Abstract
Background
Several studies suggest that airborne particulate matter (PM) is associated with infant mortality; however, most focused on short-term exposure to larger particles.
Objectives
We evaluated associations between long-term exposure to different sizes of particles [total suspended particles (TSP), PM ≤ 10 μm in aerodynamic diameter (PM10), ≤ 10–2.5 μm (PM10–2.5), and ≤ 2.5 μm (PM2.5)] and infant mortality in a cohort in Seoul, Korea, 2004–2007.
Methods
The study includes 359,459 births with 225 deaths. We applied extended Cox proportional hazards modeling with time-dependent covariates to three mortality categories: all causes, respiratory, and sudden infant death syndrome (SIDS). We calculated exposures from birth to death (or end of eligibility for outcome at 1 year of age) and pregnancy (gestation and each trimester) and treated exposures as time-dependent variables for subjects’ exposure for each pollutant. We adjusted by sex, gestational length, season of birth, maternal age and educational level, and heat index. Each cause of death and exposure time frame was analyzed separately.
Results
We found a relationship between gestational exposures to PM and infant mortality from all causes or respiratory causes for normal-birth-weight infants. For total mortality (all causes), risks were 1.44 (95% confidence interval, 1.06–1.97), 1.65 (1.18–2.31), 1.53 (1.22–1.90), and 1.19 (0.83–1.70) per interquartile range increase in TSP, PM10, PM2.5, and PM10–2.5, respectively; for respiratory mortality, risks were 3.78 (1.18–12.13), 6.20 (1.50–25.66), 3.15 (1.26–7.85), and 2.86 (0.76–10.85). For SIDS, risks were 0.92 (0.33–2.58), 1.15 (0.38–3.48), 1.42 (0.71–2.87), and 0.57 (0.16–1.96), respectively.
Conclusions
Our findings provide supportive evidence of an association of long-term exposure to PM air pollution with infant mortality.
Keywords: air pollution, Cox proportional hazards model, infant mortality, long-term effect, particulate matter, PM2.5, PM10, PM10–2.5, survival analysis, time dependent, TSP
Numerous epidemiologic studies have demonstrated associations between ambient air pollution and health outcomes, including mortality (Chen et al. 2008; Dales et al. 2004; Qian et al. 2007), hospitalizations (Dominici et al. 2006; Lin et al. 2004; Villeneuve et al. 2007; Wellenius et al. 2005), and lung function (Delfino et al. 2008; Jalaludin et al. 2000; Tang et al. 2007). Infant mortality is still a major contributor to childhood mortality (Glinianaia et al. 2004a). Infants and children are potentially susceptible because of their young immune systems, developing respiratory and other systems, and common viral infections (Bateson and Schwartz 2008; Glinianaia et al. 2004b; Koranteng et al. 2007).
Findings for air pollution and infant health are relatively consistent for particulate matter (PM) compared with other pollutants (Šrám et al. 2005). Several studies suggest that PM exposure is associated with infant mortality (Bobak and Leon 1999; Ha et al. 2003; Hajat et al. 2007; Lipfert et al. 2000; Romieu et al. 2004; Tsai et al. 2006; Woodruff et al. 1997). However, most studies focused on short-term exposure to larger particles such as total suspended particulate (TSP) or PM10 (PM ≤ 10 μm in aerodynamic diameter) and to exposures that occurred postneonatally. Research on effects of PM2.5 (≤ 2.5 μm) or coarse particles PM10–2.5 (2.5–10 μm) in infants is limited.
Only two studies evaluated associations between long-term PM2.5 exposure and infant mortality. Woodruff et al. (2006) observed an association between postneonatal respiratory mortality and PM2.5 exposure from birth to death in California. Another U.S. study did not find a relationship between PM2.5 during the first 2 months of life and infant mortality (Woodruff et al. 2008).
Smaller particles (PM2.5) may be more harmful than larger particles because they consist of different chemical components, with more combustion-related sources. Smaller particles penetrate more deeply into lung airways and are deposited in the alveolar region more often. Few studies have evaluated effects of particle size on infant mortality, although many studies in adults suggest that PM2.5 is more strongly associated with health than is PM10 (Franklin et al. 2007).
Relatively few studies have evaluated air pollution and infant mortality in Korea (Ha et al. 2003; Son et al. 2008). Moreover, these studies focused on short-term PM10 exposure postneonatally. Also, no study investigated the relationship between exposure to air pollution during pregnancy and infant mortality. We evaluated associations between long-term exposure (during pregnancy, and from birth to death or end of eligibility for outcome at 1 year of age) to different particle sizes (TSP, PM10, PM10–2.5, PM2.5) and infant mortality in a birth cohort in Seoul, Korea, for 2004–2007. We used an extended Cox proportional hazard model designed to assess long-term effects of PM while estimating cumulative lifetime exposure and average exposure during pregnancy as time-dependent variables.
Materials and Methods
Health data
We obtained linked birth and mortality records for 2004–2007 from the Korean National Statistical Office for 381,271 subjects. Birth data included reported residential address at birth, mother’s permanent residential address, sex, birth weight (grams), parents’ age (years), parents’ education (none; elementary, middle, high school; more than university; unknown), parents’ occupation (manager, expert, engineer, office worker, service job, salesperson, agriculture/fishery/forestry, technical service, mechanic, physical labor, student/unemployed/household, unknown/military), parity, birth order, gestational age (weeks), parents’ marital status (yes, no, unknown), birth month, and place of birth (home, hospital, other, unknown). Mortality data included infant’s residential address, date of death, place of death (e.g., home, hospital), sex, age, and primary and secondary causes of death.
We excluded subjects whose reported residential addresses at birth differed from the mother’s permanent residential addresses on birth certificates and subjects with different residential addresses for birth and death. For study subjects who survived, residential addresses are available only at birth. We excluded observations with incomplete birth certificate data for infant’s sex, gestational length, birth weight, and mother’s education or age. Study subjects were restricted to infants with 37–44 weeks of gestation. These criteria resulted in a loss of 5.7% of observations. To consider only deaths potentially associated with air pollution, we omitted infants who died in the neonatal period (< 28 days) because these deaths are more likely to occur in infants who had not left the hospital after birth or experienced pregnancy-related complications (Woodruff et al. 2006). After exclusions, 359,459 subjects were included. The distribution of values for variables used for analysis was similar between study subjects included in analysis and all subjects.
We classified mortality data by cause of death according to the International Classification of Diseases, 10th Revision (ICD-10) (World Health Organization 1993), from death certificate information in linked birth and mortality records. We considered total mortality as all causes of death except external causes (ICD-10 codes A00–R99), sudden infant death syndrome (SIDS; ICD-10 R95), and respiratory causes (ICD-10 J00–J99). The primary cause of death on death certificates was used.
Air pollution and meteorologic data
TSP, PM10, and PM2.5 monitoring data were obtained from 27 monitoring stations distributed evenly throughout Seoul and operated by the Department of Environment, Republic of Korea, during the whole study period. All monitors measured hourly data for all pollutants studied. We used 24-hr averages as the exposure index, by first averaging hourly values across all monitors for each day and then calculating 24-hr values. PM10–2.5 was calculated as PM10 minus PM2.5.
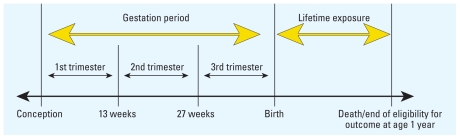
We calculated three types of long-term exposure: a) lifetime exposure, based on PM levels from birth to death or end of eligibility for outcome (1 year of age), b) gestational exposure (from conception to birth), and c) exposure for each trimester. We used length of gestation and birth date to estimate date of conception. Because data for exact day of birth were unavailable, we assigned birth day as the midpoint of the birth month. Trimesters were defined as 1–13 weeks, 14–26 weeks, and 27 weeks to birth; similar definitions have been applied elsewhere (Bell et al. 2007; Parker et al. 2005).
The National Meteorological Administration, Republic of Korea, provided hourly measurements of ambient temperature and 3-hr measurements of relative humidity for Seoul during the study period. We converted weather data into 24-hr values. To represent meteorologic conditions, we used the heat index, which is a function of temperature and relative humidity suggested by the U.S. National Weather Service (Rothfusz 1990):
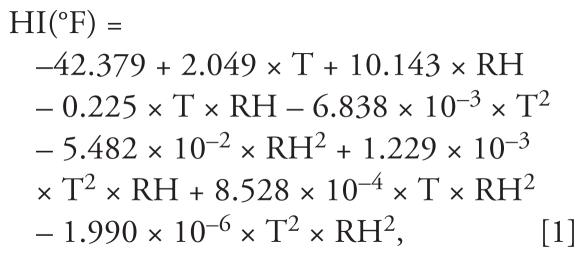 |
where HI is the heat index (°F), T is air temperature (°F), and RH is relative humidity (%). Variables for heat index, converted to degrees Celsius, were calculated for the gestation period, each trimester, and day of death in the lifetime exposure model. Similar control of weather variables was applied in previous models of air pollution exposure during pregnancy and infant outcomes (Bell et al. 2007). Data were available for 100% of days in the study period for all pollutants and weather variables.
Statistical analysis
We used an extended Cox proportional hazards model with time-dependent covariates to investigate the association of PM with infant mortality. The Cox proportional hazards model has been widely used in analysis of time-to-event data with censoring and covariates (Fisher and Lin 1999). This standard Cox proportional hazards model assumes a constant hazard ratio over time. Thus, to estimate hazards with a varying exposure period, we used an extended Cox model with time-dependent covariates, which requires no proportional hazards, because the hazards depended on time, which in this case refers to study subjects’ age. An important assumption of the extended Cox model is that the effect of a time-dependent variable Xi(t) on the survival probability at time t depends on the value of this variable at that same time t, and not on the value at an earlier or later time. The use of time-dependent survival methods ensures that effects are examined relative to other subjects for the same follow-up interval, similar to the manner in which matching is used in other analyses to establish comparability. This model has been applied previously (Platt et al. 2004; Suh et al. 2009).
We analyzed separate models for each mortality type (e.g., respiratory causes) and pollutant (e.g., PM2.5). We stratified analysis by birth weight (normal birth weight, ≥ 2,500 g; low birth weight, < 2,500 g). We created time-dependent variables for cumulative lifetime exposure (from birth to death or end of eligibility for outcome at age 1 year) and for gestational exposure (for total pregnancy and each trimester) to each pollutant (TSP, PM10, PM2.5, and PM10–2.5) (Figure 1).
Figure 1.
Structure of time-dependent variables for air pollutant exposure.
For each cause of death, air pollutant, birth weight group (normal or low birth weight), and exposure period (e.g., specific trimester), we fitted a time-dependent Cox proportional hazards model:
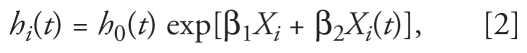 |
where h0(t) is the unspecified baseline hazard function, Xi is the vector of time-independent variables (sex, length of gestation, season of birth, mother’s age, mother’s educational level, heat index), Xi(t) is the vector of the time-dependent variable (PM), and βj (j = 1, 2) are vectors of model parameters.
Length of gestation was used as a continuous variable in weeks. Mother’s educational level was categorized as ≤ 6, 7–12, and > 12 years. To incorporate the nonlinear relationship between mother’s age and infant mortality, mother’s age was categorized as < 20, 20–24, 25–29, 30–34, 35–39, and > 39 years. Data on mother’s smoking status or alcohol use during pregnancy are not recorded on Korean birth certificates.
We performed a test of proportionality for time-fixed covariates with a few levels (e.g., mother’s educational level). For time-dependent covariates, we considered an extended Cox proportional hazards model with time-dependent covariates allowing nonproportional hazards because hazard ratios may vary across time. Analyses were conducted with SAS (version 9.2; SAS Institute Inc., Cary, NC, USA).
Results
Table 1 shows characteristics of the study population, weather, and pollutant exposures. Analysis included 359,459 subjects, 2.0% with low birth weight. There were 225 total deaths, 26 SIDS deaths, and 22 respiratory-related deaths. Most respiratory deaths were from pneumonia and pulmonary disease (72.7%). The all-cause category included a small number of respiratory deaths (10%) and SIDS (12%). The remaining deaths are mainly composed of congenital malformations of the circulatory system (33.7%), other ill-defined and unspecified causes of mortality (20%), respiratory and cardiovascular disorders specific to the perinatal period (12.6%), and other forms of heart disease (10%). The characteristics of normal-birth-weight infants were similar to those of the subjects who died (all-cause mortality) for most factors except season of birth. Low-birth-weight infants were more likely to be female and to have a shorter gestation than were normal-birth-weight infants. Gestational PM concentrations were slightly higher among infants who died compared with all normal-birth-weight infants for all particle sizes (e.g., 31.5 vs. 30.6 μg/m3 for PM2.5). TSP, PM10, PM2.5, and PM10–2.5 gestational or lifetime exposures were similar across study subjects’ sex, gestational age, mother’s age, and mother’s education. Annual levels were similar across years for PM10, PM2.5, and PM10–2.5, whereas TSP decreased slightly over the study period (97.4 μg/m3 annual average in 2004, 92.6 μg/m3 in 2007).
Table 1.
Characteristics for the birth cohort in Seoul, Korea, 2004–2007.
| Characteristic | Eligible births (n = 359,459)
|
Total mortality (all causes; n = 225) | |
|---|---|---|---|
| Normal birth weight (n = 352,405) | Low birth weight (n = 7,054) | ||
| Sex (%) | |||
| Male | 51.6 | 40.8 | 52.0 |
| Female | 48.4 | 59.2 | 48.0 |
| Age at death [years (mean ± SD)] | — | — | 0.40 ± 0.25 |
| Birth weight [kg (mean ± SD)] | 3.31 ± 0.38 | 2.30 ± 0.18 | 3.13 ± 0.49 |
| Gestational age [weeks (mean ± SD)] | 39.3 ± 1.1 | 38.1 ± 1.1 | 39.0 ± 1.1 |
| 37–38 weeks (%) | 25.2 | 67.5 | 38.7 |
| 39–40 weeks (%) | 63.4 | 30.5 | 54.7 |
| 41–42 weeks (%) | 11.3 | 2.0 | 6.6 |
| 43–44 weeks (%) | 0.1 | 0.0 | 0.00 |
| Mother’s age (%) | |||
| < 20 years | 0.2 | 0.4 | 1.3 |
| 20–24 years | 4.3 | 4.4 | 5.3 |
| 25–29 years | 36.3 | 32.5 | 34.2 |
| 30–34 years | 46.7 | 47.0 | 45.8 |
| 35–39 years | 11.2 | 13.8 | 12.0 |
| > 39 years | 1.3 | 1.9 | 1.3 |
| Educational level of mother [years (%)] | |||
| ≤ 6 | 0.3 | 0.5 | 0.4 |
| 7–12 | 33.2 | 35.4 | 39.6 |
| > 12 | 66.5 | 64.1 | 60.0 |
| Season of birth (%) | |||
| Winter | 25.1 | 24.2 | 26.2 |
| Spring | 25.7 | 25.9 | 26.7 |
| Summer | 23.7 | 24.1 | 27.6 |
| Fall | 25.5 | 25.8 | 19.5 |
| Pollution exposures during pregnancy [μg/m3 (mean ± SD)] | |||
| TSP | |||
| Gestation | 96.7 ± 6.6 | 96.3 ± 7.2 | 98.0 ± 6.5 |
| First trimester | 94.8 ± 18.6 | 94.1 ± 18.6 | 94.9 ± 18.3 |
| Second trimester | 93.1 ± 18.2 | 93.3 ± 18.3 | 95.3 ± 17.5 |
| Third trimester | 93.1 ± 20.1 | 92.8 ± 21.0 | 98.3 ± 19.2 |
| PM10 | |||
| Gestation | 61.3 ± 4.2 | 61.2 ± 4.6 | 62.2 ± 4.4 |
| First trimester | 61.7 ± 12.0 | 61.4 ± 12.0 | 61.1 ± 11.8 |
| Second trimester | 60.5 ± 11.7 | 60.6 ± 11.8 | 61.0 ± 11.0 |
| Third trimester | 60.7 ± 12.7 | 60.6 ± 13.4 | 62.9 ± 12.0 |
| PM2.5 | |||
| Gestation | 30.6 ± 2.3 | 30.5 ± 2.3 | 31.5 ± 2.5 |
| First trimester | 31.1 ± 5.1 | 30.9 ± 4.9 | 31.7 ± 5.8 |
| Second trimester | 30.1 ± 4.1 | 30.1 ± 4.1 | 30.5 ± 4.0 |
| Third trimester | 30.2 ± 4.6 | 30.1 ± 4.9 | 31.1 ± 4.5 |
| PM10–2.5 | |||
| Gestation | 30.6 ± 2.6 | 30.6 ± 2.9 | 30.7 ± 2.7 |
| First trimester | 30.6 ± 8.1 | 30.4 ± 8.1 | 29.4 ± 7.5 |
| Second trimester | 30.4 ± 8.2 | 30.5 ± 8.2 | 30.5 ± 7.7 |
| Third trimester | 30.5 ± 8.6 | 30.5 ± 9.0 | 31.8 ± 8.2 |
| Lifetime pollution exposure [μg/m3 (mean ± SD)] | |||
| TSP | 88.7 ± 14.4 | 88.8 ± 14.7 | 91.7 ± 18.5 |
| PM10 | 59.5 ± 5.7 | 59.5 ± 6.0 | 59.1 ± 11.6 |
| PM2.5 | 29.1 ± 1.7 | 29.1 ± 1.9 | 29.5 ± 4.6 |
| PM10–2.5 | 30.5 ± 4.1 | 30.5 ± 4.2 | 29.7 ± 7.5 |
As preliminary analysis, we investigated the relationship between infant mortality and covariates without including PM in the model (Table 2). Infant mortality was associated with lower birth weight, shorter gestation, older or younger mothers, and less maternal education.
Table 2.
Hazard ratios for all-cause infant mortality associated with selected nonpollution variables.
| Variable | Hazard ratio (95% CI) |
|---|---|
| Birth weight (kg) | 0.54 (0.36–0.80) |
| Child’s sex | |
| Male | Reference |
| Female | 0.91 (0.69–1.19) |
| Gestational length (weeks) | 0.85 (0.75–0.96) |
| Mother’s age (years) | |
| < 20 | Reference |
| 20–24 | 0.19 (0.05–0.69) |
| 25–29 | 0.19 (0.06–0.60) |
| 30–34 | 0.19 (0.06–0.62) |
| 35–39 | 0.21 (0.06–0.72) |
| > 39 | 0.21 (0.04–1.02) |
| Mother’s education (years) | |
| ≤ 6 | Reference |
| 7–12 | 0.75 (0.10–5.46) |
| > 12 | 0.63 (0.09–4.64) |
| Season of birth | |
| Winter | 1.07 (0.74–1.55) |
| Spring | Reference |
| Summer | 1.13 (0.78–1.64) |
| Fall | 0.83 (0.55–1.25) |
First-, second-, and third-trimester exposures can be highly correlated (Bell et al. 2007), but in this study they were not (Pearson correlation coefficients: TSP, −0.10, −0.67, and −0.05, respectively; PM10, −0.11, −0.71, and −0.09; PM2.5, −0.07, −0.31, and −0.00; PM10–2.5, −0.12, −0.74, and −0.16). We performed analyses including all trimester exposures simultaneously and including each trimester separately. Results of both models were consistent.
Table 3 shows hazard ratios for all-cause infant mortality. We stratified by birth weight to assess potential susceptibility for low- and normal-birth-weight infants separately. We observed a relationship between gestational exposures and all-cause mortality for normal-birth-weight infants for all PM measures, but results for PM10–2.5 did not reach statistical significance. For low-birth-weight infants, we did not find a statistically significant relationship between gestational PM exposure and total mortality for any PM size; however, results should be viewed in the context of the smaller sample size (n = 7,054). For normal-birth-weight infants, the trimester of PM exposure with the highest effect estimate was the third for TSP, PM10, and PM10–2.5 and the first or second for PM2.5. The only significant association by trimester exposure was for first-trimester PM2.5. In the lifetime exposure model, we observed no statistically significant associations between any PM sizes and infant mortality from all causes for both birth weight groups, except for lower risk of mortality with higher PM10–2.5. We performed sensitivity analysis with lifetime exposure for heat index also included in the lifetime exposure model; results were similar (results not shown). Statistically significant results in Table 3 are robust to analysis of all-cause mortality excluding SIDS and respiratory-related deaths (results not shown).
Table 3.
Hazard ratios for an IQR increase from Cox proportional hazards modelsa on all-cause infant mortality in a birth cohort, Seoul, Korea, 2004–2007.
| Exposure | Hazard ratio (95% CI)
|
|
|---|---|---|
| Normal birth weight (n = 352,405) | Low birth weight (n = 7,054) | |
| Gestational exposure
| ||
| TSP (IQR, 8.91 μg/m3) | ||
| All | 1.44 (1.06–1.97) | 1.69 (0.38–7.49) |
| First trimester | 1.03 (0.92–1.16) | 1.13 (0.69–1.85) |
| Second trimester | 1.03 (0.91–1.16) | 1.40 (0.89–2.18) |
| Third trimester | 1.10 (0.98–1.23) | 0.80 (0.54–1.19) |
| PM10 (IQR, 6.93 μg/m3) | ||
| All | 1.65 (1.18–2.31) | 1.48 (0.38–5.80) |
| First trimester | 1.06 (0.93–1.22) | 1.13 (0.67–1.89) |
| Second trimester | 1.04 (0.89–1.20) | 1.43 (0.85–2.41) |
| Third trimester | 1.07 (0.93–1.23) | 0.80 (0.50–1.28) |
| PM2.5 (IQR, 3.15 μg/m3) | ||
| All | 1.53 (1.22–1.90) | 1.00 (0.34–2.94) |
| First trimester | 1.15 (1.04–1.28) | 1.03 (0.63–1.69) |
| Second trimester | 1.15 (0.96–1.38) | 1.27 (0.62–2.58) |
| Third trimester | 1.06 (0.89–1.27) | 0.85 (0.47–1.54) |
| PM10–2.5 (IQR, 3.71 μg/m3) | ||
| All | 1.19 (0.83–1.70) | 1.92 (0.49–7.63) |
| First trimester | 0.94 (0.84–1.05) | 1.13 (0.76–1.69) |
| Second trimester | 0.99 (0.89–1.11) | 1.30 (0.90–1.86) |
| Third trimester | 1.05 (0.95–1.16) | 0.84 (0.58–1.21) |
| Lifetime exposure
| ||
| TSP (IQR, 18.99 μg/m3) | 1.01 (0.75–1.36) | 0.91 (0.25–3.28) |
| PM10 (IQR, 3.48 μg/m3) | 0.94 (0.87–1.02) | 0.93 (0.67–1.30) |
| PM2.5 (IQR, 1.25 μg/m3) | 1.00 (0.93–1.08) | 1.08 (0.83–1.42) |
| PM10–2.5 (IQR, 2.01 μg/m3) | 0.92 (0.85–0.99) | 0.81 (0.59–1.12) |
The 352,405 infants of normal birth weight in this analysis include 209 all-cause deaths. The 7,054 infants of low birth weight include 16 all-cause deaths.
The model included the following variables: sex, gestation period, educational level of mother, maternal age, season of birth, and heat index.
We examined the association between long-term exposure to different particle sizes and cause-specific mortality for normal-birth-weight infants (Table 4). We found associations between gestational exposure to all PM sizes and respiratory infant mortality, although PM10–2.5 results did not reach statistical significance. Estimated risks per interquartile-range (IQR) increase in gestational exposure to TSP, PM10, and PM2.5 on infant respiratory-related mortality were 3.78 [95% confidence interval (CI), 1.18–12.13], 6.20 (95% CI, 1.50–25.66), and 3.15 (95% CI, 1.26–7.85), respectively. For comparability, we calculated trimester results in Table 4 for an increment of exposure based on the IQR for gestational exposure, representing the difference between 75th and 25th percentiles. The magnitude of risk for infant respiratory-related mortality was higher than for all causes (Tables 3 and 4). The most important trimester of exposure based on the highest central estimate was the first for TSP, PM10, PM2.5, and PM10–2.5, with statistically significant associations for all but PM10–2.5.
Table 4.
Hazard ratios for an IQR increase from Cox proportional hazards modelsa on cause-specific infant mortality for normal-birth-weight infants in a birth cohort, Seoul, Korea, 2004–2007.
| Exposure | Hazard ratio (95% CI)
|
|
|---|---|---|
| Respiratory | SIDS | |
| Gestational exposure
| ||
| TSP (IQR, 8.91 μg/m3) | ||
| All | 3.78 (1.18–12.13) | 0.92 (0.33–2.58) |
| First trimester | 2.08 (1.26–3.43) | 1.02 (0.72–1.45) |
| Second trimester | 0.96 (0.64–1.45) | 0.80 (0.58–1.10) |
| Third trimester | 1.03 (0.71–1.48) | 1.00 (0.74–1.37) |
| PM10 (IQR, 6.93 μg/m3) | ||
| All | 6.20 (1.50–25.66) | 1.15 (0.38–3.48) |
| First trimester | 2.19 (1.30–3.70) | 1.04 (0.70–1.55) |
| Second trimester | 0.97 (0.59–1.60) | 0.79 (0.53–1.17) |
| Third trimester | 1.04 (0.66–1.64) | 0.95 (0.64–1.41) |
| PM2.5 (IQR, 3.15 μg/m3) | ||
| All | 3.15 (1.26–7.85) | 1.42 (0.71–2.87) |
| First trimester | 1.58 (1.14–2.19) | 1.14 (0.82–1.59) |
| Second trimester | 1.09 (0.61–1.93) | 0.89 (0.54–1.47) |
| Third trimester | 1.46 (0.79–2.68) | 0.80 (0.48–1.35) |
| PM10–2.5 (IQR, 3.71 μg/m3) | ||
| All | 2.86 (0.76–10.85) | 0.57 (0.16–1.96) |
| First trimester | 1.45 (0.99–2.14) | 0.95 (0.69–1.31) |
| Second trimester | 0.94 (0.63–1.39) | 0.81 (0.60–1.10) |
| Third trimester | 0.93 (0.66–1.32) | 1.01 (0.76–1.34) |
| Lifetime exposure
| ||
| TSP (IQR, 18.99 μg/m3) | 0.35 (0.07–1.86) | 0.81 (0.33–2.00) |
| PM10 (IQR, 3.48 μg/m3) | 0.65 (0.43–0.99) | 0.73 (0.57–0.94) |
| PM2.5 (IQR, 1.25 μg/m3) | 0.63 (0.42–0.95) | 0.88 (0.71–1.09) |
| PM10–2.5 (IQR, 2.01 μg/m3) | 0.70 (0.48–1.01) | 0.66 (0.51–0.86) |
The 352,405 infants of normal birth weight in this analysis include 22 respiratory deaths and 26 SIDS deaths.
The model included the following variables: sex, gestation period, educational level of mother, maternal age, season of birth, and heat index.
Discussion
We conducted this study to estimate effects of long-term PM exposure on infant mortality in a birth cohort in Seoul, Korea, 2004–2007. We also evaluated which particle sizes are more related to all-cause and cause-specific infant mortality. We found statistically significant relationships between gestational PM exposures and infant mortality from all causes or respiratory causes for normal-birth-weight infants.
Results from epidemiologic studies should be interpreted in the context of the exposure increment used, which in our case was smaller than in some previous studies. For example, in earlier work on infant mortality, IQR PM10 values were 42.9 μg/m3 (Ha et al. 2003), 10 μg/m3 (Lipfert et al. 2000; Woodruff et al. 2008), 67 μg/m3 (Tsai et al. 2006), and 20 μg/m3 (Romieu et al. 2004), compared with our value of 6.93 μg/m3. IQR PM2.5 values were 10 μg/m3 (Loomis et al. 1999; Woodruff et al. 2006), whereas our value was 3.15 μg/m3.
The numbers of SIDS (n = 26) and respiratory (n = 22) deaths in this analysis are small. Despite the reduced statistical power, we found statistically significant associations between PM and respiratory-related infant mortality. The survival analysis approach provides more statistical power than case-crossover analysis because all study subjects are included; therefore, this approach provides benefits in the study of rare outcomes. However, additional analysis on the impact of air pollution during pregnancy and these rare mortality outcomes is warranted because the small number of deaths limited our ability to fully evaluate these findings. Such analysis could consider a larger spatial area and/or longer time frame, which would increase the number of mortality events.
Few studies have investigated long-term PM exposure and infant mortality. Below we discuss previous studies of short-term PM exposure and infant mortality that have the same health outcome as our study but different exposure periods (e.g., we include pregnancy exposures in our study, unlike previous studies). We then briefly summarize representative previous studies of air pollution and birth outcomes that have different health end points than our study but similar exposure periods (e.g., prenatal exposure). The biological mechanisms that link PM and pregnancy outcomes likely differ by health outcome or exposure time frame; however, these studies can provide context of the overall evidence of how PM affects infant health.
The relationship with gestational PM exposure was stronger for respiratory mortality than for all-cause mortality. Previous research also found higher effects for respiratory than for all-cause mortality for short-term postnatal PM exposure. A time-series study of postneonatal infant mortality in Korea provided an adjusted relative risk of 1.14 (95% CI, 1.10–1.19) for total mortality and 2.02 (1.78–2.28) for respiratory mortality per IQR (42.9 μg/m3) in same-day PM10 (Ha et al. 2003). Woodruff et al. (2006) found an adjusted odds ratio per 10-μg/m3 increase in PM2.5 lifetime exposure (infants’ birth to death) of 1.07 (95% CI, 0.93–1.24) for total mortality and 2.13 (1.12–4.05) for respiratory-related mortality. For all-cause mortality, our analysis found risks of 1.44 (95% CI, 1.06–1.97), 1.65 (1.18–2.31), and 1.53 (1.22–1.90) per IQR increase (8.91, 6.93, 3.15 μg/m3) in gestational exposure to TSP, PM10, and PM2.5, respectively, and for respiratory mortality we found risks of 3.78 (1.18–12.13), 6.20 (1.50–25.66), and 3.15 (1.26–7.85).
We did not find statistically significant relationships between any PM size and SIDS. Previous studies found inconsistent results for this outcome. Woodruff et al. (1997) found a statistically significant relationship between PM10 during the first 2 months of life and SIDS. Another study in California did not find a relationship between PM2.5 from birth to death and SIDS (Woodruff et al. 2006). A recent U.S. study examining PM2.5, PM10, ozone, sulfur dioxide, and carbon monoxide during the first 2 months of life found that ozone may be associated with SIDS but did not observe PM effects (Woodruff et al. 2008). Further analysis is needed to investigate possible links between PM and SIDS.
We observed a positive effect on infant mortality in some lifetime exposure models (e.g., PM10–2.5 and normal-birth-weight infants for all-cause mortality). We controlled for weather and season of birth, although other temporally varying confounders may exist. Our adjustment for several maternal and individual factors that are unlikely to vary temporally are unlikely to influence results because this study focuses on a single city; however, similar studies in larger geographic areas should carefully consider such potential confounders. Our use of individual information on mother’s education as an indicator of socioeconomic status may not fully reflect full socioeconomic conditions. Actual socioeconomic status is also related to the educational level of other members of the household and many other factors such as income, occupation, housing type, the type and age of vehicles, and the air conditioning system at the residence. Further, socioeconomic conditions are a function of previous history of socioeconomic conditions such as previous income. Recent literature showed that the neighborhood environment of the mother and child has an independent influence on birth outcomes that was not explained by individual-level risk factors (Buka et al. 2003; O’Neill et al. 2003). In previous studies in Korea, area-level socioeconomic status modified the association between air pollution and health outcomes (Lee et al. 2006; Yi et al. 2010). Further study is needed to examine potential confounding and effect modification by socioeconomic status and to investigate the associations observed in this study.
Many studies suggest that air pollution exposure during pregnancy is associated with birth outcomes such as preterm birth, low birth weight, intrauterine growth restriction, and birth defects (Ballester et al. 2010; Pope et al. 2010; Ritz et al. 2002; Tsai et al. 2003). Although these studies have exposure time frames similar to those in our study, the health outcomes differ, as may the biological mechanisms. One study found a 16% increase in risk of preterm birth per 50 μg/m3 in PM10 during the first month of pregnancy (Ritz et al. 2000). The relative risk for small for gestational age was 1.03 per 1-μg/m3 increase in PM2.5 in the second trimester, with a corresponding birth weight reduction of 4.1 g (95% CI, 1.4–6.8 g) (Mannes et al. 2005). Another study found that an IQR increase in gestational exposure to PM10 and PM2.5 lowered birth weight by 8.2 g (5.3–11.1 g) and 14.7 g (12.3–17.1 g), respectively (Bell et al. 2007).
Low birth weight may have an important effect on infant health and is a significant determinant of infant mortality (McCormick 1985). Low birth weight is mainly caused by preterm birth, defined as delivery < 37 weeks of pregnancy, and fetal growth retardation (Xu et al. 2011). Many studies showed that crucial factors of infant mortality, such as low birth weight and gestational age, are associated with increased infant mortality (Callaghan et al. 2006; Donovan et al. 2010; Were and Bwibo 2009). In a study in Korea, exposure to air pollution during pregnancy was significantly associated with preterm birth (Ha et al. 2004) and low birth weight (Ha et al. 2001; Lee et al. 2003).
Prenatal exposures may enhance susceptibility and increase risk of infant mortality. Although multiple mechanisms have been proposed, the biological pathways by which PM could affect infant mortality are not fully understood. Air pollution could directly affect fetal growth through the placenta or indirectly by impairing mother’s health (Glinianaia et al. 2004b). PM is known to invoke an inflammatory response that alters blood coagulation, invoke an allergic immune response, and alter cardiac function by reducing heart rate variability (Gold et al. 2000; Liao et al. 1999; Seaton et al. 1995). All these mechanisms can occur in the fetus as well as in adults. Also, maternal exposure to PM air pollutants during pregnancy can result in reduced transplacental function, with consequent deterioration in fetal growth and development (Glinianaia et al. 2004b). The biological mechanism through which exposure to air pollution affects risk of mortality may vary by several factors, including the pollutant (e.g., chemical structure of the PM mixture), time frame of exposure (e.g., exposure during pregnancy vs. after birth), and cause of mortality. As research continues to develop our understanding of the physiologic pathways through which air pollution affects risk, future work may examine alternate model structures that incorporate this information, such as the most relevant period of exposure for the heat index or related weather variables.
Because of the structure of the available data, we assumed birth day as the day representing the midpoint of the birth month and estimated time frame for exposure based on this information. Similar approaches have been used elsewhere (Bell et al. 2007). Although this is a limitation of our study, it does not greatly affect long-term exposure estimates. The estimated date of birth cannot be > 2 weeks from the actual date of birth. Such an approach would be problematic for study of acute exposures, although for gestational exposure this approach can result in a maximum of 5% of exposure based on the incorrect time frame.
In this study, we controlled for covariates such as individual and maternal characteristics (Table 2). Our analysis showed that the infants having shorter gestation periods might be at a greater risk for infant mortality. We found that the risk of infant mortality was associated with lower educational level of mother, lower birth weight, and older or younger maternal age, which is consistent with previous research. In a study by Arntzen et al. (2004), the risk of infant mortality decreased with higher educational attainment. Gnavi and Costa (2002) reported that postneonatal mortality was strongly correlated with low maternal educational level. Adair and Popkin (1988) observed that low-birth-weight infants had a higher risk of infant mortality. We were not able to control for maternal smoking, because these data are not available on the Korean birth certificates. However, this factor is unlikely to significantly alter the effect estimates, because smoking among woman in Korea is not highly prevalent, particularly among pregnant women. The smoking level of women in Korea is the lowest (4.6%) among many countries (Organisation for Economic Cooperation and Development 2009).
Our study differs from previous work in several ways. We focused on long-term exposure and applied a survival analysis with time-dependent covariates. Many epidemiologic studies have examined associations between air pollution and infant mortality by using time-series or case-crossover studies to estimate the effects of short-term exposure to air pollution. In the time-series studies, individual data on the subjects often are not available, which limits the assessment of individual risk factors. Our analytical framework enables identification and adjustment for individual risk factors and increases the statistical power because all the subjects (deceased and alive) are included. Lepeule et al. (2006), who reported an association between air pollution and cardiorespiratory mortality, found that the results using survival analysis agreed with those obtained with the case-crossover analysis and that confidence intervals are more restricted with the Cox proportional hazards model. A study by Platt et al. (2004) supported the effectiveness of applying the extended Cox proportional hazards model for the study of fetal and infant death.
To our knowledge, this is the first study to investigate the association between long-term exposure to different sizes of particles and infant mortality in Korea. Our findings provide evidence supporting the hypothesis that long-term exposure to PM air pollution during pregnancy increases the risk of infant mortality from all causes or respiratory causes. We also suggest that using the Cox proportional hazard model with time-dependent covariates to examine the effect of air pollution in a cohort study with a time-dependent exposure has benefits over more traditionally applied approaches.
References
- Adair LS, Popkin BM. Birth weight, maturity, and proportionality in Filipino Infants. Hum Biol. 1988;60:319–339. [PubMed] [Google Scholar]
- Arntzen A, Samuelsen SO, Bakketeig LS, Stoltenberg C. Parents’ education and infant mortality 1967–1998. Tidsskr Nor Laegeforen. 2004;124(22):2904–2906. [PubMed] [Google Scholar]
- Ballester F, Estarlich M, Iniguez C, Llop S, Ramon R, Esplugues A, et al. Air pollution exposure during pregnancy and reduced birth size: a prospective birth cohort study in Valencia, Spain. Environ Health. 2010;9:6. doi: 10.1186/1476-069X-9-6. [Online 29 January 2010] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateson TF, Schwartz J. Children’s response to air pollutants. J Toxicol Environ Health. 2008;A71(3):238–243. doi: 10.1080/15287390701598234. [DOI] [PubMed] [Google Scholar]
- Bell ML, Ebisu K, Belanger K. Ambient air pollution and low birth weight in Connecticut and Massachusetts. Environ Health Perspect. 2007;115:1118–1124. doi: 10.1289/ehp.9759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobak M, Leon DA. The effect of air pollution on infant mortality appears specific for respiratory causes in the postneonatal period. Epidemiology. 1999;10(6):666–670. [PubMed] [Google Scholar]
- Buka SL, Brennan RT, Rich-Edwards JW, Raudenbush SW, Earls F. Neighborhood support and the birth weight of urban infants. Am J Epidemiol. 2003;157(1):1–8. doi: 10.1093/aje/kwf170. [DOI] [PubMed] [Google Scholar]
- Callaghan WM, MacDorman MF, Rasmussen SA, Qin C, Lackritz EM. The contribution of preterm birth to infant mortality rates in the United States. Pediatrics. 2006;118(4):1566–1573. doi: 10.1542/peds.2006-0860. [DOI] [PubMed] [Google Scholar]
- Chen G, Song G, Jiang L, Zhang Y, Zhao N, Chen B, et al. Short-term effects of ambient gaseous pollutants and particulate matter on daily mortality in Shanghai, China. J Occup Health. 2008;50(1):41–47. doi: 10.1539/joh.50.41. [DOI] [PubMed] [Google Scholar]
- Dales R, Burnett RT, Smith-Doiron M, Stieb DM, Brook JR. Air pollution and sudden infant death syndrome. Pediatrics. 2004;113(6):628–631. doi: 10.1542/peds.113.6.e628. [DOI] [PubMed] [Google Scholar]
- Delfino RJ, Staimer N, Tjoa T, Gillen D, Kleinman MT, Sioutas C, et al. Personal and ambient air pollution exposures and lung function decrements in children with asthma. Environ Health Perspect. 2008;116:550–558. doi: 10.1289/ehp.10911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominici F, Peng RD, Bell ML, Pham L, McDermott A, Zeger SL, et al. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. JAMA. 2006;295(10):1127–1134. doi: 10.1001/jama.295.10.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan EF, Besl J, Paulson J, Rose B, Iams J Ohio Perinatal Quality Collaborative. Infant death among Ohio resident infants born at 32 to 41 weeks of gestation. Am J Obstet Gynecol. 2010;203(1):58.e1–58.e5. doi: 10.1016/j.ajog.2010.01.071. [DOI] [PubMed] [Google Scholar]
- Fisher LD, Lin DY. Time-dependent covariates in the Cox proportional hazards regression model. Annu Rev Public Health. 1999;20:145–157. doi: 10.1146/annurev.publhealth.20.1.145. [DOI] [PubMed] [Google Scholar]
- Franklin M, Zeka A, Schwartz J. Association between PM2.5 and all-cause and specific-cause mortality in 27 US communities. J Expo Sci Environ Epidemiol. 2007;17(3):279–287. doi: 10.1038/sj.jes.7500530. [DOI] [PubMed] [Google Scholar]
- Glinianaia SV, Rankin J, Bell R, Pless-Mulloli T, Howel D. Does particulate air pollution contribute to infant death? A systematic review. Environ Health Perspect. 2004a;112:1365–1371. doi: 10.1289/ehp.6857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glinianaia SV, Rankin J, Bell R, Pless-Mulloli T, Howel D. Particulate air pollution and fetal health: a systematic review of the epidemiologic evidence. Epidemiology. 2004b;15(1):36–45. doi: 10.1097/01.ede.0000101023.41844.ac. [DOI] [PubMed] [Google Scholar]
- Gnavi R, Costa G. Pregnancy outcome, infant mortality and mother’s education in Piedmont from 1980 to 1995. Epidemiol Prev. 2002;26(5):225–233. [PubMed] [Google Scholar]
- Gold DR, Litonjua A, Schwartz J, Lovett E, Larson A, Nearing B, et al. Ambient pollution and heart rate variability. Circulation. 2000;101(11):1267–1273. doi: 10.1161/01.cir.101.11.1267. [DOI] [PubMed] [Google Scholar]
- Ha EH, Hong YC, Lee BE, Woo BH, Schwartz J, Christiani DC. Is air pollution a risk factor for low birth weight in Seoul? Epidemiology. 2001;12(6):643–648. doi: 10.1097/00001648-200111000-00011. [DOI] [PubMed] [Google Scholar]
- Ha EH, Lee BE, Park HS, Kim YS, Kim H, Kim YJ, et al. Prenatal exposure to PM10 and preterm birth between 1998 and 2000 in Seoul, Korea. J Prev Med Public Health. 2004;37(4):300–305. [PubMed] [Google Scholar]
- Ha EH, Lee JT, Kim H, Hong YC, Lee BE, Park HS, et al. Infant susceptibility of mortality to air pollution in Seoul, South Korea. Pediatrics. 2003;111(2):284–290. doi: 10.1542/peds.111.2.284. [DOI] [PubMed] [Google Scholar]
- Hajat S, Armstrong B, Wilkinson P, Busby A, Dolk H. Outdoor air pollution and infant mortality: analysis of daily time-series data in 10 English cities. J Epidemiol Community Health. 2007;61(8):719–722. doi: 10.1136/jech.2006.053942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalaludin BB, Chey T, O’Toole BI, Smith WT, Capon AG, Leeder SR. Acute effects of low levels of ambient ozone on peak expiratory flow rate in a cohort of Australian children. Int J Epidemiol. 2000;29(3):549–557. [PubMed] [Google Scholar]
- Koranteng S, Vargas AR, Buka I. Ambient air pollution and children’s health: a systematic review of Canadian epidemiological studies. Paediatr Child Health. 2007;12(3):225–233. [PMC free article] [PubMed] [Google Scholar]
- Lee BE, Ha EH, Park HS, Kim YJ, Hong YC, Kim H, et al. Exposure to air pollution during different gestational phases contributes to risks of low birth weight. Hum Reprod. 2003;18(3):638–643. doi: 10.1093/humrep/deg102. [DOI] [PubMed] [Google Scholar]
- Lee JT, Son JY, Kim H, Kim SY. Effect of air pollution on asthma-related hospital admissions for children by socioeconomic status associated with area of residence. Arch Environ Occup Health. 2006;61(3):123–130. doi: 10.3200/AEOH.61.3.123-130. [DOI] [PubMed] [Google Scholar]
- Lepeule J, Rondeau V, Filleul L, Dartigues JF. Survival analysis to estimate association between short-term mortality and air pollution. Environ Health Perspect. 2006;114:242–247. doi: 10.1289/ehp.8311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao D, Creason J, Shy C, Williams R, Watts R, Zweidinger R. Daily variation of particulate air pollution and poor cardiac autonomic control in the elderly. Environ Health Perspect. 1999;107:521–525. doi: 10.1289/ehp.99107521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CA, Pereira LA, Nishioka DC, Conceição GM, Braga AL, Saldiva PH. Air pollution and neonatal deaths in São Paulo, Brazil. Braz J Med Biol Res. 2004;37(5):765–770. doi: 10.1590/s0100-879x2004000500019. [DOI] [PubMed] [Google Scholar]
- Lipfert FW, Zhang J, Wyzga RE. Infant mortality and air pollution: a comprehensive analysis of U.S. data for 1990. J Air Waste Manag Assoc. 2000;50(8):1350–1366. doi: 10.1080/10473289.2000.10464168. [DOI] [PubMed] [Google Scholar]
- Loomis D, Castillejos M, Gold DR, McDonnell W, Borja-Aburto VH. Air pollution and infant mortality in Mexico City. Epidemiology. 1999;10:118–123. [PubMed] [Google Scholar]
- Mannes T, Jalaludin B, Morgan G, Lincoln D, Sheppeard V, Corbett S. Impact of ambient air pollution on birth weight in Sydney, Australia. Occup Environ Med. 2005;62:524–530. doi: 10.1136/oem.2004.014282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick MC. The contribution of low birth weight to infant mortality and childhood morbidity. N Engl J Med. 1985;312(2):82–90. doi: 10.1056/NEJM198501103120204. [DOI] [PubMed] [Google Scholar]
- Organisation for Economic Cooperation and Development. OECD Health Data 2009: Health at a Glance 2009 OECD Indicators. 2009. [accessed 7 April 2011]. Available: http://www.oecd-ilibrary.org/social-issues-migration-health/healthat-a-glance-2009_health_glance-2009-en.
- O’Neill MS, Jerrett M, Kawachi I, Levy JI, Cohen AJ, Gouveia N, et al. Workshop on Air Pollution and Socioeconomic Conditions. Health, wealth, and air pollution: advancing theory and methods. Environ Health Perspect. 2003;111:1861–1870. doi: 10.1289/ehp.6334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker JD, Woodruff TJ, Basu R, Schoendorf KC. Air pollution and birth weight among term infants in California. Pediatrics. 2005;115(1):121–128. doi: 10.1542/peds.2004-0889. [DOI] [PubMed] [Google Scholar]
- Platt RW, Joseph KS, Ananth CV, Grondines J, Abrahamowicz M, Kramer MS. A proportional hazards model with time-dependent covariates and time-varying effects for analysis of fetal and infant death. Am J Epidemiol. 2004;160:199–206. doi: 10.1093/aje/kwh201. [DOI] [PubMed] [Google Scholar]
- Pope DP, Mishra V, Thompson L, Siddiqui AR, Rehfuess EA, Weber M, et al. Risk of low birth weight and stillbirth associated with indoor air pollution from solid fuel use in developing countries. Epidemiol Rev. 2010;32(1):70–81. doi: 10.1093/epirev/mxq005. [DOI] [PubMed] [Google Scholar]
- Qian Z, He Q, Lin HM, Kong L, Liao D, Dan J, et al. Association of daily cause-specific mortality with ambient particle air pollution in Wuhan, China. Environ Res. 2007;105(3):380–389. doi: 10.1016/j.envres.2007.05.007. [DOI] [PubMed] [Google Scholar]
- Ritz B, Yu F, Chapa G, Fruin S. Effect of air pollution on preterm birth among children born in Southern California between 1989 and 1993. Epidemiology. 2000;11:502–511. doi: 10.1097/00001648-200009000-00004. [DOI] [PubMed] [Google Scholar]
- Ritz B, Yu F, Fruin S, Chapa G, Shaw GM, Harris JA. Ambient air pollution and risk of birth defects in Southern California. Am J Epidemiol. 2002;155(1):17–25. doi: 10.1093/aje/155.1.17. [DOI] [PubMed] [Google Scholar]
- Romieu I, Ramírez-Aguilar M, Moreno-Macias H, Barraza-Villarreal A, Miller P, Hernández-Cadena L, et al. Infant mortality and air pollution: modifying effect by social class. J Occup Environ Med. 2004;46(12):1210–1216. [PubMed] [Google Scholar]
- Rothfusz LP. National Weather Service Southern Region Technical Attachment. SR/SSD 90-23. Fort Worth, TX: National Weather Service; 1990. The heat index equation (or more than you ever wanted to know about heat index) [Google Scholar]
- Seaton A, MacNee W, Donaldson K, Godden D. Particulate air pollution and acute health effects. Lancet. 1995;345(8943):176–178. doi: 10.1016/s0140-6736(95)90173-6. [DOI] [PubMed] [Google Scholar]
- Son JY, Cho YS, Lee JT. Effects of air pollution on postneonatal infant mortality among firstborn infants in Seoul, Korea: case-crossover and time-series analyses. Arch Environ Occup Health. 2008;63(3):108–113. doi: 10.3200/AEOH.63.3.108-113. [DOI] [PubMed] [Google Scholar]
- Šrám RJ, Binková B, Dejmek J, Bobak M. Ambient air pollution and pregnancy outcomes: a review of the literature. Environ Health Perspect. 2005;113:375–382. doi: 10.1289/ehp.6362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh YJ, Kim H, Seo JH, Park H, Kim YJ, Hong YC, et al. Different effects of PM10 exposure on preterm birth by gestational period estimated from time-dependent survival analyses. Int Arch Occup Environ Health. 2009;82:613–621. doi: 10.1007/s00420-008-0380-7. [DOI] [PubMed] [Google Scholar]
- Tang CS, Chang LT, Lee HC, Chan CC. Effects of personal particulate matter on peak expiratory flow rate of asthmatic children. Sci Total Environ. 2007;382(1):43–51. doi: 10.1016/j.scitotenv.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Tsai SS, Chen CC, Hsieh HJ, Chang CC, Yang CY. Air pollution and postneonatal mortality in a tropical city: Kaohsiung, Taiwan. Inhal Toxicol. 2006;18(3):185–189. doi: 10.1080/08958370500434214. [DOI] [PubMed] [Google Scholar]
- Tsai SS, Yu HS, Liu CC, Yang CY. Increased incidence of preterm delivery in mothers residing in an industrialized area in Taiwan. J Toxicol Environ Health A. 2003;66(11):987–994. doi: 10.1080/15287390306396. [DOI] [PubMed] [Google Scholar]
- Villeneuve PJ, Chen L, Rowe BH, Coates F. Outdoor air pollution and emergency department visits for asthma among children and adults: a case-crossover study in northern Alberta, Canada. Environ Health. 2007;6:40–54. doi: 10.1186/1476-069X-6-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellenius GA, Schwartz J, Mittleman MA. Air pollution and hospital admissions for ischemic and hemorrhagic stroke among Medicare beneficiaries. Stroke. 2005;36(12):2549–2553. doi: 10.1161/01.STR.0000189687.78760.47. [DOI] [PubMed] [Google Scholar]
- Were FN, Bwibo NO. The contribution of very low birth weight deaths to infant mortality. East Afr Med J. 2009;86(8):374–377. doi: 10.4314/eamj.v86i8.54157. [DOI] [PubMed] [Google Scholar]
- Woodruff TJ, Darrow LA, Parker JD. Air pollution and postneonatal infant mortality in the United States, 1999–2002. Environ Health Perspect. 2008;116:110–115. doi: 10.1289/ehp.10370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff TJ, Grillo J, Schoendorf KC. The relationship between selected causes of postneonatal infant mortality and particulate air pollution in the United States. Environ Health Perspect. 1997;105:608–612. doi: 10.1289/ehp.97105608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff TJ, Parker JD, Schoendorf KC. Fine particulate matter (PM2.5) air pollution and selected causes of postneonatal infant mortality in California. Environ Health Perspect. 2006;114:786–790. doi: 10.1289/ehp.8484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. International Classification of Diseases, 10th Revision. Geneva: World Health Organization; 1993. [Google Scholar]
- Xu X, Sharma RK, Talbott EO, Zborowski JV, Rager J, Arena VC, et al. PM10 air pollution exposure during pregnancy and term low birth weight in Allegheny County, PA, 1994–2000. Int Arch Occup Environ Health. 2011;84(3):251–257. doi: 10.1007/s00420-010-0545-z. [DOI] [PubMed] [Google Scholar]
- Yi O, Kim H, Ha E. Does area level socioeconomic status modify the effects of PM10 on preterm delivery? Environ Res. 2010;110(1):55–61. doi: 10.1016/j.envres.2009.10.004. [DOI] [PubMed] [Google Scholar]