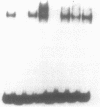
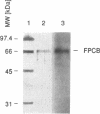
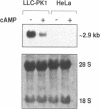
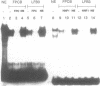
Abstract
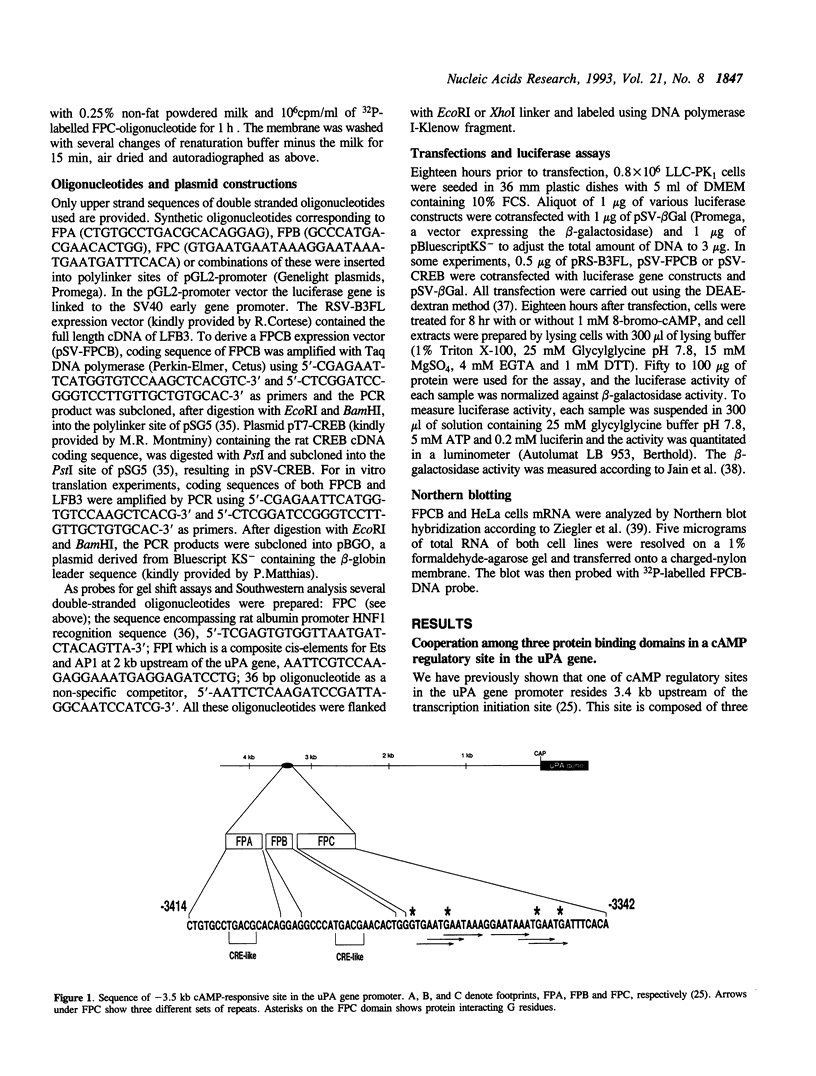
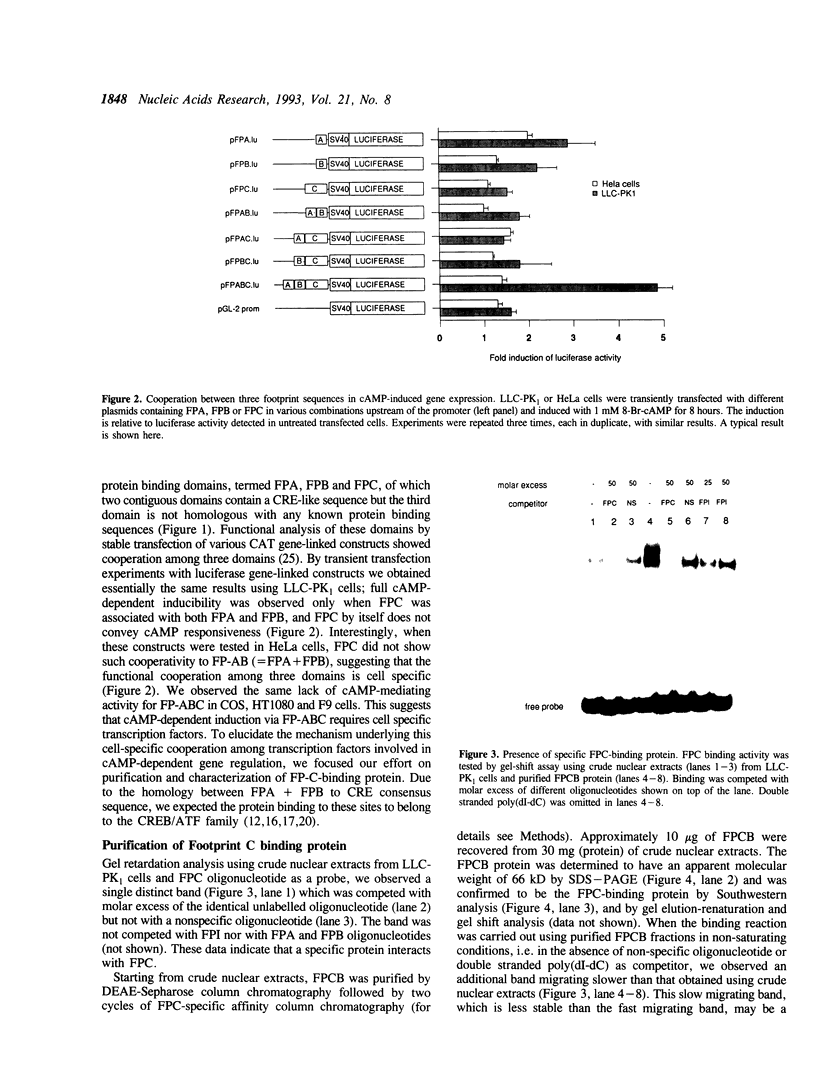
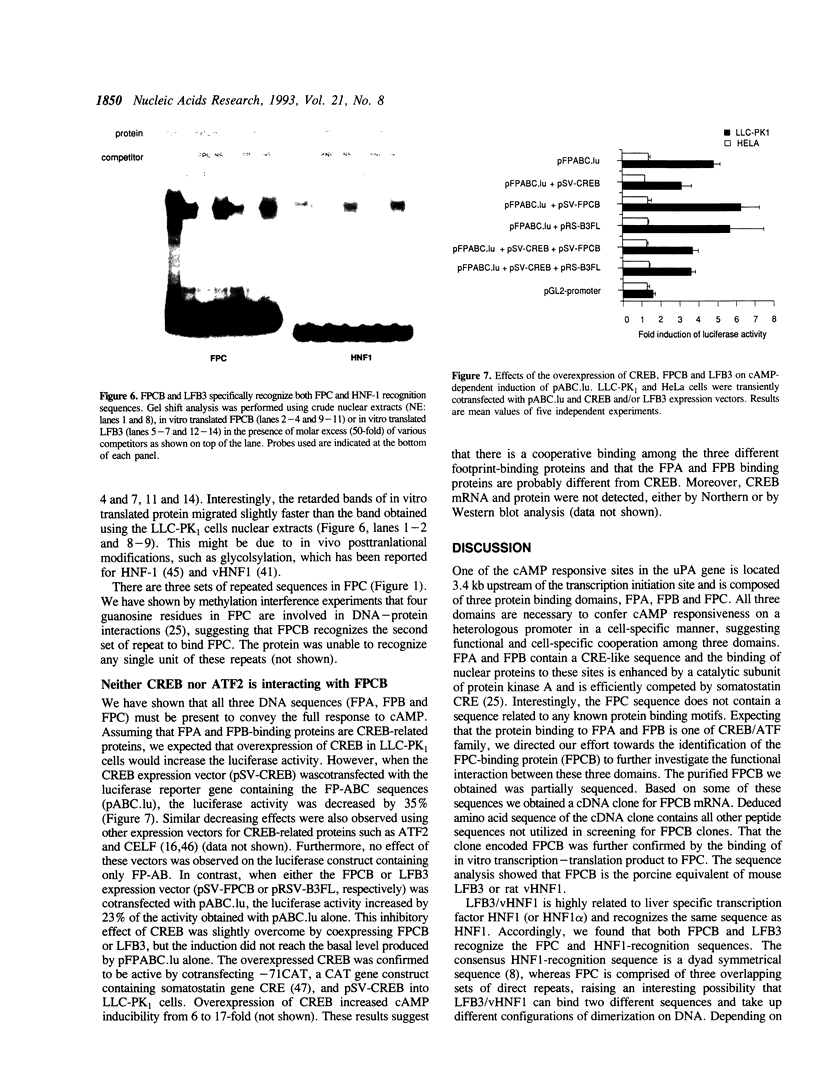
One of cAMP-regulatory sites in the porcine urokinase-type plasminogen activator (uPA) gene resides 3.4 kb upstream of the transcription initiation site and is composed of three protein binding domains, FPA, FPB and FPC. Whereas FPA and FPB contain a CRE-like sequence, the FPC sequence is not related to any known protein recognition sequences, yet all three domains are required to mediate cAMP action on a heterologous promoter. To study the functional cooperation among these three domains we purified and cloned a FPC-binding protein (FPCB) from porcine kidney derived LLC-PK1 cells. Sequence comparisons showed that FPCB is homologous to mouse LFB3 and rat vHNF1. LFB3/vHNF1 is related to a liver specific transcription factor HNF1, it recognizes the same sequence as HNF1 and is highly expressed in kidney cells. FPCB and HNF1 recognition sequences are dissimilar, nevertheless both sequences are recognized by in vitro-translated LFB3 and FPCB, indicating that binding to the two different sequences is an intrinsic character of FPCB/LFB3/vHNF1. In HeLa cells, this cAMP-responsive site was inactive whether FPCB was overexpressed or not, suggesting a requirement for an additional cell-specific factor. These results may suggest a mechanism by which hormonal control is integrated into cell-specific gene regulation.
Full text
PDF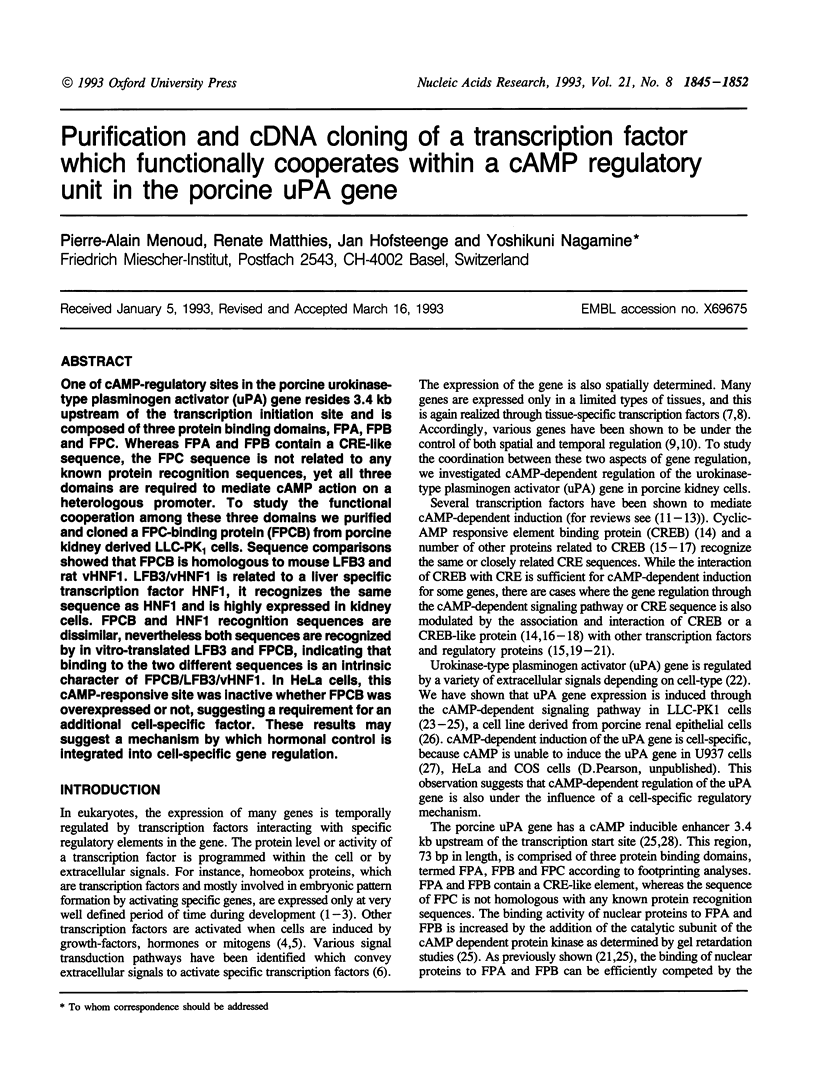




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnstiel M. L., Busslinger M., Strub K. Transcription termination and 3' processing: the end is in site! Cell. 1985 Jun;41(2):349–359. doi: 10.1016/s0092-8674(85)80007-6. [DOI] [PubMed] [Google Scholar]
- Cassady A. I., Stacey K. J., Nimmo K. A., Murphy K. M., von der Ahe D., Pearson D., Botteri F. M., Nagamine Y., Hume D. A. Constitutive expression of the urokinase plasminogen activator gene in murine RAW264 macrophages involves distal and 5' non-coding sequences that are conserved between mouse and pig. Nucleic Acids Res. 1991 Dec 25;19(24):6839–6847. doi: 10.1093/nar/19.24.6839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouard T., Blumenfeld M., Bach I., Vandekerckhove J., Cereghini S., Yaniv M. A distal dimerization domain is essential for DNA-binding by the atypical HNF1 homeodomain. Nucleic Acids Res. 1990 Oct 11;18(19):5853–5863. doi: 10.1093/nar/18.19.5853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danø K., Andreasen P. A., Grøndahl-Hansen J., Kristensen P., Nielsen L. S., Skriver L. Plasminogen activators, tissue degradation, and cancer. Adv Cancer Res. 1985;44:139–266. doi: 10.1016/s0065-230x(08)60028-7. [DOI] [PubMed] [Google Scholar]
- De Simone V., Cortese R. Transcriptional regulation of liver-specific gene expression. Curr Opin Cell Biol. 1991 Dec;3(6):960–965. doi: 10.1016/0955-0674(91)90114-e. [DOI] [PubMed] [Google Scholar]
- De Simone V., De Magistris L., Lazzaro D., Gerstner J., Monaci P., Nicosia A., Cortese R. LFB3, a heterodimer-forming homeoprotein of the LFB1 family, is expressed in specialized epithelia. EMBO J. 1991 Jun;10(6):1435–1443. doi: 10.1002/j.1460-2075.1991.tb07664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degen J. L., Estensen R. D., Nagamine Y., Reich E. Induction and desensitization of plasminogen activator gene expression by tumor promoters. J Biol Chem. 1985 Oct 15;260(23):12426–12433. [PubMed] [Google Scholar]
- Eisenberg S., Francesconi S. C., Civalier C., Walker S. S. Purification of DNA-binding proteins by site-specific DNA affinity chromatography. Methods Enzymol. 1990;182:521–529. doi: 10.1016/0076-6879(90)82041-y. [DOI] [PubMed] [Google Scholar]
- Foulkes N. S., Borrelli E., Sassone-Corsi P. CREM gene: use of alternative DNA-binding domains generates multiple antagonists of cAMP-induced transcription. Cell. 1991 Feb 22;64(4):739–749. doi: 10.1016/0092-8674(91)90503-q. [DOI] [PubMed] [Google Scholar]
- Gaunt S. J., Krumlauf R., Duboule D. Mouse homeo-genes within a subfamily, Hox-1.4, -2.6 and -5.1, display similar anteroposterior domains of expression in the embryo, but show stage- and tissue-dependent differences in their regulation. Development. 1989 Sep;107(1):131–141. doi: 10.1242/dev.107.1.131. [DOI] [PubMed] [Google Scholar]
- Green S., Issemann I., Sheer E. A versatile in vivo and in vitro eukaryotic expression vector for protein engineering. Nucleic Acids Res. 1988 Jan 11;16(1):369–369. doi: 10.1093/nar/16.1.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hai T. W., Liu F., Coukos W. J., Green M. R. Transcription factor ATF cDNA clones: an extensive family of leucine zipper proteins able to selectively form DNA-binding heterodimers. Genes Dev. 1989 Dec;3(12B):2083–2090. doi: 10.1101/gad.3.12b.2083. [DOI] [PubMed] [Google Scholar]
- Heintz N. H. Transcription factors and the control of DNA replication. Curr Opin Cell Biol. 1992 Jun;4(3):459–467. doi: 10.1016/0955-0674(92)90012-2. [DOI] [PubMed] [Google Scholar]
- Holland P. W., Hogan B. L. Expression of homeo box genes during mouse development: a review. Genes Dev. 1988 Jul;2(7):773–782. doi: 10.1101/gad.2.7.773. [DOI] [PubMed] [Google Scholar]
- Hull R. N., Cherry W. R., Weaver G. W. The origin and characteristics of a pig kidney cell strain, LLC-PK. In Vitro. 1976 Oct;12(10):670–677. doi: 10.1007/BF02797469. [DOI] [PubMed] [Google Scholar]
- Izpisúa-Belmonte J. C., Falkenstein H., Dollé P., Renucci A., Duboule D. Murine genes related to the Drosophila AbdB homeotic genes are sequentially expressed during development of the posterior part of the body. EMBO J. 1991 Aug;10(8):2279–2289. doi: 10.1002/j.1460-2075.1991.tb07764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain V. K., Magrath I. T. A chemiluminescent assay for quantitation of beta-galactosidase in the femtogram range: application to quantitation of beta-galactosidase in lacZ-transfected cells. Anal Biochem. 1991 Nov 15;199(1):119–124. doi: 10.1016/0003-2697(91)90278-2. [DOI] [PubMed] [Google Scholar]
- Kageyama R., Sasai Y., Nakanishi S. Molecular characterization of transcription factors that bind to the cAMP responsive region of the substance P precursor gene. cDNA cloning of a novel C/EBP-related factor. J Biol Chem. 1991 Aug 15;266(23):15525–15531. [PubMed] [Google Scholar]
- Karin M., Smeal T. Control of transcription factors by signal transduction pathways: the beginning of the end. Trends Biochem Sci. 1992 Oct;17(10):418–422. doi: 10.1016/0968-0004(92)90012-x. [DOI] [PubMed] [Google Scholar]
- Kerr L. D., Inoue J., Verma I. M. Signal transduction: the nuclear target. Curr Opin Cell Biol. 1992 Jun;4(3):496–501. doi: 10.1016/0955-0674(92)90017-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lazzaro D., De Simone V., De Magistris L., Lehtonen E., Cortese R. LFB1 and LFB3 homeoproteins are sequentially expressed during kidney development. Development. 1992 Feb;114(2):469–479. doi: 10.1242/dev.114.2.469. [DOI] [PubMed] [Google Scholar]
- Leonard J., Serup P., Gonzalez G., Edlund T., Montminy M. The LIM family transcription factor Isl-1 requires cAMP response element binding protein to promote somatostatin expression in pancreatic islet cells. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6247–6251. doi: 10.1073/pnas.89.14.6247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtsteiner S., Schibler U. A glycosylated liver-specific transcription factor stimulates transcription of the albumin gene. Cell. 1989 Jun 30;57(7):1179–1187. doi: 10.1016/0092-8674(89)90055-x. [DOI] [PubMed] [Google Scholar]
- Lu G. H., Schlichter D., Wicks W. D. Interaction of a nuclear factor 1-like protein with a cAMP response element-binding protein in rat liver. Int J Biochem. 1992 Mar;24(3):455–464. doi: 10.1016/0020-711x(92)90039-4. [DOI] [PubMed] [Google Scholar]
- Maguire H. F., Hoeffler J. P., Siddiqui A. HBV X protein alters the DNA binding specificity of CREB and ATF-2 by protein-protein interactions. Science. 1991 May 10;252(5007):842–844. doi: 10.1126/science.1827531. [DOI] [PubMed] [Google Scholar]
- Mayer R. E., Hendrix P., Cron P., Matthies R., Stone S. R., Goris J., Merlevede W., Hofsteenge J., Hemmings B. A. Structure of the 55-kDa regulatory subunit of protein phosphatase 2A: evidence for a neuronal-specific isoform. Biochemistry. 1991 Apr 16;30(15):3589–3597. doi: 10.1021/bi00229a001. [DOI] [PubMed] [Google Scholar]
- McGinnis W., Krumlauf R. Homeobox genes and axial patterning. Cell. 1992 Jan 24;68(2):283–302. doi: 10.1016/0092-8674(92)90471-n. [DOI] [PubMed] [Google Scholar]
- Medcalf R. L. Cell- and gene-specific interactions between signal transduction pathways revealed by okadaic acid. Studies on the plasminogen activating system. J Biol Chem. 1992 Jun 15;267(17):12220–12226. [PubMed] [Google Scholar]
- Mendel D. B., Hansen L. P., Graves M. K., Conley P. B., Crabtree G. R. HNF-1 alpha and HNF-1 beta (vHNF-1) share dimerization and homeo domains, but not activation domains, and form heterodimers in vitro. Genes Dev. 1991 Jun;5(6):1042–1056. doi: 10.1101/gad.5.6.1042. [DOI] [PubMed] [Google Scholar]
- Mendel D. B., Khavari P. A., Conley P. B., Graves M. K., Hansen L. P., Admon A., Crabtree G. R. Characterization of a cofactor that regulates dimerization of a mammalian homeodomain protein. Science. 1991 Dec 20;254(5039):1762–1767. doi: 10.1126/science.1763325. [DOI] [PubMed] [Google Scholar]
- Montminy M. R., Bilezikjian L. M. Binding of a nuclear protein to the cyclic-AMP response element of the somatostatin gene. Nature. 1987 Jul 9;328(6126):175–178. doi: 10.1038/328175a0. [DOI] [PubMed] [Google Scholar]
- Montminy M. R., Sevarino K. A., Wagner J. A., Mandel G., Goodman R. H. Identification of a cyclic-AMP-responsive element within the rat somatostatin gene. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6682–6686. doi: 10.1073/pnas.83.18.6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto R. I. Transcription factors: positive and negative regulators of cell growth and disease. Curr Opin Cell Biol. 1992 Jun;4(3):480–487. doi: 10.1016/0955-0674(92)90015-5. [DOI] [PubMed] [Google Scholar]
- Nagamine Y., Sudol M., Reich E. Hormonal regulation of plasminogen activator mRNA production in porcine kidney cells. Cell. 1983 Apr;32(4):1181–1190. doi: 10.1016/0092-8674(83)90301-x. [DOI] [PubMed] [Google Scholar]
- Nichols M., Weih F., Schmid W., DeVack C., Kowenz-Leutz E., Luckow B., Boshart M., Schütz G. Phosphorylation of CREB affects its binding to high and low affinity sites: implications for cAMP induced gene transcription. EMBO J. 1992 Sep;11(9):3337–3346. doi: 10.1002/j.1460-2075.1992.tb05412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehfuss R. P., Walton K. M., Loriaux M. M., Goodman R. H. The cAMP-regulated enhancer-binding protein ATF-1 activates transcription in response to cAMP-dependent protein kinase A. J Biol Chem. 1991 Oct 5;266(28):18431–18434. [PubMed] [Google Scholar]
- Rey-Campos J., Chouard T., Yaniv M., Cereghini S. vHNF1 is a homeoprotein that activates transcription and forms heterodimers with HNF1. EMBO J. 1991 Jun;10(6):1445–1457. doi: 10.1002/j.1460-2075.1991.tb07665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesler W. J., Vandenbark G. R., Hanson R. W. Cyclic AMP and the induction of eukaryotic gene transcription. J Biol Chem. 1988 Jul 5;263(19):9063–9066. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber E., Matthias P., Müller M. M., Schaffner W. Rapid detection of octamer binding proteins with 'mini-extracts', prepared from a small number of cells. Nucleic Acids Res. 1989 Aug 11;17(15):6419–6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronche F., Yaniv M. HNF1, a homeoprotein member of the hepatic transcription regulatory network. Bioessays. 1992 Sep;14(9):579–587. doi: 10.1002/bies.950140902. [DOI] [PubMed] [Google Scholar]
- Wahle E. The end of the message: 3'-end processing leading to polyadenylated messenger RNA. Bioessays. 1992 Feb;14(2):113–118. doi: 10.1002/bies.950140208. [DOI] [PubMed] [Google Scholar]
- Ziegler A., Hagmann J., Kiefer B., Nagamine Y. Ca2+ potentiates cAMP-dependent expression of urokinase-type plasminogen activator gene through a calmodulin- and protein kinase C-independent mechanism. J Biol Chem. 1990 Dec 5;265(34):21194–21201. [PubMed] [Google Scholar]
- Ziff E. B. Transcription factors: a new family gathers at the cAMP response site. Trends Genet. 1990 Mar;6(3):69–72. doi: 10.1016/0168-9525(90)90081-g. [DOI] [PubMed] [Google Scholar]
- von der Ahe D., Pearson D., Nagamine Y. Macromolecular interaction on a cAMP responsive region in the urokinase-type plasminogen activator gene: a role of protein phosphorylation. Nucleic Acids Res. 1990 Apr 25;18(8):1991–1999. doi: 10.1093/nar/18.8.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]