Abstract
In vivo transcranial and noninvasive cavitation detection with blood-brain barrier (BBB) opening in nonhuman primates is hereby reported. The BBB in monkeys was opened transcranically using focused ultrasound (FUS) in conjunction with microbubbles. A passive cavitation detector, confocal with the FUS transducer, was used to identify and monitor the bubble behavior. During sonication, the cavitation spectrum, which was found to be region-, pressure-, and bubble-dependent, provided real-time feedback regarding the opening occurrence and its properties. These findings demonstrate feasibility of transcranial, cavitation-guided BBB opening using FUS and microbubbles in noninvasive human applications.
Most neurological disorders and neurodegenerative diseases, including Alzheimer's and Parkinson's, remain difficult to treat because of the impermeability of the blood-brain barrier (BBB). Mechanical stress induced by the activation of microbubbles in an acoustic field is currently the only noninvasive approach to temporarily open the BBB, without damaging the surrounding tissue.1, 2 To date, BBB opening with focused ultrasound (FUS) has been achieved in different animals, including mice,2 rabbits,3 rats,4 and pigs.5 So far, this method has not been used in primates. It is nontrivial to extend this method to new species due to differences in physiology and anatomy.
It has been shown recently that a passive cavitation detector (PCD) can be used to transcranially acquire the acoustic emissions stemming from the interaction between the microbubble and the brain tissue during BBB opening in mice.6, 7 The feasibility of transcranial cavitation detection in monkeys, however, remains unknown since the thickness and attenuation of the monkey skull is around 2.5 times higher than the murine skull.8 We have recently also demonstrated that bubble size plays an important role in BBB opening.9 Both monodispersed and polydispersed microbubbles are used in this study in order to confirm the effect of bubble size in nonhuman primates.
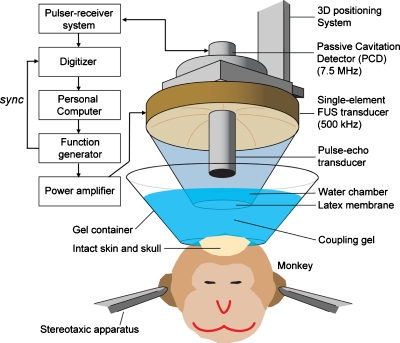
The experimental setup is shown in Fig. 1. A single-element, circular FUS transducer with a hole in its center was driven by a function generator (Agilent Technologies, Palo Alto, CA, USA) through a 50 dB power amplifier (ENI Inc., Rochester, NY, USA). The center frequency, focal depth, outer radius and inner radius of the FUS transducer were 500 kHz, 90 mm, 30 mm, and 11.2 mm, respectively. A single-element PCD (center frequency: 7.5 MHz, focal length: 60 mm, Olympus NDT, Waltham, MA, USA) through the center hole of the FUS transducer. The two transducers were aligned with the later. The PCD was connected to a digitizer (Gage Applied Technologies, Inc., Lachine, QC, Canada) through a 20 dB preamplifier (5800, Olympus NDT, Waltham, MA, USA), and used to passively acquire acoustic emissions from microbubbles.
Figure 1.
Experimental setup of in vivo FUS-induced BBB opening and transcranial cavitation detection in the monkey. A 7.5 MHz pulse-echo transducer served as the PCD.
The experiment was performed on three (n=3) male macaque monkeys with three protocols shown in Table 1. The targeting procedure has been described elsewhere by Marquet et al.10 In the case of monkey 1, the 4–5 μm microbubbles were manufactured in-house and size-isolated using differential centrifugation.11 Polydispersed Definity® microbubbles (Lantheus Medical Imaging, MA, USA) were used in the remaining two animals. The sonication was performed immediately after intravenous (IV) injection of 500 μL microbubbles for all monkeys. All methods were approved by the Institutional Animal Care and Use Committee at Columbia University and the New York State Psychiatric Institute.
Table 1.
Three protocols applied in this study.
| Monkey 1 | Monkey 2 | Monkey 3 | |
|---|---|---|---|
| P-N pressure (MPa) | 0.30, 0.45 | 0.20, 0.25, 0.30 | 0.30, 0.45 |
| Pulse length (cycles) | 5000 | 100 | 5000 |
| Microbubble | 4–5 μm | Definity | Definity |
| PRF (Hz) | 2 | 10 | 2 |
| Sampling rate (MHz) | 50 | 100 | 50 |
| Focus (below skull) | 4 cm | 1 cm | 4 cm |
| Age (yr) | 14 | 11 | 14 |
Magnetic resonance imaging (MRI) at 3.0 T (Philips Medical Systems, Andover, MA, USA) was used to confirm and quantify the BBB opening following the opening. A three-dimensional (3D) spoiled gradient T1-weighted sequence (TR∕TE=20∕1.4 ms; flip angle: 30°; NEX=2; spatial resolution: 500×500 μm2; slice thickness: 1 mm with no interslice gap) was applied after intravenous (IV) injection of gadodiamide (Omniscan®, GE Healthcare, Princeton, NJ, USA). MR imaging was performed 1 h after sonication to confirm the location of the BBB opening. A time-frequency map of the acoustic emission was generated using a customized spectrogram function [eight cycles, i.e., 16 μs, Chebyshev window; 98% overlap; 4096-point Fast Fourier Transform (FFT)] in MATLAB® (2010a, Mathworks, Natick, MA).
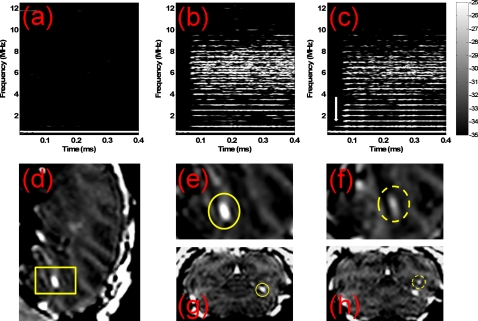
The MR images and the corresponding spectrogram of the first pulse for monkey 1 are depicted in Fig. 2. As a result of the deposition of the MRI contrast agent in the brain tissue after ultrasound exposure, the MR images indicated that the BBB was opened at 0.30 MPa [Figs. 2d, 2e, 2g] and 0.45 MPa [Figs. 2f, 2h] for the 4–5 μm bubbles. The corresponding spectrogram [Figs. 2b, 2c] showed that the broadband response, i.e., the inertial cavitation, occurred at 0.30 and 0.45 MPa. No harmonics were present in the spectrogram at 0.30 MPa without microbubble administration [Fig. 2a], which confirmed our previous findings in mice.7 The spectrogram can also be used to determine the position of the focus. The white arrow in Fig. 2c indicates that the time-point of occurrence of the second harmonic coincides with the travel distance to the skull. Therefore, harmonics higher than the third harmonic and any broadband response are due to microbubble effects [Figs. 2b, 2c].
Figure 2.
The BBB opening in Monkey 1 confirmed by 3D-MRI images. No higher harmonics or broadband response are detected at 0.30 MPa in (a) the spectrogram without microbubble administration. The corresponding spectrogram of the first pulse with microbubbles administration shows that the broadband acoustic emissions are detected at (b) 0.30 MPa and (c) 0.45 MPa. The 3D-MR images confirm that the BBB is opened at (d), (e), and (g)] 0.30 MPa and (f) and (h) 0.45 MPa with inertial cavitation. The yellow box in the sagittal plane in (d) defines a region of interest from which images in (e) and (f) were acquired. The coronal plane with BBB opening is provided at (g) 0.30 MPa and (h) 0.45 MPa. The vertical white arrow in (c) indicates that the time-point of occurrence of the second harmonic coincides with the travel distance to the skull.
Figure 2 shows that the BBB opening and the transcranial detection of cavitation spectra in monkeys are both feasible and that the cavitation spectra may differ among brain regions. The intensities of MRI contrast enhancement in the BBB opening region at 0.30 MPa and 0.45 MPa were 96.71 and 42.07, respectively. These differences may be due to a higher concentration of microbubbles in the specific sonicated region at 0.30 MPa. This would explain both the enhanced MRI contrast and the stronger broadband response.
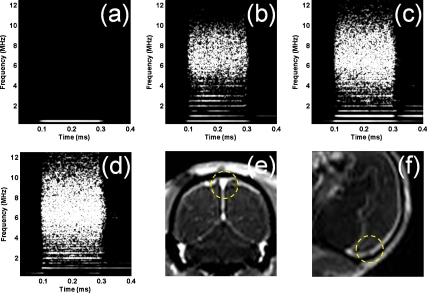
In this study, the BBB was not opened in all cases of inertial cavitation. In the case of monkey 2, given that the medial areas were targeted, the focus included the superior sagittal sinus that, due to the large volume of microbubbles circulating, resulted in a larger amplitude of the cavitation spectrum (Fig. 3). Measuring the cavitation spectrum may, therefore, be helpful to determine whether a large vessel is in the path of the FUS beam and thus predict or avoid its effects on inducing BBB opening. The exact location of the focus in the brain is difficult to predict; second, the exact location of large vessels in the brain relative to the beam is not known a priori. Hence, the relationship between the amplitude of the cavitation spectrum, the area of BBB opening, and the BBB opening threshold will provide valuable additional information regarding the presence of large vessels close to the focus. This information can thus be used to (1) predict whether opening of the BBB was obstructed due to the focal spot proximity to a large vessel and subsequent shielding and (2) adjust the targeting accordingly to achieve BBB opening, i.e., avoid shielding by large vessels.
Figure 3.
(a) The spectrogram without microbubble administration shows that all the harmonics and broadband response are from microbubbles. Spectrograms during FUS sonication with monkey 2 at (b) 0.20 MPa, (c) 0.25 MPa, (d) 0.30 MPa, and MR images with (e) coronal and (f) sagittal planes show that the broadband response is detected at all pressure but no BBB opening is induced (yellow circle).
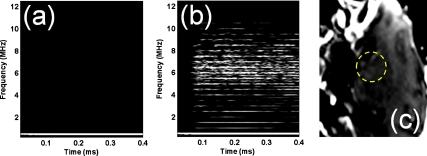
Monkeys 1 and 3 differed only with respect to the size of the microbubbles. BBB opening was only observed with the 4–5 μm microbubbles, not the Definity microbubbles (average diameter of 1.1–3.3 μm), despite the occurrence of inertial cavitation in both cases [Fig. 4b]. We have shown that the 4–5 μm bubbles result in a larger BBB opening region in mice. The capillary diameter in the murine brain is equal to 4–8 μm,12 which is two to eight times larger than the 1–2 μm diameter bubbles. The microbubble is fragmented when the radius expands to a size larger than three times its initial radius at rest (i.e., Rmax∕Rrest>3).13 Therefore, the ratio of expansion of the 4–5 μm diameter bubbles did not need to be as high as for the 1–2 μm diameter bubbles to enter into contact with the capillary wall and induce opening. Based on this finding, which complements our previously reported studies on bubble size in mice,9, 14 it is believed that the bubble size also plays an important role in the BBB opening in primates.
Figure 4.
(a) Spectrogram without microbubble administration shows that all the harmonics and broadband response correspond microbubbles. (b) Spectrogram at 0.45 MPa shows that ultraharmonic and broadband response occurred. The MR image with (c) sagittal plane, however, shows that no BBB opening is induced in the sonicated region (yellow circle).
In conclusion, the noninvasive and transcranial cavitation detection during BBB opening in nonhuman primates was shown in this study. In addition, the MRI contrast enhancement and cavitation response were shown to be region and∕or microbubble-size dependent. Inertial cavitation may not induce BBB opening when the focus overlaps with large vessels such as the superior sagittal sinus. Therefore, this technique might be used for a cavitation-guided BBB opening to better monitor the target of sonication. Further studies will be performed to optimize the application in primates and determine the correlation between the location of BBB opening and the cavitation spectrum.
Acknowledgments
This study was supported in part by the NIH NIHR01EB009041 (E.K.), NSF CAREER 0644713 (E.K.), MH059244 (V.F.), and the Kavli Institute. Additional support came from DFG 819∕1-1 to T.T. The authors appreciate Mark Borden, Ph.D., Department of Mechanical Engineering, University of Colorado and Jameel Feshitan, M.S. Department of Chemical Engineering, for providing monodispersed microbubbles; Fotios Vlachos, Ph.D., and Stephen M. Dashnaw, Department of Biomedical Engineering, Columbia University, for their help in MRI scanning.
References
- Hynynen K., McDannold N., Vykhodtseva N., and Jolesz F. A., Radiology 220, 640 (2001). 10.1148/radiol.2202001804 [DOI] [PubMed] [Google Scholar]
- Choi J. J., Pernot M., Brown T. R., Small S. A., and Konofagou E. E., Phys. Med. Biol. 52, 5509 (2007a). 10.1088/0031-9155/52/18/004 [DOI] [PubMed] [Google Scholar]
- Hynynen K., McDannold N., Martin H., Jolesz F. A., and Vykhodtseva N., Ultrasound Med. Biol. 29, 473 (2003). 10.1016/S0301-5629(02)00741-X [DOI] [PubMed] [Google Scholar]
- Liu H. L., Wai Y. Y., Chen W. S., Chen J. C., Hsu P. H., Wu X. Y., Huang W. C., Yen T. C., and Wang J. J., Ultrasound Med. Biol. 34, 598 (2008). 10.1016/j.ultrasmedbio.2008.01.011 [DOI] [PubMed] [Google Scholar]
- Xie F., Boska M. D., Lof J., Uberti M. G., Tsutsui J. M., and Porter T. R., Ultrasound Med. Biol. 34, 2028 (2008). 10.1016/j.ultrasmedbio.2008.05.004 [DOI] [PubMed] [Google Scholar]
- Tung Y. S., Choi J. J., Baseri B., and Konofagou E. E., Ultrasound Med. Biol. 36, 840 (2010a). 10.1016/j.ultrasmedbio.2010.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung Y. S., Vlachos F., Choi J. J., Deffieux T., Selert K., and Konofagou E. E., Phys. Med. Biol. 55, 6141 (2010b). 10.1088/0031-9155/55/20/007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deffieux T. and Konofagou E. E., IEEE Trans. Ultrason. Ferroelectr. Freq. Control 57, 2637 (2010). 10.1109/TUFFC.2010.1738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung Y. S., Vlachos F., Feshitan J. A., Borden M. A., and Konofagou E. E., “An in vivo study on the mechanism of the interaction between FUS and microbubbles in blood-brain barrier opening in mice,” J. Acoust. Soc. Am. (to be published). [DOI] [PMC free article] [PubMed]
- Marquet F., Tung Y. S., Teichert T., Ferrera V., and Konofagou E. E., “Noninvasive microbubble enhanced FUS BBB opening in vivo feasibility in nonhuman primates,” Plos One (to be published).
- Feshitan J. A., Chen C. C., Kwan J. J., and Borden M. A., J. Colloid Interface Sci. 329, 316 (2009). 10.1016/j.jcis.2008.09.066 [DOI] [PubMed] [Google Scholar]
- Zlokovic B. V., Neuron 57, 178 (2008). 10.1016/j.neuron.2008.01.003 [DOI] [PubMed] [Google Scholar]
- Chomas J. E., Dayton P., Allen J., Morgan K., and Ferrara K. W., IEEE Trans. Ultrason. Ferroelectr. Freq. Control 48, 232 (2001a). 10.1109/58.896136 [DOI] [PubMed] [Google Scholar]
- Choi J. J., Feshitan J. A., Baseri B., Wang S., Tung Y. S., Borden M. A., and Konofagou E. E., IEEE Trans. Biomed. Eng. 57, 145 (2010). 10.1109/TBME.2009.2034533 [DOI] [PMC free article] [PubMed] [Google Scholar]