Abstract
The intestinal immune system and the epithelium are the first line of defense in the gut. Constantly exposed to microorganisms from the environment, the gut has complex defense mechanisms to prevent infections, as well as regulatory pathways to tolerate commensal bacteria and food antigens. Intestinal pathogens have developed strategies to regulate intestinal immunity and inflammation in order to establish or prolong infection. The organisms that employ a type III secretion system use a molecular syringe to deliver effector proteins into the cytoplasm of host cells. These effectors target the host cell cytoskeleton, cell organelles and signaling pathways. This review addresses the multiple mechanisms by which the type III secretion system targets the intestinal immune response, with a special focus on pathogenic E. coli.
Keywords: gut-associated lymphoid tissue, type 3 secretion system, EPEC, Shigella
Review
The gut-associated lymphoid tissue
The intestinal lumen is exposed to the environment and therefore in continuous contact with harmless as well as pathogenic microorganisms. Thus, it is not surprising that the gut is the biggest lymphoid organ in the body and contains about 70% of the body's immune cells [1-3]. The gut-associated lymphoid tissue (GALT) uses a range of mechanisms to protect the host from pathogens, while it at the same time tolerates commensal microorganisms. Furthermore, the GALT needs to prevent the invasion of harmful agents without affecting the absorption of nutrients from the lumen.
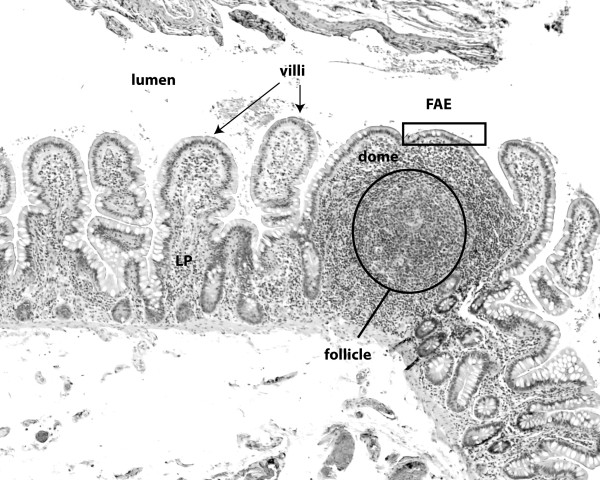
GALT is comprised of the appendix, single lymphoid follicles (Figure 1) in the small and large intestine, and the Peyer's patches (PP). The latter are clusters of follicles and have a distinct architecture with germinal centers containing B cells and follicular dendritic cells (DCs) which are surrounded by areas with T cells and macrophages [2]. PP are covered by specialized micro-folded epithelial cells, the M-cells, which make up the follicle-associated epithelium (FAE). This epithelium forms the interface between the luminal microorganisms and the immune cells of the GALT [4]. PP have no afferent lymph vessels and antigens are received directly from the intestinal lumen. After the uptake of luminal material by endocytosis and phagocytosis, the M-cells deliver antigens and microbes to antigen-presenting cells in the subepithelial dome of the PP, which subsequently present them to PP T cells [2]. PP DCs also directly sample bacteria from the intestinal lumen by sending protrusions through the epithelial layer without disrupting epithelial integrity [5]. Therefore, follicles and PP are an inductive site where microorganisms are sensed and the appropriate immune response is initiated [4]. In contrast, the lamina propria is an effector site; after activation in PP, DCs migrate to the mesenteric lymph nodes where they present antigens to B and T cells and the immune response is amplified. Via expression of homing molecules, mainly the integrin alpha4beta7 and CCR9, the lymphocytes are then able to re-enter the mucosal site where they contribute to immune defense along the entire length of the intestine [6].
Figure 1.
Follicles and PP are the inductive site for the mucosal immune response. Micrograph of a human ileal lymphoid follicle stained with hematoxylin & eosin. The follicle is covered by M-cells which form the follicle-associated epithelium (FAE). Underneath the dome area which holds dendritic cells, is a B cell follicle, surrounded by a T cell-rich zone. Adjacent to the follicle are microvilli. LP = lamina propria.
The gut has a wide range of strategies to fight infections. Amongst the non-specific mechanism are the mucus layer which traps microorganisms, the secretion of anti-microbial agents such as defensins, trefoil factors and proteases, intestinal peristalsis, and the natural microbiota which compete with pathogens for epithelial binding and nutrients [7-10]. Microorganisms are also largely prevented from epithelial attachment by secretory IgA (sIgA) which binds them in the lumen and mucus [11]. Germinal center B cells receive multiple activation and survival signals from follicular DCs in PP upon encounter with bacterial products leading to the generation of IgA secreting plasmablasts [12]. After activation and T cell-dependent class switching to IgA in the PP, B cells eventually migrate to the lamina propria where they reside as IgA secreting plasma cells [13,14]. Cellular defense mechanisms within the lamina propria are crucial in reducing and dealing with invasion of pathogens. The main cell population comprises CD4+ T lymphocytes which, depending on the cytokine milieu, respond by producing factors associated with a Th1 immune response which is crucial for the response to intracellular pathogens and stimulates phagocytosis by macrophages. A Th2 response is typically established following infection with parasites and involves production of IL-4, IL-5, IL-10, and IL-13 resulting in activation and recruitment of B cells, mast cells and eosinophils [15].
The lamina propria also contains natural killer cells which are thought to mediate intestinal homeostasis by producing IL-22 and exhibit their cytotoxic functions upon activation by T cells [16,17]. IL-22 has been shown to be an important factor in the host defense against enteral bacteria, like e.g. Citrobacter rodentium, a murine pathogen which is used to study infections with enteropathogenic E. coli (EPEC) and enterohaemorrhagic E. coli (EHEC) in humans [18]. Additional studies highlighted the importance of IFNg-producing CD4+ T cells in the defense against these bacteria [19].
Another layer of defense is located in the epithelium where a large population of mainly CD8αβ intraepithelial T lymphocytes resides in the basolateral area, between the epithelial cells [20]. Some studies demonstrated cytolytic activity of these T cells which suggest they might be involved in cancer surveillance and killing of infected cells [21].
Recognition of bacteria in the intestine
The intestinal immune system faces the constant challenge of discriminating between the commensal microbiota and pathogens. The response to the latter is usually rapid and results in the activation of innate and adaptive immune mechanisms that lead to inflammation and eradication of the pathogen, sometimes with considerable damage to the intestinal mucosa. Non-pathogenic bacteria that form the microbiota are also recognized by the GALT [22]; however, the immune response to commensals appears to be strictly controlled, and does not lead to overt inflammation. How the GALT discriminates between these two categories of microorganisms, commensals and pathogens, is complex and not fully understood. However, DCs in GALT are of paramount importance for responding to bacterial stimuli and the initiation of a tolerogenic state by promoting the expression of anti-inflammatory molecules like IL-10 and TGFbeta [23].
In the last two decades, with the discovery of toll-like receptors (TLR) and Nod-like receptors (NLR) which recognize pathogen-associated molecular patterns (PAMPs), the knowledge about the recognition of microbial structures by immune and epithelial cells has dramatically increased [24]. These receptors specifically bind ligands widely shared amongst pathogens. Well-characterised examples of such ligands are bacterial cell wall components such as peptidoglycans and lipoproteins (both binding to TLR2) or nucleic acid ligands such as bacterial CpG DNA which binds to TLR9 [25,26]. Immune recognition via pattern-recognition receptors is crucial for host defense and immune homeostasis, and dysfunction of these receptors has been shown to be associated with gut inflammatory conditions such as Crohn's disease [27]. Binding of microbial ligands to TLRs (besides TLR3) results in the activation of a pro-inflammatory MyD88-dependent pathway that leads to activation of the transcription factor NF-kappaB. Another signaling pathway that is critically involved in inflammation is the mitogen-activated protein (MAP) kinase-cascade. Although not activated by microbial ligands, this pathway is initiated by extracellular stimuli like pro-inflammatory cytokines or mitogens [28]. Both the NF-kappaB and MAPK pathway are activated in intestinal infections by pathogens which use type III secretion systems (T3SS) [29]. These pathways are also amongst the known targets for T3SS effectors. An overview on how these pathways are affected during intestinal infection with pathogens that employ the T3SS is discussed here, with special emphasis on EPEC and EHEC. These two pathogens, also known as attaching and effacing pathogens (A/E), are amongst the leading causes for diarrheal diseases. EPEC is a big health concern, especially for infants, in developing countries. An EPEC infection can be asymptomatic, but the classical feature of the infection is profuse watery diarrhea in combination with vomiting. EHEC is responsible for food-borne outbreaks of diarrheal diseases, with contaminated beef being the most common vehicle for infection. Certain EHEC strains (e.g. O157:H7) produce Shiga-like toxins which can cause potentially life threatening complications like the hemolytic-uremic syndrome (HUS). This disease is characterized by acute kidney failure, thrombocytopenia and hemolytic anemia and affects mostly children. The mortality of HUS is approximately 5-10% and it is therefore a medical emergency requiring intensive clinical care.
Sustaining colonization by preventing bacterial detachment and death of infected cells
Some of the most successful gram-negative pathogens use the type 3 secretion system (T3SS), a molecular syringe, to inject an arsenal of virulence effector proteins directly into the cytoplasm of the host cells. The effectors can then target and hijack various host cell functions for the benefit of the pathogen [30]. The increasing understanding of the variety of T3SS effectors and their functions has given rise to the idea that for every defense strategy used by the host, there might be antagonistic effector proteins. Recent data gained from research on the function of Shigella effectors, illustrate this hypothesis [31]. One of the protective mechanisms of the gut mucosa is the constant renewal and shedding of epithelial cells at the top of the villi in the small bowel and from the colon surface. If subjected to bacterial colonization, the enterocytes can undergo programmed cell death and detach from the extracellular matrix into the lumen, preventing the pathogen crossing the epithelium [32,33]. In vitro and in vivo data identified two Shigella effectors, IpaB and OspE, which counteract the intestinal epithelial turnover and exfoliation [34,35]. IpaB causes a cell cycle arrest of infected cells by interacting with Mad2L2, an inhibitor of the anaphase promoting complex (APC) which regulates the cell cycle [34]. In a rabbit ileal loop model, intestinal crypts infected with Shigella that express an IpaB mutant protein which is unable to interact with Mad2L2, have a higher number of progenitor cells and are less colonized than with the wild type strain. These findings suggest that IpaB, by blocking intestinal cell proliferation and renewal, prolongs Shigella colonization [34]. Shigella also injects the effector OspE into enterocytes which stabilizes the adhesion of intestinal cells to the extra-cellular matrix by targeting and modulating the function of integrin-linked kinase (ILK), a modulator of focal adhesion [35]. The interaction between OspE and ILK enhances the presence of beta1-integrin at the cell surface and prevents the disassembly of focal adhesions. An in vivo study performed in a guinea pig colon infection model showed reduced colonization and pathogenicity of OspE mutant bacteria. This study suggests that OspE enhances the infectivity of Shigella by preventing the exfoliation of infected intestinal cells [35].
Some EPEC and EHEC strains as well as the mouse pathogen Citrobacter rodentium might also use a similar strategy as they possess the effector EspO which has strong homology with Shigella's OspE [35,36]. The inhibition of epithelial cell detachment is an emerging theme in bacterial pathogenesis, and recent in vivo work suggests that it is a strategy shared by all bacteria that are able to bind human carcino-embryonic antigen-related cell adhesion molecules (CEACAM), e.g. Neisseria gonorrhoeae, Neisseria meningitidis, Moraxella catarrhalis, and Haemophilus influenzae [32,37].
Interestingly, some EPEC strains produce the effector Cif which blocks the cell cycle of infected cells [38]. Cif binds Nedd8, a ubiquitin-like protein and inhibits neddylated Culling-RING ligases-induced (CLRs) ubiquitination of a variety of CLR substrates, such as the cell cycle inhibitors p21waf1 and p27kip1 [39,40]. It is thus possible that in EPEC, Cif acts like Shigella's IpaB, and also prolongs colonization of the gut mucosa by preventing epithelial cell renewal.
Other work suggests that inhibition of the epithelial renewal and exfoliation could indeed be an infective strategy of EPEC and EHEC pathogens. Shames and co-workers have demonstrated a role for the effector EspZ in reducing the death and detachment of epithelial cells infected with EPEC in vitro [41]. EspZ binds the transmembrane glycoprotein CD98 and enhance its effect on beta1-integrin signaling and cell survival via activation of focal adhesion kinase. EspZ also activates the pro-survival AKT pathway, which does not seem to rely on CD98 binding [41].
A potent pro-survival activity has also been identified for NleH1 and NleH2, two effectors produced by EPEC and EHEC. NleHs inhibits apoptosis via various stimuli in epithelial cells, dependent on the binding to the anti-apoptotic Bax inhibitor-1 [42]. The mechanism by which NleH prevents cell death is independent of its kinase function and remains to be determined.
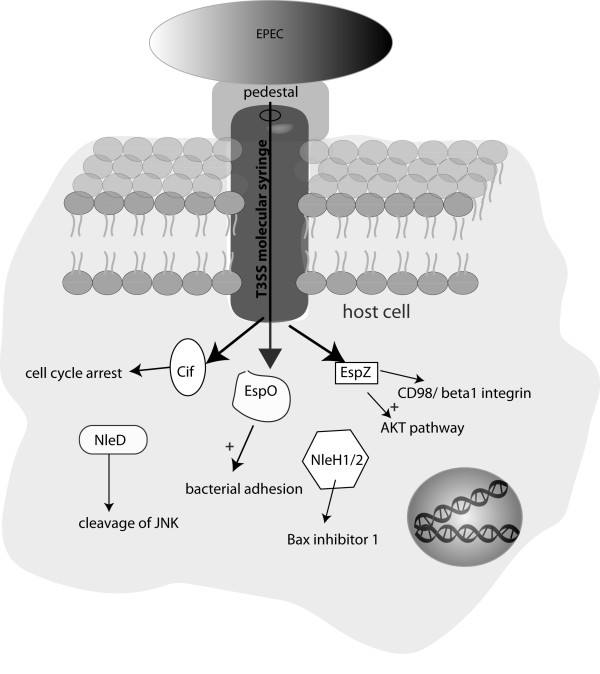
Another effector reported to be potentially involved in anti-apoptotic activity is the metalloprotease NleD which prevents JNK-mediated pro-apoptotic signaling by cleaving and inactivating JNK [43]. Apart from the effector EspZ, which is severely attenuated for virulence in the mouse model [44], an essential role for other effectors in virulence like NleHs and NleD have not been established in different animal models [45,46]. Although in vivo evidence is missing, these studies suggest that EPEC and EHEC use EspO, EspZ, NleH, and NleD to prevent or delay the exfoliation and apoptotic clearance of the targeted cells in the intestinal epithelium and to sustain bacterial colonization (Figure 2 and table 1).
Figure 2.
EPEC uses several effector proteins to promote bacterial adhesion. After binding to the epithelial cell, EPEC uses the T3SS to inject effectors into the host cell cytoplasm via a needle-like structure. Intimate adhesion to the host cell is secured by rearrangement of the actin cytoskeleton and the formation of a pedestal. Amongst the injected effectors are EspO, NleH1, NleH2, EspZ, and CiF which modulate the cell cycle and apoptotic regulation, resulting in reduced epithelial renewal and prolonged bacterial adherence.
Table 1.
Effectors of EPEC/EHEC that modulate cell detachment, pro-inflammatory signaling, and phagocytosis
| Effector | Cellular targetsa | Biochemical activity/characteristicsb | Phenotype | In vivo role |
|---|---|---|---|---|
| Inhibition of cell detachment and modulation of cell death | ||||
|
NleH1 NleH2 |
Bax inhibitor-1 (BI-1) | Binds to N-terminal amino acid 1-40 of BI-1. N-terminal aa 1-100 of NleHs not required for binding to BI-1 | Inhibition of apoptosis induced via multiple stimuli | Various roles reported in vivo. NleH reduces the level of apoptotic colonic cells in mouse model [42] |
| EspZ | CD98 | C-terminal amino acid domain 43-99 required for CD98 binding | Prevent cell detachment. Enhance activation of pro-survival FAK and AKT pathway. Binding to CD98 promotes β1-integrin activation of FAK. | Mutant espZ attenuated for colonization and hyperplasia in mice [44] |
| NleD | JNKs, p38 | Zinc metalloprotease (motif 142HExxH146) | Cleaves MAP kinases JNK and p38 in the activation loop. Reduce JNK pro-apoptotic activity | Enhance colonization in calves, no role identified in mice and lamb infection models [45,46] |
| Cif | NEDD8 | Deamidase of NEDD8 and ubiquitin | Block cell cycle at G2/M and G1/S transitions [39] | Unknown |
|
EspO/ OspO |
ILK (?) | Shigella's OspE C-terminal 68W essential for activity is conserved in EPEC/EHEC EspO/OpsO | Prevent cell detachment? | Unknown |
| Inhibition of pro-inflammatory signaling | ||||
| NleE | Unknown | C-terminal 208IDSYMK214 motif essential for activity | Inhibits TNFα, IL-1β and PRRs mediated activation of NF-kappaB and expression of pro-inflammatory cytokines in epithelial and immune cells. Acts by inhibition of IκBα phosphorylation blocking p65 nuclear translocation | Slight role in colonization and persistence reported [45,60] |
| NleC | p65, p50, c-Rel, IκBα | Zinc metalloprotease (motif 183HExxH187) N-terminal domain aa 33-64 required for p65 and p50 binding |
Cleaves p65 and p50 to inhibit NF-kappaB activation. Cleavage of c-Rel and IκBα also reported. | No role identified in mice and lamb infection model [45,46] |
| NleB | Unknown | Unknown | Inhibit TNFα-mediated NF-kappaB activation | Required for colonization and disease in mouse model [45,60] |
| NleH1 | Ribosomal protein S3 (RPS3) | Activity in N-terminal 139 amino acid (N40 and K45 required for RPS3 inhibition) | Prevent RPS3 nuclear translocation and expression of RPS3-NF-kappaB dependent pro-inflammatory genes | NleH1 EHEC mutant hypervirulent in piglet infection model [55] |
|
NleH1 and NleH2 |
Unknown | Serine-threonine kinase motif | Prevent IκBα ubiquitination and degradation | Required for colonization and reduction of inflammation in EPEC mouse model [58] |
| NleD | JNK, p38 | Zinc metalloprotease (motif 142HExxH146) | Cleaves MAP kinases JNK and p38 in the activation loop. Contributes to overall bacterial mediated inhibition of IL-8 in vitro. | Mutant not attenuated in mice, calve and lamb models [45,46]. Role in colonization in STM screen in calves |
| Inhibition of phagocytosis | ||||
| EspF | Unknown | N-term 101 amino acid for anti-phagocytic activity | Prevents PI3K-dependent phagocytosis of bacteria; Reduces uptake of EPEC bacteria in in vitro M cell model |
EspF mutant attenuated in mice model. Specific role of anti-phagocytic activity unknown [77,78] |
| EspB | Myosin proteins | Domain from amino acid 159-218 essential for myosin binding | Prevents bacterial phagocytosis via inhibition of myosin-actin interaction | Citrobacter expressing EspB mutated for myosin binding are attenuated in mouse model [74] |
| EspJ | Unknown | Unknown | Blocks FcγR and CR3-opsonophagocytosis | Role in bacterial clearance reported in mouse model [75] |
| EspH | RhoGEFs | Binds to DH-PH domain of RhoGEFs and inhibits RhoGTPase signalling | Attenuates bacteria phagocytosis and FcγR-mediated phagocytosis | EspH mutant not or slightly attenuated for colonization in mice and rabbit model [44] |
a In relation to phenotype described; b Motif or biochemical activity required for phenotype described
Modulation of proinflammatory signaling pathways
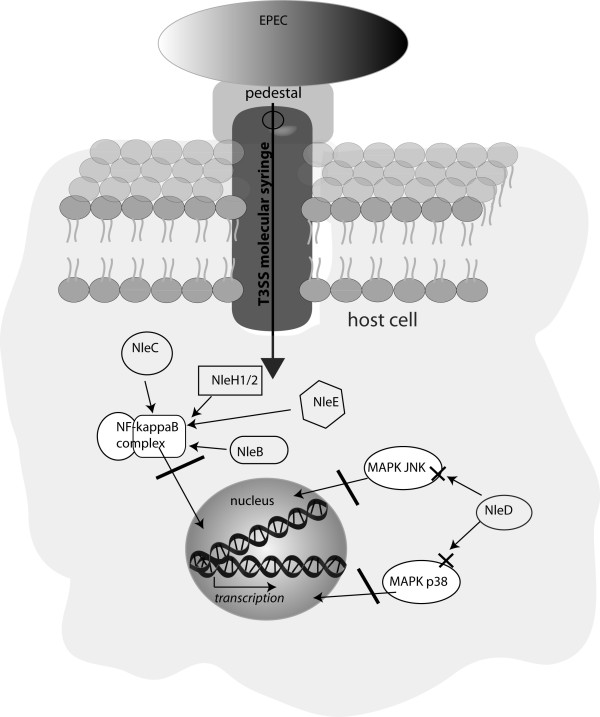
The modulation of the host immune response by effectors from Shigella, Salmonella, and Yersinia is increasingly well understood. Detailed reviews on immune modulation by these pathogens have previously been published elsewhere [30,47]. EPEC, EHEC and C. rodentium pathogens have a common set of T3SS effectors composed of seven LEE and a few non-LEE encoded effectors like NleE, NleB, and NleH and show diversity in the repertoire of other non-LEE encoded effectors. The reference strains EHEC 0157:H7 Sakai, EPEC O127:H6 strain E2348/69, EPEC O111:NM strain B171, and Citrobacter rodentium have a total of 50, 21, 28 and 29 full length effector genes, respectively [48]. Surprisingly, despite almost 20 years of research on the function of EPEC and EHEC effectors, manipulation of immune defenses in the gut had not been reported until recently; perhaps because as a pathogen which adheres to the surface of epithelial cells, it was not thought to come into contact with host immune cells. The described functions of effectors were mainly the modification of the host cell cytoskeleton in relation to the formation of intestinal attaching/effacing (A/E) lesions and the modification of epithelial tight-junctions in relation to the alteration of intestinal permeability observed during infection [49]. Some apparently contradictory results have been published concerning the pro-or anti-inflammatory activity of EPEC and EHEC. Earlier work demonstrated that the bacteria trigger a pro-inflammatory response [50,51]. However, many studies using epithelial cell lines now clearly show that whereas the bacteria induce an inflammatory response with the detection systems of the host cells, they are able to inhibit the inflammatory pathways in a T3SS-dependent manner [52,53]. By hampering the pro-inflammatory response of epithelial cells, EPEC and EHEC are likely to gain the advantage of reduced cytokine and chemokine secretion which subsequently reduces the recruitment of neutrophils into the affected site. Neutrophils are effective at killing bacteria and release a variety of anti-microbial factors; thus a reduced number and activation of these cells would prolong colonization [54]. Only very recently, anti-inflammatory activity has been demonstrated for the EPEC and EHEC effectors NleE, NleB, NleH, NleD and NleC (Figure 3 and table 1).
Figure 3.
Proinflammatory signaling pathways are a main target of EPEC effector proteins. The effectors NleC, NleH1, NleH2, NleE and NleB have been identified to target the NF-kappaB complex at different levels which eventually prevents the nuclear translocation of the p65 subunit. NleD degrades the MAP kinases JNK and p38 which results in impaired signal transduction via these pathways. By affecting these crucial inflammatory pathways, EPEC actively impairs the cell to respond to the bacterial stimulus.
NleH1 was the first effector reported to inhibit NF-kappaB in HeLa and HEK293T cell lines [55]. NleH1 and NleH2 both bind the human ribosomal protein S3 (RPS3) in the cytoplasm of infected cells. RPS3 is a non-Rel NF-kappaB subunit and interacts with p65 to increase the transcription of various pro-inflammatory genes [56]. NleH1, but not NleH2, blocks the transcription of RPS3/NF-kappaB-dependent genes by preventing the nuclear translocation of RPS3 [55]. In a gnotobiotic piglet infection model, animals infected with EHEC mutated for nleH1 died more rapidly compared to piglets infected with the wild-type or an nleH2 mutant. The hypervirulent phenotype that was caused by the nleH1 mutant, seemed to be due to a pronounced inflammatory response [55]. NleHs are auto-phosphorylated serine threonine kinases and share homology with the Shigella effector OspG which is known to inhibit NF-kappaB [57]. The RPS3-mediated inhibition of inflammation differs from OspG activity and is independent of the kinase function [55]. It was recently demonstrated that both NleH1 and NleH2 could inhibit NF-kappaB in a kinase-dependent manner [58]. NleH1 and NleH2 prevent TNFalpha-mediated NF-kappaB activation by inhibiting IkappaBalpha ubiquitination and degradation and this activity is dependent on lysine residues present in the kinase domain of both effectors [58]. Streptomycin treated mice infected with EPEC mutated for NleH1 and NleH2 were less colonized, showed higher number of neutrophil infiltration and higher serum level of KC (the mice homologue of IL-8) compared to mice infected with the wild-type, suggesting that NleH promotes colonization and is required for the modulation of the host inflammatory response [58].
NleB is encoded on the same pathogenicity island as NleE in EPEC and EHEC O157 strains, on the integrative element IE6 and the O-island 122, respectively [59]. Presence of the O Island 122 (OI122) is associated with EHEC outbreaks and the hemolytic uremic syndrome, a severe complication of an EHEC infection [60]. Citrobacter rodentium strains that have a mutated NleB effector, show an impaired ability to colonise the murine intestine and fail to induce intestinal crypt hyperplasia in the mouse model of infection [45,60]. NleB specifically inhibits NF-kappaB in response to TNFalpha stimulation in epithelial cell lines [61]. NleB's mode of action has yet to be identified but is supposed to act upstream of the IKK complex in the TNFalpha pathway, as NleB is unable to prevent NF-kappaB activation in cells following stimulation with IL-1beta or by bacterial PAMPs [61].
Four independent studies have reported that NleC is a metalloprotease that degrades the p65 NF-kappaB subunit in epithelial cell lines and contributes to the overall anti-inflammatory activity of both EPEC and EHEC strains [43,62-64]. NleC carries the zinc metalloprotease motif 183HEIIH187 which is essential for the proteolytic activity on p65. In addition to p65, NleC also cleaves the NF-kappaB p50 subunit and IkappaBalpha [62,63]. Mülhen and co-workers showed that the N-terminal motif between amino acid 33 and 64 is required for binding to p65 and p50 [62].
EPEC and EHEC produce another zinc metalloprotease, NleD, which specifically degrades MAPK JNK and p38 and contributes to the overall inhibition of IL-8 chemokine secretion by infected cells [43]. Mutation of the zinc metalloprotease motif in NleD, HEXXH, abolishes JNK cleavage.
Colonization and pathogenicity of bacteria mutated for NleC or NleD was not impaired in mice, lamb and calve infection models. Therefore the importance of each of the anti-inflammatory effectors remains to be identified [44-46]. As suggested by in vitro data which show that full IL-8 inhibition was dependent on the conjugated activity of mainly NleE and NleC, but also NleB and NleD, it is likely that the mutation of any of the effectors is compensated in vivo by the activity of the other [43,63,64]. It would be of interest to test the in vivo pathogenicity of a strain deleted for all effectors targeting NF-kappaB to assess the importance of the anti-inflammatory activity for bacterial colonization and persistence.
NleE is a T3SS effector conserved among EPEC, EHEC and Citrobacter rodentium strains. NleE is homologous to OspZ which is present in Shigella spp strains [65]. Different roles for colonization and persistence have been reported for NleE in Citrobacter rodentium mice models of infection [45,66]. Proteomic analysis of cell free Citrobacter rodentium secretion profile indicated that NleE, along with EspF and Tir, is the highest secreted effector, suggesting it plays a key role in virulence [67]. Indeed, NleE was shown to be a potent inhibitor of NF-kappaB which prevents nuclear p65 translocation in epithelial cells in response to TNFalpha and IL-1beta [61,68]. The mechanism by NleE blocks NF-kappaB signaling is not known. It was, however, suggested that NleE targets the IKK complex and prevents the phosphorylation of IKKbeta [68]. Although so far no functional domain was found that could explain NleE's mode of action, an analysis of the nleE sequence identified a motif 206IDSYMK214 of unknown function which is not sufficient but essential for NleE's anti-inflammatory activity [61].
While other research on NleE showed its inhibiting effect on NF-kappaB signaling in epithelial cells, our group provided evidence that NleE inhibits the expression and production of pro-inflammatory cytokines IL-8, TNFalpha, and IL-6 in human DCs which was due to impaired NF-kappaB p65 nuclear translocation [69]. NleE injected by EPEC was shown to drastically reduce the production of these cytokines in human monocyte-derived DCs as well as PP DCs in vitro. We further showed that EPEC injects its effectors into DCs that reach through an epithelial layer in a transwell system. These results suggest that EPEC can impair NF-kappaB signaling not only in epithelial cells, but also hampers the inflammatory response in the gut by injecting into PP DCs that sample the bacteria from the lumen. Certain EPEC strains target the FAE early on in infection [70]. The fact that EPEC can impair signaling in PP DCs when they encounter them at the FAE might explain this phenomenon.
Preventing phagocytosis
Phagocytosis is a receptor-mediated process and occurs in two different ways: via the direct binding of the particle to specific receptors at the surface of the phagocyte or via earlier opsonisation of the particle by IgG or the C3bi complement fragment [71]. IgG and C3bi subsequently bind to the FcgammaR or Complement receptor 3 (CR3), respectively, at the surface of the cell. EPEC inhibits phagocytosis in infected macrophages [72]. Furthermore, EPEC blocks both the opsonin-dependent and independent phagocytic pathways in vitro by injecting the four T3SS effectors EspF, EspB, EspJ, and EspH.
EspF from both EPEC and EHEC prevents phagocytosis by macrophages and the uptake by M cells in in vitro models (table 1) [73-77]. EspF is a multifunctional effector implicated in various others aspect of pathogenesis. Amongst them are the alteration of the intestinal epithelial tight-junctions, the effacement of the brush border microvilli, mitochondrial-dependant apoptosis, the nucleolar disruption and the targeting of various cellular proteins like the neuronal Wiskott-Aldrich syndrome protein (N-WASP), cytokeratin 18, anti-apoptotic Abcf2 or sorting nexin 9 (Snx9), a protein involved in vesicles trafficking [78]. The mechanism by which EspF prevents phagocytosis is still unknown and a study by Quitard and coworkers showing that the N-terminal 101 amino acid domain of EspF is essential suggests that the binding to proteins like N-WASP, actin or SNX9 are not required to prevent the uptake by macrophages [78]. Contradictory to its anti-phagocytic activity, a role for EspF in promoting enterocyte invasion has recently been described and was shown to depend on the interaction between EspF and SNX9 [79]. EspJ from EPEC and EHEC does not block phagocytosis of non-opsonized bacteria but prevents both the FcgammaR and CR3 opsonin-dependant phagocytosis of particles by macrophages or FcgammaR- or CR3-transfected cells [75]. The mechanism by which EspJ blocks opsonophagocytosis remains to be identified. EspB hampers phagocytosis by binding and inhibiting host myosin functions which are required for phagocytosis of non-opsonized bacteria [74]. EspH is the only effector reported to inhibit both opsono- and non-opsonophagocytosis. It binds to the DH-PH domain of several Rho GTPase exchange factors (RhoGEF), preventing activation of Rho GTPases and inducing a general inhibition of actin polymerisation which would explain the inhibition of phagocytosis [73]. The identification of anti-phagocytic activity of so far four effectors translocated by EPEC and EHEC suggest their importance for the in vivo pathogenesis. A recent paper reported that the effector EspG targets ARF6 GTPases which results in the reprogramming of endomembrane trafficking [80]. This finding raises the question whether EspG also plays a role in the general anti-phagocytic activity as ARF6 is essential for FcgammaR-mediated phagocytosis [81].
Although EPEC is a non-invasive pathogen, its mucosal uptake has been reported in various in vitro and in vivo studies [82]. The relevance of EPEC invasion for pathogenesis is not known but might play a role in persistence of the pathogen inside the host. Since EPEC and EHEC mediate disease from their luminal position, one might wonder how relevant the interaction of the bacteria with professional phagocytic cells is during infection. EPEC is known to target the FAE in the gut [70], so the inhibition of its own uptake, which was observed under in vitro conditions [76], could reflect the inhibition of its M cells transcytosis resulting in immune evasion. On the other hand, in vivo studies with Citrobacter rodentium in mice have shown that the activity of neutrophils were necessary to clear the infection, suggesting that at some point during the infection the bacteria interact with phagocytic cells [83].
Conclusion
Pathogenic bacteria have evolved alongside their hosts, developing sophisticated mechanisms and effector proteins to manipulate the host cells on multiple levels. Not only do bacteria which use the T3SS secure their attachment to epithelial cells by altering the cytoskeleton, they also actively prevent phagocytosis in the gut and impair the immune response by interfering with pro-inflammatory signaling pathways. While these modulatory strategies might not be clinically detrimental to infected individuals, the bacteria gain the advantage of facilitated and prolonged colonization in the gut. The variety of immuno-modulatory effectors in T3SS-employing pathogens might also explain why e.g. EPEC shows a tropism for the GALT, since this is the site where the bacteria encounter DCs which they modulate to hamper the initiation of the intestinal immune response.
Abbreviations
DC: dendritic cell; EHEC: enterohaemorrhagic E. coli; EPEC: enteropathogenic Escherichia coli; FAE: follicle-associated epithelium; GALT: gut-associated lymphoid tissue; PP: Peyer's patch; T3SS: type-3 secretion system.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
All authors wrote, read and approved the final manuscript.
Contributor Information
Anna Vossenkämper, Email: a.vossenkaemper@qmul.ac.uk.
Thomas T MacDonald, Email: t.t.macdonald@qmul.ac.uk.
Olivier Marchès, Email: o.marches@qmul.ac.uk.
Acknowledgements and Funding
A.V. is funded by the Medical Research Council, UK
References
- Castro GA, Arntzen CJ. Immunophysiology of the gut: a research frontier for integrative studies of the common mucosal immune system. Am J Physiol. 1993;265:G599–610. doi: 10.1152/ajpgi.1993.265.4.G599. [DOI] [PubMed] [Google Scholar]
- Jung C, Hugot JP, Barreau F. Peyer's Patches: The Immune Sensors of the Intestine. Int J Inflam. 2010;2010:823710. doi: 10.4061/2010/823710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald T. The gut is still the biggest lymphoid organ in the body. Mucosal Immunology. 2008;1:246–247. [Google Scholar]
- Neutra MR, Mantis NJ, Kraehenbuhl JP. Collaboration of epithelial cells with organized mucosal lymphoid tissues. Nat Immunol. 2001;2:1004–1009. doi: 10.1038/ni1101-1004. [DOI] [PubMed] [Google Scholar]
- Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, Bonasio R, Granucci F, Kraehenbuhl JP, Ricciardi-Castagnoli P. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol. 2001;2:361–367. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- Gorfu G, Rivera-Nieves J, Ley K. Role of beta7 integrins in intestinal lymphocyte homing and retention. Curr Mol Med. 2009;9:836–850. doi: 10.2174/156652409789105525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunliffe RN, Mahida YR. Expression and regulation of antimicrobial peptides in the gastrointestinal tract. J Leukoc Biol. 2004;75:49–58. doi: 10.1189/jlb.0503249. [DOI] [PubMed] [Google Scholar]
- Plaut AG. Trefoil peptides in the defense of the gastrointestinal tract. N Engl J Med. 1997;336:506–507. doi: 10.1056/NEJM199702133360712. [DOI] [PubMed] [Google Scholar]
- Kim YS, Ho SB. Intestinal goblet cells and mucins in health and disease: recent insights and progress. Curr Gastroenterol Rep. 2010;12:319–330. doi: 10.1007/s11894-010-0131-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerf-Bensussan N, Gaboriau-Routhiau V. The immune system and the gut microbiota: friends or foes? Nat Rev Immunol. 2010;10:735–744. doi: 10.1038/nri2850. [DOI] [PubMed] [Google Scholar]
- Macpherson AJ, Hunziker L, McCoy K, Lamarre A. IgA responses in the intestinal mucosa against pathogenic and non-pathogenic microorganisms. Microbes Infect. 2001;3:1021–1035. doi: 10.1016/S1286-4579(01)01460-5. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Maruya M, Kawamoto S, Sitnik K, Kitamura H, Agace WW, Fagarasan S. The sensing of environmental stimuli by follicular dendritic cells promotes immunoglobulin A generation in the gut. Immunity. 2010;33:71–83. doi: 10.1016/j.immuni.2010.07.003. [DOI] [PubMed] [Google Scholar]
- Spencer J, Barone F, Dunn-Walters D. Generation of Immunoglobulin diversity in human gut-associated lymphoid tissue. Semin Immunol. 2009;21:139–146. doi: 10.1016/j.smim.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Macpherson AJ, McCoy KD, Johansen FE, Brandtzaeg P. The immune geography of IgA induction and function. Mucosal Immunol. 2008;1:11–22. doi: 10.1038/mi.2007.6. [DOI] [PubMed] [Google Scholar]
- Maizels RM, Yazdanbakhsh M. Immune regulation by helminth parasites: cellular and molecular mechanisms. Nat Rev Immunol. 2003;3:733–744. doi: 10.1038/nri1183. [DOI] [PubMed] [Google Scholar]
- Cella M, Fuchs A, Vermi W, Facchetti F, Otero K, Lennerz JK, Doherty JM, Mills JC, Colonna M. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2009;457:722–725. doi: 10.1038/nature07537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama T, Kamada N, Chinen H, Okamoto S, Kitazume MT, Chang J, Matuzaki Y, Suzuki S, Sugita A, Koganei K, Hisamatsu T, Kanai T, Hibi T. Imbalance of NKp44(+)NKp46(-) and NKp44(-)NKp46(+) natural killer cells in the intestinal mucosa of patients with Crohn's disease. Gastroenterology. 2010;139:882–892. doi: 10.1053/j.gastro.2010.05.040. 892 e881-883. [DOI] [PubMed] [Google Scholar]
- Satoh-Takayama N, Vosshenrich CA, Lesjean-Pottier S, Sawa S, Lochner M, Rattis F, Mention JJ, Thiam K, Cerf-Bensussan N, Mandelboim O, Eberl G, Di Santo JP. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity. 2008;29:958–970. doi: 10.1016/j.immuni.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Shiomi H, Masuda A, Nishiumi S, Nishida M, Takagawa T, Shiomi Y, Kutsumi H, Blumberg RS, Azuma T, Yoshida M. Gamma interferon produced by antigen-specific CD4+ T cells regulates the mucosal immune responses to Citrobacter rodentium infection. Infect Immun. 2010;78:2653–2666. doi: 10.1128/IAI.01343-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabri B, Ebert E. Human CD8+ intraepithelial lymphocytes: a unique model to study the regulation of effector cytotoxic T lymphocytes in tissue. Immunol Rev. 2007;215:202–214. doi: 10.1111/j.1600-065X.2006.00481.x. [DOI] [PubMed] [Google Scholar]
- Beagley KW, Husband AJ. Intraepithelial lymphocytes: origins, distribution, and function. Crit Rev Immunol. 1998;18:237–254. doi: 10.1615/critrevimmunol.v18.i3.40. [DOI] [PubMed] [Google Scholar]
- Sanz Y, De Palma G. Gut microbiota and probiotics in modulation of epithelium and gut-associated lymphoid tissue function. Int Rev Immunol. 2009;28:397–413. doi: 10.3109/08830180903215613. [DOI] [PubMed] [Google Scholar]
- Manicassamy S, Reizis B, Ravindran R, Nakaya H, Salazar-Gonzalez RM, Wang YC, Pulendran B. Activation of beta-catenin in dendritic cells regulates immunity versus tolerance in the intestine. Science. 2010;329:849–853. doi: 10.1126/science.1188510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- Kang JY, Nan X, Jin MS, Youn SJ, Ryu YH, Mah S, Han SH, Lee H, Paik SG, Lee JO. Recognition of lipopeptide patterns by Toll-like receptor 2-Toll-like receptor 6 heterodimer. Immunity. 2009;31:873–884. doi: 10.1016/j.immuni.2009.09.018. [DOI] [PubMed] [Google Scholar]
- Hugot JP, Chamaillard M, Zouali H, Lesage S, Cezard JP, Belaiche J, Almer S, Tysk C, O'Morain CA, Gassull M, Binder V, Finkel Y, Cortot A, Modigliani R, Laurent-Puig P, Gower-Rousseau C, Macry J, Colombel JF, Sahbatou M, Thomas G. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature. 2001;411:599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- Keshet Y, Seger R. The MAP kinase signaling cascades: a system of hundreds of components regulates a diverse array of physiological functions. Methods Mol Biol. 2010;661:3–38. doi: 10.1007/978-1-60761-795-2_1. [DOI] [PubMed] [Google Scholar]
- Malladi V, Puthenedam M, Williams PH, Balakrishnan A. Enteropathogenic Escherichia coli outer membrane proteins induce iNOS by activation of NF-kappaB and MAP kinases. Inflammation. 2004;28:345–353. doi: 10.1007/s10753-004-6645-8. [DOI] [PubMed] [Google Scholar]
- Shames SR, Auweter SD, Finlay BB. Co-evolution and exploitation of host cell signaling pathways by bacterial pathogens. Int J Biochem Cell Biol. 2009;41:380–389. doi: 10.1016/j.biocel.2008.08.013. [DOI] [PubMed] [Google Scholar]
- Sasakawa C. A new paradigm of bacteria-gut interplay brought through the study of Shigella. Proc Jpn Acad Ser B Phys Biol Sci. 2010;86:229–243. doi: 10.2183/pjab.86.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Ashida H, Ogawa M, Yoshikawa Y, Mimuro H, Sasakawa C. Bacterial interactions with the host epithelium. Cell Host Microbe. 2010;8:20–35. doi: 10.1016/j.chom.2010.06.006. [DOI] [PubMed] [Google Scholar]
- Macdonald TT, Monteleone G. Immunity, inflammation, and allergy in the gut. Science. 2005;307:1920–1925. doi: 10.1126/science.1106442. [DOI] [PubMed] [Google Scholar]
- Iwai H, Kim M, Yoshikawa Y, Ashida H, Ogawa M, Fujita Y, Muller D, Kirikae T, Jackson PK, Kotani S, Sasakawa C. A bacterial effector targets Mad2L2, an APC inhibitor, to modulate host cell cycling. Cell. 2007;130:611–623. doi: 10.1016/j.cell.2007.06.043. [DOI] [PubMed] [Google Scholar]
- Kim M, Ogawa M, Fujita Y, Yoshikawa Y, Nagai T, Koyama T, Nagai S, Lange A, Fassler R, Sasakawa C. Bacteria hijack integrin-linked kinase to stabilize focal adhesions and block cell detachment. Nature. 2009;459:578–582. doi: 10.1038/nature07952. [DOI] [PubMed] [Google Scholar]
- Tobe T, Beatson SA, Taniguchi H, Abe H, Bailey CM, Fivian A, Younis R, Matthews S, Marches O, Frankel G, Hayashi T, Pallen MJ. An extensive repertoire of type III secretion effectors in Escherichia coli O157 and the role of lambdoid phages in their dissemination. Proc Natl Acad Sci USA. 2006;103:14941–14946. doi: 10.1073/pnas.0604891103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muenzner P, Bachmann V, Zimmermann W, Hentschel J, Hauck CR. Human-restricted bacterial pathogens block shedding of epithelial cells by stimulating integrin activation. Science. 2010;329:1197–1201. doi: 10.1126/science.1190892. [DOI] [PubMed] [Google Scholar]
- Marches O, Ledger TN, Boury M, Ohara M, Tu X, Goffaux F, Mainil J, Rosenshine I, Sugai M, De Rycke J, Oswald E. Enteropathogenic and enterohaemorrhagic Escherichia coli deliver a novel effector called Cif, which blocks cell cycle G2/M transition. Mol Microbiol. 2003;50:1553–1567. doi: 10.1046/j.1365-2958.2003.03821.x. [DOI] [PubMed] [Google Scholar]
- Jubelin G, Taieb F, Duda DM, Hsu Y, Samba-Louaka A, Nobe R, Penary M, Watrin C, Nougayrede JP, Schulman BA, Stebbins CE, Oswald E. Pathogenic bacteria target NEDD8-conjugated cullins to hijack host-cell signaling pathways. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1001128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samba-Louaka A, Nougayrede JP, Watrin C, Jubelin G, Oswald E, Taieb F. Bacterial cyclomodulin Cif blocks the host cell cycle by stabilizing the cyclin-dependent kinase inhibitors p21 and p27. Cell Microbiol. 2008;10:2496–2508. doi: 10.1111/j.1462-5822.2008.01224.x. [DOI] [PubMed] [Google Scholar]
- Shames SR, Deng W, Guttman JA, de Hoog CL, Li Y, Hardwidge PR, Sham HP, Vallance BA, Foster LJ, Finlay BB. The pathogenic E. coli type III effector EspZ interacts with host CD98 and facilitates host cell prosurvival signalling. Cell Microbiol. 2010;12:1322–1339. doi: 10.1111/j.1462-5822.2010.01470.x. [DOI] [PubMed] [Google Scholar]
- Hemrajani C, Berger CN, Robinson KS, Marches O, Mousnier A, Frankel G. NleH effectors interact with Bax inhibitor-1 to block apoptosis during enteropathogenic Escherichia coli infection. Proc Natl Acad Sci USA. 2010;107:3129–3134. doi: 10.1073/pnas.0911609106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baruch K, Gur-Arie L, Nadler C, Koby S, Yerushalmi G, Ben-Neriah Y, Yogev O, Shaulian E, Guttman C, Zarivach R, Rosenshine I. Metalloprotease type III effectors that specifically cleave JNK and NF-kappaB. Embo J. 2010;30:221–231. doi: 10.1038/emboj.2010.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Puente JL, Gruenheid S, Li Y, Vallance BA, Vazquez A, Barba J, Ibarra JA, O'Donnell P, Metalnikov P, Ashman K, Lee S, Goode D, Pawson T, Finlay BB. Dissecting virulence: systematic and functional analyses of a pathogenicity island. Proc Natl Acad Sci USA. 2004;101:3597–3602. doi: 10.1073/pnas.0400326101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly M, Hart E, Mundy R, Marches O, Wiles S, Badea L, Luck S, Tauschek M, Frankel G, Robins-Browne RM, Hartland EL. Essential role of the type III secretion system effector NleB in colonization of mice by Citrobacter rodentium. Infect Immun. 2006;74:2328–2337. doi: 10.1128/IAI.74.4.2328-2337.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marches O, Wiles S, Dziva F, La Ragione RM, Schuller S, Best A, Phillips AD, Hartland EL, Woodward MJ, Stevens MP, Frankel G. Characterization of two non-locus of enterocyte effacement-encoded type III-translocated effectors, NleC and NleD, in attaching and effacing pathogens. Infect Immun. 2005;73:8411–8417. doi: 10.1128/IAI.73.12.8411-8417.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhavsar AP, Guttman JA, Finlay BB. Manipulation of host-cell pathways by bacterial pathogens. Nature. 2007;449:827–834. doi: 10.1038/nature06247. [DOI] [PubMed] [Google Scholar]
- Petty NK, Bulgin R, Crepin VF, Cerdeno-Tarraga AM, Schroeder GN, Quail MA, Lennard N, Corton C, Barron A, Clark L, Toribio AL, Parkhill J, Dougan G, Frankel G, Thomson NR. The Citrobacter rodentium genome sequence reveals convergent evolution with human pathogenic Escherichia coli. J Bacteriol. 2010;192:525–538. doi: 10.1128/JB.01144-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garmendia J, Frankel G, Crepin VF. Enteropathogenic and enterohemorrhagic Escherichia coli infections: translocation, translocation, translocation. Infect Immun. 2005;73:2573–2585. doi: 10.1128/IAI.73.5.2573-2585.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czerucka D, Dahan S, Mograbi B, Rossi B, Rampal P. Implication of mitogen-activated protein kinases in T84 cell responses to enteropathogenic Escherichia coli infection. Infect Immun. 2001;69:1298–1305. doi: 10.1128/IAI.69.3.1298-1305.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez K, Huerta R, Oswald E, Garcia-Tovar C, Hernandez JM, Navarro-Garcia F. Role of EspA and intimin in expression of proinflammatory cytokines from enterocytes and lymphocytes by rabbit enteropathogenic Escherichia coli-infected rabbits. Infect Immun. 2005;73:103–113. doi: 10.1128/IAI.73.1.103-113.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruchaud-Sparagano MH, Maresca M, Kenny B. Enteropathogenic Escherichia coli (EPEC) inactivate innate immune responses prior to compromising epithelial barrier function. Cell Microbiol. 2007;9:1909–1921. doi: 10.1111/j.1462-5822.2007.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R, Tesfay S, Tomson FL, Kanteti RP, Viswanathan VK, Hecht G. Balance of bacterial pro- and anti-inflammatory mediators dictates net effect of enteropathogenic Escherichia coli on intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2006;290:G685–694. doi: 10.1152/ajpgi.00404.2005. [DOI] [PubMed] [Google Scholar]
- Mumy KL, McCormick BA. The role of neutrophils in the event of intestinal inflammation. Curr Opin Pharmacol. 2009;9:697–701. doi: 10.1016/j.coph.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Wan F, Mateo K, Callegari E, Wang D, Deng W, Puente J, Li F, Chaussee MS, Finlay BB, Lenardo MJ, Hardwidge PR. Bacterial effector binding to ribosomal protein s3 subverts NF-kappaB function. PLoS Pathog. 2009;5:e1000708. doi: 10.1371/journal.ppat.1000708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan F, Anderson DE, Barnitz RA, Snow A, Bidere N, Zheng L, Hegde V, Lam LT, Staudt LM, Levens D, Deutsch WA, Lenardo MJ. Ribosomal protein S3: a KH domain subunit in NF-kappaB complexes that mediates selective gene regulation. Cell. 2007;131:927–939. doi: 10.1016/j.cell.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Kim DW, Lenzen G, Page AL, Legrain P, Sansonetti PJ, Parsot C. The Shigella flexneri effector OspG interferes with innate immune responses by targeting ubiquitin-conjugating enzymes. Proc Natl Acad Sci USA. 2005;102:14046–14051. doi: 10.1073/pnas.0504466102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royan SV, Jones RM, Koutsouris A, Roxas JL, Falzari K, Weflen AW, Kim A, Bellmeyer A, Turner JR, Neish AS, Rhee KJ, Viswanathan VK, Hecht GA. Enteropathogenic E. coli non-LEE encoded effectors NleH1 and NleH2 attenuate NF-kappaB activation. Mol Microbiol. 2010;78:1232–1245. doi: 10.1111/j.1365-2958.2010.07400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iguchi A, Thomson NR, Ogura Y, Saunders D, Ooka T, Henderson IR, Harris D, Asadulghani M, Kurokawa K, Dean P, Kenny B, Quail MA, Thurston S, Dougan G, Hayashi T, Parkhill J, Frankel G. Complete genome sequence and comparative genome analysis of enteropathogenic Escherichia coli O127:H6 strain E2348/69. J Bacteriol. 2009;191:347–354. doi: 10.1128/JB.01238-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham ME, Lupp C, Mascarenhas M, Vazquez A, Coombes BK, Brown NF, Coburn BA, Deng W, Puente JL, Karmali MA, Finlay BB. Bacterial genetic determinants of non-O157 STEC outbreaks and hemolytic-uremic syndrome after infection. J Infect Dis. 2006;194:819–827. doi: 10.1086/506620. [DOI] [PubMed] [Google Scholar]
- Newton HJ, Pearson JS, Badea L, Kelly M, Lucas M, Holloway G, Wagstaff KM, Dunstone MA, Sloan J, Whisstock JC, Kaper JB, Robins-Browne RM, Jans DA, Frankel G, Phillips AD, Coulson BS, Hartland EL. The type III effectors NleE and NleB from enteropathogenic E. coli and OspZ from Shigella block nuclear translocation of NF-kappaB p65. PLoS Pathog. 2010;6:e1000898. doi: 10.1371/journal.ppat.1000898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhlen S, Ruchaud-Sparagano MH, Kenny B. Proteasome-independent Degradation of Canonical NF{kappa}B Complex Components by the NleC Protein of Pathogenic Escherichia coli. J Biol Chem. 2010;286:5100–5107. doi: 10.1074/jbc.M110.172254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson JS, Riedmaier P, Marches O, Frankel G, Hartland EL. A type III effector protease NleC from enteropathogenic Escherichia coli targets NF-kappaB for degradation. Mol Microbiol. 2010. [DOI] [PMC free article] [PubMed]
- Yen H, Ooka T, Iguchi A, Hayashi T, Sugimoto N, Tobe T. NleC, a type III secretion protease, compromises NF-kappaB activation by targeting p65/RelA. PLoS Pathog. 2010;6:e1001231. doi: 10.1371/journal.ppat.1001231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurawski DV, Mumy KL, Badea L, Prentice JA, Hartland EL, McCormick BA, Maurelli AT. The NleE/OspZ family of effector proteins is required for polymorphonuclear transepithelial migration, a characteristic shared by enteropathogenic Escherichia coli and Shigella flexneri infections. Infect Immun. 2008;76:369–379. doi: 10.1128/IAI.00684-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham ME, Lupp C, Vazquez A, Mascarenhas M, Coburn B, Coombes BK, Karmali MA, Puente JL, Deng W, Finlay BB. Citrobacter rodentium virulence in mice associates with bacterial load and the type III effector NleE. Microbes Infect. 2007;9:400–407. doi: 10.1016/j.micinf.2006.12.016. [DOI] [PubMed] [Google Scholar]
- Deng W, de Hoog CL, Yu HB, Li Y, Croxen MA, Thomas NA, Puente JL, Foster LJ, Finlay BB. A comprehensive proteomic analysis of the type III secretome of Citrobacter rodentium. J Biol Chem. 2010;285:6790–6800. doi: 10.1074/jbc.M109.086603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadler C, Baruch K, Kobi S, Mills E, Haviv G, Farago M, Alkalay I, Bartfeld S, Meyer TF, Ben-Neriah Y, Rosenshine I. The type III secretion effector NleE inhibits NF-kappaB activation. PLoS Pathog. 2010;6:e1000743. doi: 10.1371/journal.ppat.1000743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vossenkamper A, Marches O, Fairclough PD, Warnes G, Stagg AJ, Lindsay JO, Evans PC, Luong le A, Croft NM, Naik S, Frankel G, MacDonald TT. Inhibition of NF-kappaB signaling in human dendritic cells by the enteropathogenic Escherichia coli effector protein NleE. J Immunol. 2010;185:4118–4127. doi: 10.4049/jimmunol.1000500. [DOI] [PubMed] [Google Scholar]
- Fitzhenry RJ, Reece S, Trabulsi LR, Heuschkel R, Murch S, Thomson M, Frankel G, Phillips AD. Tissue tropism of enteropathogenic Escherichia coli strains belonging to the O55 serogroup. Infect Immun. 2002;70:4362–4368. doi: 10.1128/IAI.70.8.4362-4368.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron E, Hall A. Identification of two distinct mechanisms of phagocytosis controlled by different Rho GTPases. Science. 1998;282:1717–1721. doi: 10.1126/science.282.5394.1717. [DOI] [PubMed] [Google Scholar]
- Celli J, Olivier M, Finlay BB. Enteropathogenic Escherichia coli mediates antiphagocytosis through the inhibition of PI 3-kinase-dependent pathways. EMBO J. 2001;20:1245–1258. doi: 10.1093/emboj/20.6.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong N, Liu L, Shao F. A bacterial effector targets host DH-PH domain RhoGEFs and antagonizes macrophage phagocytosis. EMBO J. 2010;29:1363–1376. doi: 10.1038/emboj.2010.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iizumi Y, Sagara H, Kabe Y, Azuma M, Kume K, Ogawa M, Nagai T, Gillespie PG, Sasakawa C, Handa H. The enteropathogenic E. coli effector EspB facilitates microvillus effacing and antiphagocytosis by inhibiting myosin function. Cell Host Microbe. 2007;2:383–392. doi: 10.1016/j.chom.2007.09.012. [DOI] [PubMed] [Google Scholar]
- Marches O, Covarelli V, Dahan S, Cougoule C, Bhatta P, Frankel G, Caron E. EspJ of enteropathogenic and enterohaemorrhagic Escherichia coli inhibits opsono-phagocytosis. Cell Microbiol. 2008;10:1104–1115. doi: 10.1111/j.1462-5822.2007.01112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Argudo I, Sands C, Jepson MA. Translocation of enteropathogenic Escherichia coli across an in vitro M cell model is regulated by its type III secretion system. Cell Microbiol. 2007;9:1538–1546. doi: 10.1111/j.1462-5822.2007.00891.x. [DOI] [PubMed] [Google Scholar]
- Quitard S, Dean P, Maresca M, Kenny B. The enteropathogenic Escherichia coli EspF effector molecule inhibits PI-3 kinase-mediated uptake independently of mitochondrial targeting. Cell Microbiol. 2006;8:972–981. doi: 10.1111/j.1462-5822.2005.00680.x. [DOI] [PubMed] [Google Scholar]
- Holmes A, Muhlen S, Roe AJ, Dean P. The EspF effector, a bacterial pathogen's Swiss army knife. Infect Immun. 2010;78:4445–4453. doi: 10.1128/IAI.00635-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weflen AW, Alto NM, Viswanathan VK, Hecht G. E. coli secreted protein F promotes EPEC invasion of intestinal epithelial cells via an SNX9-dependent mechanism. Cell Microbiol. 2010;12:919–929. doi: 10.1111/j.1462-5822.2010.01440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selyunin AS, Sutton SE, Weigele BA, Reddick LE, Orchard RC, Bresson SM, Tomchick DR, Alto NM. The assembly of a GTPase-kinase signalling complex by a bacterial catalytic scaffold. Nature. 2011;469:107–111. doi: 10.1038/nature09593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedergang F, Colucci-Guyon E, Dubois T, Raposo G, Chavrier P. ADP ribosylation factor 6 is activated and controls membrane delivery during phagocytosis in macrophages. J Cell Biol. 2003;161:1143–1150. doi: 10.1083/jcb.200210069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddocks OD, Short AJ, Donnenberg MS, Bader S, Harrison DJ. Attaching and effacing Escherichia coli downregulate DNA mismatch repair protein in vitro and are associated with colorectal adenocarcinomas in humans. PLoS One. 2009;4:e5517. doi: 10.1371/journal.pone.0005517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spehlmann ME, Dann SM, Hruz P, Hanson E, McCole DF, Eckmann L. CXCR2-dependent mucosal neutrophil influx protects against colitis-associated diarrhea caused by an attaching/effacing lesion-forming bacterial pathogen. J Immunol. 2009;183:3332–3343. doi: 10.4049/jimmunol.0900600. [DOI] [PMC free article] [PubMed] [Google Scholar]