Abstract
Background
Understanding the factors which determine a household's or individual's risk of malaria infection is important for targeting control interventions at all intensities of transmission. Malaria ecology in Tanzania appears to have reduced over recent years. This study investigated potential risk factors and clustering in face of changing infection dynamics.
Methods
Household survey data were collected in villages of rural Muheza district. Children aged between six months and thirteen years were tested for presence of malaria parasites using microscopy. A multivariable logistic regression model was constructed to identify significant risk factors for children. Geographical information systems combined with global positioning data and spatial scan statistic analysis were used to identify clusters of malaria.
Results
Using an insecticide-treated mosquito net of any type proved to be highly protective against malaria (OR 0.75, 95% CI 0.59-0.96). Children aged five to thirteen years were at higher risk of having malaria than those aged under five years (OR 1.71, 95% CI 1.01-2.91). The odds of malaria were less for females when compared to males (OR 0.62, 95% CI 0.39-0.98). Two spatial clusters of significantly increased malaria risk were identified in two out of five villages.
Conclusions
This study provides evidence that recent declines in malaria transmission and prevalence may shift the age groups at risk of malaria infection to older children. Risk factor analysis provides support for universal coverage and targeting of long-lasting insecticide-treated nets (LLINs) to all age groups. Clustering of cases indicates heterogeneity of risk. Improved targeting of LLINs or additional supplementary control interventions to high risk clusters may improve outcomes and efficiency as malaria transmission continues to fall under intensified control.
Background
Tanzania is heavily affected by malaria which is one of the leading causes of morbidity and mortality in the country [1], accounting for over 30% of the national disease burden [2]. In order to specifically tailor and improve prevention measures targeted against the disease it is important to obtain detailed knowledge of factors associated with increased risk of malaria. Identification of the specific risk factors in a locality may provide support for existing preventative measures or the introduction of new ones and can indicate areas in which prevention activities are currently under-utilized.
The identification and quantification of heterogeneity in disease prevalence across a geographical range provides scope for targeting prevention and treatment interventions at high-prevalence or high-risk areas [3,4]. This may in turn lead to increases in the equity, efficacy and cost effectiveness of interventions. Vector-borne diseases, such as malaria, are well suited to cluster analysis, which aims to delimit hotspots of high disease prevalence. The specific habits and limited range of the anopheline vectors of malaria aid efforts to resolve spatial clusters of the disease [5].
A number of studies have used cluster analysis to identify spatial and temporal hotspots of malaria transmission in other parts of Africa [6-9]. The epidemiology of the disease in eastern Africa appears to have changed in recent years, with marked declines in malaria transmission intensity, morbidity and mortality [10-14], making a study of this kind relevant and timely. As malaria declines, continued improvements of prevention and control interventions as well as treatment distribution may increasingly rely on accurate knowledge of risk factors and an ability to delimit high-risk areas.
This study aimed to investigate changes in malaria epidemiology in Muheza district by identifying significant household risk factors from individual, behavioural and house structural parameters. In addition, the study aimed to identify spatial clustering of malaria cases. It is intended that the study outcomes will inform targeting of interventions and treatment in the district and shed light on current epidemiological patterns.
Methods
Study area
The study was conducted within Muheza district in the Tanga region of North-East Tanzania (5°1'-5°8'S, 38°46'-38°56'E). A 2001 census recorded the district population as 1,636,280 [15]. Data were collected between June and August 2010, soon after the end of the traditional long rainy season. Anopheles gambiae s.l. has been shown to be the dominant vector in this region with abundance patterns strongly correlated with seasonal rainfall [16]. Malaria transmission usually peaks shortly after the end of the rainy season with prevalence in this region traditionally considered high. Previous studies have documented greater than 40% prevalence in children and young adults [17] and associated intensive, holoendemic transmission in the area [18,19].
All children participating in the study were aged between 6 months and 13 years and resided in twenty one rural hamlets within five villages: Mlingano, Mwungano, Kwalubuye, Kibaoni and Misozwe. Villages were selected based on three criteria: that they had a population of approximately 400 residents and/or 100 children, that they were not involved with any other research programme and that they were within a logistically feasible distance of the research centre in Muheza town. Three state-recognised drug dispensaries were located within the study area: one in Misozwe and two in Kibaoni. Over 90% of all household heads in the study worked as farmers, mainly at the subsistence level.
Household survey and enumeration
Census data from 10,555 residents occupying 2,609 houses were collected during an enumeration round. Details were recorded of malaria prevention behaviours, socioeconomic status, house structure and location. GPS coordinates were taken from the front door of each residence using a GPS receiver (Garmin etrex legend®, Garmin International Inc. USA). Data were recorded using paper and personal digital assistants (PDAs) (HP iPAC 114 Classic, Hewlett-Packard Development Company, L.P. USA). Specific variables recorded were: individual-related: Age, gender, weight, height. Prevention behaviour-related: mosquito net use, type of mosquito net used (untreated, insecticide-treated net (ITN), LLIN), frequency of mosquito net use, number of holes in mosquito net, proximity of penned livestock and structurally-related: house elevation, house volume, wall and roof construction material, presence and size of eaves and the number of rooms, doors and windows.
Socioeconomic variables recorded included ownership of: a radio, a carved or iron bed, a cart, a bicycle, a car or motorbike and any livestock. A crude socioeconomic score (SES), ranging from 0 to 6, was calculated as the sum of positive responses to questions concerning the ownership of the aforementioned items. Body mass index (BMI) was calculated as weight/height2. The two age groups were based on observed differences in parasite prevalence of the two groups. All data were entered into a Microsoft Access database. The top and bottom 5% of values for each variable were extracted, double checked and verified.
Malaria prevalence survey
Biometric measurements were taken from each child. Axillary temperature was taken using a digital thermometer and the parent/guardian was questioned about their child's recent history of fever. If axillary temperature was ≥37.5°C or a history of fever was reported then a rapid diagnostic test (RDT) was administered (Paracheck® Pf device, Orchid Biomedical Systems, India) to test for Plasmodium falciparum-specific histidine rich protein II. Thick and thin blood films were made for all children. Slides were stained with Giemsa, and double read at Teule hospital, Muheza. Asexual stage parasites were counted per 200 white blood cells (WBC) and gametocytes were counted per 500 WBC. Anti-malarials (Coartem®, dispersable, artemether/lumefantrine 20 mg/120 mg) were given if RDT was positive.
Statistical analysis
Risk factors
Statistical analysis and model building were performed using STATA software (version 11, College Station, TX, USA). All variables were analysed individually for an association with malaria risk using logistic regression. All variables showing evidence for a possible association with malaria risk (p < 0.15) were included in the preliminary main-effects multivariate logistic regression model. A stepwise backwards-elimination approach was then followed to exclude any variable that showed a lack of effect on malaria risk (p > 0.05). Models were multilevel to adjust for possible clustering of cases within village and households; this gave reduced weighting to each subsequent malaria positive child recorded from a village or household after the first. For multivariable analysis untreated ITN and no mosquito net use were combined, as was insecticide-treated ITN and LLIN use. Wald tests were used to analyse the effect of removing each non-significant variable from the model. Possible interaction terms were also considered before a variable was dropped.
Clusters
Spatial analysis was performed to look for possible clustering of cases across households. A Kuldorff spatial scan statistic was obtained using the Bernoulli model [20,21] and SaTScan software (SaTScan, version 8.2.1). The software applies an infinite number of circular windows, which are plastic in both position and size, across the study area. Each distinct circle represents a possible cluster. A likelihood ratio test compares the observed prevalence of disease within the circle to the expected prevalence across the entire range to identify significant clusters of disease, providing relative risk and p-values for any clusters identified. The model was run with a maximum cluster size of 50% of the total population and p-values generated across 999 Monte Carlo replications.
Geographical
House locations were displayed using the GPS data and a geographical information system (GIS) (ArcMap, version 9.2, CA, USA). Data on administrative boundaries [22] were downloaded and added to the map as a layer. Clustering analysis statistics were also displayed and inspected using the ArcMap GIS.
Ethical approval
Ethical approval for this study was granted from the London School of Hygiene and Tropical Medicine (LSHTM) ethics committee and the National Institute for Medical Research (NIMR) Medical Research Coordination Committee (NIMR/HQ/R.8a/Vol.IX/928). Each parent/guardian and their children were informed of the purpose of the study and the nature of the clinical prevalence work. Verbal and written consent was obtained from all study participants.
Results
A total of 1438 children were included in the study, 728 (51%) male, 702 (49%) female and 8 missing values. Overall P. falciparum prevalence was 14.5% (95% CI 12.7-16.3). 43.6% (95% CI 38.5-48.8) of children did not have a mosquito net, 50.6% (95% CI 45.4- 55.7) had an LLIN, 1.1% (95% CI 0.5-2.5) an insecticide-treated net and 4.7% (95% CI 3.1-7.0) an untreated net. Children in the younger age group (6 months-4 years) were more likely to sleep under an insecticide-treated mosquito net than children in the older age group (5-13 years) (χ2 = 77.5, p < 0.001). There was no difference in the utilisation of mosquito nets between male and female children (χ2 = 0.09, p = 0.8).
Univariate analysis
Results of univariate analysis, after adjusting for possible village- and house-level clustering of cases, are shown in Table 1. Both age and gender was significantly associated with malaria risk. Children in the older age group (5-13 years) and males were associated with a significant increase in the odds of malaria infection. Use of an insecticide-treated mosquito net was associated with a decrease in the odds of malaria infection. As there was some evidence of an association between open eaves and malaria risk and also SES and malaria risk, these variables were also used in the multivariate modelling.
Table 1.
Univariate analysis of variables examined as potential risk factors for malaria in children
| Variable | n | Cases (%) | OR (95% CI)* | p-value |
|---|---|---|---|---|
| Individual | ||||
| Age group | ||||
| 6 months-4 years | 518 | 48 (9.3) | 1.0 | - |
| 5-13 years | 919 | 160 (17.4) | 2.06 (1.26-3.38) | 0.015 |
| Sex | ||||
| Male | 727 | 123 (16.9) | 1.0 | - |
| Female | 702 | 85 (12.1) | 0.68 (0.44-1.05) | 0.068 |
| Body mass index (BMI) (per additional unit) | 1436 | 208 (14.5) | 0.97 (0.90-1.05) | 0.40 |
| Socioeconomic status (per additional point score) | 1437 | 208 (14.5) | 0.87 (0.72-1.06) | 0.12 |
| Number of co-residents (per additional resident) | 1432 | 207 (14.5) | 1.05 (0.94-1.17) | 0.26 |
| Prevention behaviour | ||||
| Mosquito Net use | ||||
| None | 556 | 109 (19.6) | 1.0 | - |
| Untreated mosquito net | 59 | 8 (13.6) | 0.64 (0.22-1.88) | 0.32 |
| Insecticide-treated mosquito net | 659 | 70 (10.6) | 0.49 (0.30-0.78) | 0.013 |
| Frequency of net use | ||||
| Every night - seasonal | 235 | 29 (12.3) | 1.0 | - |
| Every night - year round | 625 | 66 (10.6) | 0.84 (0.42-1.68) | 0.52 |
| Number of holes in net (per additional hole) | 875 | 98 (11.2) | 0.98 (0.91-1.06) | 0.51 |
| Proximity of penned livestock | ||||
| No livestock penned < 20 m from house | 835 | 131 (15.7) | 1.0 | - |
| Livestock penned < 20 m from house | 594 | 77 (13.0) | 0.80 (0.50-1.27) | 0.25 |
| House related | ||||
| Elevation | 1393 | 204 (14.6) | 1.00 (1.00-1.01) | 0.28 |
| Wall material | ||||
| Cement | 327 | 41 (12.5) | 1.0 | - |
| Mud | 1089 | 161 (14.8) | 1.21 (0.68-2.14) | 0.41 |
| Wood | 9 | 3 (33.3) | 3.49 (0.31-39.29) | 0.23 |
| Other | 5 | 1 (20) | 1.74 (0.12-24.55) | 0.59 |
| Roof material | ||||
| Metal sheet | 722 | 100 (13.9) | 1.0 | - |
| Grass | 75 | 6 (8.0) | 0.54 (0.14-2.06) | 0.27 |
| Coconut palm | 588 | 97 (16.5) | 1.23 (0.77-1.97) | 0.29 |
| Other | 42 | 1 (2.4) | 0.15 (0.01-2.52) | 0.14 |
| Eaves | ||||
| Closed | 164 | 16 (9.8) | 1.0 | - |
| Open | 1261 | 189 (15.0) | 1.63 (0.73-3.63) | 0.17 |
| House size (m2) | 1428 | 207 (14.5) | 0.99 (0.99-1.00) | 0.65 |
| Number of rooms (per additional room) | 1429 | 208 (14.6) | 0.87 (0.68-1.11) | 0.19 |
| Number of windows (per additional window) | 1426 | 207 (14.5) | 0.86 (0.75-0.99) | 0.038 |
| Number of doors (per additional door) | 1430 | 208 (14.5) | 0.98 (0.66-1.46) | 0.90 |
* Robust confidence intervals adjusted for within village and within household clustering of cases
Multivariate analysis
Multivariate logistic regression analysis with an adjustment for within village and household clustering indicated that there were three variables significantly associated with malaria risk (Table 2).
Table 2.
Logistic regression model of significant variables associated with malaria risk in children
| Variable | Adjusted OR (95% CI)* | p-value |
|---|---|---|
| Insecticide-treated mosquito net use | ||
| No | 1.0 | - |
| Yes | 0.75 (0.59-0.96) | 0.030 |
| Age group | ||
| 6 months-4 years | 1.0 | - |
| 5-13 years | 1.71 (1.01-2.91) | 0.048 |
| Sex | ||
| Male | 1.0 | - |
| Female | 0.62 (0.39-0.98) | 0.044 |
* Robust confidence intervals adjusted for within village and within household clustering of cases
Risk of infection was significantly higher in children of five to thirteen years of age, compared to those aged six months to four years. There was strong evidence that sleeping under an insecticide-treated mosquito net was highly protective. The risk of malaria was lower for female than for males. There was no association between malaria risk and wall or roof construction material. There was no evidence of interaction between variables.
Cluster analysis
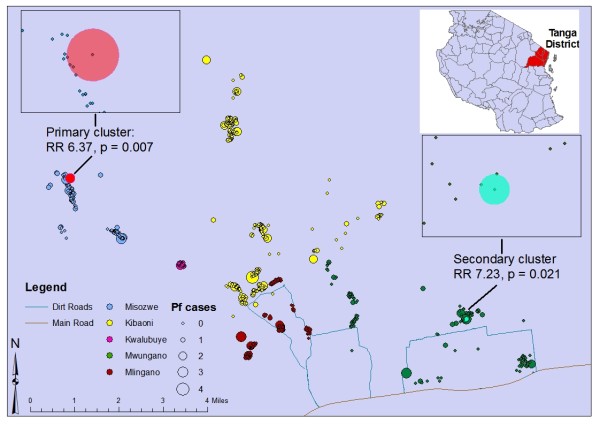
Spatial scan statistic indicated two distinct spatial clusters of malaria cases (Figure 1). In these areas the observed number of cases was significantly higher than expected (Table 3). The primary cluster consisting of four houses was located in Misozwe. A secondary, smaller cluster of two houses was identified in Mwungano.
Figure 1.
Spatial clustering of malaria cases.
Table 3.
Details of two distinct significant spatial clusters of malaria in children
| Cluster | Radius (Km) | Population (n) | Observed cases | Expected cases | Relative risk (RR) | p-value |
|---|---|---|---|---|---|---|
| Primary | 0.16 | 8 | 7 | 1.1 | 6.4 | 0.007 |
| Secondary | 0.04 | 5 | 5 | 0.7 | 7.2 | 0.021 |
Discussion
Risk factor analysis performed for this study identified a number of variables that, alone or in unison, would affect a child's risk of malaria. The child's age, gender and insecticide-treated mosquito net use were each associated with malaria risk. Use of insecticide-treated mosquito nets was highly protective against malaria whereas untreated nets were not. Older children, aged 5-13 years, and males had increased odds of malaria. Cluster analysis attempted to identify spatial hotspots of malaria where a child's risk of malaria was higher than expected for the study population as a whole. Two significant clusters of malaria were identified in two separate villages.
Age remained an important factor within the model, with older age groups being exposed to a higher risk of malaria. This finding is of particular interest as historically in Tanzania prevalence has peaked in younger age groups [23-25]. Part of this pattern is explained by the relationship between age and insecticide-treated mosquito net use. Children in the younger age group were significantly more likely to sleep under insecticide-treated mosquito nets which, in this study and many others across Africa [26], have proven to be highly protective against malaria. The observed age-prevalence patterns may be partially driven by overall increases in LLIN coverage in Tanzania [27]; shifts in the age of peak prevalence towards older children have been observed with increases in mosquito net coverage [28]. Similar shifts may be observed with reductions in the entomological inoculation rate (EIR) and the force of infection [29,30] leading children to be exposed to infective malaria inoculations less frequently meaning infections are acquired later in life. However, age-specific parasite-prevalence patterns have been demonstrated across a range of transmission intensities in this region of Tanzania before [17], suggesting that a drop in transmission intensity would be associated with reduced malaria-associated morbidity in all age groups and not a pronounced shift in age prevalence peaks. In this case a continued commitment to LLIN distribution would see further benefits for local communities [31] without risking potential changes of age-associated patterns in the clinical outcomes of malaria [32,33]. Preferential provision of mosquito net coverage to the younger age group will remain the most beneficial distribution of scarce resources as the burden of malaria-associated deaths and morbidity is likely to remain highest in the very young [34].
In this study male children were at higher risk of malaria, possibly indicating that males were associated with high exposure behaviour, although insecticide-treated mosquito net coverage was not gender-skewed. Gender as a risk factor is likely to be linked to exposure, inherent and cultural factors and has inconsistent associations with malaria risk [35,36]. Due to the nocturnal habit of the anopheline vector, data concerning the time at which children went to bed would have been an interesting variable to examine in relation to the observed age- and gender-related risk patterns. A previous study in Muheza district showed a contrasting, though non-significant, reduced risk of malaria in male children [37]. Other studies in Africa have shown no differences between gender and malaria risk [8,38] and risk fluctuating between sexes across a number of seasons [39] suggesting that distribution of risk is both spatially and temporally heterogeneous.
The protective effect of insecticide-treated mosquito net use shown in this study adds to the vast body of evidence supporting the efficacy and effectiveness of insecticide-treated mosquito nets for protection against malaria and other vector-borne diseases in this setting [26]. In this population approximately one half of the children in the study did not own or use an insecticide-treated mosquito net, increasing their risk of malaria by around 25%. This far from universal coverage is especially poignant when Tanzania is considered to be one of the continent's success stories for mass ITN and LLIN distribution [40,41]. It also gives weight to the importance in understanding the full range of risk factors for malaria in this region. This result provides scope for further reductions in transmission with scaling up of LLIN distribution and the goal of universal coverage [42].
Increased ability to target interventions in the Tanga region, a resource-limited setting, is vital. Identifying high-risk areas for disease allows policy to be tailored to give highest priority for LLIN distribution to those most at threat. An aggregation of cases within clusters may be somewhat driven by an elevated number of higher risk demographic groups residing within houses located in the clusters. Other factors not examined during this study but known to be important could also be rendering these areas as high risk [6,8,38,43,44]. Specifically, the addition of entomological data regarding the vicinity of villages and clusters to anopheline breeding sites would have provided further insight into the biological mechanisms associated with infection risk. Unfortunately there was insufficient budget to complete an enotomological risk factor analysis but it will be important to complete one for verification in future studies. Further information concerning residents' access to the health facilities available would also be useful to allow targeted improvements to treatment availability in the area. Identification of remaining high-risk areas may become more important if current declines in transmission continue, allowing resources to be targeted to areas that remain at high risk or where declines in transmission are less pronounced.
Conclusions
Identifying potential high-risk areas and the mechanisms by which the risk arises can have implications for the whole range of malaria-focussed activities, from increased surveillance to targeted interventions and treatment. In a low-income setting, such as rural Tanzania, any advances in the cost-efficiency and equitability of disease control are crucial.
Malaria epidemiology in the Tanga region has not been static over recent years [10-14]. This study provides evidence that recent declines in malaria transmission and prevalence may be affecting the age profile of malaria prevalence among children in the Muheza district, shifting the peak in risk of malaria infection to older age groups.
Clustering analysis, when combined with knowledge of specific risk factors, will assist targeting of intervention measures to specific high-risk zones which could reduce costs, increase efficacy and improve the equity of control measures for the population in question. The ability to define high-risk areas for targeting of interventions in a newly emerging region of reduced transmission intensity is vital for local elimination of transmission and the rational distribution of control interventions.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
The study was designed by MK, PW and MR. PW, MK, GM and RM collected the data. PW, MK and MR drafted the manuscript. All authors read and approved the final manuscript.
Contributor Information
Peter Winskill, Email: p.winskill@imperial.ac.uk.
Mark Rowland, Email: mark.rowland@lshtm.ac.uk.
George Mtove, Email: mtoveg2002@yahoo.co.uk.
Robert C Malima, Email: r_malima@hotmail.com.
Matthew J Kirby, Email: mattkirby.tanga@gmail.com.
Acknowledgements and Funding
This work was made possible by the cooperation of local communities in the Tanga region. The authors thank field staff from the National Institute of Medical Research (NIMR), Amani Research Centre, Muheza, Tanzania. We would also like to acknowledge the contribution made by clinical and nurse officers and slide readers from Teule hospital, Muheza. Many thanks to Dr. M-G. Basáñez and Dr. P. Lamberton for their support during manuscript preparation. This work forms part of the MSc project of PW at the London School of Hygiene and Tropical Medicine on the Biology and Control of Disease Vectors MSc course.
The study was funded by the LSHTM PRISM project.
References
- Roll Back Malaria - Key Malaria Facts. http://www.rollbackmalaria.org/keyfacts.html Accessed [02/11/10]
- National malaria medium term strategic plan 2002-2007. Dar es Salaam; 2003. Tanzania Ministry of Health. [Google Scholar]
- Hay SI, Snow RW. The malaria Atlas Project: developing global maps of malaria risk. PLoS Med. 2006;3:e473. doi: 10.1371/journal.pmed.0030473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyarango PM, Gebremeskel T, Mebrahtu G, Mufunda J, Abdulmumini U, Ogbamariam A, Kosia A, Gebremichael A, Gunawardena D, Ghebrat Y, Okbaldet Y. A steep decline of malaria morbidity and mortality trends in Eritrea between 2000 and 2004: the effect of combination of control methods. Malar J. 2006;5:33. doi: 10.1186/1475-2875-5-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter R, Mendis KN, Roberts D. Spatial targeting of interventions against malaria. Bull World Health Organ. 2000;78:1401–1411. [PMC free article] [PubMed] [Google Scholar]
- Ernst KC, Adoka SO, Kowuor DO, Wilson ML, John CC. Malaria hotspot areas in a highland Kenya site are consistent in epidemic and non-epidemic years and are associated with ecological factors. Malar J. 2006;5:78. doi: 10.1186/1475-2875-5-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudart J, Poudiougou B, Dicko A, Ranque S, Toure O, Sagara I, Diallo M, Diawara S, Ouattara A, Diakite M, Doumbo OK. Space-time clustering of childhood malaria at the household level: a dynamic cohort in a Mali village. BMC Public Health. 2006;6:286. doi: 10.1186/1471-2458-6-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooker S, Clarke S, Njagi JK, Polack S, Mugo B, Estambale B, Muchiri E, Magnussen P, Cox J. Spatial clustering of malaria and associated risk factors during an epidemic in a highland area of western Kenya. Trop Med Int Health. 2004;9:757–766. doi: 10.1111/j.1365-3156.2004.01272.x. [DOI] [PubMed] [Google Scholar]
- Mirghani SE, Nour BY, Bushra SM, Elhassan IM, Snow RW, Noor AM. The spatial-temporal clustering of Plasmodium falciparum infection over eleven years in Gezira State, The Sudan. Malar J. 2010;9:172. doi: 10.1186/1475-2875-9-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schellenberg D, Menendez C, Aponte J, Guinovart C, Mshinda H, Tanner M, Alonso P. The changing epidemiology of malaria in Ifakara Town, southern Tanzania. Trop Med Int Health. 2004;9:68–76. doi: 10.1046/j.1365-3156.2003.01161.x. [DOI] [PubMed] [Google Scholar]
- Stewart L, Gosling R, Griffin J, Gesase S, Campo J, Hashim R, Masika P, Mosha J, Bousema T, Shekalaghe S, Cook J, Corran P, Ghani A, Riley E, Drakeley C. Rapid assessment of malaria transmission using age-specific sero-conversion rates. PLoS One. 2009;4:e6083. doi: 10.1371/journal.pone.0006083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okiro EA, Hay SI, Gikandi PW, Sharif SK, Noor AM, Peshu N, Marsh K, Snow RW. The decline in paediatric malaria admissions on the coast of Kenya. Malar J. 2007;6:151. doi: 10.1186/1475-2875-6-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Meara WP, Bejon P, Mwangi TW, Okiro EA, Peshu N, Snow RW, Newton CR, Marsh K. Effect of a fall in malaria transmission on morbidity and mortality in Kilifi, Kenya. Lancet. 2008;372:1555–1562. doi: 10.1016/S0140-6736(08)61655-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mmbando BP, Vestergaard LS, Kitua AY, Lemnge MM, Theander TG, Lusingu JP. A progressive declining in the burden of malaria in north-eastern Tanzania. Malar J. 2010;9:216. doi: 10.1186/1475-2875-9-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzania NBOS: statistics for development. http://www.nbs.go.tz/index.php?option=com_content&view=article&id=103&Itemid=114 Accessed [11/11/10]
- Bodker R, Akida J, Shayo D, Kisinza W, Msangeni HA, Pedersen EM, Lindsay SW. Relationship between altitude and intensity of malaria transmission in the Usambara Mountains, Tanzania. J Med Entomol. 2003;40:706–717. doi: 10.1603/0022-2585-40.5.706. [DOI] [PubMed] [Google Scholar]
- Lusingu JP, Vestergaard LS, Mmbando BP, Drakeley CJ, Jones C, Akida J, Savaeli ZX, Kitua AY, Lemnge MM, Theander TG. Malaria morbidity and immunity among residents of villages with different Plasmodium falciparum transmission intensity in North-Eastern Tanzania. Malar J. 2004;3:26. doi: 10.1186/1475-2875-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alilio MS, Kitua A, Njunwa K, Medina M, Ronn AM, Mhina J, Msuya F, Mahundi J, Depinay JM, Whyte S, Krasnik A, Bygbjerg C. Malaria control at the district level in Africa: the case of the Muheza district in northeastern Tanzania. Am J Trop Med Hyg. 2004;71:205–213. [PubMed] [Google Scholar]
- Ronn AM, Msangeni HA, Mhina J, Wernsdorfer WH, Bygbjerg IC. High level of resistance of Plasmodium falciparum to sulfadoxine-pyrimethamine in children in Tanzania. Trans R Soc Trop Med Hyg. 1996;90:179–181. doi: 10.1016/S0035-9203(96)90129-7. [DOI] [PubMed] [Google Scholar]
- Kulldorff M. A spatial scan statistic. Communications in Statistics-Theory and Methods. 1997;26:1481–1496. doi: 10.1080/03610929708831995. [DOI] [Google Scholar]
- Kulldorff M, Nagarwalla N. Spatial Disease Clusters - Detection and Inference. Statistics in Medicine. 1995;14:799–810. doi: 10.1002/sim.4780140809. [DOI] [PubMed] [Google Scholar]
- Geonetwork. http://www.fao.org/geonetwork/srv/en/main.home Accessed [15/08/10]
- Hurt N, Smith T, Teuscher T, Tanner M. Do high levels of C-reactive protein in Tanzanian children indicate malaria morbidity. Clin Diagn Lab Immunol. 1994;1:437–444. doi: 10.1128/cdli.1.4.437-444.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T, Beck HP, Kitua A, Mwankusye S, Felger I, Fraser-Hurt N, Irion A, Alonso P, Teuscher T, Tanner M. Age dependence of the multiplicity of Plasmodium falciparum infections and of other malariological indices in an area of high endemicity. Trans R Soc Trop Med Hyg. 1999;93:15–20. doi: 10.1016/s0035-9203(99)90322-x. [DOI] [PubMed] [Google Scholar]
- Smith T, Charlwood JD, Kihonda J, Mwankusye S, Billingsley P, Meuwissen J, Lyimo E, Takken W, Teuscher T, Tanner M. Absence of seasonal variation in malaria parasitaemia in an area of intense seasonal transmission. Acta Trop. 1993;54:55–72. doi: 10.1016/0001-706X(93)90068-M. [DOI] [PubMed] [Google Scholar]
- Lengeler C. Insecticide-treated bed nets and curtains for preventing malaria. Cochrane Database Syst Rev. 2004. p. CD000363. [DOI] [PubMed]
- World Health Organisation - World Malaria Report 2009. http://whqlibdoc.who.int/publications/2009/9789241563901_eng.PDF Accessed [01/11/10]
- Smith T, Hii JL, Genton B, Muller I, Booth M, Gibson N, Narara A, Alpers MP. Associations of peak shifts in age-prevalence for human malarias with bednet coverage. Trans R Soc Trop Med Hyg. 2001;95:1–6. doi: 10.1016/S0035-9203(01)90314-1. [DOI] [PubMed] [Google Scholar]
- Egger JR, Ooi EE, Kelly DW, Woolhouse ME, Davies CR, Coleman PG. Reconstructing historical changes in the force of infection of dengue fever in Singapore: implications for surveillance and control. Bull World Health Organ. 2008;86:187–196. doi: 10.2471/BLT.07.040170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier JC, Killeen GF, Githure JI. Short report: entomologic inoculation rates and Plasmodium falciparum malaria prevalence in Africa. Am J Trop Med Hyg. 1999;61:109–113. doi: 10.4269/ajtmh.1999.61.109. [DOI] [PubMed] [Google Scholar]
- Griffin JT, Hollingsworth TD, Okell LC, Churcher TS, White M, Hinsley W, Bousema T, Drakeley CJ, Ferguson NM, Basanez MG, Ghani AC. Reducing Plasmodium falciparum malaria transmission in Africa: a model-based evaluation of intervention strategies. PLoS Med. 2010;7:e1000324. doi: 10.1371/journal.pmed.1000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow RW, Marsh K. The consequences of reducing transmission of Plasmodium falciparum in Africa. Adv Parasitol. 2002;52:235–264. doi: 10.1016/s0065-308x(02)52013-3. [DOI] [PubMed] [Google Scholar]
- Snow RW, Omumbo JA, Lowe B, Molyneux CS, Obiero JO, Palmer A, Weber MW, Pinder M, Nahlen B, Obonyo C, Newbold C, Gupta S, Marsh K. Relation between severe malaria morbidity in children and level of Plasmodium falciparum transmission in Africa. Lancet. 1997;349:1650–1654. doi: 10.1016/S0140-6736(97)02038-2. [DOI] [PubMed] [Google Scholar]
- Carneiro I, Roca-Feltrer A, Griffin JT, Smith L, Tanner M, Schellenberg JA, Greenwood B, Schellenberg D. Age-patterns of malaria vary with severity, transmission intensity and seasonality in sub-Saharan Africa: a systematic review and pooled analysis. PLoS One. 2010;5:e8988. doi: 10.1371/journal.pone.0008988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates I, Fenton C, Gruber J, Lalloo D, Medina A Lara, Squire SB, Theobald S, Thomson R, Tolhurst R. Vulnerability to malaria, tuberculosis, and HIV/AIDS infection and disease. Part 1: determinants operating at individual and household level. Lancet Infect Dis. 2004;4:267–277. doi: 10.1016/S1473-3099(04)01002-3. [DOI] [PubMed] [Google Scholar]
- Tanner M, Vlassoff C. Treatment-seeking behaviour for malaria: a typology based on endemicity and gender. Soc Sci Med. 1998;46:523–532. doi: 10.1016/S0277-9536(97)00195-0. [DOI] [PubMed] [Google Scholar]
- Bernard J, Mtove G, Mandike R, Mtei F, Maxwell C, Reyburn H. Equity and coverage of insecticide-treated bed nets in an area of intense transmission of Plasmodium falciparum in Tanzania. Malar J. 2009;8:65. doi: 10.1186/1475-2875-8-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghebreyesus TA, Haile M, Witten KH, Getachew A, Yohannes M, Lindsay SW, Byass P. Household risk factors for malaria among children in the Ethiopian highlands. Trans R Soc Trop Med Hyg. 2000;94:17–21. doi: 10.1016/S0035-9203(00)90424-3. [DOI] [PubMed] [Google Scholar]
- Giha HA, Rosthoj S, Dodoo D, Hviid L, Satti GM, Scheike T, Arnot DE, Theander TG. The epidemiology of febrile malaria episodes in an area of unstable and seasonal transmission. Trans R Soc Trop Med Hyg. 2000;94:645–651. doi: 10.1016/S0035-9203(00)90218-9. [DOI] [PubMed] [Google Scholar]
- Hanson K, Marchant T, Nathan R, Mponda H, Jones C, Bruce J, Mshinda H, Schellenberg JA. Household ownership and use of insecticide treated nets among target groups after implementation of a national voucher programme in the United Republic of Tanzania: plausibility study using three annual cross sectional household surveys. BMJ. 2009;339:b2434. doi: 10.1136/bmj.b2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magesa SM, Lengeler C, deSavigny D, Miller JE, Njau RJ, Kramer K, Kitua A, Mwita A. Creating an "enabling environment" for taking insecticide treated nets to national scale: the Tanzanian experience. Malar J. 2005;4:34. doi: 10.1186/1475-2875-4-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roll Back Malaria Partnership: Global Malaria Action Plan. http://www.rollbackmalaria.org/gmap/2-2a.html Accessed [05/11/10]
- Gunawardena DM, Wickremasinghe AR, Muthuwatta L, Weerasingha S, Rajakaruna J, Senanayaka T, Kotta PK, Attanayake N, Carter R, Mendis KN. Malaria risk factors in an endemic region of Sri Lanka, and the impact and cost implications of risk factor-based interventions. Am J Trop Med Hyg. 1998;58:533–542. doi: 10.4269/ajtmh.1998.58.533. [DOI] [PubMed] [Google Scholar]
- Staedke SG, Nottingham EW, Cox J, Kamya MR, Rosenthal PJ, Dorsey G. Short report: proximity to mosquito breeding sites as a risk factor for clinical malaria episodes in an urban cohort of Ugandan children. Am J Trop Med Hyg. 2003;69:244–246. [PubMed] [Google Scholar]