Abstract
Background
Many of researchers have focused on the emerging pathogen, Norovirus, since its first identification as the causing agent of nonbacterial acute gastroenteritis in humans. One of the virulence factors of norovirus, the great genetic diversity attributed to point mutations and recombinations, has brought forth the result of significant changes in the circulating norovirus genotype patterns.
Findings
In recognition of the necessity for tracking and monitoring of genetic diversity, a norovirus variant among the most prevalent genotype GII-4, Norovirus Hu/GII-4/CUK-3/2008/KR (CUK-3), was isolated from stool samples and analyzed on the level of whole genome sequence. Whole genome sequence analysis revealed three ORF composites of the whole genome, ORF1 (5100 bp), ORF2 (1623 bp), and ORF3 (807 bp). Each genetic relationship of CUK-3 variant analysis located the ORF1 (5,100 bp) in Cluster I, ORF2 (1623 bp) in Cluster I (2006b), ORF3 (807 bp) in Cluster I, and the whole genome sequence (about 5.1 kb) in Cluster I in the phylogenetic tree. And the phylogenetic analyses showed the same location of CUK-3 strain with the GII-4/2006b cluster in the phylogenetic tree.
Conclusions
In This study, a first concerning the full-length sequence of a NoV variant in South Korea is meaningful in that it can be used not only as a full-length NoV variant sequence standard for future comparison studies, but also as useful material for the public health field by enabling the diagnosis, vaccine development, and prediction of new emerging variants.
Keywords: Norovirus, Sequence, Phylogenetic tree
Findings
Noroviruses (NoVs) are the most important viruses that cause nonbacterial acute gastroenteritis in humans. In addition to increased susceptibility, the elderly are at increased risk for more severe disease and death, as are the very young and the immunocompromised [1,2]. They are small, and non-enveloped viruses which and belong to the family Caliciviridae, genus Norovirus. Noroviruses have a single positive-strand NoV RNA genome of about 7.6 kb in size. Three open reading frames (ORFs) have been identified in the NoV genomes. ORF1 encodes a polyprotein that is cleaved into six non-structural (NS) proteins, which carry amino acid sequence motifs conserved in NTPase, protease and RNA-dependent RNA polymerase (RdRp) [3,4]. ORF2 encodes a major structural protein, Viral Protein (VP1), which consist of two domains-the shell domain (S) and the protruding arm (P) that is again divided into two subdomains, P1 and P2. The S domain is highly conserved while the P domain is variable. P2 of the P domain is hypervariable and carries immune and cellular recognition sites [5-7]. ORF3 encodes minor capsid protein, VP2, which is rich in basic amino acids and is proposed to have a role in viral stability [8,9]
Recently, NoVs were recognized as novel emergent pathogens. The main route of transmission is suspected to be person-to-person, but food and water-borne transmission is also important [1,10,11]. According to nucleotide sequence analysis of the capsid regions, noroviruses are classified into five genogroups, GI to GV, each of which can be further divided into several clusters or genotypes [12]. Among the five genogroups, three genogroups (GI, GII and GIV) are known to cause clinical illness in humans, and genotype GII-4 has been the predominant circulating strain to the present [13]. The error-prone RNA replication and recombination between viruses is what drives noroviruses to its the great diversity. Furthermore, the accumulated mutations of the hypervariable P2 domain of the VP1 protein produced different GII-4 NoVs [14]. The most representative of the resulting variant GII-4 strain, GII-4/2006b, with 3 nucleotide insertions in the P2 domain at position 6265, emerged in the summer of 2002 which lead to a major gastroenteritis outbreak as well as an epidemic gastroenteritis worldwide in the winter of 2002/2003 [15,16]. In South Korea, gastroenteritis outbreaks by GII-4/2006b variants have been reported from September of 2007 to July of 2008 had been reported [17].
In this paper, the whole genome sequence of another isolated variant of the emerging strain type, the GII-4 variant, was analyzed and compared with other variants to reveal the genetic relationship and to predict the tendency of GII-4 variants in South Korea.
NoV positive-stool sample was isolated from patients with acute gastroenteritis in Daejeon, South Korea in November 2008. The sample was obtained from the Waterborne Virus Bank (Seoul, Korea). The stool sample was stored at -70°. The viral genomic RNA was extracted from 140 μl of 10% fecal suspension with the QIAamp® Viral RNA Mini kit (Qiagen, Hilden, Germany) in accordance with the manufacturer's instructions.
For the detection of NoV, reverse transcription PCR (RT-PCR) was performed with the One Step RT-PCR kit (Qiagen, Hilden, Germany) with primers based on the sequence of the norovirus capsid region (Table 1).
Table 1.
Primer sets used in this study
| Primer | Sequence (5'→ 3') | Polarity | Regiona | |
|---|---|---|---|---|
| Diagnosis primer sets | GIF1M | CTG CCC GAA TTY GTA AAT GAT GAT | + | 5342-5365 |
| GIRIM | CCA ACC CAR CCA TTR TAC ATY TG | - | 5649-5671 | |
| GIIF1M | GGG AGG GCG ATC GCA ATC T | + | 5049-5067 | |
| GIIRIM | CCR CCI GCA TRI CCR TTR TAC AT | - | 5367-5389 | |
| Designed primer sets | ORF1-1F | GTG AAT GAA GAT GGC GTC TAA C | + | 1-22 |
| ORF1-1R | AGT TCC ACT GCA AGG TCC TCA G | - | 999-1020 | |
| ORF1-2F | CTG AGG ACC TTG CAG TGG AAC T | + | 999-1020 | |
| ORF1-2R | ATG AGG GAA CCA GTG GTG AGA GT | - | 2018-2040 | |
| ORF1-3F | CGT GCT CGA GGC GCA TCG ATT T | + | 1821-1842 | |
| ORF1-3R | TTG TAC TCA TCA TAC TCT TCA | - | 2701-2721 | |
| ORF1-4F | CAT TGC TCG AGC ATC AGG GCT AC | + | 2038-2060 | |
| ORF1-4R | TTG ACT ATC CTC GAC CAG ATG CT | - | 3038-3060 | |
| ORF1-5F | AGC ATC TGG TCG AGG ATA GTC AA | + | 3038-3060 | |
| ORF1-5R | GTG GCA CAT ATG ACA GTG TTT CC | - | 3518-3540 | |
| ORF1-6F | GGA AAC ACT GTC ATA TGT GCC AC | + | 3518-3540 | |
| ORF1-6R | CAG TCG TTC TTC CGC ATG TGG TGC GG | - | 3935-3960 | |
| ORF1-7F | CTC AGC ACC AAG ACT AAA TTC TGG A | + | 3635-3659 | |
| ORF1-7R | TGG GCG ATG GAA TTC CAT TGA GAG G | - | 4485-4509 | |
| ORF1-8F | ATG GTT AAA TTC TCC CCA GAA CC | + | 4355-4377 | |
| ORF1-8R | CCA CCT GCA TAA CCA TTG TAC AT | - | 5367-5389 | |
| ORF2-F | ATG AAG ATG GCG TCG AAT GGC | + | 5085-5105 | |
| ORF2-R | TTA TAA TGC ACG CCT GCG CCC CGT | - | 6681-6704 | |
| ORF3-F | ATG GCT GGA GCT TTC TTT GCT | + | 6704-6724 | |
| ORF3-R | AAA GAC ACT AAA GAA AGG AAA GAT | - | 7532-7555 | |
To analyze the whole genome sequence of the detected norovirus strain, reverse transcription PCR (RT-PCR) was performed with the One Step RT-PCR kit (Qiagen, Hilden, Germany) with 10 pairs of newly designed primer sets (Table 1). Ten fragments were amplified; eight fragments for ORF1, one fragment for ORF2, and one fragment for ORF3. The PCR products were then analyzed by electrophoresis on 1.5% agarose gel and ethidium bromide staining. The amplified fragments were purified with HiYield™ Gel/PCR DNA Extraction kit (RBC, Taipei, Taiwan) from the gel. Then, the products were cloned into the pGEM-T Easy vector (Promega, USA) and were sequenced by Genotech (Daejeon, South Korea).
Sequence data analysis of the composite sequence of ten plasmids aligned with Clustal W method using the DNAStar software (DNAStar Inc.) revealed that the whole genome was composed of three ORFs, 5100 bp (ORF1), 1623 bp (ORF2), and 807 bp (ORF3).
The dendrograms were constructed with the neighbor-joining method. The nucleotide sequence data reported in this study have been deposited into GenBank (accession number; FJ514242).
A comparison of the nucleotide and amino acid sequences of the entire ORF2 with reference strains showed that CUK-3 strain had the highest sequence similarity to the NoV GII-4 reference strain X76717. The nucleotide similarity with CUK-3 was 60.1 - 88.0% with the reference strains. A protein database search (BLASTX) for the product of ORF2 yielded 59.3 - 92.0% similarity. However, the cut-off value for amino acid similarity between the CUK-3 variant and the GII-4 reference strains showed low identity (92.0%) for the genomic sequences (Table 2).
Table 2.
Nucleotide and amino acid sequence similarities between CUK-3 strain and the full length ORF2 sequences from different Genogroup GII reference strains.
| Genotype | Strain | Acession No. | Similarity (%) | |
|---|---|---|---|---|
| GII-1 | H awaii-USA94 | U07611 | 64.9a | 64.6b |
| GII-2 | Msham-GBR95 | X81879 | 62.8 | 63.9 |
| GII-3 | Toronto-CAN93 | U02030 | 64.8 | 67.4 |
| GII-4 | Bristol-GBR-93 | X76716 | 88.0 | 92.0 |
| GII-5 | Hilingd-GBR00 | AJ277607 | 64.6 | 63.9 |
| GII-6 | Seacrof-GBR00 | AJ277620 | 63.0 | 63.9 |
| GII-7 | Leeds-GBR00 | AJ277608 | 64.3 | 64.8 |
| GII-8 | Amstdam-NLD99 | AF195848 | 64.3 | 64.8 |
| GII-9 | VABeach-USA01 | AY038599 | 63.7 | 63.5 |
| GII-10 | Eufurt-DEU01 | AF421118 | 63.5 | 64.8 |
| GII-11 | SW918-JPN01 | AB074893 | 62.3 | 64.6 |
| GII-12 | Wortley-GBR00 | AJ277618 | 63.3 | 63.9 |
| GII-13 | Faytvil-USA02 | AY113106 | 64.5 | 64.3 |
| GII-14 | M7-USA03 | AY130761 | 64.3 | 64.8 |
| GII-15 | J23-USA02 | AY130762 | 60.1 | 59.3 |
| GII-16 | Tiffin-USA03 | AY502010 | 64.1 | 65.9 |
| GII-17 | CSE1-USA03 | AY502009 | 63.7 | 65.2 |
a The nucleotide sequences of the ORF2 regions starting at nucleotide 1620 were analyzed.
b The amino acid sequences of the ORF2 regions starting at amino acid 540 were analyzed.
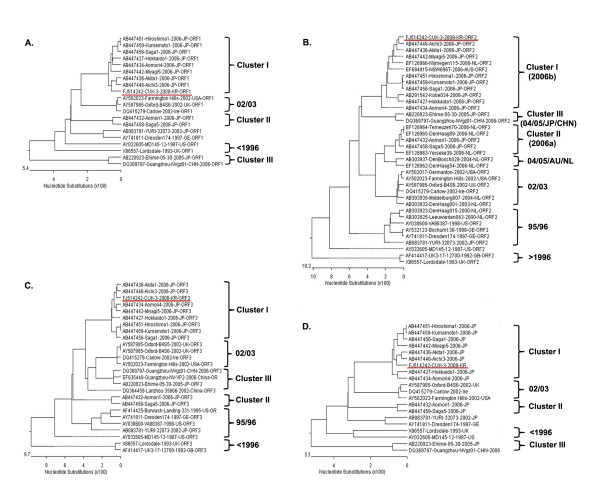
To analyze the genetic relationship between the CUK-3 variant and the other variants reported worldwide, the sequences of ORF sequences and the whole genome were processed with multiple sequence alignment. Each analysis of CUK-3 located the ORF1 (5,100 bp) in Cluster I, ORF2 (1623 bp) in Cluster I (2006b), and ORF3 (807 bp) in Cluster I as well as the whole genome sequence (about 5.1 kb) in Cluster I (Figure 1).
Figure 1.
Phylogenetic trees of the nucleotide sequences of the complete regions of NoV GII/4. Phylogenetic tree generated based on the complete sequence of the ORF1 (about 5.1 kb) (A), ORF2 (about 1.6 kb) (B), ORF3 (about 0.8 kb) (C), and near-full-length genome (7.5 kb) (D)
As shown on the case analysis of ORF1, Cluster I contains Hiroshoma1, Kumamoto1, Saga1, Hokkaodo1, Aomori4, Miayagi5, Akita1, Aichi3 and showed the highest similarity range (98.0~98.9%) (Figure 1A).
As shown on the case analysis of ORF2, Cluster I (2006b) contains emerged the GII-4 varints in Japan and showed the highest similarity range (97.7 - 99.0%), whereas Cluster II (2006a) showed low similarity range (91.7 - 92.3%) (Figure 1B).
As shown on the case analysis of ORF3, Cluster I showed the highest similarity range (97.9 - 99.3%). Interestingly, 02/03 group contains emerged the GII-4 vaiants in Europe showed more higher similarity range (93.1 - 93.6%) than Cluster II contains emerged the GII-4 variants in Japan (Figure 1C).
As shown on the case analysis of full length, the CUK-3 strain showed the highest similarity (98.3%) with the 2006 epidemic strain, Aichi3/2006/JP strain, whereas Ehime/5-30/2005/JP strain and Lordsdale/1993/UK strain showed the lowest similarity (89.2%) (Figure 1D).
The CUK-3 strain can be placed on the same branch of the phylogenetic tree as the GII-4/2006b cluster, based on the nucleotide sequence of the RdRp region and the amino acid sequence of the capsid protein (data not shown). Also, the analysis of the GDD motif (AANNTG) of the RdRp region of the GII-4 variants reported worldwide indicated that the GDD motif of CUK-3 strain was similar to that of the GII-4 variants of the epidemic strains in 2006. These strains were common in Hong Kong, Japan, and other regions of North East Asia during the same period [12,18]. Such similarities in analyses between CUK-3 and GII-4/2006b strain are evidence of the reemergence of GII-4 variant.
In Europe and Asia, most norovirus outbreaks that occurred after 2002 have been caused by GII-4 variant strains (Figure 2). In addition, biennial emergences of pandemic NoV GII-4 variants have been reported in 2002, 2004, 2006 and 2008 [12,18,19]. These GII-4 variants have also been identified in South Korea and are rapidly spreading worldwide.
Figure 2.

Partial sequence comparison of the RdRp gene. The boxed sequence is the conserved GDD motif, ANNNTG. Nucleotide positions are referenced to the Lordsdale-1993-UK sequence.
Amino acid sequences of the capsid region encoded by ORF2 was compared with a total of 39 GII-4 variants reported from various countries including US, UK, Japan, China, Germany etc. Date of 540 amino acid analyses showed the pattern of changes of two amino acid in first P1 domain, aa238 (S GII-4- >2002 → T GII-4- <2002), aa250 (Y GII-4- >2002 → F GII-4- <2002). The P2 domain showed five substitutions of amino acids, aa280 (A GII-4- >2002 → P GII-4- <2002), aa346 (A GII-4- >2002 → P GII-4- <2002), aa367 (F GII-4- >1995 → Y GII-4- >2002 → F GII-4- <2002), aa376 (Q GII-4- >2002 → E GII-4- <2002), and aa394 (G or S GII-4- >2006→ TGII-4- <2006). In addition, the second P1 domain also showed two amino acid substitutions, aa459 (L GII-4- >1995 → Q GII-4- >2002) and aa504 (P GII-4- >2002 → Q GII-4- <2002). These analytic data showed that there had already been numerous mutations, which lead to the production of a wide range of variants, already before 2002 and produced various variants. And as shown on the case of aa394 of P2 domain which inserted novel amino acid, G or S, and then substituted to T after 2006 (data not shown). Although previous studies have indicated that the sequence for the NoV capsid region is useful for the diagnosis of NoV variants, no studies have analyzed the RdRp region of the South Korean NoV GII-4 variant. This is the first report that describes the full-length sequence of a NoV GII-4 variant that was isolated from clinical samples in South Korea. We suggest that this sequence can be used as a standard for the comparison of full-length NoV GII-4 variant sequence with other strains.
The information acquired from whole genome sequencing in this study can be useful not only for a more accurate diagnoses of NoVs but also for the basic research for the elucidation genetic functions. Furthermore, it will be helpful for the prediction of newly appearing pandemic variants through comparison with GII-4 variants in neighboring countries, fundamental research for vaccine development, and eventually , for the field of public health through with the provision of new emerging strains of NoV.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
SRR and SYP conceived this study. YML, WHJ and designed and conducted the experiments. SGL and JWP analyzed the sequence data and carried out the molecular phylogenetic analysis. SGL and SYP wrote the manuscript. All authors read and approved the final manuscript.
Contributor Information
Jeong-Woong Park, Email: withgaia@sanigen.co.kr.
Sung-Geun Lee, Email: shocklsg@catholic.ac.kr.
Young-Min Lee, Email: ymlee@chungbuk.ac.kr.
Weon-Hwa Jheong, Email: purify@korea.kr.
Sangryeol Ryu, Email: sangryu@snu.ac.kr.
Soon-Young Paik, Email: paik@catholic.ac.kr.
Acknowledgements
This work was supported by Mid-career Researcher Program (2008-0061575) and Basic Science Research Program (2009-0062720) through NRF grant funded by the MEST.
References
- Donaldson EF, Lindesmith LC, Lobue AD, Baric RS. Norovirus pathogenesis: mechanisms of persistence and immune evasion in human populations. Immunol Rev. 2008;225:190–211. doi: 10.1111/j.1600-065X.2008.00680.x. [DOI] [PubMed] [Google Scholar]
- Yoon JS, Lee SG, Hong SK, Lee SA, Jheong WH, Oh SS, Oh MH, Ko GP, Lee CH, Paik SY. Molecular epidemiology of norovirus infections in children with acute gastroenteritis in South Korea in November 2005 through November 2006. J Clin Microbiol. 2008;46:1474–1477. doi: 10.1128/JCM.02282-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YF, Nomoto A, Detjen BM, Wimmer E. A protein covalently linked topoliovirus genome RNA. Proc Natl Acad Sci USA. 1977;74:59–63. doi: 10.1073/pnas.74.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueckert RR, Wimmer E. Systematic nomenclature of picornavirus proteins. J Virol. 1984;50:957–959. doi: 10.1128/jvi.50.3.957-959.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan M, Huang P, Meller J, Zhong W, Farkas T, Jiang X. Mutations within the P2 domain of norovirus capsid affect binding to human histo-blood group antigens: evidence for a binding pocket. J Virol. 2003;77:12562–12571. doi: 10.1128/JVI.77.23.12562-12571.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilssson MK, Hedlund O, Thorhagen M, Larson G, Johansen K, Ekspong A, Svensson L. Evolution of human calicivirus RNA in vivo: accumulation of mutations in the protruding P2 domain of the capsid leads to structural changes and possibly a new phenotype. J Virol. 2003;77:13117–13124. doi: 10.1128/JVI.77.24.13117-13124.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochridge VP, Jutila KI, Graff JW, Hardy ME. Epitopes in the P2 domain of norovirus VP1 recognized by monoclonal antibodies that block cell interactions. J Gen Virol. 2005;86:2799–2806. doi: 10.1099/vir.0.81134-0. [DOI] [PubMed] [Google Scholar]
- Glass PJ, White LJ, Bull JM, Leparc-Goffart I, Hardy ME, Estes MK. Norwalk virus open reading frame 3 encodes aminor structural protein. J Virol. 2000;74:6581–6591. doi: 10.1128/JVI.74.14.6581-6591.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolotti-Ciariet A, Crawford SE, Hutson AM, Estes MK. The 3' end of Norwalk virus mRNA contains determinants that regulate the expression and stability of the viral capsid protein VP1: a novel function for the VP2 protein. J Virol. 2003;77:11603–11615. doi: 10.1128/JVI.77.21.11603-11615.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney K, Menton J, Morgan JG. Carlow virus, a 2002 GII-4 variant norovirus strain from Ireland. Virol J. 2007;4:61. doi: 10.1186/1743-422X-4-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rosa G, Pourshaban M, Iaconelli M, Muscillo M. Detection of genogroup IV noroviruses in environmental and clinical samples and partial sequencing through rapid amplification of cDNA ends. Arch Virol. 2008;153:2077–2083. doi: 10.1007/s00705-008-0241-4. [DOI] [PubMed] [Google Scholar]
- Motomura K, Oka T, Yokoyama M, Nakamura H, Mori H, Ode H, Hansman GS, Katayama K, Kanda T, Tanaka T, Takeda N, Sato H. Identification of monomorphic and divergent haplotypes in the 2006-2007 norovirus GII/4 epidemic population by genome wide tracing of evolutionary history. J Virol. 2008;82:11247–11262. doi: 10.1128/JVI.00897-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale A, Mattick K, Lewis D, Estes M, Jiang X, Green J, Eglin R, Brown D. Distinct epidemiological patterns of Norwalk-like virus infection. J Med Virol. 2000;62:99–103. doi: 10.1002/1096-9071(200009)62:1<99::AID-JMV15>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Glass RI, Parashar UD, Estes MK. Norovirus gastroenteritis. N Engl J Med. 2009;361:1776–1785. doi: 10.1056/NEJMra0804575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopman B, Vennema H, Kohli E, Pothier P, Sanchez A, Negredo A, Buesa J, Schreier E, Reacher M, Brown D, Gray J, Iturriza M, Gallimore C, Bottiger B, Hedlund KO, Torvén M, von Bonsdorff CH, Maunula L, Poljsak-Prijatelj M, Zimsek J, Reuter G, Szücs G, Melegh B, Svennson L, van Duijnhoven Y, Koopmans M. Increase in viral gastroenteritis outbreaks in Europe and epidemic spread of new norovirus variant. Lancet. 2004;363:682–688. doi: 10.1016/S0140-6736(04)15641-9. [DOI] [PubMed] [Google Scholar]
- Allen DJ, Noad R, Samuel D, Gray JJ, Roy P, Iturriza-Gómara M. Characterisation of a GII-4 norovirus variant-specific surface-exposed site involved in antibody binding. Virol J. 2009;6:150. doi: 10.1186/1743-422X-6-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung JY, Han TH, Park SH, Kim SW, Hwang ES. Detection of GII-4/2006b Variant and Recombinant Noroviruses in Children With Acute Gastroenteritis, South Korea. J Med Virol. 2010;82:146–152. doi: 10.1002/jmv.21650. [DOI] [PubMed] [Google Scholar]
- Ho EC, Cheng PK, Lau AW, Wong AH, Lim WW. A typical norovirus epidemic in Hong Kong during summer of 2006 caused by a new genogroup II/4 variant. J Clin Microbiol. 2007;45:2205–2211. doi: 10.1128/JCM.02489-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu ET, Bull RA, Greening GE, Hewitt J, Lyon MJ, Marshall JA, McIver CJ, Rawlinson WD, White PA. Epidemics of gastroenteritis during 2006 were associated with the spread of norovirus GII.4 variants 2006a and 2006b. Clin Infect Dis. 2008;46:413–420. doi: 10.1086/525259. [DOI] [PubMed] [Google Scholar]