Abstract
Many of the actions of 17beta-estradiol (E2) in the central nervous system (CNS) are mediated via the classical nuclear steroid receptors, ERalpha and ERbeta, which interact with the estrogen response element to modulate gene expression. In addition to the nuclear-initiated estrogen signaling, E2 signaling in the brain can occur rapidly within minutes prior to any sufficient effects on transcription of relevant genes. These rapid, membrane-initiated E2 signaling mechanisms have now been characterized in many brain regions, most importantly in neurons of the hypothalamus and hippocampus. Furthermore, our understanding of the physiological effects of membrane-initiated pathways is now a major field of interest in the hypothalamic control of reproduction, energy balance, thermoregulation and other homeostatic functions as well as the effects of E2 on physiological and pathophysiological functions of the hippocampus. Membrane signaling pathways impact neuronal excitability, signal transduction, cell death, neurotransmitter release and gene expression. This review will summarize recent findings on membrane-initiated E2 signaling in the hypothalamus and hippocampus and its contribution to the control of physiological and behavioral functions.
Keywords: estrogen, membrane receptor, reproduction, energy homeostasis, neuroprotection, hypothalamus, hippocampus, review
2. INTRODUCTION
Estrogen receptors (ERs), ERalpha and ERbeta, were initially identified as estrogen-responsive nuclear transcriptions factors to modulate gene expression (1,2). For most of the past 30 years, the actions of estrogens (17beta-estradiol, E2) were thought to mediate only long-term transcriptional effects in the brain by either organization of neurogenesis and neural circuitry during embryonic and neonatal development or activational control of gene expression during the later stages of the life cycle (3). However, it has become quite clear that E2 has rapid, membrane-delimited effects that are independent of ER transcriptional control. These rapid effects were first identified in the uterus and hypothalamus decades ago (4,5,6), but only in the past 10–15 years has the physiological significance of these acute E2 effects been investigated (7,8,9,10,11,12).
Rapid membrane-mediated effects of E2 have been identified in the hippocampus and in various hypothalamic nuclei (7,13,14) but may occur throughout the central nervous system. Membrane-initiated estrogen signaling involves the rapid activation of various protein kinase pathways including protein kinase C (PKC), protein kinase A (PKA), phosphatidylinositol-3 kinase (PI3K), and mitogen-activated protein kinase (MAPK), to modulate signal transduction, protein phosphorylation and cation channel activity (15,16,17,18). In the hypothalamus, membrane signaling has been implicated in gonadotropin secretion and sexual behavior (19,10,20). While many aspects of reproduction are controlled by nuclear-initiated estrogen signaling (21,22), it appears that membrane E2 signaling plays a vital, modulatory role in these reproductive functions. Other hypothalamic homeostatic functions modulated by membrane signaling include energy homeostasis and thermoregulation, which appear to be modulated by a Gq-coupled membrane ER functionally characterized in hypothalamic neurons (arcuate, preoptic area). In the hippocampus, membrane E2 signaling has been implicated in the neuroprotective effects of E2 in rodent ischemia models and may have a role in memory and cognition (23,24).
3. MEMBRANE E2 SIGNALING
Nuclear-initiated estrogen signalling controls cellular functions and gene expression via the classical ERs binding to the estrogen response element (ERE) or to other promoter sites through protein-protein interactions such as Sp-1 and Fos-Jun (AP-1) and activating transcription of important oestrogen responsive genes (25). Briefly, E2 can activate transcription via complexes with other transcription factors through protein-protein interactions including pCREB, STATs, Elk-1-SRF, ATF-2-Jun and NFkappaB inducing transcription via their respective promoter sites. In membrane-initiated estrogen signalling, E2 can activate a host of rapid signaling cascades that affect cell function and modulate gene expression through other transcription factor promoter sites using membrane-associated ERs or novel G-protein coupled E2 receptors (26,27,28,29). E2 through these receptors activates multiple signalling pathways including PI3K, phospholipase C (PLC), MAPK, extracellular signal-regulated protein kinase (ERK) and protein kinase pathways (PKA, PKC, etc.) (26,30,27,28,29).
While ERalpha and ERbeta can be associated with the membrane (31), the steroid nuclear receptors do not have extracellular domains that are common for growth factor, cytokine and G protein-coupled receptors (GPCRs). In heterologous cell systems, the classical ERs are associated with the membrane via lipid anchors that attach them to membrane components or via associations with GPCR. ERs can be modified by lipids (palmitoylated) to attach to the plasma membrane and thereby interact with the membrane proteins such caveolin-1 to initiate signal transduction pathways (32,33). Another mechanism characterized in the hypothalamus is the association of ERs to other GPCRs or tyrosine kinase receptors to initiate cell signaling. Primary examples of this novel type of cell signaling is the ligand-initiated association of classical ERs with metabotropic glutamate receptors (mGluRs) (34) or interaction with the IGF-1 receptor (35) to facilitate female reproductive behavior. These associations, between ERs and other receptors, have implications for sexual behavior and positive feedback effects of E2 on GnRH release.
There are several potential candidates for novel membrane ERs including ER-X and two G-protein-coupled receptors, GPR30 and Gq-mER (36,37,38,11,30,39), which offer several targets for E2 to rapidly alter cell functions in the brain. ER-X was identified as a high-affinity, saturable estrogen receptor with sequence homology to the classical ERs that is associated with caveolar-like microdomains in developing neocortical neurones (40). E2 induces tyrosine phosphorylation of both ERK1 and ERK2 in organotypic explants of the developing cerebral cortex (36). Interestingly, the steroid pharmacology of ER-X is distinct in that 17alpha-estradiol is equipotent as E2 in activating the MAPK/ERK pathway and the receptor is insensitive to the anti-estrogen ICI 182,780 (15,40). GPR30 (GPER1), a GPCR, which binds E2 with nanomolar affinity, is involved in the rapid actions elicited by E2 in peripheral reproductive tissue (41,38) and activates the ERK pathway independently of ERalpha or ERbeta (42,30). GPR30 is expressed in the hypothalamus especially in the paraventricular nucleus, supraoptic nucleus and the preoptic area (43,44). However, the pharmacology of GPR30 in these neurones has not been characterized.
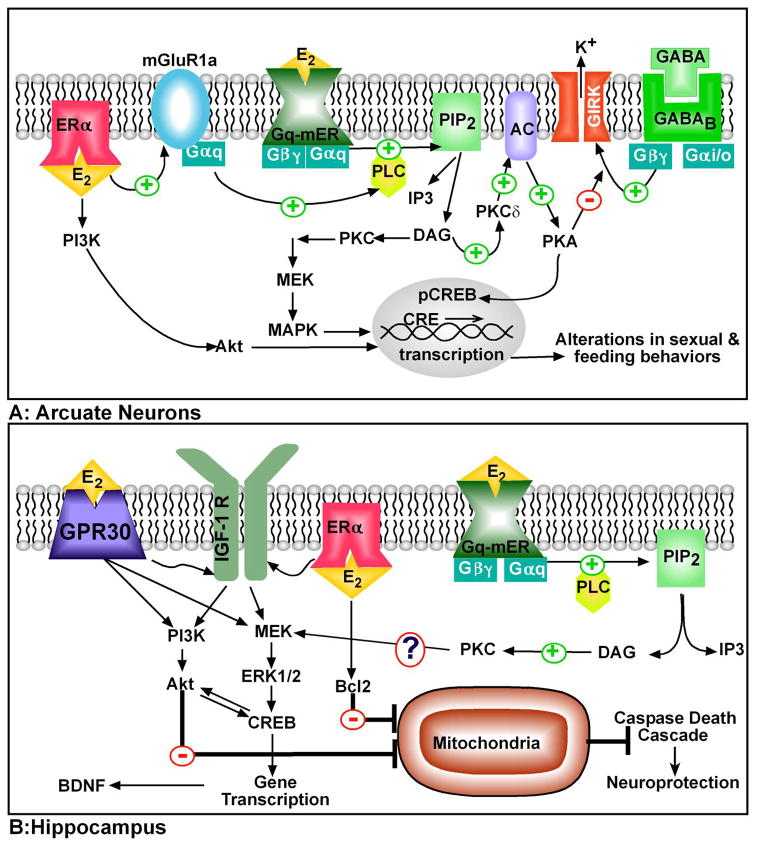
We have generated compelling electrophysiological and physiological evidence supporting the hypothesis of a hypothalamic, Gq-coupled membrane ER (Gq-mER). Using whole cell recordings from guinea pig and mouse hypothalamic slices, we have characterized a Gq-mER that activates a PLC-PKC-PKA pathway in response to E2 to significantly attenuate the potency of mu-opioid and GABAB agonists in activating an inwardly rectifying K+ (GIRK) conductance at low nanomolar concentrations (EC50 = 8 nM) in arcuate POMC neurons (See Figure 1A) (11,37,45). In GnRH neurons, E2 (EC50 = 0.6 nM) activates KATP channels via the same signaling pathway (46). Therefore, the putative Gq-mER is a novel E2-activated membrane receptor in hypothalamic (POMC, GnRH) neurons that awaits definitive characterization by the cloning of the gene.
Figure 1. Cellular models of membrane-initiated E2 signaling pathways in the brain.
A. In arcuate neurons including those that express POMC, dopamine, and GABA, E2 activates the Gq-mER leading to activation of PLC-PKC-PKA pathway. Activation of the receptor initiates the hydrolysis of membrane-bound phosphatidylinositol 4,5-biphosphate (PIP2) to IP3 and diacylglycerol (DAG) via PLC. DAG activates protein kinase Cδ (PKCδ), which through phosphorylation, upregulates adenylyl cyclase VII (AC) activity. The generation of cAMP activates PKA, which can rapidly uncouple GABAB receptors from their effector system through phosphorylation of a downstream effector molecule (e.g., G protein-coupled, inwardly rectifying K+ (GIRK) channel). The mER-mediated modulation of kinase pathways reduces the capacity of inhibitory neuromodulators such as GABA via GABAB receptor (and opioid peptides via mu-opioid receptor, not shown) to reduce neuronal excitability. The Gq-mER-mediated activation of PKA can lead to phosphorylation of cAMP-responsive element binding protein (pCREB), which can then alter gene transcription through its interaction with CREs on genes. Also, in arcuate (NPY?) neurons, ERalpha interacts with metabotropic glutamate receptor 1a (mGluR1a) to initiate Gq stimulation of PLC, which leads to MAPK-induced CREB phosphorylation. Membrane-associated ERalpha can activate PI3K/Akt signaling. B: Membrane-initiated E2 signaling treatment may act through multiple cellular pathways for its neuroprotective actions. Acute E2 administration may bind to either classical membrane ERs (ERalpha is shown) and/or GPR30. Through the membrane-associated ERs, E2 can either directly control Bcl-2 (anti-apoptotic protein) gene expression or by transactivation of IGF-1 receptors for a sustained activation of the ERK/MAPK pathway. GPR30 may block apoptotic cascades through the direct activation of either ERK/MAPK or PI3K/Akt pathways via the transactivation of receptor tyrosine kinases (IGF-1 receptors and/or Trk-B). BDNF, a target of activated CREB, is thought to bind the receptor tyrosine kinase Trk-B to activate both the MAPK and PI3K pathway to promote neuroprotection. Stimulation of the PI3K/Akt pathway by BDNF would inactivate the pro-apoptotic proteins to halt the caspase death cascade. STX treatment also offers neuroprotection from ischemia via an unknown mechanism, but a possible pathway is the PKC-activation of MAPK signaling.
4. MEMBRANE E2 SIGNALING AND REPRODUCTION
E2 is a key player in the positive and negative feedback loops on GnRH and LH secretion during the ovulatory cycle. In rodents, during negative feedback, low levels of E2 inhibit GnRH neuronal output during most of the estrous cycle. However, on the afternoon of proestrus, E2 production from maturing ovarian follicles is elevated and these peaking levels of E2 in combination with a circadian signal stimulates GnRH neurons to produce the surge of LH from pituitary gonadotropes (47). Therefore, E2 positive feedback is crucial for the LH surge to initiate ovulation. The development of an ERE-independent ERalpha signaling mouse model is a technological advance in understanding the role of nonclassical (non-ERE) signaling in E2-mediated negative and positive feedback (21). This mouse model has lost the capacity for ERalpha to bind to the ERE due to mutations in the DNA binding domain of ERalpha. Using this mouse model, the effects of E2 on positive feedback were determined to depend upon ERE-dependent signaling of ERalpha in neurons (21) presumably presynaptic to GnRH neurons since ERalpha is not expressed in GnRH neurons. However, during negative feedback, E2, in part, utilizes ERE-independent signaling to inhibit GnRH and/or LH secretion, but not synthesis (gene expression), through a rapid, p21-activated kinase (PAK1)-mediated pathway (21,48,49). Interestingly, the expression of arcuate kisspeptin, a major excitatory drive for GnRH neurons, is inhibited by E2 via an ERE-independent signaling mechanism during the negative feedback period (50).
4.1 Membrane E2 signaling and GnRH secretion
An acute direct effect of E2 on GnRH neuronal activity was first described over twenty-five years ago (5). Although the effects of membrane E2 signaling on GnRH neurons in the hypothalamus have been extensively reviewed (14,7,51,52), new insights into the multiple signaling events involved in GnRH neuronal activity and release suggest that E2 has multiple pathways to rapidly modulate these vital reproductive neurosecretory cells (53,46,54).
Studies from our lab have previously shown that in guinea pigs E2 will rapidly hyperpolarize GnRH neurons (5,55,46). Recently, in mouse GnRH neurons, high physiological doses of E2 enhanced action potential firing by modulating the intrinsic afterhyperpolarizing and afterdepolarizing potentials in PKA-dependent mechanisms involving ERbeta. Conversely, in these neurons, low physiological doses of E2 had an effect on neuronal activity via GABAergic and glutaminergic inputs suggesting that some of the inhibitory inputs of E2 occur via presynaptic mechanisms (54). In addition, an acute effect of sub-nanomolar E2 potentiates KATP channel activity via PKC and PKA pathways to maintain hyperpolarization. The KATP-controlled hyperpolarization is potentially involved in recruitment of excitatory channels that are critical for burst firing of GnRH neurons including the T-type calcium channel (53,46). Furthermore, E2 via activating ERbeta and/or GRP30 receptors rapidly potentiates high-voltage-activated Ca2+ currents (L- and R-type Ca2+ channels) suggesting that Ca2+ homeostasis is a target for membrane signaling in the GnRH neurons (56). In the primate, E2 rapidly increases firing frequency, spike density and burst duration through a membrane-delimited pathway in monkey placode GnRH neurons, although since this study did not block presynaptic inputs with TTX, the effects of E2 may not be directly on GnRH neurons (57). These studies support the hypothesis that one of the important signaling effects of E2 on GnRH function is to modulate neuronal excitability by modulating intrinsic cation channel activity.
Another membrane-initiated effect of E2 is the modulation of Ca2+ oscillations in primate and mouse GnRH neurons. Ca2+ oscillations in GnRH neurons synchronize with a periodicity of approximately 60 minutes (58,59,60) which is similar to the pulsaltile rhythm of GnRH peptide release (61,58,62). Furthermore, perfusion of primate GnRH neurons with nanomolar concentrations of E2 alters the patterns of Ca2+ oscillations via a membrane-delimited (E2-dendrimer) mechanism (63). The E2 signaling mechanism modulating the Ca2+ oscillations in primate GnRH neurons is suppressed by pertussis toxin treatment and by knockdown of GPR30 mRNA, and mimicked by the GPR30 agonist, G1 (44). In the mouse, Ca2+ oscillations are blocked by ICI 182,780 and mimicked by E2-BSA (59,60). The actions of E2 are also abrogated by PTX treatment. Wray and colleagues hypothesize that the effects of E2 are ERbeta-mediated, whereas Terasawa and colleagues hypothesize that it is GPR30-mediated since ICI 182,780 does not block the actions of E2.
Membrane-initiated E2 signaling may be involved at multiple levels of GnRH release into the median eminence and LH secretion from the pituitary during the ovulatory cycle. In the primate GnRH study, E2 stimulates the release of GnRH from cultured primate GnRH neurons via a membrane-initiated mechanism within 20 min of administration (44). At the level of the median eminence, membrane signaling events (by using E2-BSA) are implicated in the E2-induced release of GnRH from nerve terminals via activation of nitric oxide from the median eminence vascular endothelial cells (64). Conversely, membrane signaling mechanisms, potentially via ERalpha, are required for the acute suppression of LH in vitro from ovine pituitary cultures after GnRH stimulation (19,65). In an immortalized GnRH cell line, E2 via a Gi-coupled, membrane receptor-mediated mechanism inhibits cAMP production and GnRH release at picomolar concentrations (20). Collectively, these data suggest that membrane signaling is necessary for many of the pre- and postsynaptic mechanisms regulating GnRH neurons to ultimately control reproduction.
4.2 Membrane E2 signaling and sexual behavior
Both nuclear-initiated and membrane-initiated E2 signaling are involved in the regulation of sexual behavior, and specifically, lordosis, the female response to intromission by the male characterized by the arching of the spine. Lordosis behavior is heightened during estrus and is controlled by E2 through a complex hypothalamic circuitry involving the arcuate, the VMH and medial preoptic area (mPOA) (66,34). Systemic administration of E2 in ovariectomized rats activates IGF-1 receptors and induces the association between Insulin-like Growth Factor-1 (IGF-1) receptors and ERalpha in the mPOA(67,68,69). There is an interaction between the p85 subunit of phosphatidylinositol 3-kinase (PI3K) and ERalpha within 1–3 h, which leads to activation of protein kinase B/Akt, a serine/threonine kinase that has multiple downstream targets (68,69). The E2-induced activation of IGF-1 receptors augments alpha-1-adrenergic receptor signaling, and blockade of IGF-1 receptors prevents E2-induced increases in alpha-1-adrenergic receptor binding density as well as IGF-1 enhancement of noradrenergic receptor signaling, which is critical for expression of reproductive functions (67,70). The cross-talk between the E2 and IGF-1 receptor signaling pathways appears to contribute to synaptic remodeling and neuronal plasticity during the estrous cycle. Moreover, intracerebroventricular (i.c.v.) infusion of a selective competitive blocker of IGF-1 autophosphorylation (JB-1) inhibits the E2-induced LH surge and sexual behavior in ovariectomized, E2-treated rats (70). In addition, co-administration (i.c.v.) of inhibitors of PI3K (wortmannin) and MAPK (PD98059) inhibit the long-term (48 h) effects of E2 to induce the LH surge and facilitate lordosis behavior (71). Therefore, facilitation of female sexual behavior by E2 appears to involve activation of both PI3K and MAPK signal transduction pathways. The importance of growth factors for female sexual behavior is further highlighted by observations that epidermal growth factor (EGF) and also IGF-1 can, in the absence of E2 and progesterone (within 1–4 h of i.c.v. administration), induce mating behavior in rats and mice, in part, through an ERalpha-dependent mechanism (72). This relatively rapid, ligand-independent ER action is in striking contrast to the well established finding that E2 priming over a period of at least 24 h is needed for progesterone induction of female reproductive behavior (73). Therefore, the ability of both IGF-1 and E2 to induce female sexual behavior may involve complex interactions between ERalpha, the IGF-1 receptor and the PI3K p85 subunit.
Furthermore, the lordosis neural circuitry involves the actions of membrane E2 signaling via the initiation of an ERalpha-mediated pathway in the arcuate nucleus leading to the release of beta-endorphin (beta-END) in the mPOA and an internalization of the mu-opioid receptor (MOR). The internalization of MOR occurs rapidly (< 30 min of E2 treatment) and is correlated with an initial inhibition of the lordosis behavior in females (74,75). Membrane-impermeant E2 administered into the arcuate nucleus also initiates the MOR internalization (76). The short latency for the E2-induced release of beta-END and subsequent inhibition of lordosis indicates that these actions are initiated at the membrane and/or occur in the cytoplasm and not by gene expression. The transient inhibition of lordosis by E2 appears to be necessary for full sexual receptivity measured 30 h after treatment (76). Interestingly, in MOR knockout mice, females exhibit a diminished lordosis response even after E2 treatment (74,77). One question that remains is how does the transient, E2-induced activation and internalization of MOR in the mPOA facilitate full sexual receptivity 30 h later? One possibility is that activation of MOR produces a transient inhibition of mPOA neurons that rebound from a hyperpolarized state with a facilitated firing and drive to the neurons regulating sexual behavior.
Furthermore, the internalization of MOR and the inhibition of lordosis behavior by membrane-associated ERalpha-mediated signaling depend on mGluR1a signaling. ERalpha and mGluR1a are co-expressed and co-immunoprecipitated using membrane fractions from primary, hypothalamic astrocyte cultures or in HEK cells transfected with both ERalpha(-EGFP) and mGluR1a (76,78). In cultured hypothalamic astrocytes, the membrane-initiated actions of E2, at picomolar concentrations, increases free cytoplasmic Ca2+ levels from intracellular calcium stores within seconds by a membrane-associated, ERalpha-mediated mechanism interacting with mGluR1 receptors (79,80,78). The action of E2 in astrocytes is blocked by the mGluR1a antagonist, LY367385 ((S)-(+)-a-Amino-4-carboxy-2-methylbenzeneacetic acid), indicating that the interactions between ERalpha and mGluR1 are necessary for the E2-induced Ca2+ flux (76,81).
As in the blockade of E2’s actions in astrocytes, LY367385 prevents the E2-induced MOR internalization and the inhibition of lordosis behavior and an mGluR1a agonist, DHPG ((S)-3,5-dihydroxyphenylglycine), mimics the E2-induced activation/internalization of MOR in the mPOA (76). The activation of an ERalpha-mGluR1a complex initiates multiple signaling pathways including PLC-PKC and PKA, which was shown previously to stimulate lordosis behavior (82,12,83). This is similar to STX-activated Gq-mER pathway in arcuate neurons (37,11). These data collectively suggest that the membrane-initiated interactions of the ERalpha-mGluR1a complex via a PKC-mediated pathway are a component of the E2 control of MOR internalization in the mPOA and the regulation of lordosis behavior.
Finally, the membrane-mediated effects of E2 potentially modulate male sexual behavior (84). In rodents, rapid or membrane-mediated effects of E2 on male sexual behavior were initially identified in castrated rats. Peripheral injection of E2 at high doses increased genital sniffs and total mounts while decreasing the latency to mounting within 35 min of administration (85). Membrane-delimited E2-BSA, when chronically administered via cannulation directly into the medial preoptic area, significantly increased mounting behavior, intromissions and ejaculations and decreased the latency to mounting and ejaculation in castrated, dihydrotestosterone-treated rats (86) but not when chronically administered into the medial amygdale (87). In wild-type male mice, acute peripheral injections of E2 attenuate the inhibition of mounting frequency and intromission frequency by aromatase inhibitors within 10 minutes of administration. Furthermore, in aromatase knockout male mice, E2 can increase intromission frequency within 10 minutes of administration and increase mounting frequency within 30 minutes (88). Therefore, E2, within 10–30 minutes, can control male sexual behaviors potentially using similar membrane-mediated cellular mechanisms as in females.
5. MEMBRANE E2 SIGNALING AND HOMEOSTASIS
5.1. Membrane E2 signaling and energy balance
E2 controls multiple hypothalamic homeostatic functions including the regulation of energy homeostasis and core body temperature. The nuclear-initiated signaling of ERalpha is a necessary component of the regulation of energy homeostasis by E2 (defined as the balance between energy intake and energy expenditure) (89). However, there is little or no direct evidence suggesting a role for the activation of membrane-associated ERalpha signaling pathways in the control of energy homeostasis. E2 attenuates food intake within 6 h of administration into the third ventricle via cannula after an overnight fast compared to saline in mice (90). In fed rats, E2 reduces food intake in the period between 4 and 14 h after administration (91). The short-term E2-induced effects on food intake do suggest that membrane signaling events may be involved in the estrogenic regulation of energy homeostasis. Furthermore, there is compelling evidence for a novel mechanism that modulates different aspects of energy homeostasis.
A Gq-mER signaling pathway has been elucidated in hypothalamic arcuate neurons (POMC, GABA, dopamine) and was initially characterized in female guinea pigs (37,11), but has also been functionally examined in ERalpha and ERbeta knockout both male and female and in male ERalpha-beta double-knockout mice (11) as well as GPR30 knockouts (92). E2 stereospecifically activates a Gq-mER pathway and is blocked by the anti-estrogen ICI 182,780 (93,45). The steroid stereospecificity of the Gq-mER suggests a pharmacologically distinct receptor compared to ER-X. As described above, the Gq-mER activates a PLC-PKC-PKA pathway that attenuates the activation of GIRK channels by both GABAB receptors and MOR receptors. The attenuation of the inhibitory presynaptic inputs increases the excitability of anorectic POMC neurons. To target this E2 pathway, a selective agonist (STX), which is structurally similar to 4-OH tamoxifen, was developed to have no measureable binding affinity for the classical ERs (94). In fact, STX has a greater affinity (~20-fold) for the Gq-mER than E2 and has no affinity for ERalpha/beta (11).
Using a series of pharmacological agents, the signaling pathway that is activated by E2 and STX in hypothalamic neurons has been identified (See Figure 1A). The signaling mechanism begins with the binding of the ligand to a Gq-mER and the activation of G-alpha-q. Activated G-alpha-q initiates the hydrolysis of PIP2 by PLC to liberate DAG. Free DAG stimulates PKCdelta, and PKCdelta activates adenylyl cyclase (VII) to elevate cAMP levels and stimulate PKA. PKA, through phosphorylation, uncouples the inhibitory GABAB and MOR receptors from activation of GIRK channels (Figure 1A) (37). Furthermore, PKA will phosphorylate CREB (cAMP response element-binding protein) to initiate gene expression via cAMP response element (CRE) and IP3 may release calcium through the IP3 receptor on the endoplasmic reticulum. Other transcriptional pathways that the putative Gq-mER may activate include the MAPK, calcium-activated and PI3K pathways (95).
The ability of STX to mimic E2’s modulation of POMC activity led to the hypothesis that the putative Gq-mER has a role in energy homeostasis. Compelling, reproducible evidence demonstrates that STX peripheral administration mimics the effects of E2 on energy homeostasis using whole animal physiological studies (11,96,97). The E2 (STX)-induced increase in POMC neuronal activity is predicted to reduce food intake and, subsequently, the post-ovariectomy body weight gain. Indeed, STX inhibits food intake in ovariectomized guinea pigs by reducing meal frequency similarly to E2 treatment and a subsequent reduction in abdominal fat accumulation (97). Furthermore, STX administration generates new transcription in the arcuate nucleus of the STX-treated female guinea pigs (96). Several of the regulated arcuate genes are involved in the control of energy homeostasis (e.g., neuropeptide Y) and neuronal activity (e.g., Cav3.1). Therefore, Gq-mER may function in the estrogenic control of energy homeostasis, presumably through activation of POMC neurons in the arcuate nucleus, although other hypothalamic neurons may be involved.
5.2. Membrane E2 signaling, thermoregulation and bone remodeling
Another homeostatic function that E2 has a role in is the maintenance of core body temperature (Tc). In a recent publication, peripheral administration of STX reduced Tc significantly compared to the vehicle control similarly to E2 (97). The exact cellular mechanism for E2’s control of Tc is not known. A potential mechanism is the direct action of E2 on thermosensitive (GABAergic) neurons in the preoptic area of the hypothalamus (98,99,100). Previous studies in female guinea pigs have shown that mPOA GABAergic neurons respond to acute E2 treatment via a membrane-initiated pathway to attenuate GABAB autoinhibition leading to increased neuronal activity(101). It is not know if these E2-sensitive neurons are also the same warm-sensitive neurons characterized by Boulant and colleagues (102).
The Gq-mER is similar to other Gq-coupled GPCRs involved in thermoregulation such as the 5HT receptors (5HT2A/2C), which activate signaling pathways to lower Tc and are implicated in thermoregulation dysfunction caused by ovariectomy (103,104). Both the Gq-mER and 5HT2C receptors attenuate inhibitory GABAergic signals in POMC neurons (90). Selective serotonin reuptake inhibitors elevate endogenous serotonin levels and are efficacious for treating hot flushes (105) and can significantly decrease the effects of post-ovariectomy thermoregulatory dysfunction in rodents (106). These similarities imply that serotonin and E2 via a Gq-mER have similar targets sites in the hypothalamus to control Tc and other neuroendocrine and autonomic functions.
An exciting recent discovery is the efficacy of STX to mimic the effects of E2 on tibial bone density in the ovariectomized guinea pigs (97). E2 has direct effects on the osteoclast/osteoblast cells involved in bone remodeling (107,108). However, E2 via a Gq-mER may reduce bone loss, a hallmark of hypo-estrogenic states, in part, by controlling the central mechanism of bone remodeling in the hypothalamus. It is known that the preautonomic paraventricular (PVN) neurons that drive sympathetic activity are involved in bone remodeling of other central and peripheral signals (109,110). E2, potentially via the putative Gq-mER, can either directly act on these neurons or indirectly on the neurons via pathways involving arcuate POMC neurons or VMH neurons both of which are known to synapse on the preautonomic paraventricular (PVN) neurons. The sympathetic nervous system in turn controls bone remodeling via the beta 2 adrenergic receptor (Adr-beta 2) activity (110). In fact, there is an increase in bone formation and a decrease in bone reabsorption in Adr-beta 2−/− mice (111). Unlike in wild-type mice, gonadectomy of Adr-beta 2−/− mice does not alter bone mass or bone resorption parameters, indicating that increased sympathetic activity may be responsible for the bone loss in hypo-estrogenic states.
6. MEMBRANE E2 SIGNALING IN THE HIPPOCAMPUS
6.1. Memory & learning
The effects of membrane E2 signaling on hippocampal function and memory has been previously reviewed (14,24,112). Long-term potentiation (LTP) is the activity-dependent enhancement of synaptic activity (glutamate transmission) in the hippocampus (113) that can last for hours and is considered a cellular model of memory storage (112). The induction of LTP is by brief, high frequency trains of stimuli to afferents (Schaffer collaterals) of hippocampal CA1 pyramidal neurons and requires the activation of NMDA receptors. The subsequent potentiation of the strength of the synaptic signal is via non-NMDA (AMPA) receptors augmentation. E2 augments LTP rapidly (< 30 min) suggesting an membrane E2 signaling (18,114). The E2 enhancement of LTP is blocked by a tyrosine kinase inhibitor PP2 ((4-amino-5-(4-chlorophenyl)-7-(dimethylethyl)pyrazolo[3,4-d]pyrimidine), which also blocks the E2-dependent phosphorylation of NMDA receptors (115). E2, via activation of a cAMP/PKA pathway, also potentiates the non-NMDA (kainate)-mediated excitation of hippocampal CA1 pyramidal neurons (116,117) in wild-type and in ERalpha KO mice suggesting that either ERbeta or another membrane-associated E2 receptor is involved in the augmentation of LTP (118).
Furthermore, E2 exerts a positive influence on memory and higher cognitive functions both chronically (119,120,121) and acutely (122,123,124,125). Long-term hormone replacement therapy in human females either experiencing surgical or natural menopause protects against memory loss in both verbal and non-verbal memory tests and attention (126,121). In nonhuman primates, cyclical E2 replacement reversed the age-related impairment of spatial working memory and recognition memory observed in ovariectomized females (120). In acute models, E2 injection, either peripherally or centrally, in ovariectomized females will enhance various measurements of memory consolidation. Injections of E2 (5.0 μg/0.5 μl) immediately after training, but not 2 h after training, significantly potentiated memory retention after a 24 h intertrial delay potentially via interactions with cholinergic circuitry (124).
Furthermore, after a shorter intertrial delay (4 hr), E2 treatment administered immediately after training enhanced visual (object), placement memory (122,123) and inhibitory avoidance task which mimics the effects of LTP (125). Luine and colleagues (122,123) suggest that these short-term effects are the result of an “extranuclear” receptor-mediated mechanism because of the short time course (<4 hr) and the rapid effects of E2 (<30 min) on monoamine neurotransmitter levels and metabolism. However, these data are not definitive evidence for membrane-initiated estrogen signaling. The measurements of memory occur 4 hr after injection of E2, which is long enough for nuclear-initiated signaling to occur. Therefore, further experiments using membrane-delimited E2 (E2-BSA, EDC) and/or novel ligands for E2-responsive GPCR (G1, STX) are needed.
Regardless, some of the effects of E2 on these hippocampal functions are, in part, via either direct enhancement of glutamate inputs on the CA1 pyramidal cells (as described above) or indirect attenuation of GABAergic inputs (116,117,127). Although the nuclear-initiated E2 signaling events are necessary for many of these effects in the hippocampus (112), membrane E2 signaling can acutely mimic these actions on hippocampal neurons (128,129,130,117,18,114,112). In addition, E2 may modulate a host of signal transduction pathways including mobilization of calcium and increased phospholipase and protein kinase activities via G-protein coupled mechanisms (24). Membrane E2 signaling has also recently been identified as involved in the E2-induced enhancement of memory consolidation in the dorsal hippocampus via activation of ERK signaling pathway (131,132). Therefore, there are multiple mER-mediated mechanisms that contribute to learning and memory.
6.2. Neuroprotection
Due to the reduction in serum E2 after menopause, there is a significant gender difference in the incidence of stroke with the incidence of stroke higher in women as compared to men each year while premenopausal women are less likely to suffer a stroke compared to men (133,134,135). Hormone replacement, depending upon the length of time a women spends in a hypo-estrogenic state, may reduce the risk of stroke in post-menopausal women (134,135). Thus, E2 may have neuroprotective effects against ischemic injury and disease in the aging brain. There are multiple pathways involved in E2-mediated neuroprotection (136,137,138). Briefly, in the hippocampus, E2 employs both nuclear- and membrane-mediated mechanisms to augment signal transduction pathways such as PKC, PI3K/Akt and MAPK/ERK and gene expression relevant for cell survival (139,140,141,142,143). The genomic mechanisms involve both ERE-dependent and -independent transcriptional modulation that converge with the membrane-initiated effects on signaling proteins to modulate factors involved in cell death and survival such as the caspase death cascade (141,138).
While both ERalpha and ERbeta have been implicated in the neuroprotective effects of E2 (144,145) via both nuclear-and membrane-initiated mechanisms, the role of novel E2-responsive GPCRs in the neuroprotective effects of E2 has been recently examined. Using selective ligands for GPR30 (G1) and the Gq-mER (STX), Lebesgue et al. (2010) reported neuroprotective effects of both ligands in hippocampal CA1 neurons administered after a global ischemia insult during reperfusion in middle-aged rats eight weeks after ovariectomy (23). E2, G1 and STX were neuroprotective after immediate infusion via a lateral ventricle cannula with over 50% of CA1 neurons surviving compared to only 15% in the vehicle controls. G1 also mimicked potentiation of field excitatory postsynaptic potentials (LTP) by E2 in hippocampal slices from ovariectomized females. The role of GPR30 in E2’s neuroprotective effects in the hippocampus is supported by the recent findings demonstrating that robust neuroprotection is exerted by membrane-delimited E2 (E2-BSA and EDC) via the activation of an ERK-Akt-CREB-BDNF signaling mechanism (146). The ERK signaling pathway is a known effector of GPR30 actions in the hippocampus (42,30). The membrane-mediated neuroprotective effects from global ischemia are correlated with a preservation of cognitive functions (146). These data suggest that the neuroprotective effects of E2 via E2-responsive GPCRs promote cell survival after injury (See Figure 1B).
7. SUMMARY
In addition to the nuclear-initiated E2 signaling mechanisms that are often the primary driver of E2’s effects, it is now clear that membrane E2 signaling plays a modulatory role in the CNS control of physiology and behavior including reproduction, feeding, thermoregulation and learning and memory. The membrane signaling mechanisms include modulating cation channel functions and thereby neuronal excitability through multiple signaling pathways, as well as inducing changes in calcium mobilization and other signaling pathways that impinge on gene expression. There are many examples in the hypothalamus, hippocampus and other brain regions for an E2-induced up-regulation of PKC, PKA, PI3K and MAP kinase activity leading to altered neuronal functions. One of the major obstacles to our full understanding of these events is the identity and characterization of the multiple receptor types (membrane-associated ERalpha and ERbeta vs. novel GPCR) that mediate the pleiotropic effects of E2. Once all of the membrane receptors are characterized, there will be even greater progress towards understanding all of the effects of E2 on neuronal function, physiology and behavior.
Acknowledgments
The authors thank members of their laboratories who contributed to the work described herein, especially Drs. Jian Qiu, Chunguang Zhang, Anna Malyala and Ms. Martha A. Bosch. The work from the authors’ laboratories was supported by PHS grants NS 43330, NS 38809 and DK 68098.
Abbreviations
- AC
Adenylate cyclase
- ATF-2-Jun
activating transcription factor-2-Jun
- Bcl-2
B-cell lymphoma 2
- BDNF
Brain-derived neurotrophic factor
- CaM
calmodulin
- cAMP
cyclic adenosine monophosphate
- CRE
cAMP response element
- CREB
cAMP response element binding protein
- DAG
diacylglycerol
- E2
estradiol
- E2-BSA
estradiol-bovine serum albumin
- EDC
estradiol-dendrimer
- ELK-1-SRF
ETS domain-containing protein-serum response factor
- EGF
epidermal growth factor
- ER
estrogen receptor
- ERE
estrogen response element
- ERK
extracellular-signal regulated kinase
- GABA
gamma-aminobutyric acid
- GPCR
G protein-coupled receptor
- GIRK
G-protein-coupled inwardly rectifying K+ channel
- GnRH
gonadotropin releasing hormone
- Gq-mER
STX-activated, membrane estrogen receptor
- GPR30/GPER-1
G-protein-coupled estrogen receptor 1 (IUPHAR designation)
- IGF-1
insulin growth factor 1
- IP3
inositol 1,4,5-triphosphate
- KATP
ATP-sensitive K+ channel
- LTP
long term potentiation
- LH
luteinizing hormone
- mPOA
medial preoptic area
- MAPK
mitogen-activated protein kinase
- MEK
mitogen-activated protein kinase kinase
- mGluR1a
metabotropic glutamate receptor 1a
- MOR
mu-opioid receptor
- NFkappaB
nuclear factor kappa-light-chain-enhancer of activated B cells
- NPY
neuropeptide Y
- PI3K
phosphatidylinositol 3-kinase
- PIP2
phosphatidylinositol 4,5-bisphosphate
- PKA
cAMP-dependent protein kinase
- PKC
protein kinase C
- PLC
phospholipase C
- POMC
proopiomelanocortin
- STAT
signal transducers and activator of transcription
- VMH
ventromedial hypothalamus
References
- Green S, Walter P, Kumar V, Krust A, Bornert JM, Argos P, Chambon P. Human oestrogen receptor cDNA: sequence, expression and homology to v-erb-A. Nature. 1986;320:134–139. doi: 10.1038/320134a0. [DOI] [PubMed] [Google Scholar]
- Mosselman S, Polman J, Dijkema R. ERβ: Identification and characterization of a novel human estrogen receptor. FEBS Lett. 1996;392:49–53. doi: 10.1016/0014-5793(96)00782-x. [DOI] [PubMed] [Google Scholar]
- Arnold AP. The organizational-activational hypothesis as the foundation for a unified theory of sexual differentiation of all mammalian tissues. Horm Behav. 2009 Mar 5;55:570–578. doi: 10.1016/j.yhbeh.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly MJ, Moss RL, Dudley CA. The effects of microelecrophoretically applied estrogen, cortisol, and acetylcholine on medial preoptic-septal unit activity throughout the estrous cycle of the female rat. Exp Brain Res. 1977;30:53–64. doi: 10.1007/BF00237858. [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Rønnekleiv OK, Eskay RL. Identification of estrogen-responsive LHRH neurons in the guinea pig hypothalamus. Brain Res Bull. 1984;12:399–407. doi: 10.1016/0361-9230(84)90112-6. [DOI] [PubMed] [Google Scholar]
- Szego CM, Davis JS. Adenosine 3′,5′-monophosphate in rat uterus: acute elevation by estrogen. Proc Natl Acad Sci USA. 1967;58:1711–1718. doi: 10.1073/pnas.58.4.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rønnekleiv OK, Kelly MJ. Diversity of ovarian steroid signaling in the hypothalamus. Front Neuroendo. 2005;26:65–84. doi: 10.1016/j.yfrne.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Mermelstein PG, Micevych P. Nervous system physiology regulated by membrane estrogen receptors. Rev Neurosci. 2008 Jan 1;19:413–424. doi: 10.1515/revneuro.2008.19.6.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasudevan N, Pfaff DW. Non-genomic actions of estrogens and their interaction with genomic actions in the brains. Front Neuroendo. 2008;29:238–257. doi: 10.1016/j.yfrne.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Micevych P, Kuo J, Christensen A. Physiology of membrane oestrogen receptor signalling in reproduction. J Neuroendocrinology. 2008 Dec 23;21:249–256. doi: 10.1111/j.1365-2826.2009.01833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Bosch MA, Tobias SC, Krust A, Graham S, Murphy S, Korach KS, Chambon P, Scanlan TS, Rønnekleiv OK, Kelly MJ. A G protein-coupled estrogen receptor is involved in hypothalamic control of energy homeostasis. J Neurosci. 2006;26:5649–5655. doi: 10.1523/JNEUROSCI.0327-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasudevan N, Kow LM, Pfaff D. Integration of steroid hormone initiated membrane action to genomic function in the brain. Steroids. 2005;70:388–396. doi: 10.1016/j.steroids.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Roepke TA, Qiu J, Bosch MA, Rønnekleiv OK, Kelly MJ. Cross-talk between membrane-initiated and nuclear-initiated oestrogen signalling in the hypothalamus. J Neuroendocrinol. 2009;21:263–270. doi: 10.1111/j.1365-2826.2009.01846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly MJ, Rønnekleiv OK. Control of CNS neuronal excitability by estrogens via membrane-initiated signaling. Mol Cell Endocrinol. 2009;308:17–25. doi: 10.1016/j.mce.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade CB, Robinson S, Shapiro RA, Dorsa DM. Estrogen receptor (ER)alpha and ERbeta exhibit unique pharmacologic properties when coupled to activation of the mitogen-activated protein kinase pathway. Endo. 2001;142:2336–2342. doi: 10.1210/endo.142.6.8071. [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Loose MD, Rønnekleiv OK. Estrogen suppresses μ-opioid and GABAB-mediated hyperpolarization of hypothalamic arcuate neurons. J Neurosci. 1992;12:2745–2750. doi: 10.1523/JNEUROSCI.12-07-02745.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AW, Kyrozis A, Chevaleyre V, Kow L-M, Zhou J, Devidze N, Zhang Q, Etgen AM, Pfaff DW. Voltage-dependent calcium channels in ventromedial hypothalamic neurones of postnatal rats: modulation by oestradiol and phenylephrine. J Neuroendocrinology. 2008;20:188–198. doi: 10.1111/j.1365-2826.2007.01637.x. [DOI] [PubMed] [Google Scholar]
- Foy MR, Xu J, Xie X, Brinton RD, Thompson RF, Berger TW. 17 β-estradiol enhances NMDA receptor-mediated EPSPs and long-term potentiation. J Neurophysiol. 1999;81:925–929. doi: 10.1152/jn.1999.81.2.925. [DOI] [PubMed] [Google Scholar]
- Arreguin-Arevalo A, Nett TM. A nongenomic action of 17β-estradiol as the mechanism underlying the acute suppression of secretion of luteinizing hormone. Biol Repro. 2005 Mar 16;73:115–122. doi: 10.1095/biolreprod.105.040329. [DOI] [PubMed] [Google Scholar]
- Navarro CE, Saeed SA, Murdock C, Martinez-Fuentes AJ, Arora KK, Krsmanovic LZ, Catt KJ. Regulation of cyclic adenosine 3′,5′-monophosphate signaling and pulsatile neurosecretion by Gi-coupled plasma membrane estrogen receptors in immortalized gonadotrophin-releasing hormone neurons. Mol endocrinol. 2003;17:1792–1804. [PubMed] [Google Scholar]
- Glidewell-Kenney C, Hurley LA, Pfaff L, Weiss J, Levine JE, Jameson JL. Nonclassical estrogen receptor α signaling mediates negative feedback in the female mouse reproductive axis. Proc Natl Acad Sci USA. 2007;104:8173–8177. doi: 10.1073/pnas.0611514104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisel RL, Pfaff DW. RNA and protein synthesis inhibitors: effects on sexual behavior in female rats. Brain Res Bull. 1984 Jan 1;12:187–193. doi: 10.1016/0361-9230(84)90188-6. [DOI] [PubMed] [Google Scholar]
- Lebesgue D, Traub M, De Butte-Smith M, Chen C, Zukin RS, Kelly MJ, Etgen AM. Acute administration of non-classical estrogen receptor agonists attenuates ischemia-induced hippocampal neuron loss in middle-aged female rats. PLos One. 2010;5:1–8. doi: 10.1371/journal.pone.0008642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster TC. Interaction of rapid signal transduction cascades and gene expression in mediating estrogen effects on memory over the life span. Front Neuroendo. 2005 Jul 14;26:51–64. doi: 10.1016/j.yfrne.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Hammes SR, Levin ER. Extra-nuclear steroid receptors: nature and actions. Endocr Rev. 2007;28:726–741. doi: 10.1210/er.2007-0022. [DOI] [PubMed] [Google Scholar]
- Björnström L, Sjöberg M. Mechanisms of estogen receptor signaling: convergence of genomic and nongenomic actions on target genes. Mol Endo. 2005;19:833–842. doi: 10.1210/me.2004-0486. [DOI] [PubMed] [Google Scholar]
- Levin ER. Integration of the extranuclear and nuclear actions of estrogen. Mol Endocrinol. 2005;19:1951–1959. doi: 10.1210/me.2004-0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasudevan N, Pfaff DW. Membrane-initiated actions of estrogens in neuroendocrinology: emerging principles. Endocr Rev. 2007;28:1–19. doi: 10.1210/er.2005-0021. [DOI] [PubMed] [Google Scholar]
- Rønnekleiv OK, Malyala A, Kelly MJ. Membrane-initiated signaling of estrogen in the brain. Semin Reprod Med. 2007;25:165–176. doi: 10.1055/s-2007-973429. [DOI] [PubMed] [Google Scholar]
- Filardo EJ, Thomas P. GPR30: a seven-transmembrane-spanning estrogen receptor that triggers EGF release. Trends in Endocrinology and Metabolism. 2005;16:362–367. doi: 10.1016/j.tem.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Mhyre AJ, Dorsa DM. Estrogen activates rapid signaling in the brain. role of estrogen receptor α and estrogen receptor β in neurons and glia. Neurosci. 2006;138:851–858. doi: 10.1016/j.neuroscience.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Acconcia F, Ascenzi P, Bocedi A, Spisni E, Tomasi V, Trentalance A, Visca P, Marino M. Palmitoylation-dependent Estrogen Receptor {alpha} Membrane Localization: Regulation by 17{beta}-Estradiol. Mol Biol Cell. 2004 Oct 20; doi: 10.1091/mbc.E04-07-0547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razandi M, Alton G, Pedram A, Ghonshani S, Webb P, Levin ER. Identification of a structural determinant necessary for the localization and function of estrogen receptor α at the plasma membrane. Molecular and Cellular Biology. 2003;23:1633–1646. doi: 10.1128/MCB.23.5.1633-1646.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micevych P, Dominguez R. Membrane estradiol signaling in the brain. Front Neuroendo. 2009 May 3;30:315–327. doi: 10.1016/j.yfrne.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etgen AM, González-Flores O, Todd BJ. The role of insulin-like growth factor-I and growth factor-associated signal transduction pathways in estradiol and progesterone facilitation of female reproductive behaviors. Front Neuroendocrinol. 2006;27:363–375. doi: 10.1016/j.yfrne.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Toran-Allerand CD. Estrogen and the brain. beyond ER-α, ER-β and 17β-estradiol. Ann NY Acad Sci. 2005;1052:136–144. doi: 10.1196/annals.1347.009. [DOI] [PubMed] [Google Scholar]
- Qiu J, Bosch MA, Tobias SC, Grandy DK, Scanlan TS, Rønnekleiv OK, Kelly MJ. Rapid signaling of estrogen in hypothalamic neurons involves a novel G protein-coupled estrogen receptor that activates protein kinase C. J Neurosci. 2003;23:9529–9540. doi: 10.1523/JNEUROSCI.23-29-09529.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307:1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- Funakoshi T, Yanai A, Shinoda K, Kawano MM, Mizukami Y. G protein-coupled receptor 30 is an estrogen receptor in the plasma membrane. Biochem Biophys Res Commun. 2006;346:904–910. doi: 10.1016/j.bbrc.2006.05.191. [DOI] [PubMed] [Google Scholar]
- Toran-Allerand CD, Guan X, MacLusky NJ, Horvath TL, Diano S, Singh M, Connolly ES, Jr, Nethrapalli IS, Tinnikov AA. ER-X: a novel, plasma membrane-associated, putative estrogen receptor that is regulated during development and after ischemic brain injury. J Neurosci. 2002 Oct 1;22:8391–8401. doi: 10.1523/JNEUROSCI.22-19-08391.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P, Pang Y, Filardo EJ, Dong J. Identity of an Estrogen Membrane Receptor Coupled to a G-protein in Human Breast Cancer Cells. Endo. 2005 Feb 1;146:624–632. doi: 10.1210/en.2004-1064. [DOI] [PubMed] [Google Scholar]
- Filardo EJ. Epidermal growth factor receptor (EGFR) transactivation by estrogen via the G-protein-coupled receptor, GPR30: a novel signaling pathway with potential significance for breast cancer. J Steroid Biochem Mol Biol. 2002;80:231–238. doi: 10.1016/s0960-0760(01)00190-x. [DOI] [PubMed] [Google Scholar]
- Hazell GGJ, Yao ST, Roper JA, Prossnitz ER, O’Carroll A-M. Localisation of GPR30, a novel G protein-coupled oestrogen receptor, suggest multiple functions in rodent brain and peripheral tissues. J Endo. 2009 Jan 1;202:223–236. doi: 10.1677/JOE-09-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel SD, Keen KL, Baumann DI, Filardo EJ, Terasawa E. Involvement of G-protein couple receptor 30 (GPR30) in rapid action of estrogen in primate LHRH neurons. Mol Endo. 2009 Mar 23;3:349–359. doi: 10.1210/me.2008-0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagrange AH, Rønnekleiv OK, Kelly MJ. Modulation of G protein-coupled receptors by an estrogen receptor that activates protein kinase A. Mol Pharmacol. 1997;51:605–612. doi: 10.1124/mol.51.4.605. [DOI] [PubMed] [Google Scholar]
- Zhang C, Kelly MJ, Rønnekleiv OK. 17β-estradiol rapidly increases adenosine 5’-triphosphate-sensitive potassium channel activity in gonadotropin-releasing hormone neurons via a protein kinase signaling pathway. Endo. 2010 Jun 9;151:0000–0000. doi: 10.1210/en.2010-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbison AE. Multimodal influence of estrogen upon gonadotropin-releasing hormone neurons. Endocr Rev. 1998;19:302–330. doi: 10.1210/edrv.19.3.0332. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Park C, McDevitt MA, Glidewell-Kenney C, Chambon P, Weiss J, Jameson JL, Levine JE. p21-activated kinase mediates rapid estradiol-negative feedback actions in the reproductive axis. Proc Natl Acad Sci USA. 2009;106:7221–7226. doi: 10.1073/pnas.0812597106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glidewell-Kenney C, Weiss J, Hurley LA, Levine JE, Jameson JL. Estrogen receptor α signaling pathways differentially regulate gonadrotropin subunit gene expression and serum follicle-stimulating hormone in the female mouse. Endo. 2008;149:4168–4176. doi: 10.1210/en.2007-1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottsch ML, Navarro VM, Zhao Z, Glidewell-Kenney C, Weiss J, Jameson JL, Clifton DK, Levine JE, Steiner RA. Regulation of Kiss1 and dynorphin gene expression in the murine brain by classical and nonclassical estrogen receptor pathways. J Neurosci. 2009;29:9390–9395. doi: 10.1523/JNEUROSCI.0763-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly MJ, Ronnekleiv OK. Rapid membrane effects of estrogen in the central nervous system. In: Pfaff DW, editor. Hormones, Brain and Behavior. Academic Press; San Diego: 2002. pp. 361–380. [Google Scholar]
- Kelly MJ, Wagner EJ. GnRH neurons and episodic bursting activity. Trends Endocrinol Metab. 2002;13:409–410. doi: 10.1016/s1043-2760(02)00698-7. [DOI] [PubMed] [Google Scholar]
- Zhang C, Bosch MA, Levine JE, Rønnekleiv OK, Kelly MJ. Gonadotropin-releasing hormone neurons express KATP Channels that are regulated by estrogen and responsive to glucose and metabolic inhibition. J Neurosci. 2007;27:10153–10164. doi: 10.1523/JNEUROSCI.1657-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Z, Andrade J, Shupnik MA, Moenter SM. Differential regulation of gonadotropin-releasing hormone neuron activity and membrane properties by acutely applied estradiol: dependence on dose and estrogen receptor subtype. J Neurosci. 2009;29:5616–5627. doi: 10.1523/JNEUROSCI.0352-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagrange AH, Rønnekleiv OK, Kelly MJ. Estradiol-17β and μ-opioid peptides rapidly hyperpolarize GnRH neurons: A cellular mechanism of negative feedback? Endo. 1995;136:2341–2344. doi: 10.1210/endo.136.5.7720682. [DOI] [PubMed] [Google Scholar]
- Sun J, Chu Z, Moenter SM. Diurnal in vivo and rapid in vitro effects of estadiol on voltage-gated calcium channels in gonadotropin-releasing hormone neurons. The Journal of Neuroscience. 2010 Mar 17;30:3912–3923. doi: 10.1523/JNEUROSCI.6256-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe H, Terasawa E. Firing pattern and rapid modulation of activity by estrogen in primate luteinizing hormone releasing hormone-1 neurons. Endo. 2005;146:4312–4320. doi: 10.1210/en.2005-0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasawa E, Keen KL, Mogi K, Claude P. Pulsatile release of luteinizing hormone-releasing hormone (LHRH) in cultured LHRH neurons derived from the embryonic olfactory placode of the rhesus monkey. Endocrinlogy. 1999;140:1432–1441. doi: 10.1210/endo.140.3.6559. [DOI] [PubMed] [Google Scholar]
- Temple JL, Laing E, Sunder A, Wray S. Direct action of estradiol on gonadotropin-releasing hormone-1 neuronal activity via a transcription-dependent mechanism. J Neurosci. 2004 Jul 14;24:6326–6333. doi: 10.1523/JNEUROSCI.1006-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple JL, Wray S. BSA-estrogen compounds differentially alter gonadotropin-releasing hormone-1 neuronal activity. Endo. 2005 Nov 11;146:558–563. doi: 10.1210/en.2004-1117. [DOI] [PubMed] [Google Scholar]
- Levine JE, Norman RL, Gliessman PM, Oyama TT, Bangsberg DR, Spies HG. In vivo gonadotropin-releasing hormone release and serum luteinizing hormone measurements in ovariectomized, estrogen-treated Rhesus macaques. Endo. 1985;117:711–721. doi: 10.1210/endo-117-2-711. [DOI] [PubMed] [Google Scholar]
- Gearing M, Terasawa E. Luteinizing hormone releasing hormone (LHRH) neuroterminals mapped using the push-pull perfusion method inb the rhesus monkey. Brain Res Bull. 1988 Jan 1;21:117–121. doi: 10.1016/0361-9230(88)90126-8. [DOI] [PubMed] [Google Scholar]
- Abe H, Keen KL, Terasawa E. Rapid action of estrogens on intracellular calcium oscillations in primate LHRH-1neurons. Endo. 2008;149:1155–1162. doi: 10.1210/en.2007-0942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevot V, Croix D, Rialas CM, Poulain P, Fricchione GL, Stefano GB, Beauvillain JC. Estradiol coupling to endothelial nitric oxide stimulates gonadotropin-releasing hormone release from rat median eminence via a membrane receptor. Endo. 1999;140:652–659. doi: 10.1210/endo.140.2.6484. [DOI] [PubMed] [Google Scholar]
- Arreguin-Arevalo A, Ashley RL, Wagenmaker ER, Oakley AE, Karsh FJ, Nett TM. Membrane-initiated actions of estradiol (E2) in the regulation of LH secretion in ovariectomized (OVX) ewes. Reproductive Biology and Endocrinology. 2010 Aug 15;8:1–12. doi: 10.1186/1477-7827-8-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein JD, Erskine MS. Feminine sexual behavior: cellular integration of hormonal and afferent information in the rodent forebrain. In: Pfaff D, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. Academic Press; San Diego, CA U.S.A: 2002. pp. 139–214. [Google Scholar]
- Quesada A, Etgen AM. Insulin-like growth factor-1 regulation of α1-adrenergic receptor signaling is estradiol dependent in the preoptic area and hypothalamus of female rats. Endo. 2001;142:599–607. doi: 10.1210/endo.142.2.7946. [DOI] [PubMed] [Google Scholar]
- Cardona-Gómez GP, Mendez P, Garcia-Segura LM. Synergistic interaction of estradiol and insulin-like growth factor-I in the activation of PI3K/Akt signaling in the adult rat hypothalamus. Mol Brain Res. 2002 Oct 30;107:80–88. doi: 10.1016/s0169-328x(02)00449-7. [DOI] [PubMed] [Google Scholar]
- Mendez P, Azcoitia I, Garcia-Segura LM. Estrogen receptor alpha forms estrogen-dependent multimolecular complexes with insulin-like growth factor receptor and phospatidylinositol 3-kinase in the adult rat brain. Mol Brain Res. 2003;112:170–176. doi: 10.1016/s0169-328x(03)00088-3. [DOI] [PubMed] [Google Scholar]
- Quesada A, Etgen AM. Functional interactions between estrogen and insulin-like growth factor-I in the regulation of α1B-adrenoceptors and female reproductive function. J Neurosci. 2002;22:2401–2408. doi: 10.1523/JNEUROSCI.22-06-02401.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etgen AM, Acosta-Martinez M. Participation of growth factor signal transduction pathways in estradiol facilitation of female reproductive behavior. Endo. 2003;144:3828–3835. doi: 10.1210/en.2003-0157. [DOI] [PubMed] [Google Scholar]
- Apostolakis EM, Garai J, Lohmann JE, Clark JH, O’Malley BW. Epidermal growth factor activates reproductive behavior independent of ovarian steroids in female rodents. Mol Endo. 2000;14:1086–1098. doi: 10.1210/mend.14.7.0490. [DOI] [PubMed] [Google Scholar]
- Etgen AM, Ansonoff MA, Quesada A. Mechanisms of ovarian steroid regulation of norepinephrine receptor-mediated signal transduction in the hypothalamus: Implications for female reproductive physiology. Horm Behav. 2001;40:169–177. doi: 10.1006/hbeh.2001.1676. [DOI] [PubMed] [Google Scholar]
- Sinchak K, Micevych PE. Progesterone blockade of estrogen activation of mu-opioid receptors regulates reproductive behavior. J Neurosci. 2001 Aug 1;21:5723–5729. doi: 10.1523/JNEUROSCI.21-15-05723.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi T, Okere CO. Role of the supraoptic nucleus in regulation of parturition and milk ejection revisited. Microsc Res Tech. 2002 Jan 15;56:113–121. doi: 10.1002/jemt.10016. [DOI] [PubMed] [Google Scholar]
- Dewing P, Boulware MI, Sinchak K, Christensen A, Mermelstein PG, Micevych P. Membrane estrogen receptor-α interactions with metabotropic glutamate receptor 1a modulate female sexual receptivity in rats. J Neurosci. 2007;27:9294–9300. doi: 10.1523/JNEUROSCI.0592-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinchak K, Shahedi K, Dewing P, Micevych P. Sexual receptivity is reduced in the female mu-opioid receptor knockout mouse. NeuroReport. 2005 Oct 17;15:1697–1700. doi: 10.1097/01.wnr.0000181585.49130.93. [DOI] [PubMed] [Google Scholar]
- Kuo J, Hariri OR, Bondar G, Ogi J, Micevych P. Membrane estrogen receptor-α interacts with metabotropic glutamate receptor type 1a to mobilize intracellular calcium in hypothalamic astrocytes. Endo. 2009 Mar 1;150:1369–1376. doi: 10.1210/en.2008-0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kow LM, Pfaff DW. The membrane actions of estrogens can potentiate their lordosis behavior-facilitating genomic actions. Proc Natl Acad Sci U S A. 2004 Aug 17;101:12354–12357. doi: 10.1073/pnas.0404889101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micevych PE, Chaban V, Ogi J, Dewing P, Lu JKH, Sinchak K. Estradiol stimulates progesterone synthesis in hypothalamic astrocyte cultures. Endo. 2007;148:782–789. doi: 10.1210/en.2006-0774. [DOI] [PubMed] [Google Scholar]
- Boulware MI, Weick JP, Becklund BR, Kuo SP, Groth RD, Mermelstein PG. Estradiol activates group I and II metabotropic glutamate receptor signaling, leading to opposing influences on cAMP response element-binding protein. The Journal of Neuroscience. 2005;25:5066–5078. doi: 10.1523/JNEUROSCI.1427-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kow L-M, Mobbs CV, Pfaff DW. Roles of second-messenger systems and neuronal activity in the regulation of lordosis by neurotransmitters, neuropeptides, and estrogen: A review. Neurosci Biobehav Rev. 1994;18:251–268. doi: 10.1016/0149-7634(94)90028-0. [DOI] [PubMed] [Google Scholar]
- Kow LM, Pfaff DW. The membrane actions of estrogens can potentiate their lordosis behavior-facilitating genomic actions. Proc Natl Acad Sci U S A. 2004 Aug 17;101:12354–12357. doi: 10.1073/pnas.0404889101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornil CA. Rapid regulation of brain oestrogen synthesis: the behavioural roles of oestrogens and their fates. J Neuroendocrinology. 2009 Aug 1;21:217–226. doi: 10.1111/j.1365-2826.2009.01822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross E, Roselli CE. 17β-estradiol rapidly facilitates chemoinvestigation and mounting in castrated male rates. Amercian Journal of Physiology. 1999 Sep 1;:R1346–R1350. doi: 10.1152/ajpregu.1999.276.5.R1346. [DOI] [PubMed] [Google Scholar]
- Huddleston GG, Paisley JC, Graham S, Grober MS, Clancy AN. Implants of estradiol conjugated to bovine serum albumin in the male rat medial preoptic area promote copulatory behavior. Neuroendo. 2007 Aug 27;86:249–259. doi: 10.1159/000107695. [DOI] [PubMed] [Google Scholar]
- Huddleston GG, Paisley JC, Clancy AN. Effects of estrogen in the male rat medial amygdala: infusion of an aromatase inhibitor lowers mating and bovine serum albumin-conjugated estradiol implants do not promote mating. Neuroendo. 2006 Jul 5;83:106–116. doi: 10.1159/000094400. [DOI] [PubMed] [Google Scholar]
- Taziaux M, Keller M, Bakker J, Balthazart J. Sexual behavior activity tracks rapid changes in brain estrogen concentrations. The Journal of Neuroscience. 2007 Jun 13;27:6563–6572. doi: 10.1523/JNEUROSCI.1797-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geary N, Asarian L, Korach KS, Pfaff DW, Ogawa S. Deficits in E2-dependent control of feeding, weight gain, and cholecystokinin satiation in ER-alpha null mice. Endo. 2001;142:4751–4757. doi: 10.1210/endo.142.11.8504. [DOI] [PubMed] [Google Scholar]
- Qiu J, Xue C, Bosch MA, Murphy JG, Fan W, Rønnekleiv OK, Kelly MJ. Serotonin 5HT2c receptor signaling in hypothalamic POMC neurons: role in energy homeostasis in females. Mol Pharm. 2007;72:885–896. doi: 10.1124/mol.107.038083. [DOI] [PubMed] [Google Scholar]
- Gao Q, Mezei G, Nie Y, Rao Y, Choi CS, Bechmann I, Leranth C, Toran-Allerand D, Priest CA, Roberts JL, Gao X-B, Mobbs C, Shulman GI, Diano S, Horvath TL. Anorectic estrogen mimics leptin’s effect on the rewiring of melanocortin cells and Stat3 signaling in obese animals. Nature Med. 2006;13:89–94. doi: 10.1038/nm1525. [DOI] [PubMed] [Google Scholar]
- Qiu J, Rønnekleiv OK, Kelly MJ. Modulation of hypothalamic neuronal activity through a novel G-protein coupled estrogen membrane receptor. Steroids. 2008;73:985–991. doi: 10.1016/j.steroids.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weatherill PJ, Wilson APM, Nicholson RI, Davies P, Wakeling AE. Interaction of the antioestrogen ICI 164,384 with the oestrogen receptor. J Ster Bioc Mol Biol. 1988;30:263–266. doi: 10.1016/0022-4731(88)90103-3. [DOI] [PubMed] [Google Scholar]
- Tobias SC, Qiu J, Kelly MJ, Scanlan TS. Synthesis and biological evaluation of SERMs with potent nongenomic estrogenic activity. ChemMedChem. 2006;1:565–571. doi: 10.1002/cmdc.200500098. [DOI] [PubMed] [Google Scholar]
- Malyala A, Zhang C, Bryant D, Kelly MJ, Rønnekleiv OK. PI3K signaling effects in hypothalamic neurons mediated by estrogen. J Comp Neurol. 2008;506:895–911. doi: 10.1002/cne.21584. [DOI] [PubMed] [Google Scholar]
- Roepke TA, Xue C, Bosch MA, Scanlan TS, Kelly MJ, Rønnekleiv OK. Genes associated with membrane-initiated signaling of estrogen and energy homeostasis. Endo. 2008;149:6113–6124. doi: 10.1210/en.2008-0769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roepke TA, Bosch MA, Rick EA, Lee B, Wagner EJ, Seidlová-Wuttke D, Wuttke W, Scanlan TS, Rønnekleiv OK, Kelly MJ. Contribution of a membrane estrogen receptor to the estrogenic regulation of body temperature and energy homeostasis. Endo. 2010 Jan 1;151:4926–4937. doi: 10.1210/en.2010-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin JD, Saper CB, Boulant JA. Synaptic and morphological characteristics of temperature-sensitive and -insensitive rat hypothalamic neurones. J Physiol. 2001;537.2:521–535. doi: 10.1111/j.1469-7793.2001.00521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Matsumura K, Kaneko T, Kobayashi S, Katoh H, Negishi M. The rostral raphe pallidus nucleus mediates pyrogenic transmission from the preoptic area. J Neurosci. 2002 Jun 1;22:4600–4610. doi: 10.1523/JNEUROSCI.22-11-04600.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulant JA. Neuronal basis of Hammel’s model for set-point thermoregulation. J Appl Physiol. 2006;100:1347–1354. doi: 10.1152/japplphysiol.01064.2005. [DOI] [PubMed] [Google Scholar]
- Wagner EJ, Rønnekleiv OK, Bosch MA, Kelly MJ. Estrogen biphasically modifies hypothalamic GABAergic function concomitantly with negative and positive control of luteinizing hormone release. J Neurosci. 2001;21:2085–2093. doi: 10.1523/JNEUROSCI.21-06-02085.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulant JA. Hypothalamic neurons. Ann NY Acad Sci. 1998;856:108–115. doi: 10.1111/j.1749-6632.1998.tb08319.x. [DOI] [PubMed] [Google Scholar]
- Berendsen HHG, Weekers AHJ, Kloosterboer HJ. Effect of tibolone and raloxifene on the tail temperature of oestrogen-deficient rats. Eur J Pharmacol. 2001;419:47–54. doi: 10.1016/s0014-2999(01)00966-9. [DOI] [PubMed] [Google Scholar]
- Sipe K, Leventhal L, Burroughs K, Cosmi S, Johnston GH, Deecher DC. Serotonin 2A receptors modulate tail-skin temperature in two rodent models of estrogen deficiency-related thermoregulatory dysfunction. Brain Res. 2004;1028:191–202. doi: 10.1016/j.brainres.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Stearns V, Ullmer L, Lopez JF, Smith Y, Isaacs C, Hayes DF. Hot flushes. Lancet. 2002 Dec 7;360:1851–1861. doi: 10.1016/s0140-6736(02)11774-0. [DOI] [PubMed] [Google Scholar]
- Deecher DC, Alfinito PD, Leventhal L, Cosmi S, Johnston GH, Merchanthaler I, Winneker R. Alleviation of thermoregulatory dysfunction with the new serotonin and norepinephrine reuptake inhibitor desvenlafaxine succinate in ovariectomized rodent models. Endo. 2007;148:1376–1383. doi: 10.1210/en.2006-1163. [DOI] [PubMed] [Google Scholar]
- Sylvia VL, Walton J, Lopez D, Dean DD, Boyan BD, Schwartz Z. 17 beta-estradiol-BSA conjugates and 17 beta-estradiol regulate growth plate chondrocytes by common membrane associated mechanisms involving PKC dependent and independent signal transduction. J Cell Biochem. 2002;81:413–429. doi: 10.1002/1097-4644(20010601)81:3<413::aid-jcb1055>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Endoh H, Sasaki H, Maruyama K, Takeyama K, Waga I, Shimizu T, Kato S, Kawashima H. Rapid activation of MAP Kinase by estrogen in the bone cell line. Biochem Biophys Res Commun. 1997;235:99–102. doi: 10.1006/bbrc.1997.6746. [DOI] [PubMed] [Google Scholar]
- Yadav VK, Oury F, Suda N, Liu Z-W, Gao X-B, Confavreux C, Klemenhagen KC, Tanaka KF, Gingrich JA, Guo XE, Tecott LH, Mann JJ, Hen R, Horvath TL, Karsenty G. A serotonin-dependent mechanism explains the leptin regulation of bone mass, appetite, and energy expenditure. Cell. 2009;138:976–989. doi: 10.1016/j.cell.2009.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda S, Elefteriou F, Levasseur R, Liu X, Zhao L, Parker KL, Armstrong D, Ducy P, Karsenty G. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002;111:305–317. doi: 10.1016/s0092-8674(02)01049-8. [DOI] [PubMed] [Google Scholar]
- Elefteriou F, Ahn JD, Takeda S, Starbuck M, Yang X, Liu X, Kondo H, Richards WG, Bannon TW, Noda M, Clement K, Valsse C, Karsenty G. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature. 2005;434:514–520. doi: 10.1038/nature03398. [DOI] [PubMed] [Google Scholar]
- Woolley CS. Acute effects of estrogen on neuronal physiology. Annu Rev Pharmacol Toxicol. 2007;47:657–680. doi: 10.1146/annurev.pharmtox.47.120505.105219. [DOI] [PubMed] [Google Scholar]
- Bliss TVP, Lømo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foy MR. 17β-estradiol: effect on CA1 hippocampal synaptic plasticity. Neurobiol Learn Mem. 2001;76:239–252. doi: 10.1006/nlme.2001.4018. [DOI] [PubMed] [Google Scholar]
- Bi R, Broutman G, Foy MR, Thompson RF, Baudry M. The tyrosine kinase and mitogen-activated protein kinase pathways mediate multiple effects of estrogen in hippocampus. Proc Natl Acad Sci U S A. 2000;97:3602–3607. doi: 10.1073/pnas.060034497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Q, Moss RL. 17beta-estradiol potentiates kainate-induced currents via activation of the cAMP cascade. J Neurosci. 1996;16:3620–3629. doi: 10.1523/JNEUROSCI.16-11-03620.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Q, Moss RL. Novel mechanism for non-genomic action of 17beta-oestradiol on kainate-induced currents in isolated rat CA1 hippocampal neurones. J Physiol (Lond) 1998;506:745–754. doi: 10.1111/j.1469-7793.1998.745bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Q, Korach KS, Moss RL. Rapid action of 17beta-estradiol on kainate-induced currents in hippocampal neurons lacking intracellular estrogen receptors. Endo. 1999;140:660–666. doi: 10.1210/endo.140.2.6500. [DOI] [PubMed] [Google Scholar]
- Smith YR, Giordani B, Lajiness-O’Neill R, Zubieta JK. Long-term estrogen replacement is associated with improved nonverbal memory and attentional measures in postmenopausal women. Fertil Steril. 2001;76:1101–1107. doi: 10.1016/s0015-0282(01)02902-8. [DOI] [PubMed] [Google Scholar]
- Rapp PR, Morrison JH, Roberts JA. Cyclic estrogen replacement improves cognitive function in aged ovariectomized rhesus monkeys. J Neurosci. 2003 Jul 2;23:5708–5714. doi: 10.1523/JNEUROSCI.23-13-05708.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin BB. Surgical menopause, estrogen, and cognitive function in women: what do the findings tell us? Ann N Y Acad Sci. 2005;1052:3–10. doi: 10.1196/annals.1347.001. [DOI] [PubMed] [Google Scholar]
- Inagaki T, Gautreaux C, Luine V. Acute estrogen treatments facilitates recognition memory consolidation and alters monoamine levels in memory-related brain areas. Horm Behav. 2010 Jul 21;58:415–426. doi: 10.1016/j.yhbeh.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine VN, Jacome LF, MacLusky NJ. Rapid Enhancement of Visual and Place Memory by Estrogens in Rats. Endo. 2003 Jul 1;144:2836–2844. doi: 10.1210/en.2003-0004. [DOI] [PubMed] [Google Scholar]
- Packard MG. Posttraining estrogen and memory modulation. Horm Behav. 1998 May 11;34:126–139. doi: 10.1006/hbeh.1998.1464. [DOI] [PubMed] [Google Scholar]
- Rhodes ME, Frye CA. Estrogen has a mnemonic-enhancing effects in the inhibitory avoidance task. Pharmcol Biochem Behav. 2004 Jul 1;78:551–558. doi: 10.1016/j.pbb.2004.03.025. [DOI] [PubMed] [Google Scholar]
- Kurata K, Takebayashi M, Kagaya A, Morinobu S, Yamawaki S. Effect of beta-estradiol on voltage-gated Ca(2+) channels in rat hippocampal neurons: a comparison with dehydroepiandrosterone. Eur J Pharmacol. 2001 Mar 30;416:203–212. doi: 10.1016/s0014-2999(01)00880-9. [DOI] [PubMed] [Google Scholar]
- Rudick CN, Woolley CS. Estrogen regulates functional inhibition of hippocampal CA1 pyramidal cells in the adult female rat. J Neurosci. 2001 Sep 1;21:6532–6543. doi: 10.1523/JNEUROSCI.21-17-06532.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong M, Moss RL. Electrophysiological evidence for a rapid membrane action of the gonadal steroid 17β-estradiol, on CA1 pyramidal neurons of the rat hippocampus. Brain Res. 1991;543:148–152. doi: 10.1016/0006-8993(91)91057-8. [DOI] [PubMed] [Google Scholar]
- Wong M, Moss RL. Long-term and short-term electrophysiological effects of estrogen on the synaptic properties of hippocampal CA1 neurons. J Neurosci. 1992;12:3217–3225. doi: 10.1523/JNEUROSCI.12-08-03217.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong M, Moss RL. Patch-clamp analysis of direct steroidal modulation of glutamate receptor-channels. J Neuroendocrinology. 1994;6:347–355. doi: 10.1111/j.1365-2826.1994.tb00592.x. [DOI] [PubMed] [Google Scholar]
- Fernandez SM, Lewis MC, Pechenino AS, Harburger LL, Orr PT, Gresack JE, Schafe GE, Frick KM. Estradiol-induced enhancement of object memory consolidation involves hippocampal extracellular signal-regulated kinase activation and membrane-bound estrogen receptors. J Neurosci. 2008;28:8660–8667. doi: 10.1523/JNEUROSCI.1968-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis MC, Kerr KM, Orr PT, Frick KM. Estradiol-induced enhancement of object memory consolidation involves NMDA receptors and protein kinase A in the dorsal hippocampus of female C57BL/6 mice. Behav Neurosci. 2008 Jun 1;122:716–721. doi: 10.1037/0735-7044.122.3.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitka M. Studies explore stroke’s gender gap. JAMA. 2006 Apr 19;295:1755–1756. doi: 10.1001/jama.295.15.1755. [DOI] [PubMed] [Google Scholar]
- Birge SJ. Hormone therapy and stroke. Clinical Obstetrics and Gynecology. 2008 Sep 1;51:581–591. doi: 10.1097/GRF.0b013e318181df30. [DOI] [PubMed] [Google Scholar]
- Coker LH, Espeland MA, Rapp SR, Legault C, Resnick SM, Hogan P, Gaussoin S, Dailey M, Shumaker SA. Postmenopausal hormone therapy and cognitive outcomes: the women’s health initiative memory study. J Ster Bioc Mol Biol. 2010 Jan 20;118:304–310. doi: 10.1016/j.jsbmb.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebesgue D, Chevaleyre V, Zukin RS, Etgen AM. Estradiol rescues neurons from global ischemia-induced cell death: multiple cellular pathways of neuroprotection. Steroids. 2009 Jan 20;74:555–561. doi: 10.1016/j.steroids.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S, Brown CM, Wise PM. Neuroprotective effects of estrogens following ischemic stroke. Front Neuroendo. 2009 May 3;30:201–211. doi: 10.1016/j.yfrne.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant DN, Sheldahl LC, Marriott LK, Shapiro RA, Dorsa DM. Multiple pathways transmit neuroprotective effects of gonadal steroids. Endocrine. 2006;29:199–207. doi: 10.1385/ENDO:29:2:199. [DOI] [PubMed] [Google Scholar]
- Hayashi S, Ueyema T, Kajimoto T, Yagi K, Kohmura E, Saito N. Involvement of y protein kinase C in estrogen-induced neuroprotection against focal brain ischemia through G protein-coupled estrogen receptor. J Neurosci. 2005;93:883–891. doi: 10.1111/j.1471-4159.2005.03080.x. [DOI] [PubMed] [Google Scholar]
- Jover-Mengual T, Zukin RS, Etgen AM. MAPK signaling is critical to estradiol protection of CA1 neurons in global ischemia. Endo. 2007;148:1131–1143. doi: 10.1210/en.2006-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jover-Mengual T, Miyawaki T, Latuszek A, Alborch E, Zukin RS, Etgen AM. Acute estradiol protects CA1 neurons from ischemia-induced apoptotic cell death via the PI3K/Akt pathway. Brain Res. 2010 Jan 18;1321:1–12. doi: 10.1016/j.brainres.2010.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T-W, Wang JM, Chen S, Brinton RD. 17β-estradiol induced Ca2+ influx via L-type calcium channels activates the SRC/ERK/cyclic-AMP response element binding protein signal pathway and BCL-2 expression in rat hippocampal neurons: a potential initiation mechanism for estrogen-induced neuroprotection. Neurosci. 2005;135:59–72. doi: 10.1016/j.neuroscience.2004.12.027. [DOI] [PubMed] [Google Scholar]
- Zhang Q-G, Raz L, Wang R, Han D, De Sevilla L, Yang F, Vadlamudi RK, Brann DW. Estrogen attenuates ischemic oxidative damage via an estrogen receptor α-mediated inhibition of NADPH oxidase activation. J Neurosci. 2009;29:13823–13836. doi: 10.1523/JNEUROSCI.3574-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubal DB, Zhu H, Yu J, Rau SW, Shughrue PJ, Merchenthaler I, Kindy MS, Wise PM. Estrogen receptor alpha, not beta, is a critical link in estradiol-mediated protection against brain injury. Proc Natl Acad Sci U S A. 2001 Feb 13;98:1952–1957. doi: 10.1073/pnas.041483198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller NR, Jover T, Cohen HW, Zukin RS, Etgen AM. Estrogen can act via estrogen receptor α and β to protect hippocampal neurons against global ischemia-induced cell death. Endo. 2005;146:3070–3079. doi: 10.1210/en.2004-1515. [DOI] [PubMed] [Google Scholar]
- Yang LC, Zhang QG, Zhou CF, Yang F, Zhang YD, Wang RM, Brann DW. Extranuclear estrogen receptors mediate the neuroprotective effects of estrogen in the rat hippocampus. PLos One. 2010 May 7;5:1–8. doi: 10.1371/journal.pone.0009851. [DOI] [PMC free article] [PubMed] [Google Scholar]