Abstract
The cell adhesion molecule E-cadherin has been implicated in maintaining the polarized phenotype of epithelial cells and suppression of invasiveness and motility of carcinoma cells. Na,K-ATPase, consisting of an α- and β-subunit, maintains the sodium gradient across the plasma membrane. A functional relationship between E-cadherin and Na,K-ATPase has not previously been described. We present evidence that the Na,K-ATPase plays a crucial role in E-cadherin–mediated development of epithelial polarity, and suppression of invasiveness and motility of carcinoma cells. Moloney sarcoma virus-transformed Madin-Darby canine kidney cells (MSV-MDCK) have highly reduced levels of E-cadherin and β1-subunit of Na,K-ATPase. Forced expression of E-cadherin in MSV-MDCK cells did not reestablish epithelial polarity or inhibit the invasiveness and motility of these cells. In contrast, expression of E-cadherin and Na,K-ATPase β1-subunit induced epithelial polarization, including the formation of tight junctions and desmosomes, abolished invasiveness, and reduced cell motility in MSV-MDCK cells. Our results suggest that E-cadherin–mediated cell-cell adhesion requires the Na,K-ATPase β-subunit's function to induce epithelial polarization and suppress invasiveness and motility of carcinoma cells. Involvement of the β1-subunit of Na,K-ATPase in the polarized phenotype of epithelial cells reveals a novel link between the structural organization and vectorial ion transport function of epithelial cells.
INTRODUCTION
The plasma membrane of polarized epithelial cells is divided into two functionally and biochemically distinct domains, the apical and basolateral plasma membranes (Simons and Fuller, 1985). Junctional complexes such as tight junctions, adherens junctions, and desmosomes play crucial roles in the structure and function of epithelial cells. The tight junction forms a continuous belt at the boundary between the apical and lateral plasma membrane domains and selectively regulates the passage of molecules across the paracellular pathway (gate function) and passively separates molecules into the apical and basolateral plasma membrane domains (fence function) (Farquhar and Palade, 1963). The adherens junction, localized below the tight junction, consists of cell adhesion and signaling molecules and may regulate the formation of other junctional complexes (Yap et al., 1997). Desmosomes are button-like adhesion points that provide resilience and tensile strength to the epithelial monolayer (Garrod et al., 1996).
The calcium-dependent cell-cell adhesion molecule E-cadherin has been implicated in the regulation of the epithelial phenotype. E-cadherin mediates cell-cell contact between epithelial cells by homophilic interaction (Takeichi, 1990). The cytoplasmic tail of E-cadherin associates with α-, β-, and γ-catenins (Ozawa et al., 1989) and p120ctn (Shibamoto et al., 1995). α-Catenin links the E-cadherin complex to the actin cytoskeleton, which is crucial for the cell adhesion function of E-cadherin (Knudsen et al., 1995; Rimm et al., 1995). Earlier studies have shown that disruption of E-cadherin–mediated adhesive cell-cell contacts between epithelial cells prevents the assembly of tight junctions and consequently abolishes epithelial polarity (Behrens et al., 1985; Gonzalez-Mariscal et al., 1985; Gumbiner and Simons, 1986).
Loss of the polarized phenotype of carcinoma cells has been attributed to the loss of E-cadherin function. Analysis of many tumors derived from epithelial tissues suggests that loss of E-cadherin function seems to be a key event and correlates with tumor cell invasiveness (Stetler-Stevenson et al., 1993; Birchmeier and Behrens, 1994). Because reexpression of E-cadherin suppresses tumor invasiveness in vitro (Frixen et al., 1991), E-cadherin has been termed an invasion suppressor (Vleminckx et al., 1991). In spite of these circumstantial evidences the mechanism by which E-cadherin–mediated cell adhesion regulates epithelial polarity or invasion suppression is poorly understood.
The Na,K-ATPase consisting of two noncovalently linked α- and β-subunits catalyzes an ATP-dependent transport of three sodium ions out and two potassium ions into the cell per pump cycle, thereby generating a transmembrane sodium gradient. The α1-subunit (∼112 kDa; Shull et al., 1985) contains the catalytic and ligand binding sites of the enzyme. Of the four α-isoforms described in mammals (Shamraj and Lingrel, 1994; Blanco et al., 1999; Woo et al., 1999), the predominant α-isoform in kidney is α1 (Mercer, 1993).
The β1-subunit (∼55 kDa; Shull et al., 1986) is a glycosylated protein and its role in Na,K-ATPase enzyme function remains somewhat obscure. It may modulate the transport of Na+ and K+ across the membrane (Eakle et al., 1994) and facilitate the insertion of the αβ-complex into the cell membrane (Noguchi et al., 1990; Geering, 1991; Geering et al., 1996). Of the three isoforms described (Lingrel et al., 1994) the predominant isoform in kidney is β1 (Mercer, 1993).
Na,K-ATPase is a central regulator of kidney functions (Laski and Kurtzman, 1996). Therefore, we studied its levels and activity in renal cell carcinoma and found that the β1-subunit is markedly reduced in clear-cell renal cell carcinoma, an invasive and the most prevalent form of kidney cancer (Rajasekaran et al., 1999). Subsequently, we found that Moloney sarcoma virus-transformed MDCK (MSV-MDCK) cells have highly reduced Na,K-ATPase β1-subunit levels. Previous studies correlated the invasive phenotype of MSV-MDCK cells to reduced expression of E-cadherin (Behrens et al., 1989). We demonstrate that E-cadherin's cell-cell adhesion function is not sufficient to either reestablish epithelial polarity or suppress invasiveness and cell motility in these cells. In contrast, ectopic expression of both E-cadherin and Na,K-ATPase β1-subunit in MSV-MDCK cells induced junctional complexes, reestablished epithelial polarity, and suppressed invasiveness and motility, indicating that Na,K-ATPase β1-subunit function is necessary for induction of a polarized phenotype and invasion suppression of MSV-MDCK cells.
MATERIALS AND METHODS
Plasmid Constructs
Canine Na,K-ATPase β1-subunit in pKS+ was a gift from Dr. Robert Farley (University of Southern California, Los Angeles, CA). The polymerase chain reaction-amplified cDNA was subcloned into pCDNA3 (Invitrogen, Carlsbad, CA) and the sequence was confirmed by DNA sequencing. Canine Na,K-ATPase α1-subunit in plasmid bluescript was kindly provided by Dr. Askari Amir (Medical College of Ohio, Toledo, OH) and subcloned into pCDNA3. Canine E-cadherin cDNA in the eukaryotic expression vector pR2 was a gift from Dr. Lisa McConlogue (Athena Neurosciences, South San Francisco, CA). The pCB7 expression vector containing a hygromycin resistance gene was provided by Dr. Michael Roth (University of Texas Southwestern Medical Center, Dallas, TX).
Cell Lines and DNA Transfection
MDCKwt and MSV-transformed MDCK cells (DoCl1, designated here as MSV-MDCK) were obtained from American Type Culture Collection (Rockville, MD) and grown as described previously (Rajasekaran et al., 1996). The Na,K-ATPase β1-subunit cDNA was transfected into MSV-MDCK cells by using the calcium phosphate method and stable clones were selected 2 wk after addition of 450 μg/ml G418 (Geneticin; Life Technologies, Gaithersburg, MD). MSV-MDCK clones expressing the β1-subunit (MSV-NaKβ-cl 1 and MSV-NaKβ-cl 2) were identified by immunofluorescence and immunoblot analysis. MSV-MDCK vector cells transfected with pCDNA3 vector and selected in parallel with the other clones served as controls. MSV-MDCK cells expressing E-cadherin (MSV-Cad) were described previously (Rajasekaran et al., 1996). Cell lines expressing E-cadherin and Na,K-ATPase β1-subunit (MSV-Cad-NaKβ) were obtained by transfecting MSV-Cad cells with 10 μg of Na,K-ATPase β1-subunit in pCDNA3 and 2 μg of pCB7. Two stable clones (MSV-Cad-NaKβ-cl 1, MSV-Cad-NaKβ-cl 2) were selected after treatment with 250 μg/ml hygromycin; expression of E-cadherin and the β-subunit was confirmed by immunoblot and immunofluorescence analysis. MSV-Cad vector cells are MSV-Cad cells transfected with pCDNA3 and pCB7 and were selected in parallel with MSV-Cad-NaKβ cells. Cells expressing E-cadherin and Na,K-ATPase α1-subunit (MSV-Cad-NaKα) were obtained by transfecting MSV-Cad cells with 10 μg of canine Na,K-ATPase α1-subunit in pCDNA3 and 2 μg pCB7. The clones were selected with hygromycin as described above. All selected clones were maintained in culture medium containing the appropriate selection antibiotics. Revertents of MSV-MDCK cells expressing E-cadherin and Na,K-ATPase β-subunit (MSV-Cad-NaKβ rev) were obtained by maintaining MSV-Cad-NaKβ-cl 1 in the absence of G418 and hygromycin for at least 5 mo.
Antibodies
Mouse monoclonal antibodies (mAbs) raised against Na,K-ATPase α- (M7-PB-E9) and β-subunit (M17-P5-F11) recognize epitopes that are common in human, sheep, and dog and have been characterized and described previously (Abbott and Ball, 1993; Sun and Ball, 1994). Mouse mAb against canine E-cadherin was a gift from Dr. Barry Gumbiner (Memorial Sloan Kettering Institute, New York, NY). Rabbit polyclonal zonula occludens-1 (ZO-1) antibody was purchased from Zymed Laboratories (South San Francisco, CA), mouse anti-β-catenin from Transduction Laboratories (Lexington, KY) and fluorescein isothiocyanate (FITC)-conjugated phalloidin from Sigma Chemical (St. Louis, MO). FITC- and Texas Red-labeled, affinity-purified secondary antibodies were obtained from Jackson ImmunoResearch Laboratories (West Grove, PA) and horseradish peroxidase (HRP)-anti-mouse antibody and HRP-protein A from Transduction Laboratories.
Immunofluorescence and Laser Scanning Confocal Microscopy
Confluent monolayers grown on poly(d-lysine) (50 μg/ml) and laminin (50 μg/ml) (Collaborative Biomedical Products, Bedford, MA)-coated glass coverslips were fixed in ice-cold methanol and processed for immunofluorescence as described previously (Rajasekaran et al., 1996). Epifluorescence analysis was performed by using an Olympus AX 70 (Provis) microscope.
To visualize F-actin confluent monolayers were fixed with 2% paraformaldehyde, quenched with 50 mM ammonium chloride, permeabilized with 0.075% saponin (Sigma Chemical), and stained with FITC-conjugated phalloidin. Images were recorded with a 1008 × 1018 cooled charge-coupled device camera (Life Science Resources, Cambridge, United Kingdom) mounted on an Olympus AX 70 (Provis) microscope and analyzed by using Esprit software.
The relative distributions of ZO-1 and E-cadherin were examined by using a Fluoview laser scanning confocal microscope (Olympus America, Melville, NY) as described previously (Rajasekaran et al., 1996). Three-dimensional images were obtained by using the Fluoview image analysis software (version 2.1.39).
Immunoblotting
Confluent monolayers were lysed in lysis buffer (95 mM NaCl, 25 mM Tris pH 7.4, 0.5 mM EDTA, 2% SDS, 1 mM phenylmethylsulfonyl fluoride [PMSF], 5 μg/ml each of antipain, leupeptin, pepstatin), briefly sonicated, and centrifuged at 14,000 rpm in a microfuge for 10 min. Equal amounts of protein (50 or100 μg) were separated by SDS-PAGE (7.5% or 10%) and transferred to nitrocellulose membrane (Schleicher & Schuell, Keene, NH). After blocking with 10% nonfat dry milk/PBS, the blots were incubated for 2 h at room temperature (RT) with primary antibody, washed with PBS/0.3% Tween, and incubated for 1 h at RT with either HRP-conjugated anti-mouse secondary antibody or HRP-conjugated protein A. Bound antibody was detected by enhanced chemiluminescence (NEN Life Science Products, Boston, MA). Densitometric analysis was carried out with an ImageQuant software package (Molecular Dynamics, Sunnyvale, CA).
Cell Surface Biotinylation
The cell surface of subconfluent monolayers was labeled on ice with 0.5 μg/ml membrane-impermeable EZ-Link Sulfo-NHS-Biotin (Pierce, Rockford, IL) in tetraethylammonium (150 mM NaCl, 10 mM triethanolamine pH 9, 1 mM CaCl2, 1 mM MgCl2). After quenching (50 mM ammonium chloride in PBS, 0.1 mM CaCl2, 1 mM MgCl2) the cells were lysed in 0.5 ml of lysis buffer (150 mM NaCl, 20 mM Tris pH 8, 5 mM EDTA, 1% Triton X-100, 0.1% bovine serum albumin [BSA], 1 mM PMSF, 5 μg/ml each of antipain, leupeptin, pepstatin). Protein (300 μg) of each lysate was used for precipitation (16 h at 4°C) with 30 μl of Ultralink streptavidin beads (Pierce). The precipitates were washed as described previously (Rajasekaran et al., 1996) and immunoblotted (see above).
Electron Microscopy
Confluent monolayers were fixed in 2.5% glutaraldehyde/0.1 M sodium cacodylate buffer, pH 7.4, processed by conventional procedures for electron microscopy, and examined with an electron microsocope (Jeol 1200EXII) at 80 kV.
Antibody Permeability Assay
Confluent cell monolayers on glass coverslips were fixed in 2% paraformaldehyde under nonpermeabilized conditions and labeled with a monoclonal antibody against E-cadherin. The cells were washed and stained with FITC-conjugated anti-mouse secondary antibody and visualized by an Olympus AX 70 (Provis) microscope as described above.
Collagen Invasion
Ice-cold rat tail collagen, type I (∼4 mg/ml; Collaborative Biomedical Products) was added to an equal volume of 2× DMEM/20% fetal bovine serum. The pH was adjusted to ∼pH 7.4 with 1 N NaOH. In a 12-well tissue culture plate, a bottom layer of 500 μl of collagen/DMEM was polymerized at 37°C. Cells (5000) of a single cell suspension were added to 1 ml of collagen/DMEM, layered on top of the bottom gel, and allowed to polymerize at 37°C. The cells were grown for 10–14 d at 37°C, 5% CO2. Photographs were taken with an Olympus CK2 inverted phase contrast microscope.
Transwell Motility Assay
Twelve-well PET-membrane cell culture inserts with 8.0-μm pores (Becton Dickinson Labware, Franklin Lakes, NJ) were coated from the bottom with 50 μg/ml rat tail collagen type I. Single-cell suspensions were washed once with DMEM/BSA (5 mg/ml) and 100,000 cells each were plated on the coated filters. After 18-h incubation in DMEM/BSA at 37°C, 5% CO2 the filters were removed and cells attached to the bottom of the well were counted.
Wound Assay
A uniform cell-free area was created by scratching confluent monolayers with a plastic pipet tip and the wound area was inspected regularly. At each time point four photographs were taken and the distance between the two opposing edges was measured at two points on each photograph. The distance migrated in micrometers was calculated as difference of the scratch width at 0 h and that at the time point indicated.
Triton X-100 Extraction
Confluent monolayers were extracted with 200 μl of extraction buffer [50 mM NaCl, 10 mM piperazine-N,N′-bis(2-ethanesulfonic acid) pH 6.8, 3 mM MgCl2, 0.5% Triton X-100, 300 mM sucrose, 1 mM PMSF, 100 U/ml DNase] for 10 min at 4°C. The lysates were centrifuged at 4°C for 30 min at 14,000 rpm in a microfuge and the pellet was resuspended in 200 μl of 2× sample buffer. Equal volumes of each pellet and supernatant fraction were subjected to immunoblotting as described above.
Cell Aggregation Assay
Cells were washed briefly in PBS and trypsinized with 0.05% trypsin/1 mM CaCl2 to obtain single cells. Cells (200,000) in culture medium were plated on 60-mm Petri dishes and incubated on a gyratory shaker at 37°C, 5% CO2. After 3 h single cells were counted. The cell aggregation data are represented by the index (N0 − Nt)/N0 where Nt is the single cell number after the incubation time t and N0 is the cell number at the initiation of incubation.
Rubidium Transport Assay
The ouabain-sensitive ion transport was measured by determining the uptake of 86Rb+ as described by Lambrecht et al. (1998). The cells were washed once with 1.5 ml of ice-cold wash solution (144 mM NaCl, 0.5 mM CaCl2, 10 mM HEPES, pH 7.4), incubated for 10 min at 37°C with 1 ml of incubation buffer (144 mM NaCl, 10 mM HEPES pH 7.4, 0.5 mM MgCl2, 0.5 mM CaCl2, 1 mM RbCl, 1 mg/ml glucose, 1 μCi 86Rb+), and then washed three times with 1.5 ml of ice-cold wash solution. To measure the ouabain-sensitive 86Rb+ transport the cells were incubated with 50 μM ouabain for 30 min at 37°C before the experiment. The cells were dissolved with 500 μl of 0.5 M NaOH for 1 h at RT and solubilized 86Rb+ was counted. The samples were normalized to the protein content and the ouabain-sensitive Rb flux was calculated.
Sodium Measurement
Monolayers of cells treated without or with 50 μM ouabain for 2 h were rinsed three times with 10 ml of 0.25 M sucrose. The cells of five 100-mm dishes each were pooled in 0.25 M sucrose, digested with HNO3 (Ultrex II; J.T. Baker, Phillipsburg, NJ) at a final concentration of 40% at 65°C for 15 h, and diluted 1:2 with Millipore Milli-Q UF plus filtered water. The ion concentrations were measured by using an inductively coupled plasma atomic emission spectrometer (Vista Axial 730; Varian, Walnut Creek, CA). The concentrations for Na+ (588.995 nm), K+ (766.941 nm), and Mg2+ (285.213 nm) were determined and the Na+ and K+ concentrations were normalized to the total Mg2+ content (internal control).
RESULTS
Reduced Levels of E-Cadherin and β-Subunit in MSV-MDCK Cells and Establishment of MSV-MDCK Cell Lines Expressing Both E-Cadherin and β-Subunit
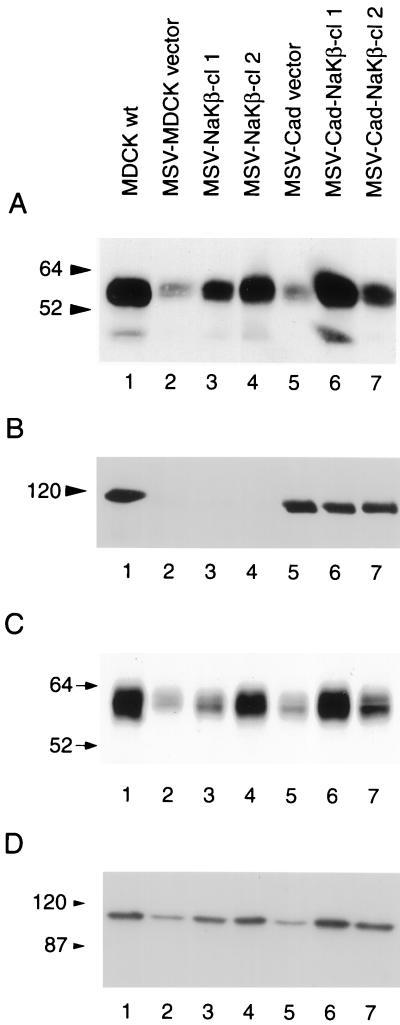
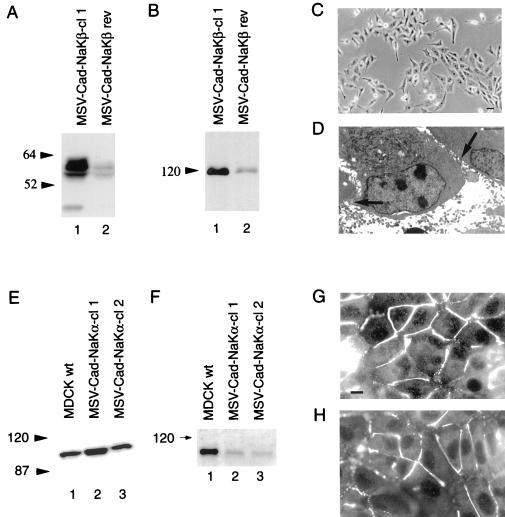
Immunoblot analysis revealed that MSV-MDCK cells have substantially reduced levels of the Na,K-ATPase β1-subunit (90% reduction) (Figure 1A, lane 2) relative to MDCKwt cells (Figure 1 A, lane 1). These cells also have highly reduced E-cadherin levels (Figure 1B, lane 2; Behrens et al., 1989). These results suggested that the invasiveness of MSV-MDCK cells may result in part from diminished levels of Na,K-ATPase β-subunit. To test whether reduced β1-subunit levels are associated with the invasive phenotype of MSV-MDCK cells, we overexpressed β1-subunit in MSV-MDCK cells and in E-cadherin-expressing MSV-MDCK (MSV-Cad vector) cells. MSV-Cad vector cells express 85% of the total E-cadherin expressed in MDCKwt cells (Figure 1B, lane 5; Rajasekaran et al., 1996). Canine β1-subunit cDNA in an eukaryotic expression vector was transfected into MSV-MDCK and MSV-Cad cells. Two stable clones that expressed β-subunit in MSV-MDCK (MSV-NaKβ-cl 1 and cl 2) (Figure 1A, lanes 3 and 4), and two β-subunit–expressing MSV-Cad clones (MSV-Cad-NaKβ-cl 1 and cl 2) (Figure 1A, lanes 6 and 7) were selected by using G418 and hygromycin, respectively. MSV-MDCK cells and MSV-Cad cells transfected with vector lacking the insert (MSV-MDCK vector; Figure 1, A and B, lane 2) and MSV-Cad vector (Figure 1, A and B, lane 5) were selected as controls for transfection. Immunoblot analysis and densitometric quantification revealed that MSV-NaKβ-cl 1 and MSV-NaKβ-cl 2 express 40 and 67% of the β-subunit level, respectively, compared with MDCKwt cells. MSV-Cad-NaKβ-cl 1 and MSV-Cad-NaKβ-cl 2 were found to express 112 and 43% of the β-subunit, respectively, relative to MDCKwt cells.
Figure 1.
Analysis of the expression of Na,K-ATPase α- and β-subunits and E-cadherin in MDCK and MSV-MDCK cells. Total cell lysates (50 μg) (A) or 100 μg (B and D) were separated on a 10% SDS-PAGE, transferred to nitrocellulose, and immunoblotted for Na,K-ATPase β-subunit (A), E-cadherin (B), and α-subunit (D) as described in MATERIALS AND METHODS. (C) Analysis of the surface expression of β-subunit by a biotinylation assay.
A cell surface biotinylation assay was used to determine the levels of β-subunit expressed on the plasma membrane (Figure 1C). The surface β-subunit level of MDCK cells was considered as 100% (Figure 1C, lane 1). MSV-Cad-NaKβ-cl 1 expressed similar levels of surface β-subunit compared with MDCKwt cells (Figure 1C, lane 6) and MSV-Cad-NaKβ-cl 2 expressed ∼60% of β-subunit on the surface compared with MDCKwt cells (Figure 1C, lane 7). MSV-NaKβ-cl 1 and MSV-NaKβ-cl 2 cells expressed 30 and 80%, respectively, of the surface β-subunit of MDCKwt cells (Figure 1C, lanes 3 and 4). MSV-MDCK vector and MSV-Cad vector cells expressed ∼15% of the surface β-subunit of MDCKwt (Figure 1C, lanes 2 and 5). Cells that contained high levels of total β-subunit (MSV-NaKβ-cl 2 and MSV-Cad-NaKβ-cl 1) expressed higher levels of surface β-subunit (compare lanes 4 and 6 of Figure 1, A and C).
In contrast to the levels of β1-subunit, the α1-subunit level did not reveal a drastic reduction in MSV-MDCK cells (60% reduction, Figure 1D, lane 2). The α1-subunit levels increased 2- to 3-fold in cells overexpressing β-subunit (Figure 1D, lanes 3 and 4 and 6 and 7). MSV-MDCK (Figure 1D, lane 2) and MSV-Cad cells (Figure 1D, lane 5) transfected with the vector alone showed similar α-subunit levels. This result indicates that repletion of β-subunit expression increases the level of α-subunit in MSV-MDCK cells.
MSV-MDCK Cells Expressing Both E-Cadherin and β-Subunit Show a Polarized Epithelial Phenotype
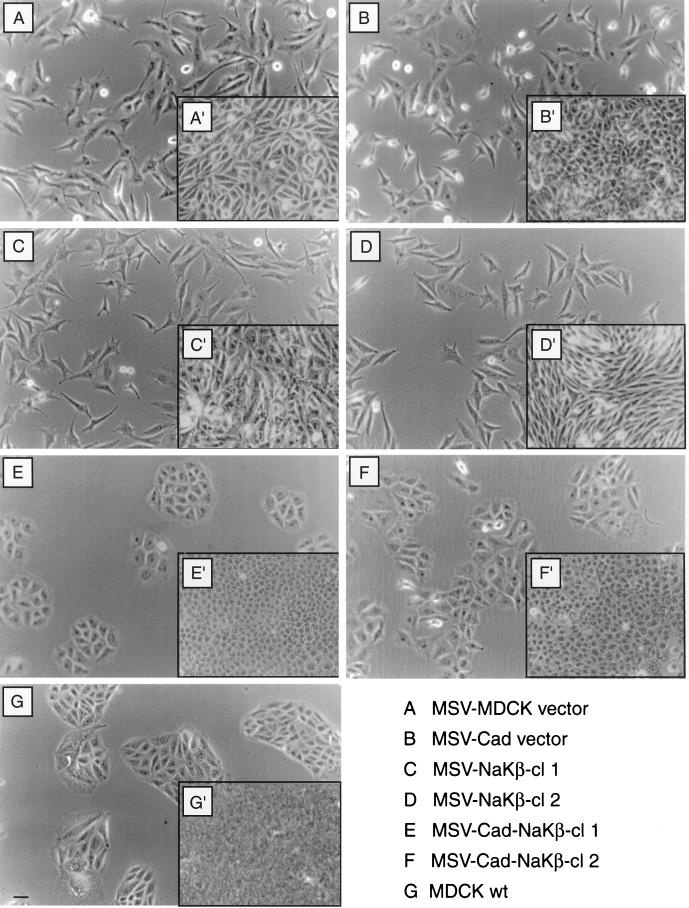
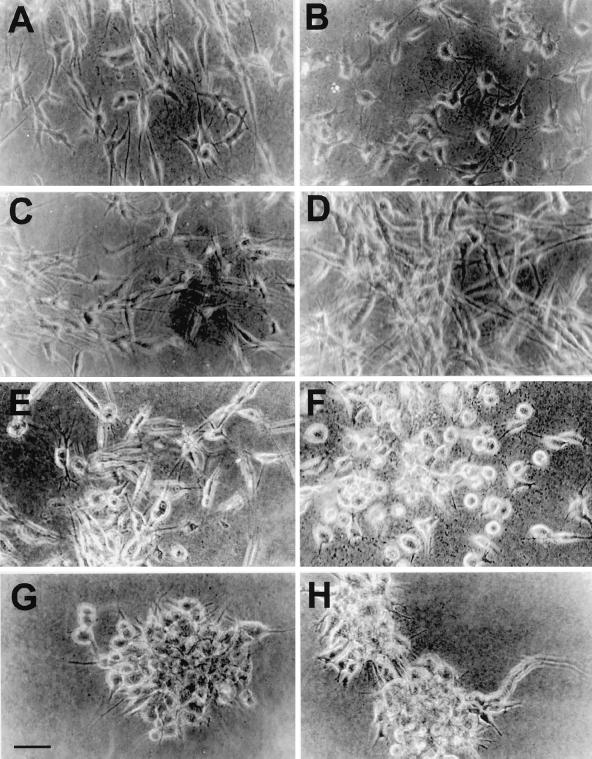
MSV-MDCK cells expressing both β-subunit and E-cadherin grew as compact colonies (Figure 2, E and F) comparable to MDCKwt cells (Figure 2G). MSV-Cad vector cells (Figure 2B) or MSV-NaKβ cells (Figure 2, C and D) showed a fibroblastic phenotype and grew as single cells similar to MSV-MDCK vector cells (Figure 2A). In addition, confluent MDCKwt cells and both clones of MSV-Cad-NaKβ cells formed honeycomb-like monolayers (Figure 2, E′, F′, and G′). However, MSV-MDCK, MSV-Cad vector, and MSV-NaKβ cells grew loosely attached to each other as revealed by increased intercellular space between cells (Figure 2, A′–D′). These results indicated that repletion of E-cadherin and β-subunit of Na,K-ATPase expression in MSV-MDCK cells induces an epithelial phenotype.
Figure 2.
Phase contrast microscopy of MDCK cells and MSV-MDCK cells expressing E-cadherin and Na,K-ATPase β-subunit. Phase contrast micrographs of cells grown at low cell density (A–G) and of confluent monolayers (A′–G′). MDCKwt cells (G) and MSV-MDCK cells expressing E-cadherin and the β-subunit of Na,K-ATPase, MSV-Cad-NaKβ-cl 1 (E), and MSV-Cad-NaKβ-cl 2 (F) grow as compact colonies at low density and form tight monolayers at confluence (G′, E′, and F′). MSV-MDCK cells expressing either E-cadherin (B and B′) or the β-subunit, MSV-NaKβ-cl 1 (C and C′) and MSV-NaKβ-cl 2 (D and D′), alone grow with a fibroblastic phenotype similar to MSV-MDCK vector cells (A and A′). Bar, 50 μm.
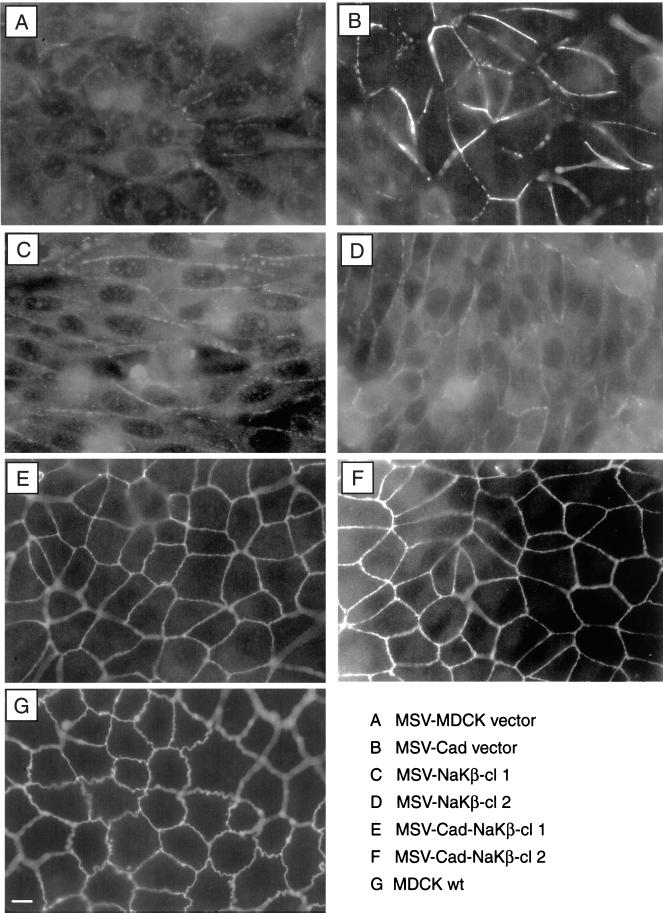
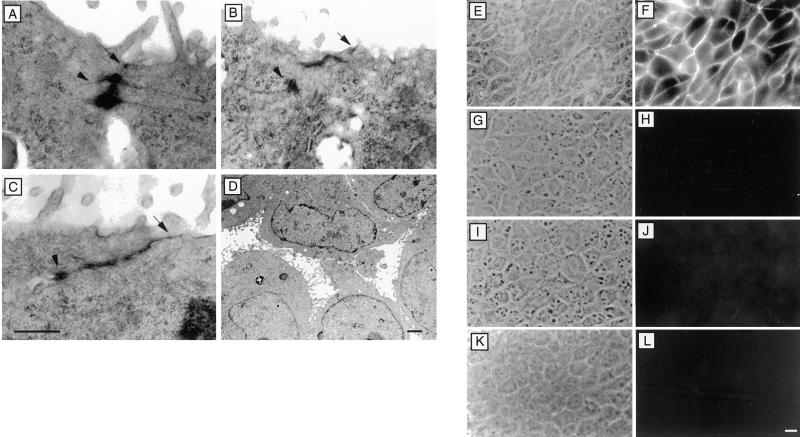
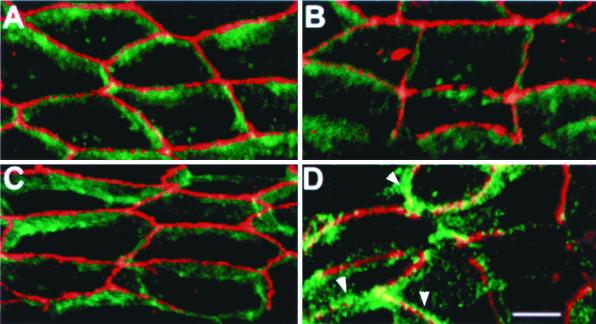
A distinguishing characteristic of the polarized epithelial phenotype is the presence of tight junctions. Immunofluorescence staining of ZO-1, a tight junction protein, revealed a continuous staining pattern in both clones of MSV-Cad-NaKβ cells (Figure 3, E and F), which was comparable to MDCKwt cells (Figure 3G). In MSV-MDCK vector and MSV-NaKβ cells most of the ZO-1 staining was localized intracellularly (Figure 3, A, C, and D). In MSV-Cad vector cells ZO-1 was localized on the plasma membrane, however, the staining pattern was discontinuous (Figure 3B; Rajasekaran et al., 1996), indicating that the presence of E-cadherin alone is sufficient for the initial translocation of ZO-1 to the plasma membrane. Consistent with the ZO-1 staining pattern, transmission electron microscopy revealed tight junctions in MSV-Cad-NaKβ cells and MDCKwt cells (Figure 4, A–C, arrows). In addition, these cells also had distinct desmosomes (Figure 4, A–C, arrowheads). MSV-Cad vector cells (Figure 4D) or MSV-NaKβ cells (our unpublished results) did not show tight junctions. The presence of tight junctions in MSV-Cad-NaKβ cells was further confirmed by a macromolecular permeability assay. Because MSV-Cad-NaKβ cells did not grow well on polycarbonate filters we were unable to measure transepithelial electrical resistance, a commonly used method to assess the function of tight junctions. Therefore, we tested the impermeability to macromolecules of confluent monolayers grown on glass coverslips. Anti E-cadherin antibody was added to the apical surface and the tight junction permeability to the antibody was detected by staining the cells with a secondary antibody under nonpermeabilized conditions (Figure 4). MDCKwt (Figure 4, K and L) cells and both clones of MSV-Cad-NaKβ cells (Figure 4, G and H and I and J) were impermeable to anti-E-cadherin antibody, indicating the presence of functional tight junctions. In contrast, MSV-Cad cells showed clear E-cadherin staining on the plasma membrane, indicating the absence of tight junctions (Figure 4, E and F).
Figure 3.
Immunofluorescence of ZO-1 in MDCK cells and MSV-MDCK cells expressing E-cadherin and Na,K-ATPase β-subunit. MSV-MDCK vector (A), MSV-NaKβ-cl 1 (C), and MSV-NaKβ-cl 2 (D) cells show ZO-1 mainly localized to the cytoplasm. Expression of E-cadherin in MSV-MDCK cells (B) results in the translocation of ZO-1 to the plasma membrane. However, these cells do not show a continuous ZO-1 ring pattern on the plasma membrane like MSV-Cad-NaKβ-cl 1 (E), MSV-Cad-NaKβ-cl 2 (F), and MDCKwt (G) cells. Bar, 10 μm.
Figure 4.
Transmission electron microscopy and passage of macromolecules through confluent monolayers of MDCK cells and MSV-MDCK cells expressing E-cadherin and Na,K-ATPase β-subunit. MSV-MDCK cells expressing both E-cadherin and the Na,K-ATPase β-subunit, MSV-Cad-NaKβ-cl 1 (A) and MSV-Cad-NaKβ-cl 2 (B), form tight junctions (arrows) and desmosomes (arrowheads). Tight junctions and desmosomes of MDCKwt cells are shown in C. MSV-MDCK cells expressing only E-cadherin, MSV-Cad vector cells (D), form neither tight junctions nor desmosomes. Bar, A–C, 0.5 μm; bar, D, 2 μm. Confluent monolayers were fixed under nonpermeabilizing conditions with paraformaldehyde. The cells were incubated from the apical side with anti-E-cadherin antibody and FITC-conjugated secondary antibody. The absence of tight junctions in MSV-Cad vector cells results in clear staining of E-cadherin on the plasma membrane (F). In MSV-MDCK cells expressing E-cadherin and Na,K-ATPase β-subunit, MSV-Cad-NaKβ-cl 1 (H) and MSV-Cad-NaKβ-cl 2 (J), as well as in MDCKwt cells (L), the antibody is not able to pass through tight junction and therefore does not stain the basolaterally localized E-cadherin. E, G, I, and K are phase contrast micrographs of the same areas depicted in F, H, J, and L, respectively. Bar, 10 μm.
The presence of tight junctions correlates with the polarized distribution of apical and basolateral plasma membrane markers in epithelial cells. Three-dimensional images of monolayers of MDCKwt (Figure 5C), MSV-Cad-NaKβ (Figure 5, A and B), and MSV-Cad vector (Figure 5D) cells, double-labeled with antibodies against ZO-1 (red) and E-cadherin (green), were generated from serial optical sections obtained by laser scanning confocal microscopy. We showed previously that latitudinal rotation of the image by 56° enables clear visualization of the apical and basolateral regions of the monolayer in MDCK cells (Rajasekaran et al., 1996). MSV-Cad-NaKβ cells and MDCKwt cells showed that E-cadherin is distinctly localized to the basolateral membrane. In addition, these cells also showed distinct basolateral localization of Na,K-ATPase α- and β-subunit and apical localization of GP135, an apical marker (our unpublished results). In contrast, MSV-Cad vector cells were not polarized and E-cadherin was found all over the cell surface (Figure 5D, arrowheads). In MSV-Cad-NaKβ and MDCKwt cells the apicolateral staining of ZO-1 can be distinctly separated from the basolateral staining of E-cadherin. In contrast, in MSV-Cad cells, ZO-1 staining colocalized with E-cadherin staining in the apical adherens junction region, indicating that the tight junction components are not separated from the basolateral membrane (Figure 5D; Rajasekaran et al., 1996). These results demonstrated that coexpression of E-cadherin and β-subunit of Na,K-ATPase in MSV-MDCK cells results in the establishment of epithelial polarization. Neither of these proteins when expressed alone was sufficient to induce polarization of MSV-MDCK cells.
Figure 5.
Confocal microscope images showing the distribution of E-cadherin and ZO-1 in MSV-MDCK cells expressing both E-cadherin and Na,K-ATPase β-subunit, MDCK, and MSV-MDCK cells expressing E-cadherin alone. Confluent monolayers of MSV-Cad-NaKβ-cl 1 (A), MSV-Cad-NaKβ-cl 2 (B), MDCKwt (C), and MSV-Cad vector (D) cells were grown on glass coverslips and fixed in cold methanol. Cells were labeled with a polyclonal antibody against ZO-1 and a monoclonal antibody against E-cadherin followed by Texas Red-conjugated anti-rabbit and fluorescein-conjugated anti-mouse antibodies. Serial optical sections of the monolayers were recorded at 0.5-μm intervals. Three-dimensional images were created and rotated 56° latitudinally by using Fluoview image analysis software. Note the distinct staining pattern of ZO-1 (red) and E-cadherin (green) in MSV-Cad-NaKβ-cl 1, MSV-Cad-NaKβ-cl 2, and MDCKwt cells, and the colocalization of these proteins (yellow) in MSV-Cad vector cells. E-cadherin is also seen above the ZO-1 staining (arrows) in MSV-Cad vector cells. Bar, 5 μm.
Epithelial Phenotype Is Lost in a Reverted Clone of MSV-Cad-NaKβ-Cells
To further confirm that the phenotypic reversion of MSV-MDCK cells is due to repletion of both E-cadherin and β-subunit and not a transfection artifact, MSV-Cad-NaKβ-cl 1 was maintained in the absence of G418 and hygromycin used to select this clone. This growth condition resulted in a dramatic reduction of both β-subunit levels (80% reduction) (Figure 6A) and E-cadherin levels (90% reduction) (Figure 6B) in the reverted clone (MSV-Cad-NaKβ rev). These cells were fibroblastic and grew like MSV-MDCK vector cells both at low density (Figure 6C) and when at confluence (our unpublished results). In addition, transmission electron microscopy did not show tight junctions in these cells (Figure 6D). These results indicate that the phenotypic reversion of MSV-MDCK cells is due to the near normal levels of both E-cadherin and β-subunit and that these levels are crucial to induce and maintain the polarized phenotype of MSV-MDCK cells.
Figure 6.
Loss of Na,K-ATPase β-subunit and E-cadherin expression in a reverted clone of MSV-MDCK cells expressing Na,K-ATPase β-subunit and E-cadherin, and characterization of MSV-MDCK cells expressing E-cadherin and Na,K-ATPase α-subunit. (A) Total cell lysates (50 μg) were separated on a 10% SDS-PAGE and immunoblotted for Na,K-ATPase β-subunit as described in MATERIALS AND METHODS. Reversion of MSV-Cad-NaKβ-cl 1 cells (MSV-Cad-NaKβ rev) results in a drastic reduction of β-subunit expression. (B) Total cell lysates (100 μg) were separated on a 10% SDS-PAGE and immunoblotted for E-cadherin. MSV-Cad-NaKβ rev cells show also a dramatic reduction of E-cadherin protein levels. (C) Loss of E-cadherin and β-subunit expression results in the reversion to a fibroblastic phenotype. Bar, 50 μm. (D) Transmission electron microscopy reveals that MSV-Cad-NaKβ rev cells form neither tight junctions nor desmosomes (arrows). Bar, 0.5 μm. (E) Immunoblot analysis: total cell lysates (100 μg) of MSV-Cad-NaKα cells and MDCKwt cells were separated on a 10% SDS-PAGE and immunoblotted for Na,K-ATPase α-subunit as described in MATERIALS AND METHODS. (F) Cell surface biotinylation: Cells were biotinylated and immunoblotted for Na,K-ATPase α-subunit. (G and H) Immunofluorescence staining of ZO-1. In MSV-Cad-NaKα-cl 1 (G) and in MSV-Cad-NaKα-cl 2 (H) cells, ZO-1 is localized on the plasma membrane but does not show a continuous ring-like staining pattern. Bar, 10 μm.
Enhanced Expression of the α-Subunit Does Not Alter the Fibroblastic Phenotype of MSV-Cad Cells
To test whether overexpression of Na,K-ATPase α-subunit induces an epithelial phenotype, MSV-Cad cells were transfected with the canine α1-subunit cDNA in an eukaryotic expression vector and two stable clones expressing 200 and 100% of the α-subunit expressed in MDCKwt cells were selected (Figure 6E). Cell surface biotinylation experiments revealed that only ∼20% of the total α-subunit present in these cells was expressed on the cell surface (Figure 6F). Consistent with the biotinylation data, immunofluorescence microscopy revealed that most of the α-subunit was localized in large intracellular vesicles (our unpublished results). These results are consistent with published reports indicating that the β-subunit is crucial for the surface expression of the α-subunit (Geering, 1991; Geering et al., 1996). MSV-Cad-NaKα cells were fibroblastic like MSV-Cad vector cells (our unpublished results) and ZO-1 localization in these clones was discontinuous similar to MSV-Cad vector cells (Figure 6, G and H).
Taken together, our results demonstrate that expression of E-cadherin alone is not sufficient to induce epithelial polarity and that the β-subunit plays a crucial role in the induction and maintenance of the polarized phenotype of MSV-MDCK cells.
Repletion of β-Subunit in MSV-Cad Cells Abolishes Their Invasive Behavior
In MSV-MDCK cells their invasive behavior has been attributed to a reduced level of E-cadherin expression (Behrens et al., 1989). However, in an in vitro collagen gel invasion assay commonly used to monitor the invasiveness of carcinoma cells (Hordijk et al., 1997), we found that MSV-Cad vector cells (Figure 7B) were as invasive as MSV-MDCK vector cells (Figure 7A). These cells grew in a dispersed manner and showed invasion throughout the matrix, indicating that forced expression of E-cadherin alone is not sufficient to inhibit their invasive behavior. MSV-NaKβ cells (Figure 7, C and D), MSV-Cad-NaKα cells (Figure 7E) and MSV-Cad-NaKβ rev cells (Figure 7F) were invasive and grew in a dispersed manner in the collagen gel. In contrast, the two clones of MSV-Cad-NaKβ cells grew predominantly as colonies without invading the matrix (Figure 7, G and H), indicating that repletion of both E-cadherin and β-subunit inhibits the invasiveness of MSV-MDCK cells.
Figure 7.
Invasion of collagen matrix by MSV-MDCK cells and by MSV-MDCK cells expressing E-cadherin and Na,K-ATPase β-subunit or Na,K-ATPase α-subunit. Single-cell suspensions were plated into a collagen matrix and invasiveness was monitored as described in MATERIALS AND METHODS. MSV-Cad vector (B), MSV-NaKβ-cl 1 (C), and MSV-NaKβ-cl 2 (D) cells grow, invading throughout the collagen matrix like MSV-MDCK vector cells (A). Similarly, MSV-Cad-NaKα-cl 1 cells (E) and MSV-Cad-NaKβ rev cells (F) grow, invading throughout the collagen matrix. MSV-Cad-NaKβ-cl 1 (G) and MSV-Cad-NaKβ-cl 2 (H) cells grow predominantly as colonies without invading the collagen matrix. Bar, 25 μm.
MSV-MDCK Cells Expressing Both E-Cadherin and β-Subunit Show Reduced Motility
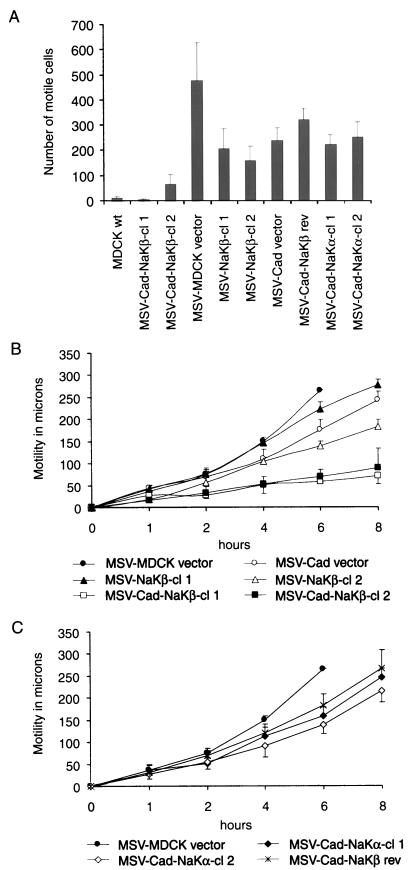
Invasive carcinoma cells are characterized by their increased cell motility. Therefore, we determined whether the reduced invasiveness of MSV-Cad-NaKβ cells is accompanied by a reduced cell motility. Two independent methods, a Transwell cell motility assay (Figure 8A) and a wound assay (Figure 8, B and C) were used to determine the motility. The Transwell cell motility assay clearly showed that MSV-Cad-NaKβ cells were significantly (p < 0.01) less motile than either MSV-MDCK vector or MSV-MDCK cells overexpressing either E-cadherin or β-subunit alone (Figure 8A). Also, MSV-Cad-NaKβ rev and MSV-Cad-NaKα cells were significantly more motile than MSV-Cad-NaKβ cells. Consistent with the Transwell cell motility assay results, the wound assay also revealed a significantly (p < 0.01) reduced motility of MSV-Cad-NaKβ cells compared with MSV-MDCK vector, MSV-Cad vector, and MSV-NaKβ-cells (Figure 8B). MSV-Cad-NaKβ rev cells and the MSV-Cad-NaKα clones, likewise, were significantly more motile than MSV-Cad-NaKβ cells (Figure 8C). In both motility assays, a correlation between the levels of β-subunit expression and cell motility was observed. MSV-Cad-NaKβ-cl 1 cells that express more β-subunit were less motile than MSV-Cad-NaKβ-cl 2 cells. Similarly, MSV-NaKβ-cl 2 cells were less motile than MSV-NaKβ-cl 1 cells. Interestingly, MSV-Cad and MSV-NaKβ cells were less motile than MSV-MDCK cells transfected with the vector. A two-way analysis of variance comparing logarithms of motile cell numbers confirmed a significant (p < 0.01) effect of E-cadherin or β-subunit expression alone and an interaction effect that is greater (p < 0.03) than multiplicative. This suggests that E-cadherin or β-subunit expression alone may reduce the motility of carcinoma cells to some extent and that overexpression of both E-cadherin and β-subunit acts synergistically to significantly reduce tumor cell motility.
Figure 8.
Transwell motility assay and wound assay of MSV-MDCK cells expressing E-cadherin and Na,K-ATPase α- or β-subunit. (A) Migration of MDCK cells and MSV-MDCK cells expressing E-cadherin and Na,K-ATPase α- or β-subunit across porous filter membranes. The error bars show the SD of the mean of triplicate determinations done in duplicates. (B) Migration of MSV-MDCK cells expressing E-cadherin and Na,K-ATPase β-subunit in the wound assay. Note that MSV-MDCK vector cells closed the wound already after 6 h. The error bars show the SD of the mean of three independent determinations done in quadruplicates. (C) Migration of MSV-MDCK cells expressing E-cadherin and Na,K-ATPase α-subunit and of reverted MSV-Cad-NaKβ cells in the wound assay. The error bars show the SD of the mean of three independent determinations done in quadruplicates.
Expression of β-Subunit Results in a More Stable Association of E-Cadherin to the Submembranous Actin Cytoskeleton
An actin-based cytoskeleton regulates epithelial polarization (Jou and Nelson, 1998), cell motility, and invasiveness of transformed epithelial cells (Carlier and Pantaloni, 1997; Hordijk et al., 1997; Keely et al., 1997). Therefore, we determined the organization of actin by FITC-phalloidin staining in MSV-MDCK cells expressing either E-cadherin, β-subunit, or both. MSV-Cad-NaKβ (Figure 9, A and B) and MDCKwt cells (Figure 9C) showed a continuous submembranous cortical actin compared with MSV-Cad vector cells in which the actin ring was discontinuous (Figure 9D, arrows). MSV-MDCK, MSV-NaKβ, MSV-Cad-NaKα, and MSV-Cad-NaKβ rev cells showed a discontinuous cortical actin cytoskeleton similar to that of MSV-Cad vector cells (our unpublished results). These results indicated that enhanced expression of both E-cadherin and β-subunit in MSV-MDCK cells results in the formation of a cortical actin cytoskeleton similar to MDCKwt cells.
Figure 9.
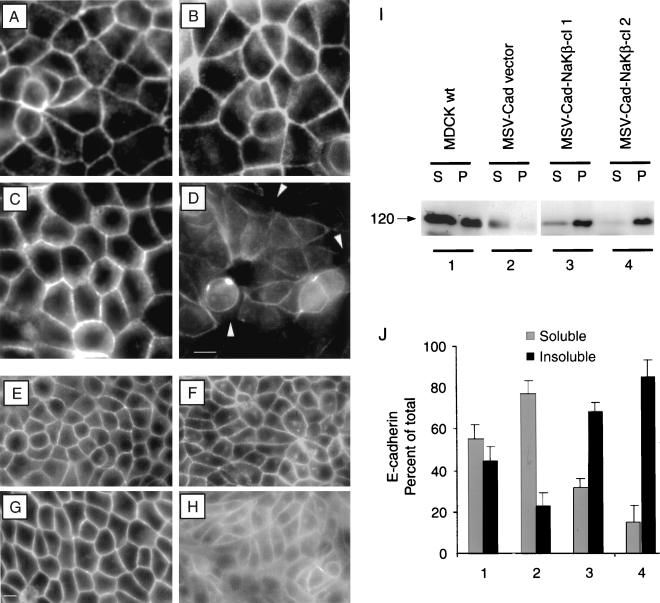
F-actin and E-cadherin staining and detergent solubility of E-cadherin, α- and β-subunit in MSV-MDCK cells expressing E-cadherin and Na,K-ATPase β-subunit. (A) MSV-Cad-NaKβ-cl 1 and MSV-Cad-NaKβ-cl 2 (B) cells show an intense cortical actin ring similar to MDCKwt cells (C). Similarly, E-cadherin localization in MSV-Cad-NaKβ-cl 1 (E), MSV-Cad-NaKβ-cl 2 (F), and MDCKwt cells (G) were nearly identical. Overexpression of E-cadherin alone in MSV-MDCK cells did not reveal a similar staining pattern of cortical actin (D) and E-cadherin (H). Bar, A–D, 20 μm; bar, E–H, 10 μm. (I) Confluent monolayers were extracted with Triton X-100 and soluble and insoluble fractions were obtained by centrifugation. Equal volumes of supernatant (S) and pellet (P) fraction were separated on a 10% SDS-PAGE and immunoblotted with anti-E-cadherin antibody and visualized by enhanced chemiluminescence. (J) Relative levels of E-cadherin in soluble and insoluble fractions were quantified by densitometric analysis. Bars indicate the SD of three independent determinations.
We then tested whether the change in the cortical actin cytoskeleton organization resulted in an increased stabilization of E-cadherin on the plasma membrane in MSV-Cad-NaKβ cells. Immunofluorescence staining of E-cadherin in MSV-Cad-NaKβ cells (Figure 9, E and F) was almost indistinguishable from MDCKwt cells (Figure 9G), whereas MSV-Cad vector cells did not show such an E-cadherin-staining pattern (Figure 9H). The observed well-organized cortical actin and intense E-cadherin staining on the plasma membrane suggested that E-cadherin might be more stably associated with the actin cytoskeleton in MSV-Cad-NaKβ cells compared with MSV-Cad vector cells. E-cadherin function in cell-cell adhesion is dependent on its stable association with the actin cytoskeleton (Hirano et al., 1987; Nagafuchi and Takeichi, 1988). Detergent extraction and quantification of E-cadherin in the soluble and insoluble fractions have been widely used to assess the strength of interaction of E-cadherin with the actin cytoskeleton (Hordijk et al., 1997; Piepenhagen and Nelson, 1998). Triton X-100 extraction of confluent monolayers of MDCKwt and MSV-Cad-NaKβ cells revealed that 50–90% of the E-cadherin was detergent insoluble, indicating an increased association with the actin cytoskeleton (Figure 9, I and J, lanes 1, 3, and 4). In contrast, only 23% of E-cadherin was detergent insoluble in MSV-Cad vector cells (Figure 9, I and J, lane 2). Because MSV-Cad vector cells start to pile up after 48 h of growth, the detergent solubility experiments were carried out at this time point. However, in MDCK cells, at 24 and 48 h still a large amount of E-cadherin is extracted in the soluble fraction. In fact, it takes ∼5 d to significantly reduce E-cadherin in the soluble fraction of MDCK cells (Shore and Nelson, 1991). However, in MSV-Cad-NaKβ-cl 1 and MSV-Cad-NaKβ-cl 2 cells a significant amount of E-cadherin is detergent insoluble at 48 h, indicating that the association of E-cadherin with cortical actin cytoskeleton takes place much faster in MSV-Cad-NaKβ cells than in MDCK cells. Similarly, α- and β-subunits of Na,K-ATPase revealed high detergent insolubility in MDCKwt and MSV-Cad-NaKβ-clones (our unpublished results). These results suggest that the E-cadherin–mediated cell adhesion machinery is weakly associated with the cortical actin cytoskeleton in cells expressing low levels of Na,K-ATPase β-subunit. An increased expression of β-subunit in E-cadherin–expressing MSV-MDCK cells appears to increase the strength of the E-cadherin–mediated cell adhesion system, perhaps by stabilizing the association of the E-cadherin complex with the cortical actin cytoskeleton.
Reduced Association of E-Cadherin to the Actin Cytoskeleton Does Not Compromise the Adhesive Behavior of MSV-Cad Cells
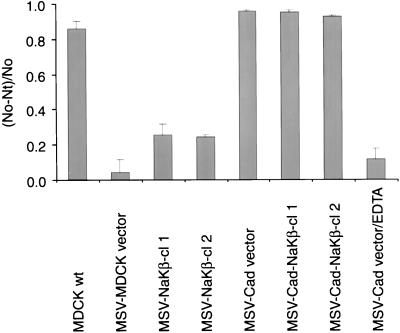
We then tested whether the reduced association of E-cadherin to the actin cytoskeleton alters the cell adhesion function of E-cadherin expressed in these cells by using a cell aggregation assay commonly used to monitor the cell-cell contact function of cell adhesion molecules (Nagafuchi and Takeichi, 1988; Lu et al., 1998; Noe et al., 1999). In this assay, a defined number of single cells in growth medium is plated on a plastic Petri dish (which will not support cell attachment) with growth media and incubated on a gyratory shaker at 37°C for few hours. Cells that can adhere will form aggregates, whereas cells that lack this ability will remain as single cells. The number of single cells was determined as described in MATERIALS AND METHODS. MSV-MDCK cells transfected with the vector alone showed no aggregation after 3 h of incubation (Figure 10). In contrast, MSV-Cad, and MDCKwt as well as both clones of MSV-Cad-NaKβ cells aggregated similarly to ∼95% (Figure 10). The aggregation of MSV-Cad cells was found to be dependent on the presence of calcium because depletion of calcium inhibited the aggregation of these cells. This result indicated that a reduced association of E-cadherin to the actin cytoskeleton does not compromise the calcium-dependent adhesive function of E-cadherin in MSV-Cad cells. Surprisingly, both of the MSV-NaKβ clones clearly showed increased aggregation compared with the MSV-MDCK vector cells (Figure 10). Because these latter cells lack detectable E-cadherin expression (Figure 1B, lanes 3 and 4) the aggregation observed with these cells should be due to the ectopic expression of the β-subunit. Aggregation of MSV-NaKβ cells is not due to transfection artifacts because MSV-MDCK vector cells that were transfected and selected similarly like MSV-NaKβ cells did not show any aggregation.
Figure 10.
Aggregation of MDCK and MSV-MDCK cells expressing E-cadherin and β-subunit of Na,K-ATPase. Single cells (200,000) were plated in regular culture medium in a plastic Petri dish and incubated at 37°C on a gyratory shaker (80 rpm) for 3 h. Note MSV-Cad vector cells aggregated similar to MDCKwt and MSV-Cad-NaKβ cells. MSV-Cad vector cells were incubated in calcium free medium containing 2.5 mM EDTA to test the calcium dependence of cell aggregation in these cells. Note in the absence of calcium, the aggregation of MSV-Cad vector cells is inhibited by more than 85% compared with cells incubated in the presence of calcium. The aggregation index was calculated as described in MATERIALS AND METHODS. SE bars indicate the average of three independent experiments done in triplicates.
Expression of β-Subunit of Na,K-ATPase Reduces the Intracellular Sodium Levels in MSV-MDCK Cells
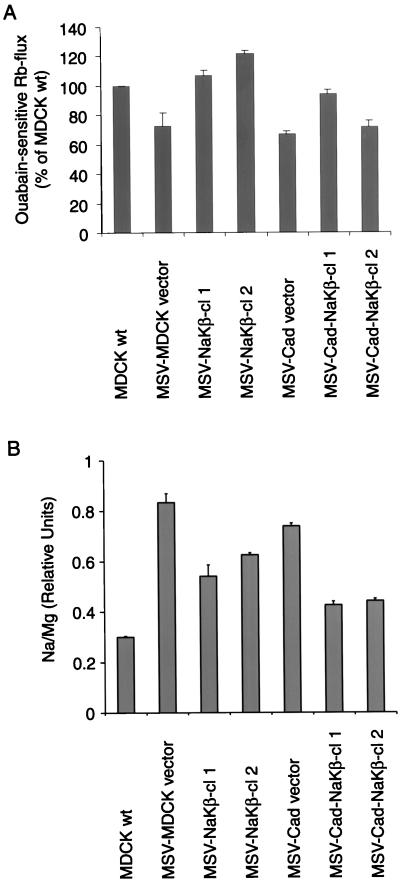
It seemed possible that MSV-MDCK cells would have reduced Na,K-ATPase activity due to their reduced levels of α- and β-subunits and that the ectopic expression of the β-subunit would increase the Na,K-ATPase activity in these transformed cells. The increased Na,K-ATPase activity resulting from β-subunit expression might be involved in the regulation of the polarized phenotype or the decreased cell motility in E-cadherin and β-subunit–expressing MSV-MDCK cells. We measured the ouabain-sensitive influx of 86Rb+ to monitor the Na,K-ATPase activity (Reznik et al., 1985; Lambrecht et al., 1998) as described in MATERIALS AND METHODS. The 86Rb+ uptake was comparable in MSV-MDCK vector and MSV-Cad vector cells, which express similar levels of α- and β-subunits. However, in spite of highly reduced levels of Na,K-ATPase α- and β-subunits (Figure 1) the Na,K-ATPase activity in these cells was only 25% less than that of MDCKwt cells (p < 0.01) (Figure 11A). Comparison of the 86Rb+ uptake among MSV-MDCK vector, MSV-NaKβ-cl 1, MSV-NaKβ-cl 2, MSV-Cad vector, MSV-Cad-NaKβ-cl 1, and MSV-Cad-NaKβ-cl 2 cells did not demonstrate any significant correlation of Na,K-ATPase transport activity with either the α-subunit (Spearman rank correlation rS = 0.26) or β-subunit levels (rS = 0.49).
Figure 11.
Analysis of Na,K-ATPase function in MSV-MDCK cells expressing E-cadherin and β-subunit of Na,K-ATPase. (A) Ouabain-sensitive 86Rb+ uptake. The 86Rb+ uptake assay was performed as described in MATERIALS AND METHODS and the ouabain-sensitive 86Rb+ flux was normalized to total protein. The bars indicate the SE of two independent determinations done in triplicates. (B) Determination of the intracellular Na+ levels by atomic emission spectrometry. The intracellular sodium concentrations were measured at 588.995 nm and normalized to the Mg2+ content of the cells. The error bars show the SE of the mean of two independent determinations.
Although the 86Rb+-uptake rates were near normal, the reduced number of sodium pumps on the plasma membrane in MSV-MDCK cells might have resulted in an increased intracellular sodium content, thereby increasing the activity of the enzyme. To test this possibility, we determined the intracellular sodium levels in these different cell lines. To remove the extracellular sodium, the cells were extensively washed in 0.25 M sucrose before analysis. Therefore, the obtained sodium levels should represent the intracellular sodium content ([Na]i). This analysis revealed an [Na]i of 12 mM for MDCKwt cells, which is a reasonable value for intracellular sodium. Treatment of MDCKwt cells with the Na,K-ATPase inhibitor ouabain increased ([Na]i by >10-fold and was accompanied by a dramatic decrease in the potassium content of these cells (our unpublished results). Ouabain treatment of MSV-MDCK cells expressing either β-subunit or E-cadherin or both increased the sodium content by >10-fold with corresponding decreases in the potassium content (our unpublished results). Thus, the ouabain-sensitive changes in the levels of intracellular sodium and potassium validated our ionic measurements by using atomic emission spectrometry. The sodium levels in MSV-MDCK cells expressing either β-subunit or E-cadherin or both β-subunit and E-cadherin are shown in Figure 11B. The [Na]i of MSV-MDCK vector cells was 2.8-fold higher than that of MDCKwt cells (p < 0.05). The [Na]i of MSV-Cad vector cells was comparable to that of MSV-MDCK vector cells. In cells transfected with β-subunit (MSV-NaKβ-cl 1 and -cl 2), [Na]i levels were reduced compared with MSV-MDCK vector cells, but the differences were only borderline statistically significant. Coexpression of E-cadherin and Na,K-ATPase β-subunit further decreased [Na]i compared with MSV-MDCK vector cells (p < 0.05), and this difference appeared larger than the effect due to the expression of β-subunit alone. The intracellular potassium levels in all these cell lines were similar (our unpublished results). These results indicate that the Na,K-ATPase β-subunit expression in MSV-MDCK cells results in the reduction of the intracellular sodium levels.
DISCUSSION
Epithelial Polarization Requires E-Cadherin and the β-Subunit of Na,K-ATPase
In this study, we demonstrate that Na,K-ATPase β1-subunit is required for epithelial polarization and suppression of cell invasion and motility in E-cadherin–expressing MSV-MDCK cells. Because MSV-MDCK cells expressing E-cadherin alone were nonpolarized, invasive, and motile, and the expression of Na,K-ATPase β1-subunit in these cells induced a polarized phenotype, abolished invasiveness, and reduced cell motility, we suggest that kidney epithelial cell polarity and tumor invasion suppression are likely regulated by a functional synergism between E-cadherin and Na,K-ATPase. Our conclusions are based on multiple independent controls that show a relationship between the polarized phenotype and β-subunit and E-cadherin expression. 1) Replenishing Na,K-ATPase β-subunit expression in two independent clones expressing E-cadherin induces a polarized phenotype and suppresses invasion and motility. 2) Expression of Na,K-ATPase α-subunit in two independent clones of E-cadherin–expressing MSV-MDCK cells did not induce a polarized phenotype. 3) E-cadherin–expressing MSV-MDCK cells transfected with vector alone did not show an altered phenotype. 4) Two independent clones of MSV-MDCK cells expressing β-subunit alone did not show any change in phenotype. 5) Using a reverted clone, we show that near normal expression levels of E-cadherin and β-subunit are crucial to maintain the polarized phenotype and reduce invasiveness and motility of MSV-MDCK cells. Clearly, we recognize that MSV-transformation of MDCK cells may result in multiple genetic and biochemical changes, including loss of E-cadherin and β-subunit expression. However, our results indicate that replenishing both E-cadherin and β-subunit expression is sufficient to significantly reverse the Moloney sarcoma virus-induced transformed phenotype of MDCK cells.
Overexpression of β-subunit in MSV-MDCK and MSV-Cad cells increased the levels and surface expression of the α-subunit (our unpublished results). In contrast, ectopic expression of Na,K-ATPase α-subunit in MSV-Cad cells resulted predominantly in its intracellular localization. Repletion of β-subunit expression in MSV-MDCK and MSV-Cad cells might have resulted in the more efficient transport of the α-subunit from the endoplasmic reticulum to the plasma membrane and stabilization of the α/β heterodimer on the cell surface as previously reported in Xenopus oocytes (Geering, 1991; Geering et al., 1996). MSV-NaKβ cells that lack E-cadherin expression did not show a polarized phenotype despite higher α-subunit levels, indicating that E-cadherin is also crucial for the development of the polarized phenotype. Our results thus indicate that both E-cadherin– and the β-subunit–mediated α/β-heterodimer function is crucial for the development of a polarized phenotype in MSV-MDCK cells.
Lack of tight junctions and desmosomes in MSV-MDCK cells expressing E-cadherin alone and the presence of these junctions in MSV-MDCK cells expressing both E-cadherin and β-subunit suggest that E-cadherin's cell-cell adhesion function alone is not sufficient to induce junctional complexes in kidney epithelial cells. In a previous report, we showed that in MSV-MDCK cells ZO-1 is predominantly localized to the cytoplasm. E-cadherin expression in these cells resulted in the translocation of ZO-1 to the plasma membrane. However, these cells showed a discontinuous ZO-1 staining pattern and failed to make functional tight junctions (Rajasekaran et al., 1996). In polarized MDCK cells the adherens junction is localized below the tight junction, and in cells expressing functional tight junctions the ZO-1 staining can be clearly separated from the E-cadherin staining by using three-dimensional laser scanning confocal microscopy. In MSV-Cad cells, ZO-1 colocalizes with E-cadherin in the adherens junction region (Figure 5D; Rajasekaran et al., 1996), indicating the absence of tight junctions. In contrast, the ZO-1 staining can be clearly separated from the lateral E-cadherin staining in MDCK and MSV-Cad-NaKβ cells (Figure 5, A and B), indicating the presence of tight junctions. These results suggest that functional E-cadherin is essential for initial events that trigger the translocation of ZO-1 to the plasma membrane, whereas the β-subunit–mediated α/β-heterodimer function is crucial for events that regulate the formation of an undisrupted ZO-1 ring, functional tight junctions, and, consequently, the polarized epithelial phenotype. Therefore, we propose that the tight junction development might consist of two major steps: 1) E-cadherin–dependent early signaling events necessary for the targeting of tight junction proteins to the adherens junction region, and 2) E-cadherin–independent events that regulate the separation of tight junction components from the adherens junction region to form functional tight junctions. It is possible that the α/β-heterodimer itself by strengthening the cell-cell contact or the ionic gradient generated by the Na,K-ATPase (see below) might play a role in the regulation of events that separate tight junction components from the adherens junction region to form functional tight junctions.
Suppression of Invasiveness and Motility of Carcinoma Cells Requires E-Cadherin and the β-Subunit of Na,K-ATPase
E-cadherin's association with the actin cytoskeleton is dramatically increased in E-cadherin– and β-subunit–expressing MSV-MDCK, indicating that the β-subunit expression may directly or through Na,K-ATPase activity strengthen the cell-cell contact mediated by E-cadherin (see below). In addition, these cells show junctional complexes such as tight junctions and desmosomes, which might further increase and stabilize the adhesive contact among cells and consequently reduce invasion and motility. Recently, desmosomal adhesion induced by expressing desmosomal proteins in L929 fibroblasts has been shown to inhibit invasion through a collagen gel (Tselepis et al., 1998). These results indicate that junctional complexes themselves might have a role in restricting invasive behavior of carcinoma cells. We suggest that increased cell-cell contact established due to the formation of tight junctions and desmosomes in MSV-Cad-NaKβ cells may simply restrain cells, thereby preventing the cell movement necessary for invasion. It is also possible that increased cell-cell contact might counterbalance the effect of invasion and motility promoting factors that are induced upon cellular transformation as previously proposed by Vleminckx et al. (1991).
Role of the Actin Cytoskeleton in E-Cadherin– and β-Subunit–induced Polarity, Invasion Suppression, and Motility
E-cadherin–mediated cell adhesion induces an extensive reorganization of the cortical actin cytoskeleton that is important in generating a polarized epithelial phenotype (Rodriguez-Boulan and Nelson, 1989; Adams and Nelson, 1998). MDCKwt and MSV-Cad-NaKβ cells showed a well-organized cortical actin cytoskeleton, and E-cadherin in these cells was significantly less detergent soluble compared with MSV-Cad cells. Similarly, most of the α- and β-subunit was present in the insoluble fraction in MSV-Cad-NaKβ cells compared with MSV-Cad cells (our unpublished results), indicating that replenishing β-subunit expression results in an increased stabilization of the α/β-heterodimer on the plasma membrane. The α-subunit of Na,K-ATPase has been shown to associate with the ankyrin-spectrin cytoskeleton, which in turn associates with the cortical actin cytoskeleton (Nelson and Veshnock, 1987; Morrow et al., 1989; Bennett and Gilligan, 1993). We suggest that efficient transport of the α-subunit to the cell surface upon overexpression of β-subunit in MSV-Cad cells may result in increased recruitment of actin at the subplasma membrane region and facilitate association and stabilization of the E-cadherin/α-β catenin complex with the submembranous actin cytoskeleton. Previous reports suggested that E-cadherin initiates an actin-based membrane cytoskeleton at cell-cell contact regions that stabilizes the Na,K-ATPase (McNeil et al., 1990; Piepenhagen and Nelson; 1998). Our results indicate that the Na,K-ATPase itself may be involved in strengthening a submembranous cortical actin cytoskeleton that stabilizes E-cadherin expression on the plasma membrane that may then augment the strength of cell-cell contacts, resulting in the induction of the epithelial phenotype and suppression of motility and invasion. A macromolecular complex containing E-cadherin, catenins, Na,K-ATPase, together with actin binding proteins and cortical actin may facilitate the stabilization of cell-cell contacts necessary to maintain a polarized epithelial phenotype and restrict cell motility and invasiveness.
Na,K-ATPase β-Subunit's Regulatory Functions in Epithelial Cells
Ectopic expression of Na,K-ATPase β-subunit in MSV-MDCK cells reduced [Na]i toward normal levels and coexpression of E-cadherin and β-subunit strengthened this effect by some unknown mechanism(s). The presence of functional tight junctions and desmosomes in MSV-Cad-NaKβ cells suggests that near normal [Na]i maintained by Na,K-ATPase and E-cadherin function may be crucial for the formation and maintenance of epithelial tight junctions and desmosomes and thus the polarization of epithelial cells. These results are consistent with a notion that a synergism might exist between the functions of Na,K-ATPase β-subunit and E-cadherin to induce and maintain the polarized phenotype in epithelial cells. The precise mechanism by which Na,K-ATPase β-subunit may function synergistically with E-cadherin to regulate the polarized epithelial phenotype is not known. The β-subunit may increase the α-subunit transport to the cell surface. The α/β-heterodimer by recruiting more actin may stabilize E-cadherin expression on the plasma membrane and strengthen cell-cell contacts necessary to maintain the polarized epithelial phenotype. Alternatively, stable association of the α-subunit with the cortical actin cytoskeleton mediated by β-subunit and E-cadherin in epithelial cells may be necessary to maintain normal intracellular sodium levels and thus the polarized epithelial phenotype. In addition, the β-subunit itself may act as a cell adhesion molecule and function synergistically with E-cadherin to establish the epithelial phenotype. In fact, based on the glycosylation pattern, a role for the β-subunit in cell adhesion has been suggested (Treuheit et al., 1993). Furthermore, the β2-isoform initially designated as adhesion molecule on glia functions as adhesion molecule in glia cells (Gloor et al., 1990).
Whatever be the mechanism, the β-subunit appears to have a crucial role in maintaining structure and function of polarized epithelial cells. The vectorial transport function (directional transport of molecules across an epithelial cell layer) of epithelial cells largely depends on the transepithelial flow of sodium ions (Na+) from the apical to the basolateral side. The Na+-gradient resulting from this enzyme's activity is essential for the functioning of other Na+-coupled transport systems. In consequence, Na,K-ATPase is a crucial enzyme that regulates vectorial transport in epithelial cells. The β-subunit of Na,K-ATPase is essential for the plasma membrane expression of the catalytic α-subunit. Vectorial transport is dependent on the structural organization (polarized epithelial phenotype) of epithelial cells. We now show that E-cadherin and Na,K-ATPase β-subunit are required to maintain the polarized epithelial phenotype. Thus, the Na,K-ATPase β-subunit is an excellent candidate for linking structure and function of polarized epithelial cells. It is conceivable that reduced β-subunit expression in carcinoma may therefore lead to alteration of both structure and function of epithelial cells.
ACKNOWLEDGMENTS
We thank Drs. Ernest Wright, Alexander Van der Bliek, and Gregory Payne for valuable suggestions. We thank Dr. Joel Pardee for encouragement and support. We are very grateful to Dr. Elliot Landaw for statistical analysis of the data. We thank Ric Grambo (Biomedical Technology Research and Instructional Productions, University of California Los Angeles) and Carol Appleton for help in the preparation of illustrations. We thank Dr. Robert Farley for the canine Na,K-ATPase β-subunit cDNA, Dr. Askari Amir for the canine Na,K-ATPase α-subunit cDNA, Dr. Lisa McConlogue for the E-cadherin cDNA, and Dr. Mike Roth for the pCB7 expression vector. This work is primarily supported by a grant-in-aid award from the American Heart Association (Western States Affiliate) to A.K.R and in part by National Institutes of Health Grant CA-67113 (to A.P.S.). Metal analysis was supported by a National Science Foundation Grant DBI-0077378 (to J.F.H.). S.A.R is supported by a Fellowship from Toohey Foundation. A.K.R is a member of the Jonsson Comprehensive Cancer Center.
REFERENCES
- Abbott A, Ball WJ., Jr The epitope for the inhibitory antibody M7-PB-E9 contains Ser-646 and Asp-652 of sheep Na,K-ATPase α-subunit. Biochemistry. 1993;32:3511–3518. doi: 10.1021/bi00064a040. [DOI] [PubMed] [Google Scholar]
- Adams CL, Nelson WJ. Cytomechanics of cadherin-mediated cell-cell adhesion. Curr Opin Cell Biol. 1998;10:572–577. doi: 10.1016/s0955-0674(98)80031-8. [DOI] [PubMed] [Google Scholar]
- Behrens J, Birchmeier W, Goodman SL, Imhof BA. Dissociation of Madin-Darby canine kidney epithelial cells by the monoclonal antibody anti-Arc-1: mechanistic aspects and identification of the antigen as a component related to uvomorulin. J Cell Biol. 1985;101:1307–1315. doi: 10.1083/jcb.101.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens J, Mareel MM, Van Roy FM, Birchmeier W. Dissecting tumor cell invasion: epithelial cells acquire invasive properties after the loss of uvomorulin-mediated cell-cell adhesion. J Cell Biol. 1989;108:2435–2447. doi: 10.1083/jcb.108.6.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett V, Gilligan D. The spectrin-based membrane skeleton and micron-scale organization of the plasma membrane. Annu Rev Cell Biol. 1993;9:27–66. doi: 10.1146/annurev.cb.09.110193.000331. [DOI] [PubMed] [Google Scholar]
- Birchmeier W, Behrens J. Cadherin expression in carcinomas: role in the formation of cell junctions and the prevention of invasiveness. Biochim Biophys Acta. 1994;1198:11–26. doi: 10.1016/0304-419x(94)90003-5. [DOI] [PubMed] [Google Scholar]
- Blanco G, Melton RJ, Sanchez G, Mercer RW. Functional characterization of a testes-specific α-subunit isoform of the sodium/potassium adenosinetriphosphatase. Biochemistry. 1999;38:13661–13669. doi: 10.1021/bi991207b. [DOI] [PubMed] [Google Scholar]
- Carlier M-F, Pantaloni D. Control of actin dynamics in cell motility. J Mol Biol. 1997;269:459–467. doi: 10.1006/jmbi.1997.1062. [DOI] [PubMed] [Google Scholar]
- Eakle KA, Kabalin MA, Wang S, Farley RA. The influence of β-subunit structure on the stability of Na,K-ATPase complexes and interaction with K+ J Biol Chem. 1994;269:6550–6557. [PubMed] [Google Scholar]
- Farquhar MG, Palade GE. Junctional complexes in various epithelia. J Cell Biol. 1963;17:375–412. doi: 10.1083/jcb.17.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frixen UH, Behrens J, Sachs M, Eberle G, Voss B, Warda A, Lochner D, Birchmeier W. E-Cadherin-mediated cell-cell adhesion prevents invasiveness of human carcinoma cells. J Cell Biol. 1991;113:173–185. doi: 10.1083/jcb.113.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrod D, Chidgey M, North A. Desmosomes: differentiation, development, dynamics, and disease. Curr Opin Cell Biol. 1996;5:670–678. doi: 10.1016/s0955-0674(96)80108-6. [DOI] [PubMed] [Google Scholar]
- Geering K. The functional role of the β-subunit in the maturation and intracellular transport of Na,K-ATPase. FEBS Lett. 1991;285:189–193. doi: 10.1016/0014-5793(91)80801-9. [DOI] [PubMed] [Google Scholar]
- Geering K, Beggah A, Good P, Girardet S, Roy S, Schaer D, Jaunin P. Oligomerization and maturation of Na,K-ATPase: functional interaction of the cytoplasmic NH2 terminus of the β subunit with the α subunit. J Cell Biol. 1996;133:1193–1204. doi: 10.1083/jcb.133.6.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloor S, Antonicek H, Sweadner KJ, Pagliusi S, Frank R, Moos M, Schachner M. The adhesion molecule on glia (AMOG) is a homologue of the beta subunit of the Na,K-ATPase. J Cell Biol. 1990;110:165–174. doi: 10.1083/jcb.110.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Mariscal L, Chavez de Ramirez B, Cereijido M. Tight junction formation in cultured epithelial cells (MDCK) J Memb Biol. 1985;86:113–125. doi: 10.1007/BF01870778. [DOI] [PubMed] [Google Scholar]
- Gumbiner B, Simons K. A functional assay for proteins involved in establishing an epithelial occluding barrier: identification of a uvomorulin-like polypeptide. J Cell Biol. 1986;102:457–468. doi: 10.1083/jcb.102.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano S, Nose A, Hatta K, Kawakami A, Takeichi M. Calcium-dependent cell-cell adhesion molecules (cadherins): subclass specificities and possible involvement of actin bundles. J Cell Biol. 1987;105:2501–2510. doi: 10.1083/jcb.105.6.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hordijk PL, ten Kloster JP, Van der Kammen RA, Michiels F, Oomen LCJM, Collard JG. Inhibition of invasion of epithelial cells by Tiam1-Rac signaling. Science. 1997;278:1464–1466. doi: 10.1126/science.278.5342.1464. [DOI] [PubMed] [Google Scholar]
- Jou TS, Nelson WJ. Effects of regulated expression of mutant RhoA and Rac1 small GTPases on the development of epithelial (MDCK) cell polarity. J Cell Biol. 1998;142:85–100. doi: 10.1083/jcb.142.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keely PJ, Westwick JK, Whitehead IP, Channing JD, Parise LV. Cdc42 and Rac1 induce integrin-mediated cell motility and invasiveness through PI(3)K. Nature. 1997;390:632–636. doi: 10.1038/37656. [DOI] [PubMed] [Google Scholar]
- Knudsen KA, Peralta Soler A, Johnson KR, Wheelock MJ. Interaction of alpha-actinin with the cadherin/catenin cell-cell adhesion complex via alpha-catenin. J Cell Biol. 1995;130:67–77. doi: 10.1083/jcb.130.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrecht N, Corbett Z, Bayle D, Karlish SJD, Sachs G. Identification of the site of inhibition by omeprazole of a α-β fusion protein of the H,K-ATPase using site-directed mutagenesis. J Biol Chem. 1998;273:13719–13728. doi: 10.1074/jbc.273.22.13719. [DOI] [PubMed] [Google Scholar]
- Lingrel JB, Van Huysse J, O‘Brien W, Jewell-Motz E, Askew R, Schultheis P. Structure-function studies of the Na,K-ATPase. Kidney Int. 1994;45(suppl 44):S32–S39. [PubMed] [Google Scholar]
- Laski ME, Kurtzman NA. The renal adenosine triphosphatase: functional integration and clinical significance. Miner Electrolyte Metab. 1996;22:410–422. [PubMed] [Google Scholar]
- Lu Q, Paredes M, Zhanh J, Kosik KS. Basal extracellular signal-regulated kinase activity modulates cell-cell and cell-matrix interactions. Mol Cell Biol. 1998;18:3257–3265. doi: 10.1128/mcb.18.6.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil H, Ozawa M, Kemler R, Nelson WJ. Novel function of the cell adhesion molecule uvomurulin as an inducer of cell surface polarity. Cell. 1990;62:309–316. doi: 10.1016/0092-8674(90)90368-o. [DOI] [PubMed] [Google Scholar]
- Mercer RW. Structure of the Na,K-ATPase. Int Rev Cytol. 1993;137C:139–168. [PubMed] [Google Scholar]
- Morrow JS, Cianci CD, Ardito T, Mann AS, Kashgarian M. Ankyrin links fodrin to the alpha subunit of Na,K-ATPase in Madin-Darby canine kidney cells and in intact renal tubule cells. J Cell Biol. 1989;108:455–465. doi: 10.1083/jcb.108.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagafuchi A, Takeichi M. Cell binding function of E-cadherin is regulated by the cytoplasmic domain. EMBO J. 1988;7:3679–3684. doi: 10.1002/j.1460-2075.1988.tb03249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson WJ, Veshnock PJ. Ankyrin binding to (Na+,K+)ATPase and implications for the organization of membrane domains in polarized cells. Nature. 1987;328:533–536. doi: 10.1038/328533a0. [DOI] [PubMed] [Google Scholar]
- Noe V, Willems J, Vandekerckhove J, Van Roy FV, Bruyneel E, Mareel M. Inhibition of adhesion and induction of epithelial cell invasion by HAV-containing E-cadherin-specific peptides. J Cell Sci. 1999;112:127–135. doi: 10.1242/jcs.112.1.127. [DOI] [PubMed] [Google Scholar]
- Noguchi S, Higashi K, Kawamura M. A possible role of the β-subunit of (Na,K)-ATPase in facilitating correct assembly of the α-subunit into the membrane. J Biol Chem. 1990;265:15991–15995. [PubMed] [Google Scholar]
- Ozawa M, Baribault H, Kemler R. The cytoplasmic domain of the cell adhesion molecule uvomorulin associates with three independent proteins structurally related in different species. EMBO J. 1989;8:1711–1717. doi: 10.1002/j.1460-2075.1989.tb03563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piepenhagen PA, Nelson WJ. Biogenesis of polarized epithelial cells during kidney development in situ: roles of E-cadherin mediated cell-cell adhesion and membrane cytoskeleton organization. Mol Biol Cell. 1998;11:3161–3177. doi: 10.1091/mbc.9.11.3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasekaran SA, Ball WJ, Jr, Bander NH, Liu H, Pardee JD, Rajasekaran AK. Reduced expression of β-subunit of Na,K-ATPase in human clear-cell renal cell carcinoma. J Urol. 1999;162:574–580. [PubMed] [Google Scholar]
- Rajasekaran AK, Hojo M, Huima T, Rodriguez-Boulan E. Catenins and Zonula Occludens-1 form a complex during early stages in the assembly of tight junctions. J Cell Biol. 1996;132:451–463. doi: 10.1083/jcb.132.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznik VM, Shapiro RJ, Mendoza SA. Vasopressin stimulates DNA synthesis and ion transport in quiescent epithelial cells. Am J Physiol. 1985;249:C267–C270. doi: 10.1152/ajpcell.1985.249.3.C267. [DOI] [PubMed] [Google Scholar]
- Rimm DL, Koslov ER, Kebriaei P, Cianci CD, Morrow JS. α1 (E)-Catenin is an actin-binding and -bundling protein mediating the attachment of F-actin to the membrane adhesion complex. Proc Natl Acad Sci USA. 1995;92:8813–8817. doi: 10.1073/pnas.92.19.8813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Boulan E, Nelson WJ. Morphogenesis of the polarized epithelial cell phenotype. Science. 1989;245:718–725. doi: 10.1126/science.2672330. [DOI] [PubMed] [Google Scholar]
- Shamraj OI, Lingrel JB. A putative fourth Na+,K+-ATPase α-subunit gene is expressed in testis. Proc Natl Acad Sci USA. 1994;91:12952–12956. doi: 10.1073/pnas.91.26.12952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibamoto S, Hayakawa M, Takeuchi K, Hori T, Miyazawa K, Kitamura N, Johnson KR, Wheelock MJ, Matsuyoshi N, Takeichi M, Ito F. Association of p120, a tyrosine kinase substrate, with E-cadherin/catenin complexes. J Cell Biol. 1995;128:949–957. doi: 10.1083/jcb.128.5.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore EM, Nelson WJ. Biosynthesis of the cell adhesion molecule uvomorulin (E-cadherin) in Madin-Darby canine kidney epithelial cells. J Biol Chem. 1991;266:19672–19680. [PubMed] [Google Scholar]
- Shull GE, Lane LK, Lingrel JB. Amino acid sequence of the β-subunit of the Na+,K+-ATPase deduced from a cDNA. Nature. 1986;321:429–431. doi: 10.1038/321429a0. [DOI] [PubMed] [Google Scholar]
- Shull GE, Schwartz A, Lingrel JB. Amino-acid sequence of the catalytic subunit of the (Na++K+)ATPase deduced from a complementary DNA. Nature. 1985;316:691–695. doi: 10.1038/316691a0. [DOI] [PubMed] [Google Scholar]
- Simons K, Fuller S. Cell surface polarity in epithelia. Annu Rev Cell Biol. 1985;1:243–288. doi: 10.1146/annurev.cb.01.110185.001331. [DOI] [PubMed] [Google Scholar]
- Stetler-Stevenson WG, Aznavoorian S, Liotta LA. Tumor cell interactions with the extracellular matrix during invasion and metastasis. Annu Rev Cell Biol. 1993;9:541–573. doi: 10.1146/annurev.cb.09.110193.002545. [DOI] [PubMed] [Google Scholar]
- Sun Y, Ball WJ., Jr Identification of antigenic sites on the Na+/K+ -ATPase β-subunit: their sequences and the effects of thiol reduction upon their structure. Biochim Biophys Acta. 1994;1207:236–248. doi: 10.1016/0167-4838(94)00074-3. [DOI] [PubMed] [Google Scholar]
- Takeichi M. Cadherins: a molecular family important in selective cell-cell adhesion. Annu Rev Biochem. 1990;59:237–252. doi: 10.1146/annurev.bi.59.070190.001321. [DOI] [PubMed] [Google Scholar]
- Treuheit MJ, Costello CE, Kirley TL. Structures of the complex glycans found on the beta-subunit of (Na,K)-ATPase. J Biol Chem. 1993;268:13914–13919. [PubMed] [Google Scholar]
- Tselepis C, Chidgey M, North A, Garrod D. Desmosomal adhesion inhibits invasive behavior. Proc Natl Acad Sci USA. 1998;95:8064–8069. doi: 10.1073/pnas.95.14.8064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vleminckx K, Vakaet L, Jr, Mareel M, Fiers W, Van Roy F. Genetic manipulation of E-cadherin expression by epithelial tumor cells reveals an invasion suppressor role. Cell. 1991;66:107–119. doi: 10.1016/0092-8674(91)90143-m. [DOI] [PubMed] [Google Scholar]
- Woo AL, James PF, Lingrel JB. Characterization of the fourth α isoform of the Na,K-ATPase. J Membr Biol. 1999;169:39–44. doi: 10.1007/pl00005899. [DOI] [PubMed] [Google Scholar]
- Yap AS, Brieher WM, Gumbiner BM. Molecular and functional analysis of cadherin-based adherens junctions. Annu Rev Cell Dev Biol. 1997;13:119–146. doi: 10.1146/annurev.cellbio.13.1.119. [DOI] [PubMed] [Google Scholar]