Abstract
Astrocytes have been shown to release factors that affect various aspects of neuronal development. We have previously shown that the acetylcholine analogue carbachol, by activating muscarinic M3 receptors in rat astrocytes, increases their ability to promote neuritogenesis in hippocampal neurons. This effect was mediated by an increased expression and release by astrocytes of several permissive factors, a most relevant of which was fibronectin. In the present study we investigated the signal transduction pathways involved in these effects of carbachol in astrocytes. Results show that multiple pathways are involved in the effects of carbachol on astrocyte-mediated increases in fibronectin expression and neuritogenesis. These include the phospholipase D pathway, leading to sequential activation of protein kinase C (PKC) ζ, p70S6 kinase and nuclear factor-κB; the phosphoinositide- 3 kinase pathway; and the PKC ε pathway leading to activation of mitogen activated protein kinase. These pathways were shown to mediate the effect of carbachol on neurite outgrowth as well as the increased expression of fibronectin, further substantiating the important role of the latter in astrocyte-mediated neuritogenesis. Interference with these signaling pathways would be expected to impair astrocyte-neurons communication leading to impaired neuronal development.
Keywords: Astrocytes, hippocampal neurons, neuritogenesis, fibronectin, muscarinic receptors, signal transduction
1. Introduction
Neurite outgrowth is a fundamental event in brain development, as well as in regeneration of damaged nervous tissue (Kiryushko et al. 2004). Astrocytes express and/or release factors, such as fibronectin or laminin, which can promote neurite outgrowth (Costa et al. 2002; Tom et al. 2004). Limited information exist on the stimuli that may cause astrocytes to release neurite promoting factors, two of which have been reported to be thyroid hormone (T3) and vasoactive intestinal peptide (Martinez and Gomes, 2002; Trentin et al. 2003; Blondel et al. 2000).
Astrocytes have been shown to express cholinergic muscarinic receptors (Hosli and Hosli, 1993; Guizzetti et al. 1996); the acetylcholine analogue carbachol, by activating muscarinic M3 receptors in cortical or hippocampal astrocytes, promotes neurite outgrowth in hippocampal neurons (Guizzetti et al. 2008). Indeed astrocytes, stimulated for 24 h with carbachol (which is then washed-out), significantly increase neuritogenesis in hippocampal neurons upon a further 24 h co-culture. The main effect is an increase in the length of the longest neurite, identified as the axon by Tau-1 staining, and an increase in minor neurite length. These effects were found to be mediated by the release by carbachol-stimulated astrocytes of various neuritogenic factors, such as the extracellular matrix proteins fibronectin and laminin. Experiments with function-blocking antibodies indicated a primary role for fibronectin in carbachol-induced, astrocyte-mediated neuritogenesis in hippocampal neurons (Guizzetti et al. 2008). By activating muscarinic M3 receptors, carbachol was found to increase the synthesis, expression, and release of fibronectin in astrocytes (Guizzetti et al. 2008; Moore et al. 2009).
Muscarinic M3 receptors have been shown to activate a variety of signal transduction pathways (Caulfield, 1993), and over the years, we have characterized most of such signaling pathways in astroglial cells (Fig. 1). One pathway involves the activation of phospholipase D (PLD), the enzyme which hydrolyzes phosphatidylcholine, thereby generating choline and phosphatidic acid (PA) (Guizzetti and Costa, 2000; Guizzetti et al. 2004). PA activates the atypical protein kinase C ζ(PKC ζ), which in turn phosphorylates p70S6 kinase and nuclear factor κB (NF-κB) (Guizzetti and Costa, 2000; 2002; Guizzetti et al. 2003; 2004). The p70S6 kinase can also be activated by the phosphatidylinositol-3-kinase (PI-3K) pathway, which is also stimulated by muscarinic M3 receptors in astroglial cells (Guizzetti and Costa, 2001). Finally, muscarinic receptors activate phospholipase C, which hydrolyzes phosphatidylinositol bisphosphate to generate inositol 1,4,5-trisphosphate and diacylglycerol (DAG); the former mobilizes calcium from intracellular stores, while the latter activates classical and novel PKCs (Caulfield, 1993). In astrocytes, muscarinic M3 receptors cause an increase in intracellular calcium (Catlin et al. 2000), but do not appear to activate classical, calcium-and DAG-dependent PKCs such as PKC α (Guizzetti et al. 1998). In contrast, the novel, DAG-dependent PKC ε was activated by muscarinic M3 receptors in astroglial cells, and this PKC in turn activates mitogen-activated protein kinase (MAPK) (Yagle et al. 2001).
Fig. 1.
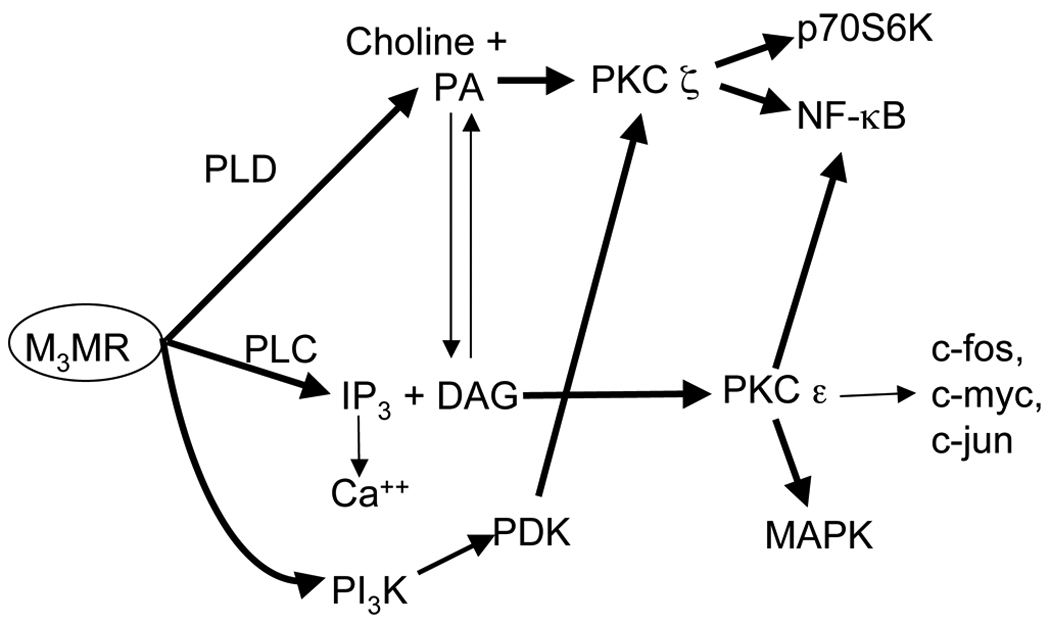
Schematic representation of pathways activated by M3 muscarinic receptors (M3MR) in astroglial cells. Shown in bold are those found in the present study to be involved in the neuritogenic effect of carbachol-stimulated astrocytes on hippocampal neurons. PLC, phospholipase C; PLD, phospholipase D; PA, phosphatidic acid; DAG, diacylglycerol; IP3, inositol 1,4,5-trisphosphate; PKC, protein kinase C; NF-κB, nuclear factor-κB; MAPK, mitogen activated protein kinase; PI-3K, phosphoinositide -3 kinase; PDK, phosphoinositide-dependent kinase.
The aim of the present study was to investigate the signal transduction pathway(s) involved in the neuritogenic action of carbachol in astrocytes, by assessing their effects on neurite outgrowth and fibronectin expression.
2. Material and Methods
2.1. Materials
Alexa fluor-555 and Alexa fluor-594 secondary antibodies, Hoechst 33342, tissue culture medium, serum and B27 supplements were from Invitrogen (Carlsbad, CA). Tissue culture vessels were from Corning (Acton, MA), while glass coverslips were from Fisher Scientific (Federal Way, WA). The anti-βIII-tubulin antibody was from Chemicon International (Temecula, CA). Rapamycin, wortmannin, Gö6976 [12-(2-cyanoethyl)-6,7,12,13-tetrahydro-13-methyl-5-oxo-5H-indolo(2,3-a)pyrrolo(3,4-c)-carbazole], U0126 [1,4-diamino-2,3-dicyano-1,4-bis(2-aminophenylthio)butadiene], PD98059 (2’-amino-3’-metoxyflavone), LY294002 (2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one), GF109203X (bisindolylmaleimide I), and Bay 11-7082 {(E)3-[(4-methylphenyl)sulfonyl]-2-propenenitrile}were purchased from Calbiochem/EMD Biosciences (San Diego, CA). The peptide SN50, its negative control SN50 mock (SN50M), and the myristoylated pseudosubstrates to PKC ζ and PKC α/β were purchased from Enzo Life Sciences (Plymouth Meeting, PA). All other chemicals and antibodies were from Sigma-Aldrich (St. Louis, MO). Time-pregnant Sprague-Dawley rats were purchased from Taconic Farms (Hudson, NY).
2.2. Primary cultures of hippocampal neurons
Hippocampal neurons from E21 rat fetuses were prepared as previously described in detail (Guizzetti et al. 2008; VanDeMark et al. 2009). Briefly, rat hippocampi were removed and dissected into 1 to 3 mm3 pieces in Hank’s balanced salt solution (HBSS), and treated with papain (2 mg/ml in HBSS) in the presence of DNase (40 µg/ml) and MgCl (5 mM) for 3 min at 37°C. The tissue was spun down, and resuspended in Neurobasal medium supplemented with 10% fetal bovine serum (FBS), 30 mM glucose, 3 mM GlutaMAX, 1% gentamycin, 0.5% fungizone, and DNase (40 µg/ml). Tissue was further dissociated by repeated passages through a Pasteur pipette, and cells were filtered through a nylon mesh of 40 µm pore size. Cells were then spun down and resuspended in Neurobasal medium. For quantitative morphological analysis of neurite outgrowth, neurons (1 × 104 cells/coverslip) were plated in glass coverslips, coated overnight with 100 ug/ml poly-D-lysine to support neuron attachment, to which 3–4 beads of paraffin were previously affixed. After 30 min incubation in Neurobasal/B27 medium to allow for neurons to attach, the glass coverslips were inverted in 24-well plates above the astrocyte monolayer, the paraffin drops preventing direct neuron-astrocyte contact.
2.3. Primary culture of astrocytes
Primary cultures of cortical astrocytes from E21 rat fetuses were prepared as previously described in detail (Guizzetti et al. 1996; 2003; 2008). Briefly, rat cortices were dissected mechanically dissociated and incubated with trypsin, followed by trituration, repeated washing and filtering. After counting, cells were plated at a density of 107 cells/75 cm2 tissue culture dish, pre-coated with poly-D-lysine, and grown in DMEM containing 10% FBS, 100 U/ml penicillin, and 100 µg/ml streptomycin at 37°C in 5%CO2/95%O2. After nine days in culture, astrocytes were plated for experiments in 24-well plates for astrocyte-neuron co-culture experiments, or on glass coverslips for immunocytochemistry experiments (2.5 × 105 cells/well or coverslip); three-four days later, cells were serum-deprived for 24 h before treatments.
2.4. Treatments of astrocyte-neuron co-cultures
Astrocytes were treated with carbachol in the absence or presence of inhibitors of various signal transduction pathways (as indicated in Results) for 24 h. The treatments were then washed out, and neurons were added to the astrocyte monolayer for an additional 24 h.
2.5. Quantitative morphological analysis of neurite outgrowth
Morphological analysis has been previously described in detail (Guizzetti et al. 2008). Briefly, at the end of each co-incubation, neurons plated on coverslips were fixed in 4% formaldehyde, permeabilized in 0.2% Triton X-100, labeled with an anti-βIII-tubulin antibody followed by an Alexa fluor-555 secondary antibody, and mounted on microscope slides. Only stage 3 pyramidal neurons were selected for analysis. Stage 3 pyramidal neurons were those with three or more extensions, a cell body diameter of 10 to 15 µm, two to five undifferentiated neurites, and a single axon with length >40 µm (Dotti et al. 1988). Neurons whose processes were intermingled with those of neighboring cells were excluded from the analysis. Neurite length was measured from the point of emergence at the cell body to the tip of each segment. Images were obtained with a NIKON Labphot 2A microscope and projected to a Spot RT Slider cooled CCD digital camera. Quantification of the morphological parameters was carried out using MetaMorph 6.1 analysis software. At least 20 cells per treatment were analyzed in each experiment. As we had previously shown that the longest neurite, identified as the axon by the axonal marker Tau-1, was the most affected by carbachol-treated astrocytes (Guizzetti et al. 2008), axonal length was measured in the present study. All analyses were carried out in a blind fashion.
2.6. Astrocyte immunocytochemistry
At the end of the incubation, astrocytes plated on glass coverslips were fixed in 4% formaldehyde without permeabilization, for immunostaining of fibronectin, as previously described in detail (Guizzetti et al. 2008). After blocking nonspecific binding sites with 10% goat serum, astrocytes were incubated with an anti-fibronectin antibody, followed by an Alexa fluor-594 secondary antibody. Nuclei were stained with Hoechst 33342 (5 ug/ml) for 5 min at room temperature. Coverslips were then mounted on microscope slides and analyzed with a Zeiss Meta confocal microscope (Keck imaging Center, University of Washington). Images from 21 planes 0.5 um thick were acquired per each field; the total stack size was 11 um. Fluorescence intensity was quantified using MetaMorph software as the sum of the integrated intensities for each plane.
2.7. Statistical analysis
Data resulted from at least three separate determinations and were analyzed by one-way analysis of variance followed by the Bonferroni post hoc test, using Kalidagraph software. Data derived from the quantitative morphometric analysis represented the mean of at least 60 determinations and were thus considered normally distributed.
3. Results
Signal transduction pathways known to be activated by muscarinic M3 receptor in astrocytes and shown in Fig. 1, were investigated with regard to their possible involvement in the effects of carbachol in astrocytes leading to a neuritogenic action on hippocampal neurons. As indicated, rat cortical astrocytes were incubated for 24 h in the absence or presence of carbachol (1 mM) and a number of pharmacological inhibitors of the signaling pathways illustrated in Fig. 1. After a complete washout, astrocytes were co-cultured for an additional 24 h with hippocampal neurons plated on a glass coverslip, which were then stained for morphological assessment of neurite outgrowth. Length of the longest extension (the axon, as determined previously by Tau-1 staining; Guizzetti et al. 2008) was measured in all experiments.
We had already shown an involvement of PLD in the effect of carbachol, as 1-butanol and ethanol, two alcohols which undergo a transphosphatidylation reaction thereby inhibiting PLD activity (Seidler et al. 1996), blocked the neuritogenic action of carbachol-stimulated astrocytes (Guizzetti et al. 2010). In contrast, the inactive alcohol analog tert-butanol was devoid of effect (Guizzetti et al. 2010).
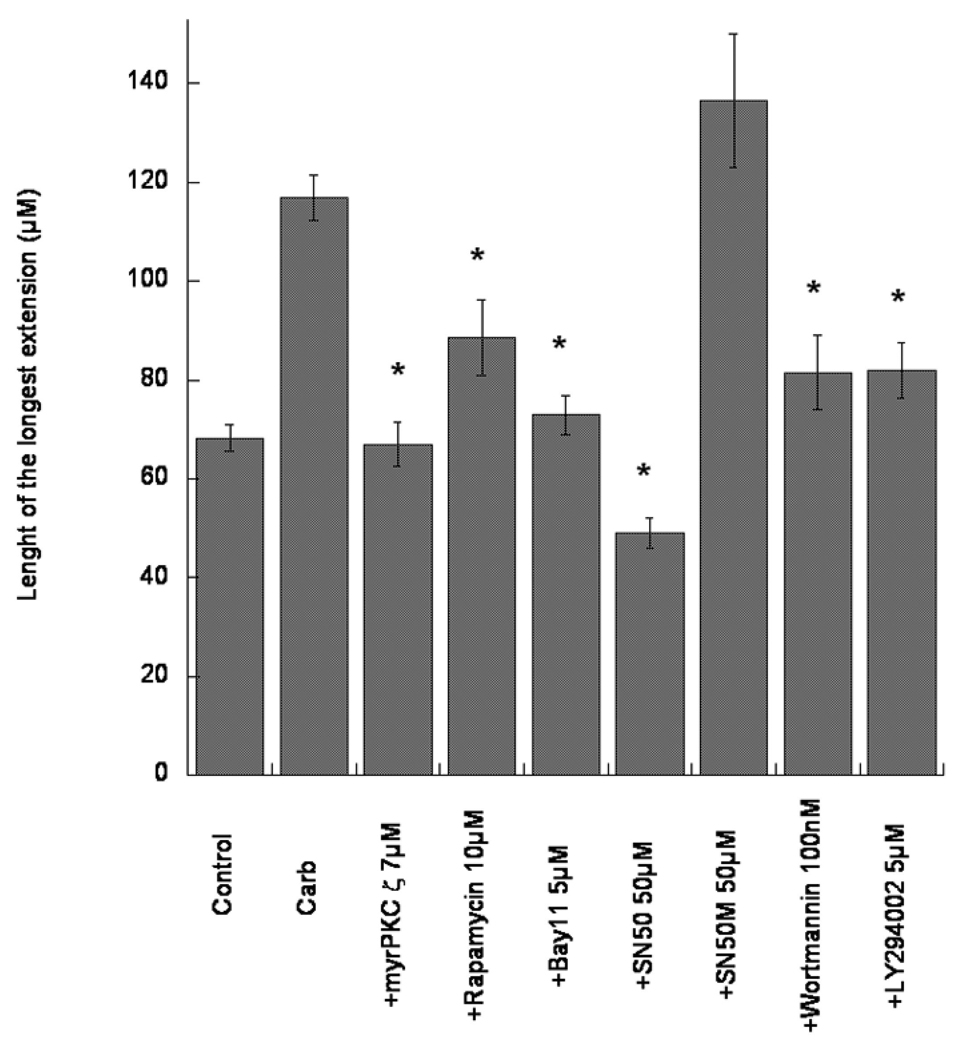
Here we show that downstream events in the PLD pathway are similarly involved in the effect of carbachol. Concentrations of all compounds used to inhibit different signal transduction pathways are indicated in parenthesis, as well as in the Figures, and were based on previously published findings (Guizzetti and Costa 2000; 2001; 2002; Guizzetti et al. 1998; 2002; 2003, 2004). A myristoylated peptide (7 µM) corresponding to the pseudosubstrate of PKC ζ, inhibited the effect of carbachol, and so did rapamycin (10 µM), an inhibitor of the mammalian target for rapamycin (mTOR), an upstream activator of p70S6 kinase (Fig. 2). An involvement of NF-κB in the effect of carbachol is also suggested; Bay-11-7082 (5 µM), an inhibitor of IκB phosphorylation, which prevents its dissociation from NF-κB, as well as SN50 (50 µM), which inhibits the translocation of the p65 subunit of NF-κB to the nucleus (Guizzetti et al. 2003), inhibited neurite outgrowth stimulated by carbachol-treated astrocytes (Fig. 2). In contrast, the mock, inactive peptide inhibitor SN50M (50 µM), which does not affect p65 translocation (Guizzetti et al. 2003), had no effect on carbachol/astrocytes-mediated neuritogenesis (Fig. 2). A contribution by the PI-3K pathways and by Akt, which can also activate p70S6 kinase, is also suggested, as two PI-3K inhibitors, wortmannin (100 nM) and LY294002 (5 µM) inhibited the effect of carbachol (Fig. 2).
Fig. 2.
Effect of carbachol-treated astrocytes on neurite outgrowth in hippocampal neurons. Rat cortical astrocytes were treated with carbachol (Carb; 1 mM) in the absence of presence of inhibitors of various signal transduction pathways (PKC ζ, p70S kinase, NF-kB, PI-3K) at the indicated concentrations, for 24 h. After complete wash-out, astrocytes were co-cultured with rat hippocampal neurons for 24 h. Neurons were then stained with a neuron-specific βIII-tubulin antibody. Pictures were taken with a digital camera attached to a fluorescence microscope. Shown is the quantification of the longest neurite (the axon, as determined by Tau-1 staining; Guizzetti et al. 2008) carried out using MetaMorph software. Results represent the mean (± S.E.) of three separate experiments and 60–80 cells per treatment. * Significantly different from carbachol alone, p<0.01.
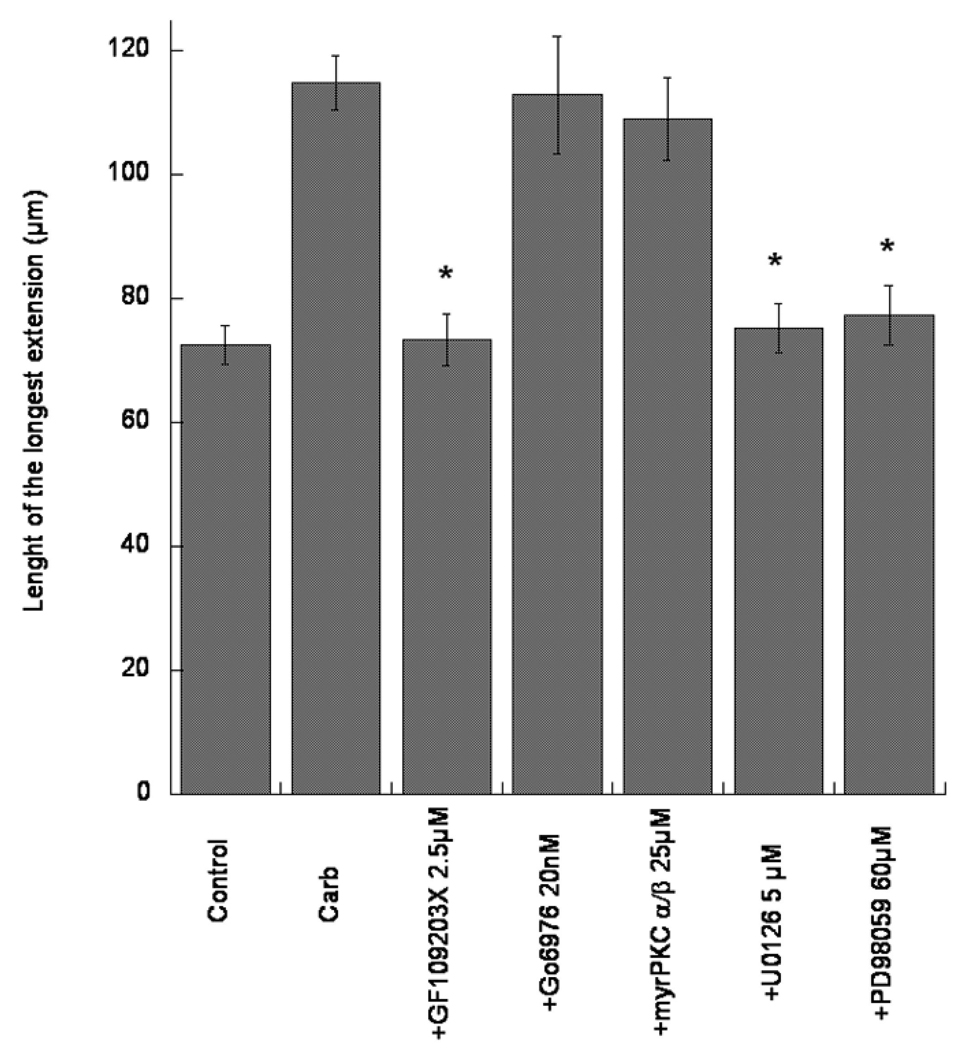
The pan-PKC inhibitor GF109203X (2.5 µM), which inhibits both classical and novel PKCs inhibited the effect of carbachol (Fig. 3). However, two inhibitors of classical PKCs [Gö6976 (20 nM; an inhibitor of PKC α and βI), and a myristoylated pseudosubstrate of PKC α/β (25 µM)] were ineffective at antagonizing the effects of carbachol on neurite outgrowth (Fig. 3). This suggests a direct involvement of novel PKCs, but not of classical PKCs in the effect of carbachol. In agreement with this finding, the novel PKC ε, but not the classical PKC α, is activated by carbachol in astroglial cells (Guizzetti et al. 1998). A downstream effector of PKC ε is Erk 1/2 MAPK, and an involvement of MAPK is indeed suggested by the results obtained with U0126 (5 µM) and PD98059 (60 µM), two inhibitors of MEK (the kinase that directly phosphorylates MAPK) (Fig. 3). None of the inhibitors, when tested alone in astrocytes (in the absence of carbachol) had any effect on neuritogenesis (not shown).
Fig. 3.
Effect of carbachol-treated astrocytes on neurite outgrowth in hippocampal neurons. Rat cortical astrocytes were treated with carbachol (Carb; 1 mM) in the absence or presence of inhibitors of various signal transduction pathways (PKC, MAPK) at the indicated concentrations, for 24 h. After complete wash-out, astrocytes were co-cultured with rat hippocampal neurons for 24 h. Neurons were then stained with a neuron-specific βIII-tubulin antibody. Pictures were taken with a digital camera attached to a fluorescence microscope. Shown is the quantification of the longest neurite (the axon, as determined by Tau-1 staining; Guizzetti et al. 2008) carried out using MetaMorph software. Results represent the mean (± S.E.) of three separate experiments and 60–80 cells per treatment. * Significantly different from carbachol alone, p<0.01.
The effect of carbachol-stimulated astrocytes on neurite outgrowth in hippocampal neurons has been ascribed primarily to an increased synthesis, expression and release of fibronectin (Guizzetti et al. 2008; Moore et al. 2009). Indeed, carbachol (1 mM for 24 h) was shown to increase fibronectin immunofluorescense measured by confocal microscopy, to cause assembly of fibronectin in organized structures, fibrils, to increase fibronectin protein (by Western blot) and mRNA (by quantitative RT-PCR) levels, and to increase levels of fibronectin in the medium (Guizzetti et al. 2008). Additionally, levels of membrane-anchored fibronectin and of fibronectin in the astrocyte medium were still higher than control 24 h after carbachol wash-out (Guizzetti et al. 2008). Finally, a fibronectin function-blocking antibody was shown to antagonize the effect of carbachol-treated astrocytes on neurite outgrowth (Guizzetti et al. 2008), thus substantiating a central role for fibronectin in the observed neuritogenic effects of carbachol-treated astrocytes.
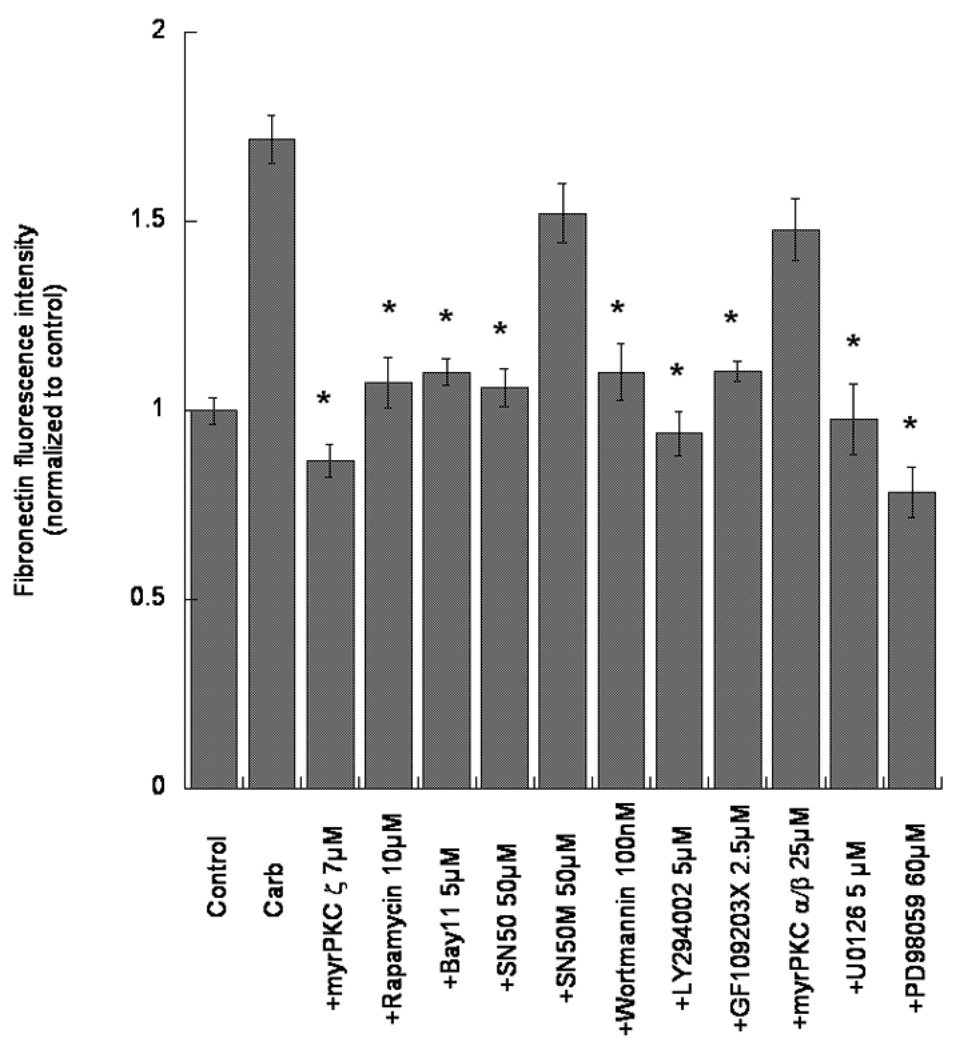
We thus sought to determine whether the same pathways examined in the first part of this study were also involved in carbachol-induced increase in fibronectin expression. As shown in Figg. 4 and 5, all compounds that inhibited neurite outgrowth in hippocampal neurons promoted by carbachol-treated astrocytes, also inhibited carbachol-induced increase in fibronectin expression. The involvement of PLD in this process has also been previously shown (Guizzetti et al. 2010). Astrocytes incubated with inhibitors in the absence of carbachol did not show significant differences in fibronectin immunostaining in comparison with control astrocytes (not shown).
Fig. 4.
Effects of pharmacological inhibitors of signal transduction pathways activated by muscarinic M3 receptors in astrocytes on carbachol-mediated fibronectin expression. Astrocytes were treated with 24 h with carbachol (Carb; 1 mM) in the absence or presence of the indicated inhibitors at the shown concentrations. At the end of the incubation, cells were fixed and the total extracellular fibronectin levels were quantified by immunocytochemistry coupled with confocal microscopy. Bars represent the normalized mean (± S.E.) of triplicate coverslips (10 field per coverslip) from three separate experiments. * Significantly different from carbachol alone, p<0.01.
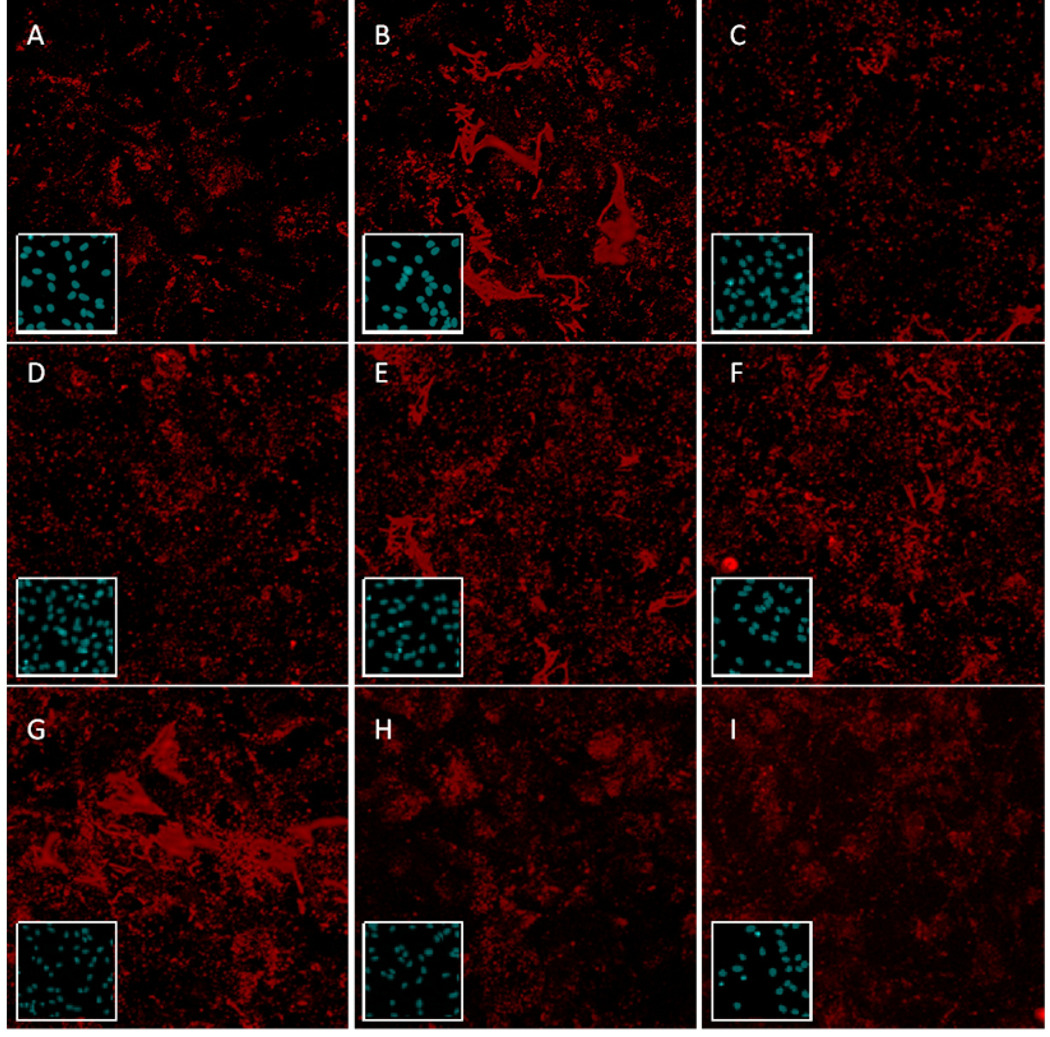
Fig. 5.
Representative images of the effects of pharmacological inhibitors of signal transduction pathways activated by muscarinic M3 receptors in astrocytes on carbachol-mediated fibronectin expression. Astrocytes were treated for 24 h in the absence (A) or presence (B-I) of carbachol (1 mM), together with no inhibitors (A, B), U0126 (5 µM, C), wortmannin (100 nM, D), rapamycin (10 µM, E), a pseudosubstrate for PKC ζ (7 µM, F), a pseudosubstrate for PKC α/β (25 µM, G), SN50 (50 µM, H), or Bay-11-7082 (5 µM, I). At the end of the incubation, cells were fixed and the total extracellular fibronectin levels were visualized by immunocytochemistry coupled with confocal microscopy. Representative images are shown for each inhibitor. Inset shows Hoechst 33342 nuclear staining for each corresponding image.
4. Discussion
Astrocyte-neuronal interactions are emerging as most important events for brain development (Gomes et al. 2001; Ullian et al. 2004). Our previous research had determined that carbachol-treated astrocytes increase neuritogenesis in hippocampal neurons and that this effect was mediated in particular by an increased synthesis, expression and release, on part of astrocytes, of the extracellular matrix protein fibronectin (Guizzetti et al. 2008). Extensive research in the past decade had also shown that activation of muscarinic M3 receptors in astroglial cells stimulates a number of signal transduction pathways (see Fig. 1). Aim of the present investigation was to identify the signal transduction pathways that are involved in the ability of carbachol to promote astrocytes’ neuritogenic action in hippocampal neurons.
The results of this study show an involvement of multiple signaling pathways in the effect of carbachol. With the use of pharmacological inhibitors, tested at concentrations that are believed to have a high, though not complete degree of specificity, we identified upstream and downstream components of muscarinic receptor-activated signaling in astrocytes, that lead to an increased neuritogenesis and an increased expression of fibronectin. We had previously shown an involvement of PLD in such effects (Guizzetti et al. 2010), and we provide further evidence that downstream effectors in this pathway (i.e. PKC ζ, p70S6 kinase, NF-kB; see Fig. 1) are similarly involved. We also show that another signaling pathway leading to PKC ζ activation (the PI-3K/Akt/PDK pathway) also plays a role in the effect of carbachol (Fig. 1).
A third pathway, consisting in activation of PLC with the formation of DAG and activation of PKCs was also partially involved, in that only a novel PKC (PKC ε) was found to play a role, while a classical PKC (PKC α) was not. This was supported by the finding of an involvement of MAPK, which in astrocytes has been shown to be downstream of PKC ε but not of PKC α (Yagle et al. 2001).
A limited number of studies had previously investigated signaling pathway in astrocytes leading to increased neuritogenesis and fibronectin expression. Martinez and Gomes (2002) found that T3, through the release of epidermal growth factor, increased the neuritogenic effect of astrocytes on cerebellar neurons and fibronectin expression in astrocytes. Both effects were shown to be mediated by the MAPK and PI-3K pathways. In another study, guanine derivatives (guanosine -5’-monophosphate and guanosine) were found to increase fibronectin organization (i.e. its assembly in organized structures, fibrils), but not its levels of expression, in astrocytes, by activating the PKC and MAPK pathways (Decker et al. 2007). Interestingly, the lack of increase in fibronectin expression levels upon this treatment did not lead to an increase neuritogenic action of astrocytes on cerebellar neurons (Decker et al. 2007), in contrast to the findings obtained when fibronectin levels are increased (Martinez and Gomes, 2002; Guizzetti et al. 2008; 2010; the present study).
Intracellular signaling pathways involved in an increase of fibronectin expression have been investigated in a number of cell types upon different stimuli. Endothelin-1-stimulated fibronectin expression in human optic nerve head astrocytes was reported to be independent of PKC and MAPK (He et al. 2007), at difference with the findings reported by others (Martinez and Gomes, 2002; the present study). The increase in fibronectin caused in renal proximal tubular epithelial cells by high glucose or high insulin was reported to involve activation of MAPK, PI-3K and p70S6 kinase (Mariappan et al. 2008; Lee et al. 2010). In human fibroblasts, transforming growth factor-β was found to cause a PKC- and MAPK-mediated increase in fibronectin (Mulsow et al. 2005), and an involvement of PKC in fibronectin expression was reported in human mesangial cells upon treatment with anti-DNA antibodies (Yung et al. 2009). In Madin-Darby canine kidney cells, hepatocyte growth factor caused an increase of fibronectin expression that was mediated by MAPK (Liu et al. 2007), while in human hepatic stellate cells acetaldehyde-induced increase in fibronectin expression was shown to involve activation of p70S6 kinase (Svegliati-Baroni et al. 2001). Overall, these studies indicate that increased fibronectin expression caused by various agents is modulated by multiple pathways, as we found in the case of carbachol.
In summary, we found that activation of muscarinic M3 receptors in astrocytes increases their neuritogenic action on hippocampal neurons through an increased expression of fibronectin, substantiating our previous findings (Guizzetti et al. 2008; 2010). Such effects involved activation of several signaling pathways that have been shown to be involved in similar effects induced in a variety of cells by different stimuli. Given the proposed role of astrocytes in fostering neuronal differentiation (Blondel et al. 2000; Martinez and Gomes, 2002; Trentin et al. 2003; Guizzetti et al. 2008), any interference by chemicals with these signal transduction pathways would be expected to cause adverse developmental effects. Indeed, ethanol, a known human developmental neurotoxicant, was found to impair carbachol-induced fibronectin expression in astrocytes, and to inhibit neurite outgrowth in hippocampal neurons produced by carbachol-treated astrocytes, by inhibiting PLD (Guizzetti et al. 2010).
Acknowledgments
We thank Khoi Dao for her assistance in some of the experiments.
Grant support: This work was supported by grants from the National Institutes of Health (R01 AA08154 and P30 ES07033).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Blondel O, Collin C, McCarran WJ, Zhu S, Zamostiano R, Gozes I, Brenneman DE, McKay RDG. A glia-derived signal regulating neuronal differentiation. J. Neurosci. 2000;20:8012–8020. doi: 10.1523/JNEUROSCI.20-21-08012.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buznikov GA. The action of neurotransmitters and related substances on early embryogenesis. Pharmacol. Ther. 1984;25:23–59. doi: 10.1016/0163-7258(84)90023-8. [DOI] [PubMed] [Google Scholar]
- Catlin MC, Kavanagh TJ, Costa. LG. Muscarinic rceptor-induced calcium responses in astroglia. Cytometry. 2000;41:123–132. [PubMed] [Google Scholar]
- Caulfield MP. Muscarinic receptors - characterization, coupling and function. Pharmacol. Ther. 1993;58:319–379. doi: 10.1016/0163-7258(93)90027-b. [DOI] [PubMed] [Google Scholar]
- Costa S, Planchenault T, Charriere-Bertrand C, Mouchel Y, Fages C, Juliano S, Lefrancois T, Barlovatz-Meimon G, Tardy M. Astroglial permissivity of neurite outgrowth in neuron-astrocyte co-cultures depends on regulation of laminin bioavailability. Glia. 2002;37:105–113. doi: 10.1002/glia.10015. [DOI] [PubMed] [Google Scholar]
- Decker H, Francisco SS, Mendes-de-Aguilar CBN, Romao LF, Boeck CR, Trentin AG, Moura-Neto V, Tasca CI. Guanine derivatives modulate extracellular matrix proteins organization and improve neuron-astrocyte co-culture. J. Neurosci. Res. 2007;85:1943–1951. doi: 10.1002/jnr.21332. [DOI] [PubMed] [Google Scholar]
- Dotti CG, Sullivan CA, Banker GA. The establishment of polarity by hippocampal neurons in culture. J. Neurosci. 1988;8:1454–1468. doi: 10.1523/JNEUROSCI.08-04-01454.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes FC, Spohr TC, Martinez R, Moura Nieto V. Cross-talk between neurons and glia: highlights on soluble factors. Braz. J. Med. Biol. Res. 2001;34:611–620. doi: 10.1590/s0100-879x2001000500008. [DOI] [PubMed] [Google Scholar]
- Guizzetti M, Costa LG. Possible role of protein kinase C ζ in muscarinic receptor-induced proliferation of astrocytoma cells. Biochem. Pharmacol. 2000;60:1457–1466. doi: 10.1016/s0006-2952(00)00468-8. [DOI] [PubMed] [Google Scholar]
- Guizzetti M, Costa LG. Activation of phosphatidylinositol 3 kinase by muscarinic receptors in astrocytoma cells. Neuroreport. 2001;12:1639–1642. doi: 10.1097/00001756-200106130-00025. [DOI] [PubMed] [Google Scholar]
- Guizzetti M, Costa LG. Effect of ethanol on protein kinase C ζ and p70S6 kinase activation by carbachol: a possible mechanism for ethanol-induced inhibition on glial cell proliferation. J. Neurochem. 2002;82:38–46. doi: 10.1046/j.1471-4159.2002.00942.x. [DOI] [PubMed] [Google Scholar]
- Guizzetti M, Costa P, Peters J, Costa LG. Acetylcholine as a mitogen: muscarinic receptor-mediated proliferation of rat astrocytes and human astrocytoma cells. Eur. J. Pharmacol. 1996;297:265–273. doi: 10.1016/0014-2999(95)00746-6. [DOI] [PubMed] [Google Scholar]
- Guizzetti M, Wei M, Costa LG. The role of protein kinase C α and ε isozymes in DNA synthesis induced by muscarinic receptors in a glial cell line. Eur. J. Pharmacol. 1998;359:223–233. doi: 10.1016/s0014-2999(98)00620-7. [DOI] [PubMed] [Google Scholar]
- Guizzetti M, Bordi F, Dieguez-Acuna FJ, Vitalone A, Madia F, Woods JS, Costa LG. Nuclear factor kB activation by muscarinic receptors in astroglial cells: effect of ethanol. Neuroscience. 2003;120:941–950. doi: 10.1016/s0306-4522(03)00401-9. [DOI] [PubMed] [Google Scholar]
- Guizzetti M, Thompson BD, Kim Y, VanDeMark K, Costa LG. Role of phospholipase D signaling in ethanol-induced inhibition of carbachol-stimulated DNA synthesis of 1321N1 astrocytoma cells. J. Neurochem. 2004;90:646–653. doi: 10.1111/j.1471-4159.2004.02541.x. [DOI] [PubMed] [Google Scholar]
- Guizzetti M, Moore NH, Giordano G, Costa LG. Modulation of neuritogenesis by astrocyte muscarinic receptors. J. Biol. Chem. 2008;283:31884–31897. doi: 10.1074/jbc.M801316200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guizzetti M, Moore NH, Giordano G, VanDeMark KL, Costa LG. Ethanol inhibits neuritogenesis induced by astrocytic muscarinic receptors. Glia. 2010;58:1395–1406. doi: 10.1002/glia.21015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S, Prasanna G, Yorio T. Endothelin-1-mediated signaling in the expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in astrocytes. Invest. Ophtalmol.Vis. Sci. 2007;48:3737–3745. doi: 10.1167/iovs.06-1138. [DOI] [PubMed] [Google Scholar]
- Hosli E, Hosli L. Receptors for neurotransmitters on astrocytes in the mammalian nervous system. Prog. Neurobiol. 1993;40:477–506. doi: 10.1016/0301-0082(93)90019-o. [DOI] [PubMed] [Google Scholar]
- Kiryushko D, Berezin V, Bock E. Regulators of neurite outgrowth: role of cell adhesion molecules. Ann. N.Y. Acad. Sci. 2004;1014:140–154. doi: 10.1196/annals.1294.015. [DOI] [PubMed] [Google Scholar]
- Lee MJ, Feliers D, Sataranatarajan K, Mariappan MM, Li M, Barnes JL, Choudhury GG, Kasinath BS. Resveratrol ameliorates high glucose-induced protein synthesis in glomerular epithelial cells. Cell. Signal. 2010;22:65–70. doi: 10.1016/j.cellsig.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton SA, Kater SB. Neurotransmitter regulation of neuronal outgrowth, plasticity and survival. Trends Neurosci. 1989;12:265–270. doi: 10.1016/0166-2236(89)90026-x. [DOI] [PubMed] [Google Scholar]
- Liu Z, Greco AJ, Hellman NE, Spector J, Robinson J, Tang OT, Lipshutz JH. Intracellular signaling via ERK/MAPK completes the pathway for tubulogenic fibronectin in MDCK cells. Biochem. Biophys. Res. Comm. 2007;353:793–798. doi: 10.1016/j.bbrc.2006.12.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariappan MM, Shetty M, Sataranatarajan K, Choudhury GG, Kasinath BS. Glycogen synthase kinase 3β is a novel regulator of high glucose- and high insulin-induced extracellular matrix protein syntheis in renal proximal tubular epithelial cells. J. Biol. Chem. 2008;283:30566–30575. doi: 10.1074/jbc.M801756200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez R, Gomes FCA. Neuritogenesis induced by tyroid hormone-treated astrocytes is mediated by epidermal growth factor/mitogen-activated protein kinase-phosphatidylinositol 3-kinase pathways and involves modulation of extracellular matrix proteins. J. Biol. Chem. 2002;277:49311–49318. doi: 10.1074/jbc.M209284200. [DOI] [PubMed] [Google Scholar]
- Moore NH, Costa LG, Shaffer SA, Goodlett DR, Guizzetti M. Shotgun proteomics implicates extracellular matrix proteins and protease systems in neuronal development induced by astrocyte cholinergic stimulation. J. Neurochem. 2009;108:891–908. doi: 10.1111/j.1471-4159.2008.05836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulsow JJW, Watson RWG, Fitzpatrick JM, O’Connel PR. Transforming growth factor-β promotes pro-fibrotic behavior by serosal fibroblasts via PKC and Erk 1/2 mitogen activated protein kinase cell signaling. Ann. Surg. 2005;242:880–889. doi: 10.1097/01.sla.0000189606.58343.cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler L, Kaszkin M, Kinzel V. Primary alcohols and phosphatidylcholine metabolism in rat brain synaptosomal membranes via phospholipase D. Pharmacol. Toxicol. 1996;78:249–253. doi: 10.1111/j.1600-0773.1996.tb00213.x. [DOI] [PubMed] [Google Scholar]
- Svegliati-Baroni G, Ridolfi F, Di Sario A, Saccomanno S, Bendia E, Benedetti A, Greenwel P. Intracellular signaling pathways involved in acetaldehyde-induced collagen and fibronectin gene expression in human hepatic stellate cells. Hepatology. 2001;33:1130–1140. doi: 10.1053/jhep.2001.23788. [DOI] [PubMed] [Google Scholar]
- Tom VJ, Doller CM, Malouf AT, Silver J. Astrocyte-associated fibronectin is critical for axonal regeneration in adult white matter. J. Neurosci. 2004;24:9282–9290. doi: 10.1523/JNEUROSCI.2120-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trentin AG, Aguiar CBNM, Garcez RC, Alvarez-Silva M. Thyroid hormone modulates the extracellular matrix organization and expression in cerebellar astrocytes: effect on astrocyte adhesion. Glia. 2003;42:359–369. doi: 10.1002/glia.10228. [DOI] [PubMed] [Google Scholar]
- Ullian EM, Christopherson KS, Barres BA. Role for glia in synaptogenesis. Glia. 2004;47:209–216. doi: 10.1002/glia.20082. [DOI] [PubMed] [Google Scholar]
- VanDeMark KL, Guizzetti M, Giordano G, Costa LG. The activation of M1 muscarinic receptor signaling induces differentiation in pyramidal hippocampal neurons. J. Pharmacol. Exp. Ther. 2009;329:532–542. doi: 10.1124/jpet.108.150128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagle K, Lu H, Guizzetti M, Moeller T, Costa LG. Activation of mitogen-activated protein kinase by muscarinic receptors in astroglial cells: role in DNA synthesis and effects of ethanol. Glia. 2001;35:111–120. doi: 10.1002/glia.1076. [DOI] [PubMed] [Google Scholar]
- Yung S, Zhang Q, Zhang CZ, Chan KW, Lui SL, Chan TM. Anti-DNA antibody induction of protein kinase C phosphorylation and fibronectin synthesis in human and murine lupus and the effect of mycophenolic acid. Arthr. Rheumat. 2009;60:2071–2082. doi: 10.1002/art.24573. [DOI] [PubMed] [Google Scholar]