] [Google Scholar]
- Huttenlocher A, Ginsberg MH, Horwitz AF. Modulation of cell migration by integrin-mediated cytoskeletal linkages and ligand-binding affinity. J Cell Biol. 1996;134:1551–1562. doi: 10.1083/jcb.134.6.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher A, Lakonishok M, Kinder M, Wu S, Truong T, Knudsen KA, Horwitz AF. Integrin and cadherin synergy regulates contact inhibition of migration and motile activity. J Cell Biol. 1998;141:515–526. doi: 10.1083/jcb.141.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher A, Palecek SP, Lu Q, Zhang W, Mellgren RL, Lauffenburger DA, Ginzberg MH, Horwitz AF. Regulation of cell migration by the calcium-dependent protease Calpain. J Biol Chem. 1997;272:32719–32722. doi: 10.1074/jbc.272.52.32719. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Ilic D, Furuta Y, Kanazawa S, Takeda N, Sobue K, Nakatsuji N, Nomura S, Fujimoto J, Okada M, Yamamoto T, S. Aizawa S. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature. 1995;377:539–544. doi: 10.1038/377539a0. [DOI] [PubMed] [Google Scholar]
- Jay PY, Pham PA, Wong SA, Elson EL. A mechanical function of myosin II in cell motility. J Cell Sci. 1995;108:387–393. doi: 10.1242/jcs.108.1.387. [DOI] [PubMed] [Google Scholar]
- Klemke RK, Cai S, Giannini AL, Gallagher PJ, de Lanerolle P, Cheresh DA. Regulation of cell motility by mitogen-activated protein kinase. J Cell Biol. 1997;137:481–492. doi: 10.1083/jcb.137.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemke RK, Leng J, Molander R, Brooks PC, Vuori K, Cheresh DA. CAS/Crk coupling serves as a “molecular switch” for induction of cell migration. J Cell Biol. 1998;140:961–972. doi: 10.1083/jcb.140.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozma R, Ahmed S, Best A, Lim L. The Ras-related protein Cdc42Hs and bradykinin promote formation of peripheral actin microspikes and filopodia in Swiss 3T3 fibroblasts. Mol Cell Biol. 1995;15:1942–1952. doi: 10.1128/mcb.15.4.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauffenburger DA, Horwitz AF. Cell migration: a physically integrated molecular process. Cell. 1996;84:1–20. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- Legrand C, Gilles C, Zahm JM, Polette M, Buisson AC, Kaplan H, Birembaut P, Tournier JM. Airway epithelial cell migration dynamics. MMP-9 role in cell-extracellular matrix remodeling. J Cell Biol. 1999;146:517–529. doi: 10.1083/jcb.146.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu PC, Ye H, Maeda M, Azar DT. Immunolocalization and gene expression of matrilysin during corneal wound healing. Invest Ophthalmol Vis Sci. 1999;40:20–27. [PubMed] [Google Scholar]
- Maheshwari G, Wells A, Griffith LG, Lauffenburger DA. Biophysical integration of effects of epidermal growth factor and fibronectin on fibroblast migration. Biophys J. 1999;76:2814–2823. doi: 10.1016/S0006-3495(99)77435-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks PW, Hendey B, Maxfield FR. Attachment to fibronectin or vitronectin makes human neutrophil migration sensitive to alterations in cytosolic free calcium concentration. J Cell Biol. 1991;112:149–158. doi: 10.1083/jcb.112.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobes CD, Hall A. Rho, Rac, and Cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- Nobes CD, Hall A. Rho GTPases control polarity, protrusion, and adhesion during cell movement. J Cell Biol. 1999;144:1235–1244. doi: 10.1083/jcb.144.6.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palecek SP, Cox EA, Huttenlocher A, Lauffenburger DA, Horwitz AF. Integrin adhesion in cell migration. In: Bittar EE, Garrod DR, North AJ, Chidgey MAJ, editors. Advances in Molecular and Cell Biology. Vol. 28. Stamford, CT: JAI Press; 1999. pp. 367–388. [Google Scholar]
- Palecek SP, Huttenlocher A, Horwitz AF, Lauffenburger DA. Physical and biochemical regulation of integrin release during rear detachment of migrating cells. J Cell Sci. 1998;111:929–940. doi: 10.1242/jcs.111.7.929. [DOI] [PubMed] [Google Scholar]
- Palecek SP, Loftus JC, Ginsberg MH, Lauffenburger DA, Horwitz AF. Integrin-ligand binding properties govern cell migration speed through cell-substratum adhesiveness. Nature. 1997;385:537–540. doi: 10.1038/385537a0. [DOI] [PubMed] [Google Scholar]
- Price LS, Leng J, Schwartz MA, Bokoch GM. Activation of Rac and Cdc42 by integrins mediates cell spreading. Mol Biol Cell. 1998;9:1863–1871. doi: 10.1091/mbc.9.7.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren XD, Kiosses WB, Schwartz MA. Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO J. 1999;18:578–585. doi: 10.1093/emboj/18.3.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley AJ, Allen WE, Peppelenbosch M, Jones GE. Rho family proteins and cell migration. Biochem Soc Symp. 1999;65:111–123. [PubMed] [Google Scholar]
- Ridley AJ, Hall A. The small GTP-binding protein Rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Rooprai HK, Van Meter T, Rucklidge GJ, Hudson L, Everall IP, Pilkington GJ. Comparative analysis of matrix metalloproteinases by immunocytochemistry, immunohistochemistry and zymography in human primary brain tumors. Int J Oncol. 1998;13:1153–1157. doi: 10.3892/ijo.13.6.1153. [DOI] [PubMed] [Google Scholar]
- Rottner K, Hall A, Small JV. Interplay between Rac and Rho in the control of substrate contact dynamics. Curr Biol. 1999;9:640–648. doi: 10.1016/s0960-9822(99)80286-3. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E, Hayman EG, Pierschbacher M, Engvall E. Fibronectin: purification, immunochemical properties, and biological activities. Methods Enzymol. 1982;82:803–831. doi: 10.1016/0076-6879(82)82103-4. [DOI] [PubMed] [Google Scholar]
- Sander EE, ten Klooster JP, van Delft S, van der Kammen RA, Collard JG. Rac down-regulates Rho activity: reciprocal balance between both GTPases determines cellular morphology and migratory behavior. J Cell Biol. 1999;147:1009–1022. doi: 10.1083/jcb.147.5.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, del Carmen M, Ovejero, Hou P, Heegaard M, Kumegawa NT, Foged, Delaisse JM. Identification of the membrane-type matrix metalloproteinase MT1-MMP in osteoclasts. J Cell Sci. 1997;110:589–596. doi: 10.1242/jcs.110.5.589. [DOI] [PubMed] [Google Scholar]
- Trinkaus JP. Cells into Organs. 2nd ed. Englewood Cliffs, New Jersey: Prentice-Hall; 1984. [Google Scholar]
- van Leeuwen FN, Kain HE, Kammen RA, Michiels F, Kranenburg OW, Collard JG. The guanine nucleotide exchange factor Tiam1 affects neuronal morphology: opposing roles for the small GTPases Rac and Rho. J Cell Biol. 1997;139:797–807. doi: 10.1083/jcb.139.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zondag GC, Evers EE, ten Klooster JP, Janssen L, van der Kammen RA, Collard JG. Oncogenic Ras down-regulates. Rac activity, which leads to increased Rho activity and epithelial-mesenchymal transition. J Cell Biol. 2000;149:775–782. doi: 10.1083/jcb.149.4.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.