Abstract
Background
In recent decades, young men in some industrialized areas have reportedly experienced a decrease in semen quality.
Objective
We examined effects of perinatal dioxin exposure on sperm quality and reproductive hormones.
Methods
We investigated sperm quality and hormone concentrations in 39 sons (mean age, 22.5 years) born between 1977 and 1984 to mothers exposed to dioxin after the accident in Seveso, Italy (1976), and 58 comparisons (mean age, 24.6 years) born to mothers exposed only to background dioxin. Maternal dioxin levels at conception were extrapolated from the concentrations measured in 1976 serum samples.
Results
The 21 breast-fed sons whose exposed mothers had a median serum dioxin concentration as low as 19 ppt at conception had lower sperm concentration (36.3 vs. 86.3 million/mL; p = 0.002), total count (116.9 vs. 231.1; p = 0.02), progressive motility (35.8 vs. 44.2%; p = 0.03), and total motile count (38.7 vs. 98 million; p = 0.01) than did the 36 breast-fed comparisons. The 18 formula-fed exposed and the 22 formula-fed and 36 breast-fed comparisons (maternal dioxin background 10 ppt at conception) had no sperm-related differences. Follicle-stimulating hormone was higher in the breast-fed exposed group than in the breast-fed comparisons (4.1 vs. 2.63 IU/L; p = 0.03) or the formula-fed exposed (4.1 vs. 2.6 IU/L; p = 0.04), and inhibin B was lower (breast-fed exposed group, 70.2; breast-fed comparisons, 101.8 pg/mL, p = 0.01; formula-fed exposed, 99.9 pg/mL, p = 0.02).
Conclusions
In utero and lactational exposure of children to relatively low dioxin doses can permanently reduce sperm quality.
Keywords: breast-feeding, dioxin, environmental endocrine disrupters, human sperm impairment, human sperm quality, perinatal exposure, reproductive hormones, TCDD
For the past 50 years, scientists have debated the issue of a reported decline in human semen quality, especially in Western countries (Carlsen et al. 1992; Swan et al. 2000). There is now a general consensus that human sperm quality has declined over time in different areas of the world (Auger et al. 1995; Jørgensen et al. 2001; Swan et al. 2003), especially in younger men (López-Teijòn et al. 2008; Zheng et al. 1997), with indications of concurrent male sub- or infertility (Bonde et al. 1998; Paasch et al. 2008; Zheng et al. 1997).
No clear causes have been identified; however, selected widespread persistent environmental endocrine-disrupting chemicals, such as 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and other dioxin-like chemicals, are suspected to be potential etiologic agents, as demonstrated in experimental animals, but no definitive data are available for men.
Recently, we showed that exposure in infancy to low doses of dioxin induces a sperm concentration reduction of about 25%; however, no reduction is observed if similar exposures occur during adulthood (Mocarelli et al. 2008). These data are consistent with animal models, which have demonstrated that the most sensitive times for these effects were exposures while in utero and during breast-feeding (Faqui et al. 1998).
In infants, after 4–5 months of breast-feeding, dioxin serum concentrations increase up to 2-fold (Abraham et al. 1998). In that period, there is a hormonal surge (Forest et al. 1973; Quigley 2002), defined as “neonatal minipuberty,” in which an increase of follicle-stimulating hormone (FSH) and inhibin B (Andersson et al. 1998) induces and reflects a strong proliferation of Sertoli cells (Cortes et al. 1987), the number of which determines how many germ cells can develop into spermatozoa (Sharpe et al. 2003). In addition to exposure during in utero development, exposure to TCDD within this developmental window could induce a direct or indirect toxic effect (i.e., imbalance of the hormonal equilibrium), impairing such proliferation and reducing sperm production in adults.
In this study, we assessed the association between human dioxin exposure in utero and during breast-feeding and adverse human adult male reproductive outcomes, as measured in men whose mothers were exposed to dioxin as a result of the trichlorophenol plant explosion near Seveso, Italy, in July 1976 (Mocarelli et al. 1991; Needham et al. 1997–1998).
Materials and Methods
Participants
All 78 men, 18–26 years old, born between March 1977 and January 1984 to 73 Caucasian women who lived in the most dioxin-polluted areas near Seveso, Italy, in 1976 (Eskenazi et al. 2004; Mocarelli et al. 1991; Needham et al. 1997–1998) were invited to participate. Thirty-nine of these men, who are the sons of 36 of the 73 women (mean age, 24.8 ± 5.6 years at the date of accident), actually participated; 21 were breast-fed, and 18 were formula-fed (Table 1). This further subdivision allowed us to separate the exposed men into two groups: men who had been exposed in utero only (those formula-fed and not breast-fed), and men who had been exposed perinatally—that is, both in utero and during nursing (breast-fed) (Table 1).
Table 1.
Characteristics of study participants according to dioxin exposure of the mother and to the type of nursing.
| Characteristic | Exposed group | Comparison group |
|---|---|---|
| Recruited for the study (n) | 78 | 123 |
| Refused (n) | 37 | 62 |
| Interested (n) | 41 | 61 |
| Compliance (%) | 53 | 50 |
| Excluded from the study (n)a | ||
| Varicocele | 1 | 2 |
| Varicocele and cryptorchidism | 1 | 1 |
| Participants (n) | 39 | 58 |
| Age [years (mean ± SD)] | 22.5 ± 2.2 | 24.6 ± 2.0 |
| Age of mothers at conception [years (mean ± SD)] | 28.2 ± 5.4 | 28.1 ± 4.8 |
| Nursing | ||
| Breast-fed [n (%)] | 21 (54) | 36 (62) |
| Duration of breast-feeding [months (mean)]b | 4.06 | 5.03 |
| Formula-fed [n (%)] | 18 (46) | 22 (38) |
| BMI (kg/m2) [n (%)] | ||
| < 25 | 33 (85) | 48 (83) |
| 25–30 | 6 (15) | 10 (17) |
| > 30 | — | — |
| Days of abstinencec (mean ± SD) | ||
| All | 4.2 ± 1.57 | 4.2 ± 1.77 |
| Breast-fed | 3.9 ± 1.58 | 4.4 ± 1.79 |
| Formula-fed | 4.5 ± 1.56 | 4.0 ± 1.77 |
Excluded for self-reported causes (questionnaire) or because of pathologic results of clinical laboratory tests (see “Materials and Methods”).
Data on duration of breast-feeding were missing for four comparisons.
Days without ejaculation before semen sampling.
The exposure status of the mothers of the exposed men (either participating or refusing) was verified by measuring dioxin levels in their serum, which was collected soon after the explosion. There were no significant differences in median TCDD serum concentrations in mothers of refusing men compared with the mothers of participating ones (36 vs. 51.7 ppt). Twenty exposed mothers breast-fed 21 sons, and 17 exposed mothers formula-fed 18 sons. Of these mothers, three each had two sons: One mother had a son in 1977 who was formula-fed, and another in 1981 who was breast-fed; one mother had a son in 1978 and another in 1980, both of whom were breast-fed; and one mother had a son in 1977 and another in 1983, both of whom were formula-fed. Sons of dioxin-exposed women whose spouses were also exposed, as well as all men with reported diseases (Table 1), were excluded.
As a control, a comparison group of 123 consecutive healthy volunteer permanent blood donors (in Italy, blood donors have medical examinations performed every 3 months) of the same age and similar socioeconomic status as the exposed men, but whose mothers had not lived in the TCDD-contaminated areas, were invited to participate; they lacked additional accidental dioxin exposure (but did have the background exposure), and they were comparable in terms of breast-feeding and formula feeding. Fifty-eight of these men actually participated; 36 were breast-fed, and 22 were formula-fed (Table 1).
All participants completed a questionnaire on health, socioeconomic status, smoking and drinking habits, and working conditions. Participants also provided information on their weight at birth and how long they nursed as infants. They were screened for hidden diseases by clinical laboratory tests (aspartate aminotransferase, alanine aminotransferase, γ-glutamyl transferase, total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, C-reactive protein, glucose, creatinine, complete blood cell count and differential, hemoglobin, hepatitis B surface antigen, hepatitis B core antibody, hepatitis C, urine analysis) and donated blood and semen samples.
The study protocol was approved by institutional human subjects committee (Istituto di Ricovero e Cura a Carattere Scientifico Don Gnocchi, Milano, Italy). All study participants gave written informed consent.
Laboratory data
Semen samples were collected from participants as a postmasturbatory at-home semen sample, which was transported to the Desio Hospital laboratory at about 37°C and kept at that temperature until examination, which occurred within 1 hr from ejaculation. Blind tests were performed by the same two technicians according to the World Health Organization (WHO 1999) recommendations. Sperm percentages of A + B grades of motility, that is, progressive motility, were assessed at 400× magnification on a microscope heating stage (37°C) in duplicate, and concentration was measured using a Bürker-Türk chamber at phase contrast (400×). Morphology was evaluated by the same observer on 300 Papanicolaou-stained sperm per slide (David et al. 1975; Jouannet et al. 1988).
For serum hormone analysis, fasting blood samples were obtained on the same morning as the semen collection. An aliquot of serum was stored frozen at −80°C, and hormone levels were measured in large batches in order to reduce interassay variability. FSH, inhibin B, serum 17-β-estradiol (E2), and luteinizing hormone (LH) were measured according to established immunometric methods. Testosterone was measured by radioimmunoassay. Quality control protocols were strictly applied for all tests using Westgard’s multirule (Westgard QC 2009). Intra- and interassay coefficients of variation were, respectively, 2.7% and 4.5% for FSH, 4.2% and 6.7% for inhibin B, 2.5% and 4.6% for E2, 1.8% and 3.4% for LH, and 3.4% and 6.6% for testosterone. Sensitivity was 0.1 IU/L for FSH, 7.0 pg/mL for inhibin B, 15 pg/mL for E2, 0.05 mIU/mL for LH, and 0.02 ng/mL for testosterone.
All serum TCDD measurements taken from maternal serum samples stored frozen since 1976–1977 were conducted by isotope-dilution high-resolution mass spectrometry at the Centers for Disease Control and Prevention (Atlanta, GA, USA) (Patterson et al. 1987). The estimated individual maternal serum TCDD concentration at the time of conception was calculated for 20–42 years of age by using the TCDD serum concentration in 1976–1977 and the TCDD half-life for women of that age, estimated to be an average of 4 years (range, 2.1–6.7 years) (Kreuzer et al. 1997). For the equation and an example of calculation, see Supplemental Material (doi:10.1289/ehp.1002134). Serum samples collected in 2002–2003, both from the case men and from their comparisons, were also analyzed for dioxin.
Statistical analysis
A general database was established and maintained using SAS software (version 8.2; SAS Institute Inc., Cary, NC, USA). Sperm and hormone data were fitted with a general linear model that included group, lactation class, and group × lactation class interaction as terms. Covariates included age at time of tests, length of abstinence in days, smoking habits (the total number of cigarettes smoked per day during months of habitual smoking), chemical exposures (mostly organic solvents, adhesives, paints, colors, and powders), body mass index (BMI) at the moment of the study, alcohol use (grams per day), educational level, and employment status. Scale transformations were applied to approximate normal distribution and homoscedasticity. Sperm concentration, total sperm count, progressive motile sperm count, and E2, testosterone, and FSH concentrations were log transformed, whereas semen volume and LH and inhibin B concentrations were square root transformed. Results were expressed as back transformation of least squares means (i.e., the means adjusted for all the terms in the model). Two types of comparisons were made: among groups within lactation class, and among lactation classes within group.
Results
Dioxin exposure
In 1976, the median serum TCDD concentrations in women whose eligible sons did and did not participate in the study were similar (51.7 vs. 36 ppt). We assumed the serum TCDD concentrations in the comparison group mothers at conception (Table 2) were 10 ppt in 1976–1977 (Eskenazi et al. 2004). All mothers at the time were also exposed to a background of dioxin-like chemicals [polychlorinated dibenzofurans (PCDFs) and some polychlorinated biphenyls (PCBs)], constituting a total of about 90 ppt of dioxin toxic equivalent (TEQ) concentration, which is added to the concentration of TCDD (Eskenazi et al. 2004).
Table 2.
Serum TCDD concentrations (percentile distribution) of mothers at exposure in 1976 and extrapolated values at conception of their sons.
| Percentile (ppt)a |
|||||
|---|---|---|---|---|---|
| Mothers | 5th | 25th | Median | 75th | 95th |
| At exposure (July 1976) | |||||
| All mothers (n = 36) | 17.0 | 26.6 | 51.7 | 115.0 | 321.0 |
| Mothers who breast-fed (n = 20) | 17.0 | 25.2 | 46.8 | 115.0 | 321.0 |
| Mothers who formula-fed (n = 17) | 19.0 | 29.1 | 55.7 | 87.6 | 301.0 |
| At conception (1976–1983) | |||||
| All mothers | 11.8 | 16.2 | 26.0 | 58.9 | 232.3 |
| Mothers who breast-fedb | 11.8 | 13.1 | 19.0 | 58.9 | 117.1 |
| Mothers who formula-fedc | 17.0 | 20.6 | 27.9 | 54.6 | 240.3 |
Mothers of comparisons were considered as exposed to average background TCDD level of 10 ppt in 1976 (Eskenazi et al. 2004).
Values are parts per trillion TCDD concentration expressed on serum lipid basis.
After breast-feeding for 4–5 months, the TCDD value in the child roughly doubles compared with the conception value (Abraham et al. 1998).
The formula-fed men were exposed to dioxin only in utero.
Median maternal TCDD concentrations (Table 2) measured in serum collected in 1976 and extrapolated to conception levels for mothers who breast-fed or formula-fed their children were 19.0 and 27.9 ppt, respectively, and were not statistically different. Serum TCDD concentrations in sons after 4–5 months of breast-feeding are considered 2-fold those of the mothers (Abraham et al. 1998).
Serum TCDD concentrations measured at the time of this study in the exposed breast- and formula-fed groups (average, 2.4 ppt and 1.1 ppt, respectively) and their respective comparison groups (average, 1.8 ppt and 1.0 ppt, respectively) were not statistically different.
Semen quality
About 50% of both the exposed and comparison group men recruited for this study complied with semen examination (Table 1). Figure 1 and Table 3 list variables for exposed men and their comparisons. The 39 men exposed in utero and during nursing had significantly decreased sperm concentration (p = 0.01), total sperm count (p = 0.03), and number of total motile sperm (p = 0.05) relative to the 58 men in the comparison group (Table 3, Figure 1A).
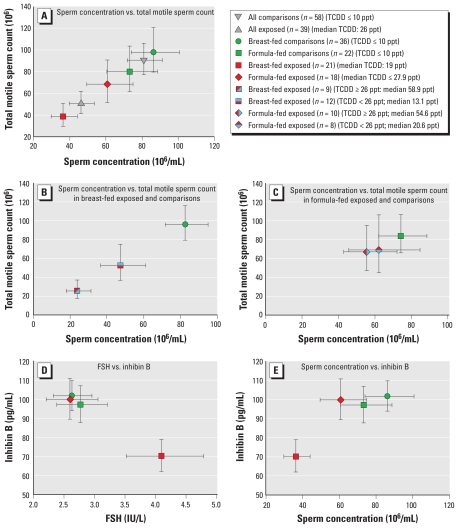
Figure 1.
Differences in sperm variables, FSH, and inhibin B between in utero and breast- or formula-fed men exposed to dioxin and comparisons. (A) Lower sperm concentration and the lower total motility of all TCDD-exposed men (median maternal TCDD at conception, 26 ppt) with respect to comparisons was related mainly to breast-feeding (see also Table 3). (B) The nine breast-fed exposed men belonging to the two highest quartiles (median maternal TCDD at conception, 58.9 ppt) had both lower median sperm concentration (23.8 × 106/mL) and lower motility (p = 0.0003, p = 0.003, respectively) than did the 36 breast-fed comparisons (82.5 × 106/mL). Also, the 12 exposed breast-fed men belonging to the two lowest quartiles (median maternal TCDD at conception, 13.1 ppt) had lower (p = 0.05) sperm concentration (47.3 × 106/mL) than did the 36 breast-fed comparisons (82.5 × 106/mL). (C) Sperm variables did not statistically differ among all the formula-fed men exposed to more or less than 26 ppt of TCDD and the formula-fed comparisons. (D) The effect of dioxin through breast-feeding on FSH (increasing) and inhibin B (decreasing) values, compared with exposed formula-fed and comparison men, was significant and permanent. (E) The low sperm concentration of TCDD in exposed breast-fed men was strongly correlated to low inhibin B concentration (a marker of Sertoli cells). The formula-fed exposed men and comparisons had normal inhibin B concentration.
Table 3.
Differences in sperm and hormone data between men exposed to dioxin in utero (i.e., formula-fed) or also through breast-feeding and same-age comparisons.
| Breast-fed
|
Formula-fed
|
p-Value,a breast-fed versus formula-fed
|
||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | Exposed group (n = 39) | Comparison group (n = 58) | Exposed group (n = 21) | Comparison group (n = 36) | Exposed group (n = 18) | Comparison group (n = 22) | Exposed group | Comparison group |
| Semen volume (mL)
| ||||||||
| Median (25th–75th percentile) | 3.0 (2.2–3.8) | 3.3 (2.4–4.3) | 3.0 (2.3–3.8) | 3.4 (2.1–4.2) | 3.0 (2.0–3.8) | 3.0 (2.4–4.4) | ||
| Adjusted meanb (−/+ SE)c | 3.2 (3.0/3.4) | 3.0 (2.8/3.2) | 3.4 (3.1/3.7) | 2.9 (2.6/3.1) | 3.0 (2.7/3.3) | 3.2 (2.9/3.5) | ||
| p-Valuea | 0.60 | 0.21 | 0.68 | 0.39 | 0.38 | |||
| Sperm concentration (106/mL)
| ||||||||
| Median (25th–75th percentile) | 61.5 (32.1–100.0) | 88.7 (54.6–133.1) | 54.4 (22.1–68.5) | 99.6 (58.2–141.6) | 81.1 (37.2–107.3) | 81.8 (41.4–96.1) | ||
| Adjusted meanb (−/+ SE)c | 46.2 (39.8/53.6) | 81.0 (71.9/91.1) | 36.3 (29.8/44.2) | 86.3 (74.1/100.6) | 60.8 (49.4/74.9) | 73.2 (60.5/88.6) | ||
| p-Value | 0.01 | 0.002 | 0.53 | 0.07 | 0.51 | |||
| Sperm total count (106)
| ||||||||
| Median (25th–75th percentile) | 143.9 (78.1–336.2) | 230.6 (162.6–422.3) | 108.2 (57.0–209.0) | 270.8 (178.1–431.8) | 222.0 (118.3–349.2) | 214.1 (134.4–359.7) | ||
| Adjusted meanb (−/+ SE)c | 139.2 (119.3/162.4) | 229.9 (203.4/259.9) | 116.9 (95.1/143.6) | 231.1 (197.0/271.2) | 171.4 (137.9/213.1) | 227.0 (185.9/277.1) | ||
| p-Value | 0.03 | 0.02 | 0.36 | 0.19 | 0.94 | |||
| Sperm progressive motility (%)d | ||||||||
| Median (25th–75th percentile) | 39.0 (33.0–47.0) | 41.5 (34.0–50.0) | 37.0 (31.0–44.0) | 43.5 (38.5–51.5) | 40.0 (37.0–48.0) | 36.5 (32.0–49.0) | ||
| Adjusted meanb (−/+ SE)c | 38.7 (36.3/40.9) | 41.5 (39.8/43.2) | 35.8 (33.0/38.5) | 44.2 (42.0/46.3) | 41.7 (38.8/44.6) | 37.4 (34.8/40.1) | ||
| p-Value | 0.47 | 0.03 | 0.30 | 0.13 | 0.06 | |||
| Progressive motile sperm count (106)e | ||||||||
| Median (25th–75th percentile) | 58.5 (24.8–141.2) | 98.1 (65.9–193.9) | 44.4 (21.1–81.2) | 111.7 (75.0–197.1) | 87.2 (42.6–143.2) | 94.9 (41.7–150.2) | ||
| Adjusted meanb (−/+ SE)c | 50.6 (41.4/61.8) | 90.5 (77.1/106.2) | 38.7 (29.6/50.6) | 98.0 (79.7/120.6) | 68.5 (51.6/90.8) | 80.0 (61.7/103.6) | ||
| p-Value | 0.05 | 0.01 | 0.70 | 0.13 | 0.55 | |||
| FSH (IU/L)
| ||||||||
| Median (25th–75th percentile) | 3.2 (2.1–5.3) | 2.7 (1.9–4.7) | 3.7 (2.9–6.4) | 2.6 (1.8–4.7) | 2.7 (1.6–3.8) | 3.2 (2.3–4.7) | ||
| Adjusted meanb (−/+ SE)c | 3.3 (3.0/3.7) | 2.7 (2.4/2.9) | 4.1 (3.5/4.8) | 2.6 (2.3/3.0) | 2.6 (2.2/3.1) | 2.8 (2.4/3.2) | ||
| p-Value | 0.23 | 0.03 | 0.79 | 0.04 | 0.79 | |||
| Inhibin B (pg/mL)
| ||||||||
| Median (25th–75th percentile) | 77.6 (49.3–114.7) | 99.6 (86.4–129.7) | 73.2 (49.3–89.9) | 105.7 (87.6–126.7) | 87.0 (50.2–133.2) | 96.0 (83.4–133.2) | ||
| Adjusted meanb (−/+ SE)c | 83.2 (76.4/90.3) | 100.0 (94.0/106.2) | 70.2 (62.0/78.9) | 101.8 (94.0/109.8) | 99.9 (89.5/110.8) | 97.2 (87.8/107.0) | ||
| p-Value | 0.13 | 0.01 | 0.85 | 0.02 | 0.72 | |||
p-Values refer to differences adjusted by abstinence time (days), BMI, smoking status and total number of cigarettes per day during months of habitual smoking, age at the time of test, chemical substances exposure, alcohol use (g/day), and education level for sperm data. Hormone data were not adjusted for education level, employment status, and abstinence time.
Values derived from back-transformation of log (sperm concentration, sperm total count, progressive motile sperm count, and FSH) and square-root transformation (semen volume and inhibin B).
Values indicate adjusted mean minus the SE/adjusted mean plus the SE.
Considered as A + B grades of progressive motility of sperm, according to WHO (1999).
Considered as A + B grades of progressive motility of sperm per total sperm count, according to WHO (1999).
A stronger significant effect appeared (Table 3) when comparing the 21 breast-fed exposed sons and the 36 breast-fed comparisons (sperm concentration, p = 0.002; total sperm count, p = 0.02; total motile sperm count, p = 0.01; progressive motility, p = 0.03; see Figure 1A). In contrast, we observed no statistical differences between the 18 formula-fed exposed men and the 22 formula-fed comparisons or between the 36 breast-fed and 22 formula-fed comparisons (Table 3). On the other hand, we observed an almost significant (p = 0.07) sperm concentration decrease in the 21 breast-fed exposed men compared with the 18 formula-fed exposed men. Figure 1A summarizes these observations, with TCDD values.
In examining any differences in outcome, we found that the nine breast-fed men belonging to the two highest quartiles of TCDD serum concentrations (median TCDD, 58.9 ppt) did show lower sperm concentration (p = 0.0003) and total motility (p = 0.003) than the 36 breast-fed comparisons (Figure 1B); in the equivalent formula-fed groups, we observed no statistical differences between the 10 exposed men (median TCDD, 54.6 ppt) and the 22 comparisons (Figure 1C). We observed similar effects for total sperm motility (Figure 1B,C). For the men in the two lowest quartiles, who had serum TCDD concentrations < 26 ppt (median TCDD, 13.1 ppt), the only difference was a lower sperm concentration (p = 0.05) in the 12 exposed breast-fed men with respect to the 36 comparison breast-fed men (Figure 1B).
These data also showed that a much lower semen concentration was present in the breast-fed men with the highest exposure, but the dose–response model did not reach statistical significance; we observed only a tendency, possibly because of the small number of cases. We did not observe this result with the formula-fed exposed men.
We observed no statistically significant difference for sperm morphology parameters between exposed and comparison groups.
Hormones
The 21 breast-fed exposed men had higher FSH concentrations than did both the 36 breast-fed comparison men (p = 0.03) and the 18 formula-fed exposed men (p = 0.04; Table 3), whereas inhibin B was significantly decreased (p = 0.01 and p = 0.02, respectively). Figure 1D,E, illustrates the relationships between inhibin B and FSH, and inhibin B and sperm concentration in the different conditions.
We observed no statistically significant differences for E2, testosterone, and LH between exposed and comparison groups.
Discussion
The results of this study clearly indicate that continuous exposure of males starting from low concentrations of dioxin before and after birth due to the mother being exposed during the Seveso accident, and then due to breast-feeding during “neonatal minipuberty,” results in a permanent impairment of the reproductive system (reduction of about 50% of sperm concentration and total sperm count and about 20% of sperm progressive motility, and increase of FSH with decrease of inhibin B) in young adulthood.
This impairment is not seen in males who were born to similarly exposed Seveso mothers who did not breast-feed. In fact, this group of men had sperm counts similar to those of the breast-fed and formula-fed comparison group.
The breast-fed exposed men had lower sperm variables than did the breast-fed comparison and formula-fed exposed men. In the latter case, statistical significance was not reached, probably because of the small number of cases. These observations on semen quality are considerably reinforced by corresponding changes in hormone levels: decreased inhibin B and increased FSH found in the breast-fed exposed group.
We had the rather unique opportunity to discriminate TCDD exposure with respect to background levels. Men exposed to rather high maternal serum TCDD concentrations at birth, such as 54.6 ppt (the 75th percentile of formula-fed exposed children), plus the then background value of 90 ppt of dioxin-like chemicals, did not show differences compared with the breast- and formula-fed comparison group members.
At the age of 4–5 months, formula-fed infants almost double their birth weight and halve the dioxin concentration, to about 27 ppt for the formula-fed exposed group and 5 ppt for the formula-fed comparison group, whereas the breast-fed comparison group approximately doubles the concentration to 20 ppt because of background dioxin exposure. On the other hand, the median of 19 ppt of dioxin in the breast-fed exposed group increases with breast-feeding to about 40 ppt. Our data show that impaired semen quality in adulthood results from exposure in utero, as well as continuous exposure through breast-feeding during “neonatal minipuberty,” with adverse effects starting with a dioxin exposure range of 19–40 ppt and higher (the concentrations of background dioxin-like chemicals must also be added). Similar doses of TCDD administered to rhesus monkeys during pregnancy and lactation have recently been shown to produce lower semen quality in their young adult offspring (Arima et al. 2009). It would be interesting to investigate the effect of TCDD administration only during lactation.
Our observations show for the first time that continuous exposure of the developing human male in utero, and even more so during lactation, the “neonatal minipuberty period,” to modest elevations of dioxin above background exposure levels can permanently impair later adult sperm production.
In fact, the only similar data (Guo et al. 2000) are related to in utero and nursing exposure of children to high doses of burned PCBs/PCDFs, but not to TCDD. That study demonstrated a reduction in sperm motility, but not density, as well as reduced egg penetration ability. Unfortunately, data concerning the specific concentration of the toxicants in maternal blood at conception were not available.
Mechanism of action
Dioxin and the structurally related PCDF chemicals mediate their toxic effect (but with different potencies) mainly as a ligand to the same aryl hydrocarbon receptor (AHR)/AHR nuclear translocator (ARNT) complex that binds to the xenobiotic responsive element (XRE; also called dioxin-responsive element, DRE) on target DNA. The widely expressed AHR/ARNT, when ligand activated, interacts with several transcription factors, steroids, and growth factors and possibly plays a critical constitutive role, especially in early tissue development. Through targeted gene networks, AHR/ARNT regulates or directly intervenes in reproductive system development (Schultz et al. 2003).
As our study shows, the untimely interference of dioxin in utero and within the first 4–5 months of life (the “neonatal minipuberty”) (Forest et al. 1973; Quigley 2002) results in a permanent impairing effect. It is during this time that the human testes almost double their volume (Cortes et al. 1987), due in great part to Sertoli cell proliferation, which determines spermatogenic potential (Sharpe et al. 2003). An indication of possible Sertoli cell number (or functionality) reduction is given by the decrease of inhibin B, a marker of their activity, associated with the decrease of sperm concentration, not only in the exposed breast-fed with respect to the comparison breast-fed group, but also in the exposed breast-fed compared with the exposed formula-fed group. The increase of FSH associated with the decrease of inhibin B (as observed here) has been reported to be a very sensitive marker for impaired spermatogenesis in men (von Eckardstein et al. 1999).
It is well established that sons born to mothers who smoked heavily in pregnancy have a 20–50% reduction in sperm concentration in adulthood (Jensen et al. 2005; Jensen et al. 2004; Ramlau-Hansen et al. 2007), reduced blood levels of inhibin B, and increased FSH (Storgaard et al. 2003), compared with unexposed males. No information is provided about smoking during breast-feeding in these studies, but it is likely that exposure to the chemicals in tobacco smoke occurred both prenatally and postnatally, similar to our dioxin study. This is relevant to our data because a common mechanism of the phenomenon we observed and the one due to smoking could be the action on XRE (Kasai et al. 2006) of some polycyclic aromatic hydrocarbon (PAH) compounds contained in cigarette smoke, using the same AHR as dioxin.
It has also been shown that PAHs have a negative impact on rat and human Sertoli cell function (Coutts et al. 2007; Raychoudhury and Kubinski 2003).
Relevance to general population and individuals
These observed effects of perinatal dioxin exposure on lowering adult sperm counts might also explain, in part, the wider semen quality reduction reported in young men in some industrialized regions, such as in Spain, Denmark, and Germany (Andersen et al. 2000; López-Teijòn et al. 2008; Paasch et al. 2008; Zheng et al. 1997). The men observed in these studies were in fact born in the 1970s and 1980s to mothers born in the 1950s and 1960s, a period when environmental concentrations of dioxin and dioxin-like chemicals peaked (Hagenmaier and Walczok 1996). The bioaccumulation of these compounds with age is well demonstrated, and a concurrent effect of maternal smoking could also contribute to this phenomenon.
Because of environmental policies instituted throughout most of Europe and the United States, TCDD and total TEQ concentrations in women 20–40 years of age have recently decreased sharply, to less than 2 ppt and 12 ppt, respectively (Päpke 1998; Patterson et al. 2008). Thus, current serum dioxin levels in infants in these areas, even after being increased 2- to 3-fold because of breast-feeding, are far from the concentrations that would yield adverse effects.
Conclusions
Our data indicate for the first time that environmental chemical exposure to dioxin continuously throughout the perinatal period permanently reduces semen quality and sperm counts in young men and demonstrates that the male reproductive system is dramatically sensitive to the action of dioxin, starting from rather low doses (~ 19 ppt) in utero and doubled by breast-feeding during “neonatal minipuberty,” resulting in permanent impairment in the adult (reduction of ~ 50% of sperm concentration and total sperm count and ~ 20% of sperm progressive motility, with increased FSH and decreased inhibin B).
Although our findings are derived from examining a unique cohort of men exposed perinatally to supranormal dioxin levels, they may have relevance to the wider population of young men in industrialized countries and may partly explain the reported widespread occurrence of low sperm counts in young men in Europe. Our findings also raise concern for those areas of the world in which rapid industrial development may cause widespread distribution of environmental endocrine-disrupting chemicals, such as dioxin and dioxin-like chemicals.
Furthermore, our data should encourage studies of semen quality in men of appropriate ages, comparing breast-fed and formula-fed groups, especially if this could be linked to proximity to polluted areas at the time of perinatal life.
Footnotes
Supplemental Material is available online (doi:10.1289/ehp.1002134 via http://dx.doi.org/).
We are indebted to all the people of the Seveso area for making this study possible through their civic example of courage, their responsible actions in a dramatic situation, and their great cooperation 30 years after the accident. Thanks to these generous people, “Seveso” is a sentinel for evaluating the ongoing effect of dioxin and dioxin-like chemicals on world health. We also thank their family physicians. We thank E. Acmet, L. Basso, and M. Solaro (University Department of Laboratory Medicine, Hospital of Desio) and the staff of our laboratories for their assistance. Special thanks to B. Eskenazi and M. Warner (University of California–Berkeley) for information regarding some of the exposed mothers from our common Seveso Women’s Health Study. We also thank J. Auger (Cochin Hospital, Paris, France) for his knowledge of and assistance with sperm morphology classification. Last but not least, we would like to remember a great friend and scientist, Larry L. Needham, who passed away last October. He will be greatly missed by all of us.
This study was supported by grant 2896 from Regione Lombardia, Milano, Italy, and by the Centers for Disease Control and Prevention.
References
- Abraham K, Päpke O, Gross A, Kordonouri O, Wiegand U, Wahn U, et al. Time course of PCDD/PCDF/PCB concentrations in breast-feeding mothers and their infants. Chemosphere. 1998;37:1731–1741. doi: 10.1016/s0045-6535(98)00238-0. [DOI] [PubMed] [Google Scholar]
- Andersen AG, Jensen TK, Carlsen E, J⊘rgensen N, Andersson AM, Krarup T, et al. High frequency of sub-optimal semen quality in an unselected population of young men. Hum Reprod. 2000;15:366–372. doi: 10.1093/humrep/15.2.366. [DOI] [PubMed] [Google Scholar]
- Andersson A-M, Toppari J, Haavisto A-M, Petersen JH, Simell T, Simell O, et al. Longitudinal reproductive hormone profiles in infants: peak of inhibin B levels in infant boys exceeds levels in adult men. J Clin Endocrinol Metab. 1998;83:675–681. doi: 10.1210/jcem.83.2.4603. [DOI] [PubMed] [Google Scholar]
- Arima A, Kato H, Ooshima Y, Tateishi T, Inoue A, Muneoka A, et al. In utero and lactational exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) induces a reduction in epididymal and ejaculated sperm number in rhesus monkeys. Reprod Toxicol. 2009;28:485–502. doi: 10.1016/j.reprotox.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Auger J, Kunstmann JM, Czyglik F, Jouannet P. Decline in semen quality among fertile men in Paris during the past 20 years. N Engl J Med. 1995;322:281–285. doi: 10.1056/NEJM199502023320501. [DOI] [PubMed] [Google Scholar]
- Bonde JP, Ernst E, Jensen TK, Hjollund NHI, Kolstad H, Hentiksen TB, et al. Relation between semen quality and fertility: a population-based study of 430 first-pregnancy planners. Lancet. 1998;352:1172–1177. doi: 10.1016/S0140-6736(97)10514-1. [DOI] [PubMed] [Google Scholar]
- Carlsen E, Giwercman A, Kieding N, Skakkebaek N. Evidence for decreasing quality of semen during past 50 years. BMJ. 1992;305:609–613. doi: 10.1136/bmj.305.6854.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes D, Muller J, Skakkebaek NE. Proliferation of Sertoli cells during development of the human testis assessed by stereological methods. Int J Androl. 1987;10:589–596. doi: 10.1111/j.1365-2605.1987.tb00358.x. [DOI] [PubMed] [Google Scholar]
- Coutts SM, Fulton N, Anderson RA. Environmental toxicant-induced germ cell apoptosis in the human fetal testis. Hum Reprod. 2007;22:2912–2918. doi: 10.1093/humrep/dem300. [DOI] [PubMed] [Google Scholar]
- David G, Boisson JP, Czyglik F, Jouanet P, Gernigon C. Anomalies morphologiques du spermatozoïde human. 1. Proposition pour un système de classification. J Gynecol Obstet Biol Reprod. 1975;4(suppl 1):17–36. [Google Scholar]
- Eskenazi B, Mocarelli P, Warner M, Needham L, Patterson DG, Jr, Samuels S, et al. Relationship of serum TCDD concentrations and age at exposure of female residents of Seveso, Italy. Environ Health Perspect. 2004;112:22–27. doi: 10.1289/ehp.6573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faqui AS, Dalsenter PR, Merker HJ, Chahoud I. Reproductive toxicity and tissue concentration of low doses of 2,3,7,8-tetrachlorodibenzo-p-dioxin in male offspring rats exposed throughout pregnancy and lactation. Toxicol Appl Pharmacol. 1998;150:383–392. doi: 10.1006/taap.1998.8433. [DOI] [PubMed] [Google Scholar]
- Forest MG, Cathiard AM, Bertrand JA. Evidence of testicular activity in early infancy. J Clin Endocrinol Metab. 1973;37:148–151. doi: 10.1210/jcem-37-1-148. [DOI] [PubMed] [Google Scholar]
- Guo YL, Hsu PC, Hsu CC, Lambert GH. Semen quality after prenatal exposure to polychlorinated biphenyls and dibenzofurans. Lancet. 2000;356:1240–1241. doi: 10.1016/S0140-6736(00)02792-6. [DOI] [PubMed] [Google Scholar]
- Hagenmaier H, Walczok M. Time trends in levels, patterns and profiles for PCDD/PCDF in sediment cores of Lake Constance. Organohalogen Compounds. 1996;28:101–104. [Google Scholar]
- Jensen MS, Mabeck LM, Toft G, Thulstrup AM, Bonde JP. Lower sperm counts following prenatal tobacco exposure. Hum Reprod. 2005;20:2559–2566. doi: 10.1093/humrep/dei110. [DOI] [PubMed] [Google Scholar]
- Jensen TK, J⊘rgensen N, Punab M, Haugen TB, Souminen J, Zilaitiene B, et al. Association of in utero exposure to maternal smoking with reduced semen quality and testis size in adulthood: a cross-sectional study of 1,770 young men from the general population in five European countries. Am J Epidemiol. 2004;159:49–58. doi: 10.1093/aje/kwh002. [DOI] [PubMed] [Google Scholar]
- J⊘rgensen N, Andersen A-G, Eustache F, Irvine DS, Suominen J, Petersen JH, et al. Regional differences in semen quality in Europe. Hum Reprod. 2001;16:1012–1019. doi: 10.1093/humrep/16.5.1012. [DOI] [PubMed] [Google Scholar]
- Jouannet P, Ducot B, Feneux D, Spira A. Male factors and the likelihood of pregnancy in infertile couples. 1. Study of sperm characteristics. Int J Androl. 1988;11:379–394. doi: 10.1111/j.1365-2605.1988.tb01011.x. [DOI] [PubMed] [Google Scholar]
- Kasai A, Hiramatsu N, Hayakawa K, Yao J, Maeda S, Kitamura M. High levels of dioxin-like potential in cigarette smoke evidence by in vitro and in vivo biosensing. Cancer Res. 2006;66:7143–7150. doi: 10.1158/0008-5472.CAN-05-4541. [DOI] [PubMed] [Google Scholar]
- Kreuzer PE, Csanády GA, Baur C, Kessler W, Päpke O, Greim H, et al. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) and congeners in infants: a toxicokinetic model of human lifetime body burden by TCDD with special emphasis on its uptake by nutrition. Arch Toxicol. 1997;71:383–400. doi: 10.1007/s002040050402. [DOI] [PubMed] [Google Scholar]
- López-Teijòn M, Elbaile M, Alvarez JG. Geographical differences in semen quality in a population of young healthy volunteers from the different regions of Spain. Andrologia. 2008;40:318–328. doi: 10.1111/j.1439-0272.2008.00862.x. [DOI] [PubMed] [Google Scholar]
- Mocarelli P, Gerthoux PM, Patterson DG, Milani S, Limonta G, Bertona M, et al. Dioxin exposure, from infancy through puberty, produces endocrine disruption and affects human semen quality. Environ Health Perspect. 2008;116:70–77. doi: 10.1289/ehp.10399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocarelli P, Needham LL, Marocchi A, Patterson DG, Jr, Brambilla P, Gerthoux PM, et al. Serum concentrations of 2,3,7,8-tetrachlorodibenzo-p-dioxin and test results from selected residents of Seveso, Italy. Toxicol Environ Health. 1991;32:357–366. doi: 10.1080/15287399109531490. [DOI] [PubMed] [Google Scholar]
- Needham LL, Gerthoux PM, Patterson DG, Jr, Brambilla P, Turner WE, Beretta C, et al. Serum dioxin levels in Seveso, Italy, population in 1976. Teratog Carcinog Mutagen. 1997–1998;17:225–240. [PubMed] [Google Scholar]
- Paasch U, Salzbrunn A, Glander HJ, Plambeck K, Salzbrunn H, Grunewald S, et al. Semen quality in sub-fertile range for a significant proportion of young men from the general German population: a co-ordinated, controlled study of 791 men from Hamburg and Leipzig. Int J Androl. 2008;31:93–102. doi: 10.1111/j.1365-2605.2007.00860.x. [DOI] [PubMed] [Google Scholar]
- Päpke O. PCDD/PCDF: human background data for Germany, a 10-year experience. Environ Health Perspect. 1998;106:723–731. doi: 10.1289/ehp.106-1533397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson DG, Jr, Hampton L, Lapeza CR, Jr, Belser WT, Green V, Alexander L, et al. High-resolution gas-chromatography/high-resolution mass spectrometric analysis of human serum on a whole weight and lipid basis for 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) Anal Chem. 1987;59:2000–2005. doi: 10.1021/ac00142a023. [DOI] [PubMed] [Google Scholar]
- Patterson DG, Jr, Turner WE, Caudill SP, Needham LL. Total TEQ reference range (PCDDs, PCDFs, cPCBs, mono-PCBs) for the US population 2001–2002. Chemosphere. 2008;73:261–277. doi: 10.1016/j.chemosphere.2007.08.074. [DOI] [PubMed] [Google Scholar]
- Quigley CA. Editorial: the postnatal gonadotropin and sex steroid surge—insights from the androgen insensitivity syndrome. J Clin Endocrinol Metab. 2002;87:24–28. doi: 10.1210/jcem.87.1.8265. [DOI] [PubMed] [Google Scholar]
- Ramlau-Hansen CH, Thulstrup AM, Storgaard L, Toft G, Olsen J, Bonde JP. Is prenatal exposure to tobacco smoking a cause of poor semen quality? A follow-up study. Am J Epidemiol. 2007;165:1372–1379. doi: 10.1093/aje/kwm032. [DOI] [PubMed] [Google Scholar]
- Raychoudhury SS, Kubinski D. Polycyclic aromatic hydrocarbon-induced cytotoxicity in cultured rat Sertoli cells involves differential apoptotic response. Environ Health Perspect. 2003;111:33–38. doi: 10.1289/ehp.5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz R, Suominen J, Värre T, Hakovirta H, Parvinen M, Toppari J, et al. Expression of aryl hydrocarbon receptor and aryl hydrocarbon receptor nuclear translocator messenger ribonucleic acids and proteins in rat and human testis. Endocrinology. 2003;144:767–776. doi: 10.1210/en.2002-220642. [DOI] [PubMed] [Google Scholar]
- Sharpe RM, McKinnel C, Kivlin C, Fisher JS. Proliferation and functional maturation of Sertoli cells, and their relevance to disorders of testis function in adulthood. Reproduction. 2003;125:769–784. doi: 10.1530/rep.0.1250769. [DOI] [PubMed] [Google Scholar]
- Storgaard L, Bonde JP, Ernst E, Spanò M, Andersen CY, Frydenberg M, et al. Does smoking during pregnancy affect sons’ sperm counts? Epidemiology. 2003;14:278–286. [PubMed] [Google Scholar]
- Swan SH, Brazil C, Drobnis EZ, Liu F, Kruse RL, Hatch M, et al. Geographic differences in semen quality of fertile U.S. males. Environ Health Perspect. 2003;111:414–420. doi: 10.1289/ehp.5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan SH, Elkin EP, Fenster L. The question of declining sperm density revisited: an analysis of 101 studies published 1934–1996. Environ Health Perspect. 2000;108:961–966. doi: 10.1289/ehp.00108961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Eckardstein S, Simoni M, Bergmann M, Weinbauer GF, Gassner P, Schepers AG, et al. Serum inhibin B in combination with serum follicle-stimulating hormone (FSH) is a more sensitive marker than serum FSH alone for impaired spermatogenesis in men, but cannot predict the presence of sperm in testicular tissue samples. J Clin Endocrin Metab. 1999;84:2496–2501. doi: 10.1210/jcem.84.7.5855. [DOI] [PubMed] [Google Scholar]
- Westgard QC. Westgard Rules. 2009. [accessed 4 February 2010]. Available: http://www.westgard.com/westgard-rules.
- WHO (World Health Organization) Laboratory Manual for the Examination of Human Semen and Sperm-Cervical Mucus Interaction. 4th ed. Cambridge, UK: Cambridge University Press; 1999. [Google Scholar]
- Zheng Y, Bonde JP, Ernst E, Mortensen JT, Egense J. Is semen quality related to the year of birth among Danish infertility clients? Int J Epidemiol. 1997;26:1289–1297. doi: 10.1093/ije/26.6.1289. [DOI] [PubMed] [Google Scholar]