Abstract
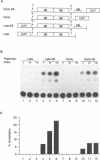
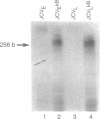
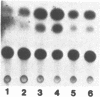
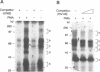
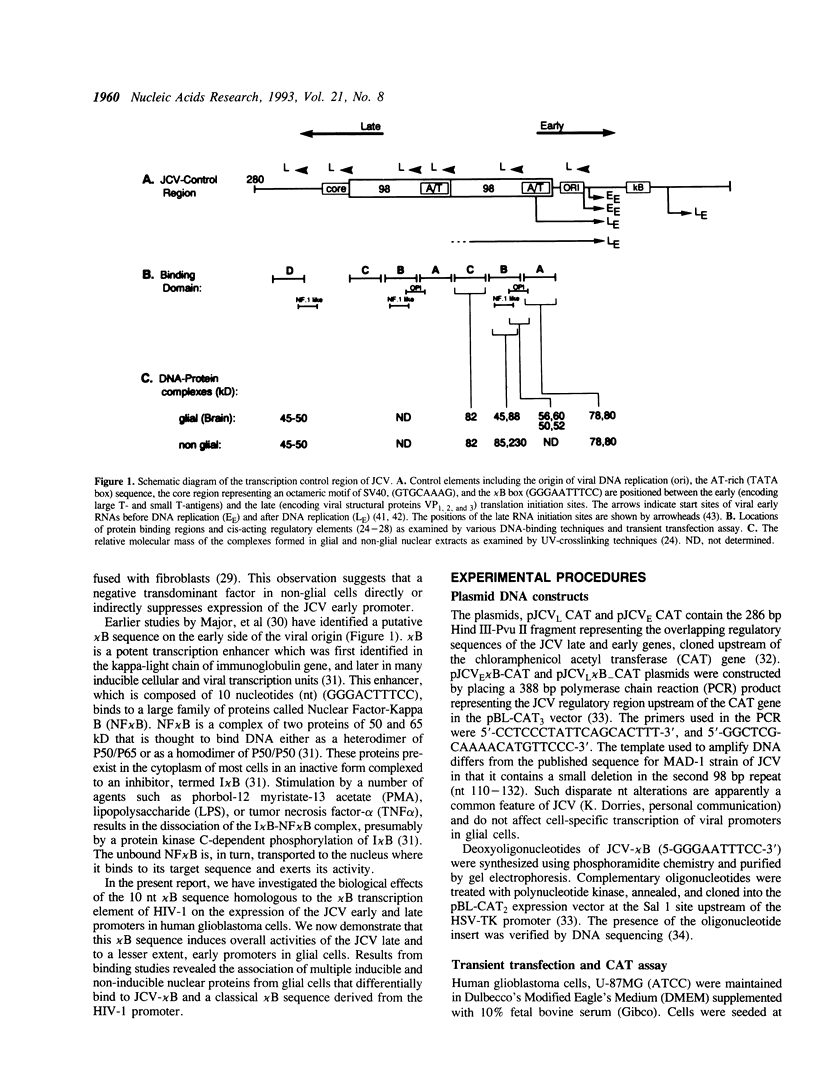
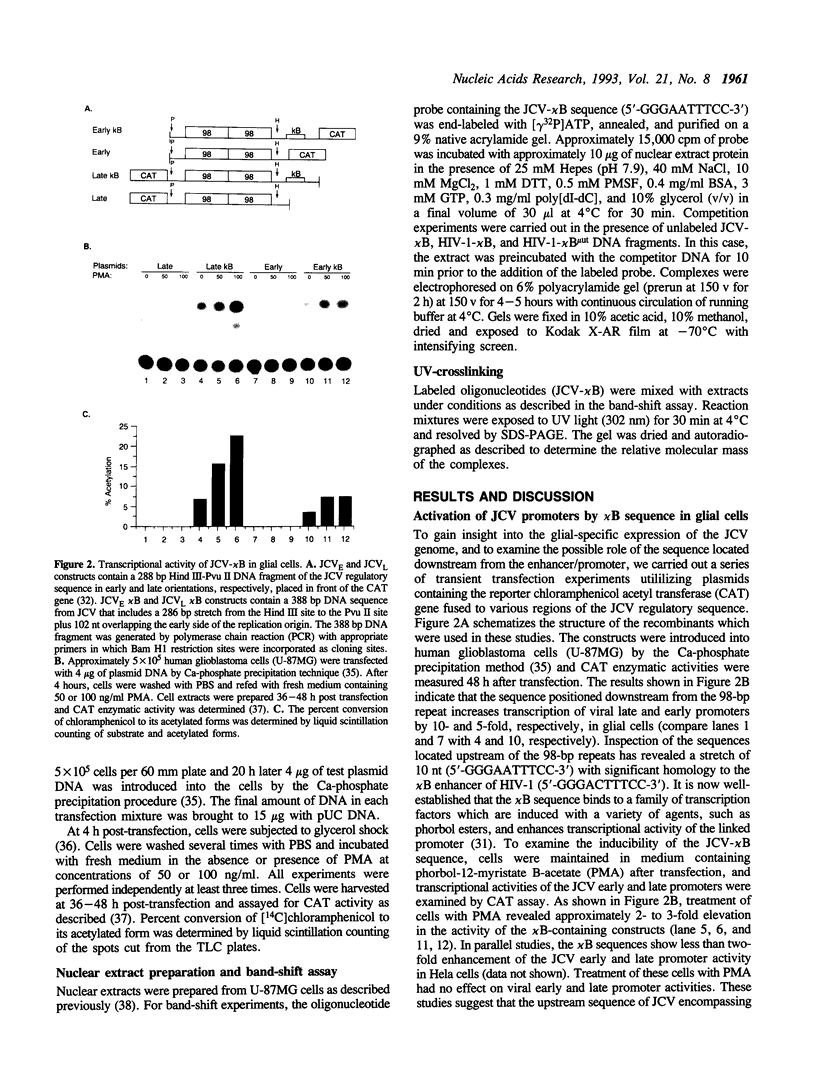
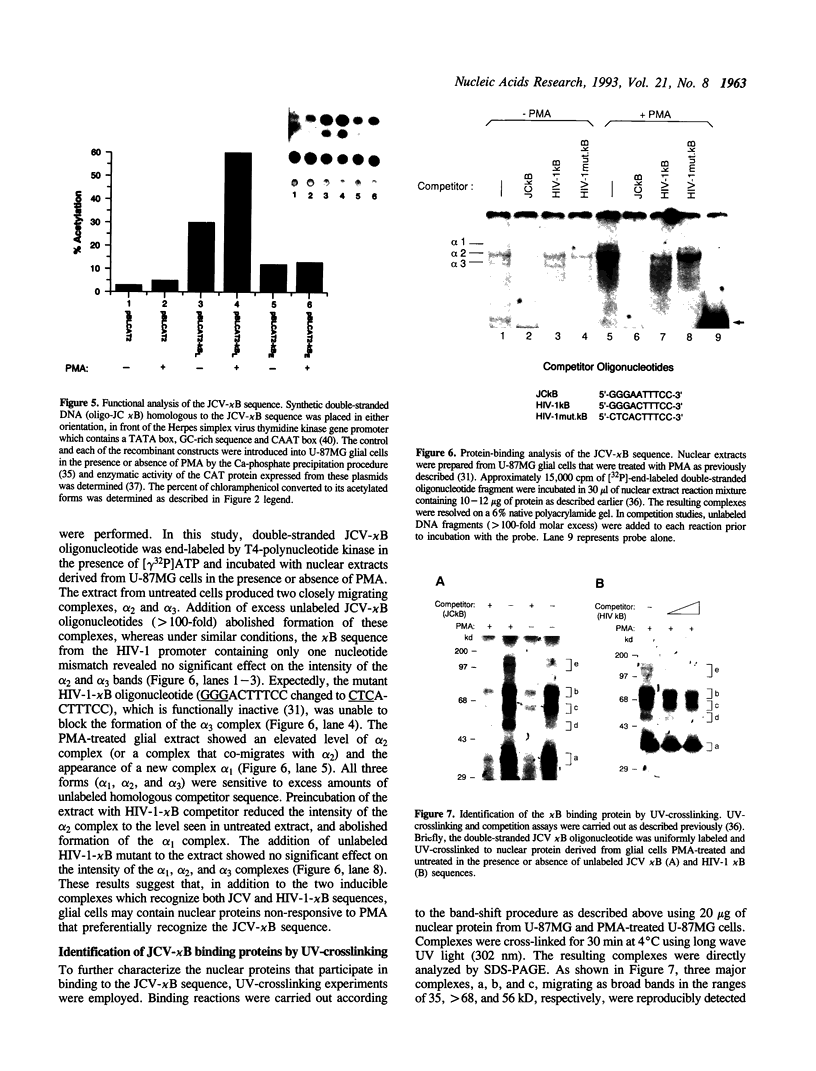
Studies on the regulation of the human neurotropic virus (JCV) promoter, have been focused primarily on the 98 bp tandem repeat sequence which confers glial-specificity to viral gene expression. We demonstrate that a distinct regulatory element outside of the 98 bp region, which spans a stretch of 10 nucleotides (nt) (5'-GGGAATTTCC-3') increases transcriptional activity of JCV late (JCVL), and early (JCVE) promoters in glial cells. Sequence analysis of this motif reveals extensive homology to the kappa B sequence of HIV-1 (5'-GGGACTTTCC-3'). A DNA fragment corresponding to the 10 nt sequence of JCV exhibits transcriptional activity when placed upstream of the test promoter in glial cells. The induction mediated by this regulatory motif is moderately enhanced in response to phorbol 12-myristate 13-acetate (PMA) in glial cells. Band-shift and UV-crosslinking experiments suggest that glial cells constitutively produce proteins that specifically interact with the JCV kappa B, but not the HIV-1 kappa B motif. Treatment of cells with PMA results in formation of new complexes that are sensitive to the kappa B sequences derived from the JCV and HIV-1 genomes. These results suggest that the kappa B sequence located in the JCV genome may play a role in transcriptional regulation of JCV gene expression by interacting with inducible and uninducible nuclear proteins from glial cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASTROM K. E., MANCALL E. L., RICHARDSON E. P., Jr Progressive multifocal leuko-encephalopathy; a hitherto unrecognized complication of chronic lymphatic leukaemia and Hodgkin's disease. Brain. 1958 Mar;81(1):93–111. doi: 10.1093/brain/81.1.93. [DOI] [PubMed] [Google Scholar]
- Ahmed S., Rappaport J., Tada H., Kerr D., Khalili K. A nuclear protein derived from brain cells stimulates transcription of the human neurotropic virus promoter, JCVE, in vitro. J Biol Chem. 1990 Aug 15;265(23):13899–13905. [PubMed] [Google Scholar]
- Amemiya K., Traub R., Durham L., Major E. O. Interaction of a nuclear factor-1-like protein with the regulatory region of the human polyomavirus JC virus. J Biol Chem. 1989 Apr 25;264(12):7025–7032. [PubMed] [Google Scholar]
- Baeuerle P. A. The inducible transcription activator NF-kappa B: regulation by distinct protein subunits. Biochim Biophys Acta. 1991 Apr 16;1072(1):63–80. doi: 10.1016/0304-419x(91)90007-8. [DOI] [PubMed] [Google Scholar]
- Beggs A. H., Frisque R. J., Scangos G. A. Extinction of JC virus tumor-antigen expression in glial cell--fibroblast hybrids. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7632–7636. doi: 10.1073/pnas.85.20.7632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigenbaum L., Khalili K., Major E., Khoury G. Regulation of the host range of human papovavirus JCV. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3695–3698. doi: 10.1073/pnas.84.11.3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First annual meeting of the Society for Experimental Neuropathology. October 1, 1988, Philadelphia, PA. Abstracts. Ann Neurol. 1988 Sep;24(3):471–482. doi: 10.1002/ana.410240330. [DOI] [PubMed] [Google Scholar]
- Frisque R. J., Bream G. L., Cannella M. T. Human polyomavirus JC virus genome. J Virol. 1984 Aug;51(2):458–469. doi: 10.1128/jvi.51.2.458-469.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Howley P. M., Rentier-Delrue F., Heilman C. A., Law M. F., Chowdhury K., Israel M. A., Takemoto K. K. Cloned human polyomavirus JC DNA can transform human amnion cells. J Virol. 1980 Dec;36(3):878–882. doi: 10.1128/jvi.36.3.878-882.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney S., Natarajan V., Salzman N. P. Mapping 5' termini of JC virus late RNA. J Virol. 1986 Apr;58(1):216–219. doi: 10.1128/jvi.58.1.216-219.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney S., Natarajan V., Selzer G., Salzman N. P. Mapping 5' termini of JC virus early RNAs. J Virol. 1986 May;58(2):651–654. doi: 10.1128/jvi.58.2.651-654.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney S., Natarajan V., Strike D., Khoury G., Salzman N. P. JC virus enhancer-promoter active in human brain cells. Science. 1984 Dec 14;226(4680):1337–1339. doi: 10.1126/science.6095453. [DOI] [PubMed] [Google Scholar]
- Kerr D., Khalili K. A recombinant cDNA derived from human brain encodes a DNA binding protein that stimulates transcription of the human neurotropic virus JCV. J Biol Chem. 1991 Aug 25;266(24):15876–15881. [PubMed] [Google Scholar]
- Khalili K., Feigenbaum L., Khoury G. Evidence for a shift in 5'-termini of early viral RNA during the lytic cycle of JC virus. Virology. 1987 Jun;158(2):469–472. doi: 10.1016/0042-6822(87)90224-8. [DOI] [PubMed] [Google Scholar]
- Khalili K., Khoury G., Brady J. Spacing between simian virus 40 early transcriptional control sequences is important for regulation of early RNA synthesis and gene expression. J Virol. 1986 Dec;60(3):935–942. doi: 10.1128/jvi.60.3.935-942.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalili K., Rappaport J., Khoury G. Nuclear factors in human brain cells bind specifically to the JCV regulatory region. EMBO J. 1988 Apr;7(4):1205–1210. doi: 10.1002/j.1460-2075.1988.tb02932.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London W. T., Houff S. A., Madden D. L., Fuccillo D. A., Gravell M., Wallen W. C., Palmer A. E., Sever J. L., Padgett B. L., Walker D. L. Brain tumors in owl monkeys inoculated with a human polyomavirus (JC virus). Science. 1978 Sep 29;201(4362):1246–1249. doi: 10.1126/science.211583. [DOI] [PubMed] [Google Scholar]
- Luckow B., Schütz G. CAT constructions with multiple unique restriction sites for the functional analysis of eukaryotic promoters and regulatory elements. Nucleic Acids Res. 1987 Jul 10;15(13):5490–5490. doi: 10.1093/nar/15.13.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Major E. O., Amemiya K., Elder G., Houff S. A. Glial cells of the human developing brain and B cells of the immune system share a common DNA binding factor for recognition of the regulatory sequences of the human polyomavirus, JCV. J Neurosci Res. 1990 Dec;27(4):461–471. doi: 10.1002/jnr.490270405. [DOI] [PubMed] [Google Scholar]
- Major E. O., Amemiya K., Tornatore C. S., Houff S. A., Berger J. R. Pathogenesis and molecular biology of progressive multifocal leukoencephalopathy, the JC virus-induced demyelinating disease of the human brain. Clin Microbiol Rev. 1992 Jan;5(1):49–73. doi: 10.1128/cmr.5.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight S. L., Kingsbury R. Transcriptional control signals of a eukaryotic protein-coding gene. Science. 1982 Jul 23;217(4557):316–324. doi: 10.1126/science.6283634. [DOI] [PubMed] [Google Scholar]
- Nagashima K., Yasui K., Kimura J., Washizu M., Yamaguchi K., Mori W. Induction of brain tumors by a newly isolated JC virus (Tokyo-1 strain). Am J Pathol. 1984 Sep;116(3):455–463. [PMC free article] [PubMed] [Google Scholar]
- Osborn J. E., Robertson S. M., Padgett B. L., ZuRhein G. M., Walker D. L., Weisblum B. Comparison of JC and BK human papovaviruses with simian virus 40: restriction endonuclease digestion and gel electrophoresis of resultant fragments. J Virol. 1974 Mar;13(3):614–622. doi: 10.1128/jvi.13.3.614-622.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHARDSON E. P., Jr Progressive multifocal leukoencephalopathy. N Engl J Med. 1961 Oct 26;265:815–823. doi: 10.1056/NEJM196110262651701. [DOI] [PubMed] [Google Scholar]
- Small J. A., Scangos G. A., Cork L., Jay G., Khoury G. The early region of human papovavirus JC induces dysmyelination in transgenic mice. Cell. 1986 Jul 4;46(1):13–18. doi: 10.1016/0092-8674(86)90855-x. [DOI] [PubMed] [Google Scholar]
- Tada H., Lashgari M. S., Khalili K. Regulation of JCVL promoter function: evidence that a pentanucleotide "silencer" repeat sequence AGGGAAGGGA down-regulates transcription of the JC virus late promoter. Virology. 1991 Jan;180(1):327–338. doi: 10.1016/0042-6822(91)90037-c. [DOI] [PubMed] [Google Scholar]
- Tada H., Lashgari M., Rappaport J., Khalili K. Cell type-specific expression of JC virus early promoter is determined by positive and negative regulation. J Virol. 1989 Jan;63(1):463–466. doi: 10.1128/jvi.63.1.463-466.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto K. K., Howley P. M., Miyamura T. JC human papovavirus replication in human amnion cells. J Virol. 1979 Apr;30(1):384–389. doi: 10.1128/jvi.30.1.384-389.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D. L., Padgett B. L., ZuRhein G. M., Albert A. E., Marsh R. F. Human papovavirus (JC): induction of brain tumors in hamsters. Science. 1973 Aug 17;181(4100):674–676. doi: 10.1126/science.181.4100.674. [DOI] [PubMed] [Google Scholar]
- Wroblewska Z., Wellish M., Gilden D. Growth of JC virus in adult human brain cell cultures. Arch Virol. 1980;65(2):141–148. doi: 10.1007/BF01317325. [DOI] [PubMed] [Google Scholar]
- ZURHEIN G., CHOU S. M. PARTICLES RESEMBLING PAPOVA VIRUSES IN HUMAN CEREBRAL DEMYELINATING DISEASE. Science. 1965 Jun 11;148(3676):1477–1479. doi: 10.1126/science.148.3676.1477. [DOI] [PubMed] [Google Scholar]
- Zu Rhein G. M. Association of papova-virions with a human demyelinating disease (progressive multifocal leukoencephalopathy). Prog Med Virol. 1969;11:185–247. [PubMed] [Google Scholar]
- Zu Rhein G. M., Varakis J. N. Perinatal induction of medulloblastomas in Syrian golden hamsters by a human polyoma virus (JC). Natl Cancer Inst Monogr. 1979 May;(51):205–208. [PubMed] [Google Scholar]