Abstract
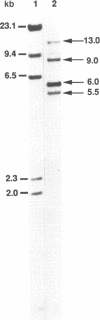
Fibrillarin is a nucleolar protein which is associated with small nucleolar RNAs, and is required for pre-rRNA processing. We have cloned and characterized the gene encoding fibrillarin in the fission yeast Schizosaccharomyces pombe and we have followed its expression under various conditions. Fission yeast fibrillarin is a 305 amino-acid protein which appears to be highly conserved throughout evolution. In Xenopus, human or Saccharomyces cerevisiae, a single fibrillarin mRNA is detected while, in S. pombe a single copy gene encodes different mRNAs which differ at the 3' ends. Under normal growth conditions, two mRNAs of 1.1 and 1.35 kb are detected with the 1.1 kb being the most abundant. Both the total amount and relative abundance of these two mRNAs are strongly affected by exposure to low temperature, namely the 1.1 kb mRNA almost disappears while the 1.35 kb is less markedly diminished. A new species of 3.2 kb accumulates in the cell, which contains an unusually long 3' untranslated region of 2 kb. We have found that exposure of the cells to a cold shock has a profound effect on 3' end formation in S.pombe since the transcription of several other mRNAs is also capable of skipping the normal 3' end site to terminate at a further downstream site.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amaldi F., Pierandrei-Amaldi P. Translational regulation of the expression of ribosomal protein genes in Xenopus laevis. Enzyme. 1990;44(1-4):93–105. doi: 10.1159/000468750. [DOI] [PubMed] [Google Scholar]
- Aris J. P., Blobel G. cDNA cloning and sequencing of human fibrillarin, a conserved nucleolar protein recognized by autoimmune antisera. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):931–935. doi: 10.1073/pnas.88.3.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachvarova R. F. A maternal tail of poly(A): the long and the short of it. Cell. 1992 Jun 12;69(6):895–897. doi: 10.1016/0092-8674(92)90606-d. [DOI] [PubMed] [Google Scholar]
- Bally M., Hughes J., Cesareni G. SnR30: a new, essential small nuclear RNA from Saccharomyces cerevisiae. Nucleic Acids Res. 1988 Jun 24;16(12):5291–5303. doi: 10.1093/nar/16.12.5291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caizergues-Ferrer M., Mariottini P., Curie C., Lapeyre B., Gas N., Amalric F., Amaldi F. Nucleolin from Xenopus laevis: cDNA cloning and expression during development. Genes Dev. 1989 Mar;3(3):324–333. doi: 10.1101/gad.3.3.324. [DOI] [PubMed] [Google Scholar]
- Caizergues-Ferrer M., Mathieu C., Mariottini P., Amalric F., Amaldi F. Developmental expression of fibrillarin and U3 snRNA in Xenopus laevis. Development. 1991 May;112(1):317–326. doi: 10.1242/dev.112.1.317. [DOI] [PubMed] [Google Scholar]
- Dandekar T., Tollervey D. Cloning of Schizosaccharomyces pombe genes encoding the U1, U2, U3 and U4 snRNAs. Gene. 1989 Sep 30;81(2):227–235. doi: 10.1016/0378-1119(89)90183-2. [DOI] [PubMed] [Google Scholar]
- Eng F. J., Warner J. R. Structural basis for the regulation of splicing of a yeast messenger RNA. Cell. 1991 May 31;65(5):797–804. doi: 10.1016/0092-8674(91)90387-e. [DOI] [PubMed] [Google Scholar]
- Fantes P. Epistatic gene interactions in the control of division in fission yeast. Nature. 1979 May 31;279(5712):428–430. doi: 10.1038/279428a0. [DOI] [PubMed] [Google Scholar]
- Frohman M. A., Dush M. K., Martin G. R. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatermann K. B., Teletski C., Gross T., Käufer N. F. A ribosomal protein gene family from Schizosaccharomyces pombe consisting of three active members. Curr Genet. 1989 Dec;16(5-6):361–367. doi: 10.1007/BF00340715. [DOI] [PubMed] [Google Scholar]
- Girard J. P., Lehtonen H., Caizergues-Ferrer M., Amalric F., Tollervey D., Lapeyre B. GAR1 is an essential small nucleolar RNP protein required for pre-rRNA processing in yeast. EMBO J. 1992 Feb;11(2):673–682. doi: 10.1002/j.1460-2075.1992.tb05099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm C., Kohli J., Murray J., Maundrell K. Genetic engineering of Schizosaccharomyces pombe: a system for gene disruption and replacement using the ura4 gene as a selectable marker. Mol Gen Genet. 1988 Dec;215(1):81–86. doi: 10.1007/BF00331307. [DOI] [PubMed] [Google Scholar]
- Henríquez R., Blobel G., Aris J. P. Isolation and sequencing of NOP1. A yeast gene encoding a nucleolar protein homologous to a human autoimmune antigen. J Biol Chem. 1990 Feb 5;265(4):2209–2215. [PubMed] [Google Scholar]
- Hughes J. M., Ares M., Jr Depletion of U3 small nucleolar RNA inhibits cleavage in the 5' external transcribed spacer of yeast pre-ribosomal RNA and impairs formation of 18S ribosomal RNA. EMBO J. 1991 Dec;10(13):4231–4239. doi: 10.1002/j.1460-2075.1991.tb05001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes J. M., Konings D. A., Cesareni G. The yeast homologue of U3 snRNA. EMBO J. 1987 Jul;6(7):2145–2155. doi: 10.1002/j.1460-2075.1987.tb02482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey T., Sadhale P., Platt T., Proudfoot N. Homologous mRNA 3' end formation in fission and budding yeast. EMBO J. 1991 Nov;10(11):3503–3511. doi: 10.1002/j.1460-2075.1991.tb04914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irniger S., Egli C. M., Braus G. H. Different classes of polyadenylation sites in the yeast Saccharomyces cerevisiae. Mol Cell Biol. 1991 Jun;11(6):3060–3069. doi: 10.1128/mcb.11.6.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob S. T., Terns M. P., Hengst-Zhang J. A., Vulapalli R. S. Polyadenylation of mRNA and its control. Crit Rev Eukaryot Gene Expr. 1990;1(1):49–59. [PubMed] [Google Scholar]
- Jansen R. P., Hurt E. C., Kern H., Lehtonen H., Carmo-Fonseca M., Lapeyre B., Tollervey D. Evolutionary conservation of the human nucleolar protein fibrillarin and its functional expression in yeast. J Cell Biol. 1991 May;113(4):715–729. doi: 10.1083/jcb.113.4.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass S., Tyc K., Steitz J. A., Sollner-Webb B. The U3 small nucleolar ribonucleoprotein functions in the first step of preribosomal RNA processing. Cell. 1990 Mar 23;60(6):897–908. doi: 10.1016/0092-8674(90)90338-f. [DOI] [PubMed] [Google Scholar]
- Kenan D. J., Query C. C., Keene J. D. RNA recognition: towards identifying determinants of specificity. Trends Biochem Sci. 1991 Jun;16(6):214–220. doi: 10.1016/0968-0004(91)90088-d. [DOI] [PubMed] [Google Scholar]
- Lapeyre B., Bourbon H., Amalric F. Nucleolin, the major nucleolar protein of growing eukaryotic cells: an unusual protein structure revealed by the nucleotide sequence. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1472–1476. doi: 10.1073/pnas.84.6.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapeyre B., Mariottini P., Mathieu C., Ferrer P., Amaldi F., Amalric F., Caizergues-Ferrer M. Molecular cloning of Xenopus fibrillarin, a conserved U3 small nuclear ribonucleoprotein recognized by antisera from humans with autoimmune disease. Mol Cell Biol. 1990 Jan;10(1):430–434. doi: 10.1128/mcb.10.1.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H. D., Zagorski J., Fournier M. J. Depletion of U14 small nuclear RNA (snR128) disrupts production of 18S rRNA in Saccharomyces cerevisiae. Mol Cell Biol. 1990 Mar;10(3):1145–1152. doi: 10.1128/mcb.10.3.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maicas E., Pluthero F. G., Friesen J. D. The accumulation of three yeast ribosomal proteins under conditions of excess mRNA is determined primarily by fast protein decay. Mol Cell Biol. 1988 Jan;8(1):169–175. doi: 10.1128/mcb.8.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley J. L., Proudfoot N. J., Platt T. RNA 3'-end formation. Genes Dev. 1989 Dec;3(12B):2218–2222. doi: 10.1101/gad.3.12b.2218. [DOI] [PubMed] [Google Scholar]
- Mayer S. A., Dieckmann C. L. Yeast CBP1 mRNA 3' end formation is regulated during the induction of mitochondrial function. Mol Cell Biol. 1991 Feb;11(2):813–821. doi: 10.1128/mcb.11.2.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno S., Klar A., Nurse P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1991;194:795–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- Porter G. L., Brennwald P. J., Holm K. A., Wise J. A. The sequence of U3 from Schizosaccharomyces pombe suggests structural divergence of this snRNA between metazoans and unicellular eukaryotes. Nucleic Acids Res. 1988 Nov 11;16(21):10131–10152. doi: 10.1093/nar/16.21.10131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potashkin J. A., Derby R. J., Spector D. L. Differential distribution of factors involved in pre-mRNA processing in the yeast cell nucleus. Mol Cell Biol. 1990 Jul;10(7):3524–3534. doi: 10.1128/mcb.10.7.3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presutti C., Ciafré S. A., Bozzoni I. The ribosomal protein L2 in S. cerevisiae controls the level of accumulation of its own mRNA. EMBO J. 1991 Aug;10(8):2215–2221. doi: 10.1002/j.1460-2075.1991.tb07757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raué H. A., Planta R. J. Ribosome biogenesis in yeast. Prog Nucleic Acid Res Mol Biol. 1991;41:89–129. doi: 10.1016/s0079-6603(08)60007-0. [DOI] [PubMed] [Google Scholar]
- Russell P., Nurse P. cdc25+ functions as an inducer in the mitotic control of fission yeast. Cell. 1986 Apr 11;45(1):145–153. doi: 10.1016/0092-8674(86)90546-5. [DOI] [PubMed] [Google Scholar]
- Sachs A. The role of poly(A) in the translation and stability of mRNA. Curr Opin Cell Biol. 1990 Dec;2(6):1092–1098. doi: 10.1016/0955-0674(90)90161-7. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Schimmang T., Tollervey D., Kern H., Frank R., Hurt E. C. A yeast nucleolar protein related to mammalian fibrillarin is associated with small nucleolar RNA and is essential for viability. EMBO J. 1989 Dec 20;8(13):4015–4024. doi: 10.1002/j.1460-2075.1989.tb08584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thekkumkara T. J., Livingston W., 3rd, Kumar R. S., Sen G. C. Use of alternative polyadenylation sites for tissue-specific transcription of two angiotensin-converting enzyme mRNAs. Nucleic Acids Res. 1992 Feb 25;20(4):683–687. doi: 10.1093/nar/20.4.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollervey D. A yeast small nuclear RNA is required for normal processing of pre-ribosomal RNA. EMBO J. 1987 Dec 20;6(13):4169–4175. doi: 10.1002/j.1460-2075.1987.tb02763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollervey D., Lehtonen H., Carmo-Fonseca M., Hurt E. C. The small nucleolar RNP protein NOP1 (fibrillarin) is required for pre-rRNA processing in yeast. EMBO J. 1991 Mar;10(3):573–583. doi: 10.1002/j.1460-2075.1991.tb07984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollervey D., Tessars G., Lührmann R. Immunoprecipitation distinguishes non-overlapping groups of snRNPs in Schizosaccharomyces pombe. Nucleic Acids Res. 1990 Sep 11;18(17):5207–5212. doi: 10.1093/nar/18.17.5207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsay Y. F., Thompson J. R., Rotenberg M. O., Larkin J. C., Woolford J. L., Jr Ribosomal protein synthesis is not regulated at the translational level in Saccharomyces cerevisiae: balanced accumulation of ribosomal proteins L16 and rp59 is mediated by turnover of excess protein. Genes Dev. 1988 Jun;2(6):664–676. doi: 10.1101/gad.2.6.664. [DOI] [PubMed] [Google Scholar]
- Wang Y. C., Rubenstein P. A. Choice of 3' cleavage/polyadenylation site in beta-tropomyosin RNA processing is differentiation-dependent in mouse BC3H1 muscle cells. J Biol Chem. 1992 Feb 5;267(4):2728–2736. [PubMed] [Google Scholar]
- Warner J. R., Mitra G., Schwindinger W. F., Studeny M., Fried H. M. Saccharomyces cerevisiae coordinates accumulation of yeast ribosomal proteins by modulating mRNA splicing, translational initiation, and protein turnover. Mol Cell Biol. 1985 Jun;5(6):1512–1521. doi: 10.1128/mcb.5.6.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner J. R. Synthesis of ribosomes in Saccharomyces cerevisiae. Microbiol Rev. 1989 Jun;53(2):256–271. doi: 10.1128/mr.53.2.256-271.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner J. R. The nucleolus and ribosome formation. Curr Opin Cell Biol. 1990 Jun;2(3):521–527. doi: 10.1016/0955-0674(90)90137-4. [DOI] [PubMed] [Google Scholar]
- Wickens M. How the messenger got its tail: addition of poly(A) in the nucleus. Trends Biochem Sci. 1990 Jul;15(7):277–281. doi: 10.1016/0968-0004(90)90054-f. [DOI] [PubMed] [Google Scholar]
- Zagorski J., Tollervey D., Fournier M. J. Characterization of an SNR gene locus in Saccharomyces cerevisiae that specifies both dispensible and essential small nuclear RNAs. Mol Cell Biol. 1988 Aug;8(8):3282–3290. doi: 10.1128/mcb.8.8.3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaret K. S., Sherman F. DNA sequence required for efficient transcription termination in yeast. Cell. 1982 Mar;28(3):563–573. doi: 10.1016/0092-8674(82)90211-2. [DOI] [PubMed] [Google Scholar]
- de Sauvage F., Kruys V., Marinx O., Huez G., Octave J. N. Alternative polyadenylation of the amyloid protein precursor mRNA regulates translation. EMBO J. 1992 Aug;11(8):3099–3103. doi: 10.1002/j.1460-2075.1992.tb05382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]