Abstract
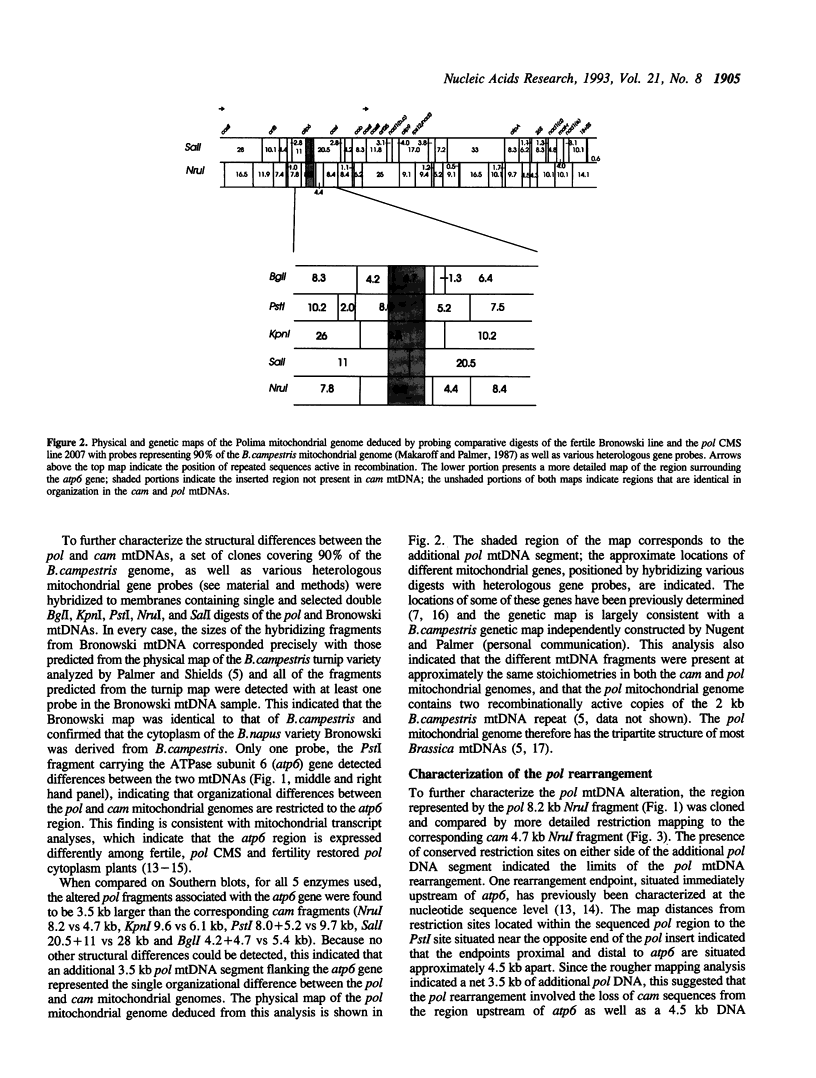
Comparison of the physical maps of male fertile (cam) and male sterile (pol) mitochondrial genomes of Brassica napus indicates that structural differences between the two mtDNAs are confined to a region immediately upstream of the atp6 gene. Relative to cam mtDNA, pol mtDNA possesses a 4.5 kb segment at this locus that includes a chimeric gene that is cotranscribed with atp6 and lacks an approximately 1kb region located upstream of the cam atp6 gene. The 4.5 kb pol segment is present and similarly organized in the mitochondrial genome of the common nap B.napus cytoplasm; however, the nap and pol DNA regions flanking this segment are different and the nap sequences are not expressed. The 4.5 kb CMS-associated pol segment has thus apparently undergone transposition during the evolution of the nap and pol cytoplasms and has been lost in the cam genome subsequent to the pol-cam divergence. This 4.5 kb segment comprises the single DNA region that is expressed differently in fertile, pol CMS and fertility restored pol cytoplasm plants. The finding that this locus is part of the single mtDNA region organized differently in the fertile and male sterile mitochondrial genomes provides strong support for the view that it specifies the pol CMS trait.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Conklin P. L., Wilson R. K., Hanson M. R. Multiple trans-splicing events are required to produce a mature nad1 transcript in a plant mitochondrion. Genes Dev. 1991 Aug;5(8):1407–1415. doi: 10.1101/gad.5.8.1407. [DOI] [PubMed] [Google Scholar]
- Fauron C. M., Havlik M., Brettell R. I. The mitochondrial genome organization of a maize fertile cmsT revertant line is generated through recombination between two sets of repeats. Genetics. 1990 Feb;124(2):423–428. doi: 10.1093/genetics/124.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa H., Nakajima K. Different organization and altered transcription of the mitochondrial atp6 gene in the male-sterile cytoplasm of rapeseed (Brassica napus L.). Curr Genet. 1992 Feb;21(2):153–159. doi: 10.1007/BF00318475. [DOI] [PubMed] [Google Scholar]
- Hanson M. R. Plant mitochondrial mutations and male sterility. Annu Rev Genet. 1991;25:461–486. doi: 10.1146/annurev.ge.25.120191.002333. [DOI] [PubMed] [Google Scholar]
- Köhler R. H., Horn R., Lössl A., Zetsche K. Cytoplasmic male sterility in sunflower is correlated with the co-transcription of a new open reading frame with the atpA gene. Mol Gen Genet. 1991 Jul;227(3):369–376. doi: 10.1007/BF00273925. [DOI] [PubMed] [Google Scholar]
- Levings C. S., 3rd The Texas cytoplasm of maize: cytoplasmic male sterility and disease susceptibility. Science. 1990 Nov 16;250(4983):942–947. doi: 10.1126/science.250.4983.942. [DOI] [PubMed] [Google Scholar]
- Makaroff C. A., Palmer J. D. Extensive mitochondrial specific transcription of the Brassica campestris mitochondrial genome. Nucleic Acids Res. 1987 Jul 10;15(13):5141–5156. doi: 10.1093/nar/15.13.5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer J. D., Herbon L. A. Plant mitochondrial DNA evolves rapidly in structure, but slowly in sequence. J Mol Evol. 1988 Dec;28(1-2):87–97. doi: 10.1007/BF02143500. [DOI] [PubMed] [Google Scholar]
- Rasmussen J., Hanson M. R. A NADH dehydrogenase subunit gene is co-transcribed with the abnormal Petunia mitochondrial gene associated with cytoplasmic male sterility. Mol Gen Genet. 1989 Jan;215(2):332–336. doi: 10.1007/BF00339738. [DOI] [PubMed] [Google Scholar]
- Rottmann W. H., Brears T., Hodge T. P., Lonsdale D. M. A mitochondrial gene is lost via homologous recombination during reversion of CMS T maize to fertility. EMBO J. 1987 Jun;6(6):1541–1546. doi: 10.1002/j.1460-2075.1987.tb02398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siculella L., Palmer J. D. Physical and gene organization of mitochondrial DNA in fertile and male sterile sunflower. CMS-associated alterations in structure and transcription of the atpA gene. Nucleic Acids Res. 1988 May 11;16(9):3787–3799. doi: 10.1093/nar/16.9.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M., Brown G. G. Suppression of cytoplasmic male sterility by nuclear genes alters expression of a novel mitochondrial gene region. Plant Cell. 1991 Dec;3(12):1349–1362. doi: 10.1105/tpc.3.12.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small I., Suffolk R., Leaver C. J. Evolution of plant mitochondrial genomes via substoichiometric intermediates. Cell. 1989 Jul 14;58(1):69–76. doi: 10.1016/0092-8674(89)90403-0. [DOI] [PubMed] [Google Scholar]
- Temple M., Makaroff C. A., Mutschler M. A., Earle E. D. Novel mitochondrial genomes in Brassica napus somatic hybrids. Curr Genet. 1992 Sep;22(3):243–249. doi: 10.1007/BF00351732. [DOI] [PubMed] [Google Scholar]
- Wise R. P., Pring D. R., Gengenbach B. G. Mutation to male fertility and toxin insensitivity in Texas (T)-cytoplasm maize is associated with a frameshift in a mitochondrial open reading frame. Proc Natl Acad Sci U S A. 1987 May;84(9):2858–2862. doi: 10.1073/pnas.84.9.2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young E. G., Hanson M. R. A fused mitochondrial gene associated with cytoplasmic male sterility is developmentally regulated. Cell. 1987 Jul 3;50(1):41–49. doi: 10.1016/0092-8674(87)90660-x. [DOI] [PubMed] [Google Scholar]