Abstract
Voltage-gated sodium channels (VGSCs) play an important role in the control of membrane excitability. We previously reported that the excitability of nociceptor was increased by hypotonic stimulation. The present study tested the effect of hypotonicity on tetrodotoxin-sensitive sodium current (TTX-S current) in cultured trigeminal ganglion (TG) neurons. Our data show that after hypotonic treatment, TTX-S current was increased. In the presence of hypotonicity, voltage-dependent activation curve shifted to the hyperpolarizing direction, while the voltage-dependent inactivation curve was not affected. Transient Receptor Potential Vanilloid 4 receptor (TRPV4) activator increased TTX-S current and hypotonicity-induced increase was markedly attenuated by TRPV4 receptor blockers. We also demonstrate that inhibition of PKC attenuated hypotonicity-induced inhibition, whereas PKA system was not involved in hypotonic-response. We conclude that hypotonic stimulation enhances TTX-S current, which contributes to hypotonicity-induced nociception. TRPV4 receptor and PKC intracellular pathway are involved in the increase of TTX-S current by hypotonicity.
Keywords: hypotonicity, tetrodotoxin-sensitive sodium current, TRPV4 receptor, intracellular signaling pathway, nociception
Findings
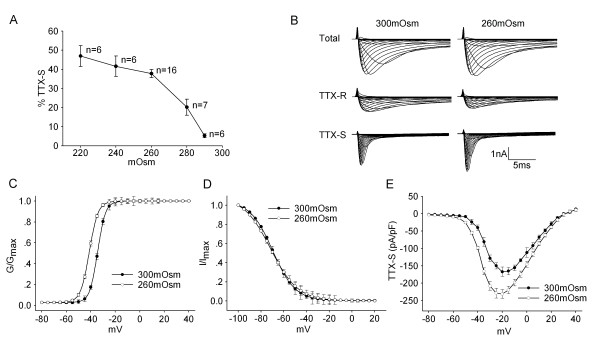
Osmotic balance is of great significance for maintaining the internal environment homeostasis. Many pathological processes, in accompany with the changes in osmolality (such as the facial or intraoral edema which is not contained within a rigid physical restraint), are painful. Both in vitro and in vivo experiments have proved that hypotonic stimuli can induce nociception or pain-related behavior [1,2]. We previously reported that hypotonic stimulation caused an increase of action potential (AP) generation in small to medium-sized trigeminal ganglion (TG) neurons that are likely to be nociceptive in nature, resulting in the hyperexcitability of nociceptors [3]. Voltage-gated sodium channels (VGSCs), providing an inward current that underlies the upswing of an AP, contribute to the control of membrane excitability and underlie AP generation [4]. In nociceptors, VGSCs are pharmacologically separated into tetrodotoxin-sensitive (TTX-S) and tetrodotoxin-resistant (TTX-R) channels [5]. Our recent study found that TTX-R current was decreased by hypotonic stimulation [6] and this result seemingly can not explain hypotonicity-induced hyperexcitability of TG neurons. However, the modulation of VGSCs varies between laboratories and models. The selective up-regulation of TTX-S channel is detected in the pain caused by nerve injury or in inflammatory pain [7,8]. This phenomenon indicates that TTX-S channel may also play an important role in the pain sensation. Therefore, we tested the effect of hypotonic stimulation on TTX-S current in cultured small- to medium-sized TG neurons which have characteristics of nociceptors. Voltage-gated sodium current was measured first in the absence of TTX to get the total sodium current and then in the presence of TTX to obtain the TTX-R current. TTX-S current was obtained by subtracting the latter from the former. We found that TTX-S current was increased by 37.74 ± 2.12% from -160.89 ± 14.11 pA/pF to -220.18 ± 18.57 pA/pF (n = 16, paired t-test, P < 0.05) when the external solution was changed from isotonicity (300mOsm) to hypotonicity (260mOsm) (Figure 1A and 1B). Hypotonicity-induced increase was largely reversible and TTX-S current recovered to -163.75 ± 12.13 pA/pF after hypotonicity was washed out for 3 min. We also found that the voltage-dependent activation curve (G-V curve) markedly shifted to the hyperpolarizing direction in the presence of hypotonic stimulation (paired t-test, P < 0.05) (Figure 1D). Unlike the activation function, the voltage-dependent inactivation curve (inactivation-voltage curve) did not markedly shift before and during hypotonic treatment (paired t-test, P > 0.05) (Figure 1E).
Figure 1.
Effect of hypotonicity on TTX-S current in TG neurons. A. Plot of current densities as function of hypotonic stimuli shows that the increase of TTX-S current was conspicuous at the largest osmotic gradient. B. The typical recordings show that TTX-S current was increased by hypotonicity. Upper: total sodium current, middle: TTX-R current obtained during application of 300nM TTX, below: TTX-S current obtained by subtracting TTX-R current from total. C. Comparison of voltage-current relationship (I-V curve) for TTX-S current in isotonic and hypotonic solution. D. TTX-S current was converted to a conductance and fitted to a Boltzman function. The mid-point of activation (V0.5) was significantly more negative in hypotonic than in isotonic solution (-41.17 ± 1.09 mV vs. -34.41 ± 2.16 mV, n = 9, pared t-test, P < 0.05). However, the slope factor (k) was not significantly different between isotonic and hypotonic solution (3.54 ± 0.23 vs. 3.29 ± 0.89, n = 9, pared t-test, P > 0.05). E. Unlike G-V curve, the inactivation-voltage curve did not shift before and during hypotonic treatment. V0.5 were -69.66 ± 2.17 mV and -71.55 ± 3.21 mV (n = 10, paired t-test, P > 0.05), k were -9.86 ± 1.81 and -11.25 ± 1.03 (n = 10, paired t-test, P > 0.05) for 300mOsm and 260mOsm respectively.
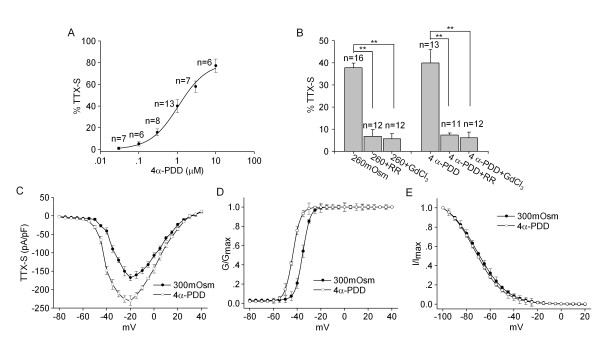
Transient receptor potential vanilloid subtype 4 (TRPV4) is a member of TRP super family which can be activated by multiple stimuli including hypotonicity [9-11]. As an important osmotic cellular sensor which is present in nociceptors, TRPV4 is now receiving accumulating attention concerning nociception [12]. Recent studies support an involvement of TRPV4 in anisotonicity-induced nociception [13,14]. Consistently, our recent study demonstrated that TRPV4 mediated the increase of APs number by hypotonic stimulation in TG neurons [3]. To test whether TRPV4 receptors may be involved in hypotonicity-induced modulation of TTX-S current, the agonist of TRPV4 receptor 4α-PDD was firstly used. After exposed to 4α-PDD (1 μM) for 3 min, TTX-S current was reversibly increased by 39.93 ± 6.04% from -167.46 ± 9.09 pA/pF to -230.10 ± 12.61 pA/pF (n = 13, paired t-test, P < 0.01). The increase was recoverable after 4α-PDD was washed out. 4α-PDD caused a hyperpolarizing shift in G-V curve (paired t-test, P < 0.05) (Figure 2D), but had no effect on inactivation-voltage curve (paired t-test, P > 0.05) (Figure 2E). The concentration-dependent increase of TTX-S current by 4α-PDD is shown in Figure 2A. The dose-response curve was fitted by Hill equation with EC50 being 1.07 μM.
Figure 2.
Involvement of TRPV4 receptor in hypotonicity-induced increase of TTX-S current. A. The plot shows the increase of TTX-S current by TRPV4 receptor agonist 4α-PDD at concentrations of 0.03-30 μM. The dose-response curve fits to Hill equation with EC50 being 1.07 μM and n being 1.08. B. The increase of TTX-S current by hypotonicity was markedly attenuated by TRPV4 receptor antagonists RR and GdCl3. Additionally, 4α-PDD-induced response was also significantly blocked by RR and GdCl3. In the presence of RR or GdCl3, the increase of TTX-S current by 4α-PDD (1 μM) was attenuated from 39.93 ± 6.04% to 7.36 ± 0.91% (unpaired t-test, P < 0.01) and to 6.11 ± 2.57% (unpaired t-test, P < 0.01), respectively. C. I-V curve shows the voltage-current relationship of TTX-S current before and during 4α-PDD (1 μM) treatment. D. In the presence of 4α-PDD (1 μM), G-V curve shifted to the hyperpolarizing direction, with V0.5 being -35.33 ± 1.32 mV and -43.67 ± 3.72 mV (n = 8, paired t-test, P < 0.05), k being 3.31 ± 0.98 and 3.11 ± 0.68 (n = 8, paired t-test, P > 0.05) before and during 4α-PDD treatment, respectively. E. There was no significant difference in inactivation-voltage curve before and during 4α-PDD treatment. V0.5 were -71.19 ± 4.15 mV and -72.72 ± 3.94 mV (n = 9, paired t-test, P > 0.05), k were -10.01 ± 1.05 and -10.94 ± 1.41 (n = 9, paired t-test, P > 0.05) for control and 4α-PDD group, respectively.
To further determine whether TRPV4 receptor was involved in the effects of hypotonicity, TRPV4 receptor blockers ruthenium red (RR) and GdCl3 were used to determine how they would affect the increase of TTX-S current under hypotonic condition. In isotonic condition, after exposure to 10 μM RR or 100 μM GdCl3 for 3 min, TTX-S current was reduced from -166.07 ± 10.05 pA/pF to -135.77 ± 12.03 pA/pF (n = 8, paired t-test, P < 0.05) and from -165.89 ± 7.41 pA/pF to -140.33 ± 6.98 pA/pF (n = 9, paired t-test, P < 0.05), respectively. Upon pre-incubation with RR or GdCl3, the increase of TTX-S current by hypotonicity was reduced from 37.74 ± 2.12% to 6.82 ± 3.00% (unpaired t-test, P < 0.01) and to 5.67 ± 2.33% (unpaired t-test, P < 0.01), respectively. Here, it was noted that after pre-application of RR or GdCl3 for 3 min, the increase of TTX-S current by 4α-PDD was also significantly blocked (unpaired t-test, P < 0.01 in each case) (Figure 2B). Taken together, these data suggested that the increase of TTX-S current by hypotonic stimulation might be mediated through TRPV4 receptor.
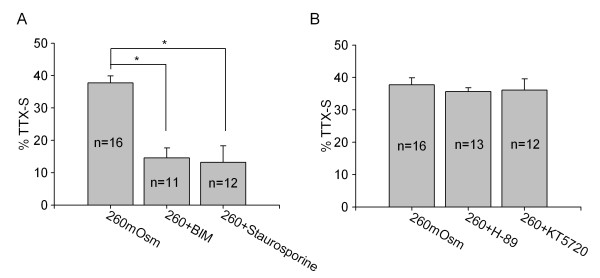
We then tested some intracellular signaling pathways, including PKA and PKC system, to determine whether they were involved in hypotonicity-induced increase of TTX-S current. These two signaling pathways were chosen because they have been proved to be the important intracellular signal pathways modulating VGSCs [4]. We firstly compared the effect of each pathway on TTX-S current in isotonic solution. Agonist of PKA system 8-Br-cAMP (1 mM), and of PKC system phorbol-12, 13-dibutyrate (PMA, 1 μM) inhibited TTX-S current (P < 0.05 in each case). Consistently, antagonists of PKA pathway H-89 (10 μM) and KT5720 (1 μM), and of PKC pathway Bisindolylmaleimide II (BIM, 1 μM) and staurosporine (1 μM) enhanced TTX-S current (P < 0.05 in each case) (see additional file 1). Here we found that pre-treatment with PKC antagonists BIM or staurosporine markedly attenuated hypotonicity-induced increase of TTX-S current from 37.74 ± 2.12% to 14.55 ± 3.09% and to 13.17 ± 5.10% (unpaired t-test, P < 0.05 in each case), respectively. However, in the presence of H-89 or KT5720, TTX-S current was increased 35.63 ± 1.17% and 36.09 ± 3.47% by hypotonicity, respectively (unpaired t-test, P > 0.05 in each case) (Figure 3).
Figure 3.
Role of PKA and PKC system in hypotonicity-induced increase of TTX-S current. A. In the presence of PKC antagonist BIM or staurosporine, hypotonicity-induced increase of TTX-S current was markedly blocked from 37.74 ± 2.12% to 14.55 ± 3.09% (unpaired t-test, P < 0.05) and to 13.17 ± 5.10% (unpaired t-test, P < 0.05), respectively. B. Hypotonicity-induced response was not affected by PKA antagonists and TTX-S current was increased 35.63 ± 1.17% (unpaired t-test, P > 0.05) and 36.09 ± 3.47% (unpaired t-test, P > 0.05) by hypotonicity in the presence of H-89 or KT5720, respectively.
Nociceptors are primary afferent neurons that response to noxious stimulus and transmit the information to the central nervous system to produce pain. As VGSCs have an essential role in the biophysical properties of nociceptors, changes in both channel function and expression can lead to electrical instability of neurons, which is observed in many kinds of pain condition. Two sodium channels, a fast-inactivating TTX-S channel and a slow-inactivating TTX-R channel, predominate in small nociceptive sensory neurons. TTX-S channels are necessary for the transduction in many Aδ-fibers, whereas C-fibers responses are either wholly or partly dependent on TTX-R channels [15]. There is strong evidence that altered sodium channels play an important role in both inflammatory and neuropathic pain [7,8,16]. In the present study, a hyperpolarizing shift in the activation curve of TTX-S current was observed in the hypotonic solution, which is likely responsible for the enhancement of TTX-S current. Combined with previous study concerning TTX-R current [6], the present results support the hypothesis that sodium channel subtypes are differentially altered by hypotonic stimulation. In fact, this differential modulation of sodium channel has been observed in the pain following nerve damage, in which there is a down-regulation of TTX-R channels, but an up-regulation of TTX-S channels [7,16-19]. The enhancement of capsaicin-evoked current and trafficking of TRPV1 receptor have been noted in anisotonic stimulation [20] and more experiments need to be performed to further test whether hypotonicity had effect on TTX-S channels expression.
TRPV4 receptor is a polymodal receptor that is activated by hypotonicity, mechanical stimuli, warm heat, phorbol ester, low pH, anadamide and its LOX metabolite arachidonic acid etc. [21]. TRPV4 receptors are distributed in sensory ganglia as well as in free nerve endings and cutaneous A- and C- fiber terminals, suggesting the role in pain sensation. This idea was supported by the studies that the hypotonicity-induced increased nociceptor excitability and pain-related behavior was not present in TRPV4 knockout mice [2,3]. Besides this, activation of TRPV4 receptor promotes the release of pain-related neuropeptides (substance P and calcitonin gene-related peptide) from the central projections of primary afferents in the spinal cord [22]. In our previous report, TRPV4 receptor is involved in the modulation of ion channels, such as TTX-R current [6], voltage-gated potassium [23] and calcium channels [24]. The present study demonstrated that TRPV4 agonist 4α-PDD mimicked the effect of hypotonicity with an increase of TTX-S current and a hyperpolarizing shift of G-V curve. Additionally, the enhancement of TTX-S current by hypotonicity and 4α-PDD was markedly attenuated by TRPV4 antagonist RR and GdCl3. These results indicated that TRPV4 receptor was likely responsible for the modulation of TTX-S current by hypotonicity. Sodium currents are regulated by multiple signal transduction cascades. In the present study, we examined the contribution of PKA and PKC signaling pathways to the increase of TTX-S current by hypotonic stimulation. By pre-incubating the antagonists of protein kinases, we found that hypotonicity-induced increase of TTX-S current was markedly blocked by PKC antagonists but unaffected by antagonism of PKA system, which implied that PKC system was selectively involved in the hypotonic-response (Figure 3). Here, it was noted that hypotonicity-induced increase of TTX-S current was not reserved by PKC antagonists completely, indicating that other factors might contribute to the modulation. Recent study reports that Nav1.7 TTX-S channel can be modulated by ERK1/2 mitogen-activated protein kinase which is an important intracellular signaling pathway regulating multiple voltage-gated ion channels in dorsal root ganglion neurons [25]. Whether ERK1/2 pathway was involved in the increase of TTX-S current by hypotonicity needs to be proved in the future study.
In small primary sensor neurons, both TTX-S and TTX-R sodium current are important for generation of action potentials. TTX-S current which has much faster activation and inactivation kinetics than TTX-R current activates during the initial depolarization of AP, rapidly declines before the action potential reaches its peak value and remains near zero during the repolarization. On the other hand, TTX-R current does not inactivation completely during the AP and carries the majority of inward current following during the shoulder [26]. Therefore, sodium channels, which were previously thought to be simply responsible for the rising phase of an action potential, have been shown to play multiple roles in the generation of AP. Besides TTX-R current, high voltage-gated calcium current (IHVA) [24], another important contribution to the AP shoulder [26], was also inhibited by hypotonic stimulation, while voltage-gated potassium current was enhanced by hypotonicity. The above modulation would accelerate the repolarization of AP and affect the shape of the shoulder. The present results demonstrated that TTX-S current was increased by hypotonicity, which would facilitate the depolarization during an AP. In addition, the decrease of calcium influx makes it have less chance to activate calcium-activated potassium current which plays an important role in reducing the repetitive activity [27]. Therefore, hypotonicity-induced changes in the excitability and firing pattern of TG neurons may be mediated through the modulation of multiple ion channels on the cell membrane. Our present framework provides the possibility of pharmacologically targeting specific channel subtypes in treating hypotonicity-induced nociception.
Methods and materials
Cell culture
TG neurons from male Sprague-Dawley rats (180-200 g) were cultured as described previously [6]. Briefly, trigeminal ganglia were dissected aseptically and washed in cold (4°C) modified Hank's Balanced Salt solution (mHBSS). The ganglia were incubated in mHBSS at 37°C for 20-40 min with 0.1% collagenase (Type XI-S), triturated with a fire-polished glass pipette and finally incubated at 37°C for 10 min with 10 μg/ml DNase I (Type IV) in F-12 medium (Life Technologies, Gaithersburg, MD). Then they were centrifuged for 3 times at 5 × 1500 rpm/min and cultured in F-12 supplemented with 10% fetal bovine serum at 37°C for 24 h in a water saturated atmosphere with 5% CO2. The cell diameter (μm) was measured with a calibrated eyepiece under phase contrast illumination. Care of animals conformed to standards established by the National Institutes of Health. All animal protocols were approved by the Nanjing Medical University Animal Care and Use Committee. All efforts were made to minimize animal suffering and to reduce the number of animals used.
Patch clamp recording
All experiments were carried out at room temperature (22-23°C). Whole-cell patch clamp recordings were obtained using an Axopatch-200B patch clamp amplifier (Axon Instruments, Foster City, CA) and the output was digitized with a Digidata 1322A converter (Axon Instruments). The sampling rate was 10 kHz and filtered at 5 kHz. The capacitance and series resistance were compensated ≥90%. Data obtained from neurons in which uncompensated series resistance resulted in voltage-clamp errors > 5 mV were not taken in further analysis. Liquid junction potentials were compensated before patching. When the osmolality of external solutions was changed from isotonicity to hypotonicity, measurements of the changes in liquid junction potentials were less than 2 mV and were not corrected. The glass pipettes (No. 64-0817(G85150T-3), Warner Instruments Inc., Hamden, CT, USA) with resistance of 1-3 MW when filled with pipette solution were used. In voltage-clamp experiment, the holding potential was -80 mV. The G-V curve of sodium current was measured by 20 ms depolarizing pulses from -80 to +40 mV stepping by 5 mV with interval of 2s. The inactivation-voltage curve was obtained by double pulses: precondition pulses (20 ms) were from -100 to +20 mV in 5 mV steps and following 0 mV test pulse (20 ms) with interval of 4s.
Solutions
For sodium current recording, pipette solution contained (in mM) CsCl 130, NaCl 10, CaCl2 1, MgCl2 2, EGTA 10, HEPES 10, Tris-ATP 5 at pH 7.3 and osmolality 300mOsm. The external solution was composed of (in mM): NaCl 30, KCl 5, MgCl2 3, TEA-Cl 20, Choline-Cl 35, 4-AP 3, D-Mannitol 106, HEPES 10 at pH 7.4 and osmolality 300mOsm. 300nM TTX was added in the external solution to separate TTX-S current. Hypotonic external solutions were obtained by adjusting the concentration of D-Mannitol. The osmolality was measured using a vapor pressure osmometer (Model 3300, Advanced Instruments, Norwood, MA).
Data analysis
Data were analyzed using pClamp (Axon Instruments) and SigmaPlot (SPSS Inc., Chicago, IL, USA) software. All data were presented as mean ± S.E.M. and the significance was indicated as P < 0.05 (*) and P < 0.01(**) tested by paired or unpaired Student's t-tests. The amplitude of sodium current was calculated as peak current. G-V curve and inactivation-voltage curve were fitted by Boltzmann functions, which G/Gmax = 1/(1 + exp (V0.5 - Vm)/k) or I/Imax = 1/(1 + exp (V0.5 - Vm)/k), with V0.5 being membrane potential (Vm) at which 50% of activation or inactivation was observed and k being the slope of the function. The dose-response curve was fitted by Hill equation, in which Ipeak = Ipeakmax/[1+(EC50/C)n], with n as the Hill coefficient, and EC50 as the concentration producing 50% increase.
Chemicals
Cell culture materials were purchased from GIBCO (Life Technologies, Rockville, MD, USA). Others came from Sigma Chemical Company.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
LC conceived and designed the study. LL and CJL performed the experiments. Manuscript was written by LC and LgC. The final manuscript was read and approved by all authors.
Supplementary Material
Table S1 Effect of second messenger systems on TTX-S current.
Contributor Information
Lin Li, Email: biolilin@yahoo.com.cn.
Changjin Liu, Email: tjchangjinliu@hotmail.com.
Lei Chen, Email: chenltj@hotmail.com.
Ling Chen, Email: lingchen@njmu.edu.cn.
Acknowledgements
This work was supported by National Natural Science Foundation of China (No. 30900577) and Science and Technology Projects of Jiangsu Province (No. BK2009416).
References
- Alessandri-Haber N, Yeh JJ, Boyd AE, Parada CA, Chen X, Reichling DB, Levine JD. Hypotonicity induces TRPV4-mediated nociception in rat. Neuron. 2003;39:497–511. doi: 10.1016/S0896-6273(03)00462-8. [DOI] [PubMed] [Google Scholar]
- Chen X, Alessandri-Haber N, Levine JD. Marked attenuation of inflammatory mediator-induced C-fiber sensitization for mechanical and hypotonic stimuli in TRPV4-/- mice. Mol Pain. 2007;3:31–37. doi: 10.1186/1744-8069-3-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Liu C, Liu L. Osmolality-induced tuning of action potentials in trigeminal ganglion neurons. Neurosci Lett. 2009;452:79–83. doi: 10.1016/j.neulet.2009.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahine M, Ziane R, Vijayaragavan K, Okamura Y. Regulation of Na v channels in sensory neurons. Trends Pharmacol Sci. 2005;26:496–502. doi: 10.1016/j.tips.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Wood JN, Baker M. Voltage-gated sodium channels. Curr Opin Pharmacol. 2001;1:17–21. doi: 10.1016/S1471-4892(01)00007-8. [DOI] [PubMed] [Google Scholar]
- Chen L, Liu C, Liu L, Cao X. Changes in osmolality modulate voltage-gated sodium channels in trigeminal ganglion neurons. Neurosci Res. 2009;64:199–207. doi: 10.1016/j.neures.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black JA, Cummins TR, Plumpton C, Chen YH, Hormuzdiar W, Clare JJ, Waxman SG. Upregulation of a silent sodium channel after peripheral, but not central, nerve injury in DRG neurons. J Neurophysiol. 1999;82:2776–2785. doi: 10.1152/jn.1999.82.5.2776. [DOI] [PubMed] [Google Scholar]
- Black JA, Liu S, Tanaka M, Cummins TR, Waxman SG. Changes in the expression of tetrodotoxin-sensitive sodium channels within dorsal root ganglia neurons in inflammatory pain. Pain. 2004;108:237–247. doi: 10.1016/j.pain.2003.12.035. [DOI] [PubMed] [Google Scholar]
- Todaka H, Taniguchi J, Satoh J, Mizuno A, Suzuki M. Warm temperature-sensitive transient receptor potential vanilloid 4 (TRPV4) plays an essential role in thermal hyperalgesia. J Biol Chem. 2004;279:35133–35138. doi: 10.1074/jbc.M406260200. [DOI] [PubMed] [Google Scholar]
- Liedtke W. TRPV channels' role in osmotransduction and mechanotransduction. Handb Exp Pharmacol. 2007;179:473–487. doi: 10.1007/978-3-540-34891-7_28. full_text. [DOI] [PubMed] [Google Scholar]
- Liedtke W. Molecular mechanisms of TRPV4-mediated neural signaling. Ann N Y Acad Sci. 2008;1144:42–52. doi: 10.1196/annals.1418.012. [DOI] [PubMed] [Google Scholar]
- Levine JD, Alessandri-Haber N. TRP channels: targets for the relief of pain. Biochim Biophys Acta. 2007;1772:989–1003. doi: 10.1016/j.bbadis.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Alessandri-Haber N, Joseph E, Dina OA, Liedtke W, Levine JD. TRPV4 mediates pain-related behavior induced by mild hypertonic stimuli in the presence of inflammatory mediator. Pain. 2005;118:70–79. doi: 10.1016/j.pain.2005.07.016. [DOI] [PubMed] [Google Scholar]
- Alessandri-Haber N, Yeh JJ, Boyd AE, Parada CA, Chen X, Reichling DB, Levine JD. Hypotonicity induces TRPV4-mediated nociception in rat. Neuron. 2003;39:497–511. doi: 10.1016/S0896-6273(03)00462-8. [DOI] [PubMed] [Google Scholar]
- Baker MD, Wood JN. Involvement of Na+ channels in pain pathways. Trends Pharmacol Sci. 2001;22:27–31. doi: 10.1016/S0165-6147(00)01585-6. [DOI] [PubMed] [Google Scholar]
- Dib-Hajj SD, Fjell J, Cummins TR, Zheng Z, Fried K, LaMotte R, Black JA, Waxman SG. Plasticity of sodium channel expression in DRG neurons in the chronic constriction injury model of neuropathic pain. Pain. 1999;83:591–600. doi: 10.1016/S0304-3959(99)00169-4. [DOI] [PubMed] [Google Scholar]
- Abdulla FA, Smith PA. Changes in Na+ channel currents of rat dorsal root ganglion neurons following axotomy and axotomy-induced autotomy. J Neurophysiol. 2002;88:2518–2529. doi: 10.1152/jn.00913.2001. [DOI] [PubMed] [Google Scholar]
- Sleeper AA, Cummins TR, Dib-Hajj SD, Hormuzdiar W, Tyrrell L, Waxman SG, Black JA. Changes in expression of two tetrodotoxin resistant sodium channels and their currents in dorsal root ganglion neurons after sciatic nerve injury but not rhizotomy. J Neurosci. 2000;20:7279–7289. doi: 10.1523/JNEUROSCI.20-19-07279.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman SG, Kocsis JD, Black JA. Type III sodium channel mRNA is expressed in embryonic but not adult spinal sensory neurons, and is reexpressed following axotomy. J Neurophysiol. 1994;72:466–470. doi: 10.1152/jn.1994.72.1.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Chen L, Liedtke W, Simon SA. Changes in osmolality sensitize the response to capsaicin in trigeminal sensory neurons. J Neurophysiol. 2007;97:2001–2015. doi: 10.1152/jn.00887.2006. [DOI] [PubMed] [Google Scholar]
- O'Neil RG, Heller S. The mechanosensitive nature of TRPV channels. Pflugers Arch. 2005;451:193–203. doi: 10.1007/s00424-005-1424-4. [DOI] [PubMed] [Google Scholar]
- Grant AD, Cottrell GS, Amadesi S, Trevisani M, Nicoletti P, Materazzi S, Altier C, Cenac N, Zamponi GW, Bautista-Cruz F, Lopez CB, Joseph EK, Levine JD, Liedtke W, Vanner S, Vergnolle N, Geppetti P, Bunnett NW. Protease-activated receptor 2 sensitizes the transient receptor potential vanilloid 4 ion channel to cause mechanical hyperalgesia in mice. J Physiol. 2007;578:715–733. doi: 10.1113/jphysiol.2006.121111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Liu C, Liu L. The modulation of voltage-gated potassium channels by anisotonicity in trigeminal ganglion neurons. Neuroscience. 2008;154:482–495. doi: 10.1016/j.neuroscience.2008.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Liu C, Liu L. Changes in osmolality modulate voltage-gated calcium channels in trigeminal ganglion neurons. Brain Res. 2008;1208:56–66. doi: 10.1016/j.brainres.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamboulian S, Choi JS, Ahn HS, Chang YW, Tyrrell L, Black JA, Waxman SG, Dib-Hajj SD. ERK1/2 mitogen-activated protein kinase phosphorylates sodium channel Na(v)1.7 and alters its gating properties. J Neurosci. 2010;30:1637–1647. doi: 10.1523/JNEUROSCI.4872-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair NT, Bean BP. Roles of tetrodotoxin (TTX)-sensitive Na+ current, TTX-resistant Na+ current, and Ca2+ current in the action potentials of nociceptive sensory neurons. J Neurosci. 2002;22:10277–10290. doi: 10.1523/JNEUROSCI.22-23-10277.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz A, Gruss M, Vogel W. Properties and functions of calcium-activated K+ channels in small neurons of rat dorsal root ganglion studied in a thin slice preparation. J Physiol. 1998;513:55–69. doi: 10.1111/j.1469-7793.1998.055by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Effect of second messenger systems on TTX-S current.