Summary
Dampening of insulin/insulin like growth factor-1 (IGF1) signaling results in extension of lifespan in invertebrate as well as murine models. The impact of this evolutionarily conserved pathway on modulation of human lifespan remains unclear. We previously identified two IGF1R mutations (Ala-37-Thr and Arg-407-His) that are enriched in Ashkenazi Jewish centenarians as compared to younger controls and are associated with reduced activity of the IGF1 receptor as measured in immortalized lymphocytes. To determine whether these human longevity-associated IGF1R mutations affect IGF1 signaling, we engineered mouse embryonic fibroblasts (MEFs) expressing the different human IGF1R variants in a mouse Igf1r null background. The results indicate that MEFs expressing the human longevity-associated IGF1R mutations attenuated IGF1 signaling, as demonstrated by significant reduction in phosphorylation of both IGF1R and AKT after IGF1 treatment, in comparisons to MEFs expressing the wild type IGF1R. The impaired IGF1 signaling caused by the IGF1R mutations resulted in reduced induction of the major IGF1-activated genes in MEFs, including EGR1, mCSF, IL3Rα, and TDAG51. Furthermore, the IGF1R mutations caused a delay in cell cycle progression after IGF1 treatment, indicating a dysfunctional physiological response to a cell proliferation signal. These results demonstrate that the human longevity-associated IGF1R variants are reduced-function mutations, implying that dampening of IGF1 signaling may be a longevity mechanism in humans.
Keywords: human longevity, IGF1 signaling, genetic variation, gene expression
In recent years, there have been significant advances in our understanding of the pathways that modulate lifespan. The best characterized of all is the insulin/insulin like growth factor-1 signaling (IIS) pathway. Complete or partial loss-of-function mutations in genes encoding components of the IIS pathway result in extension of lifespan in yeast, worms, flies and mice (Kenyon 2010). This remarkable conservation throughout evolution suggests that altered IIS may also influence human lifespan. Recently, common genetic variations in the IIS candidate loci, including FOXO3A (Willcox et al. 2008; Flachsbart et al. 2009; Pawlikowska et al. 2009; Soerensen et al. 2010) and AKT1 (Pawlikowska et al. 2009), have been associated with human longevity. However, association studies typically leave open the question of whether an associated genetic variant is functionally important or can serve only as a genetic marker with the functional locus co-inherited on the polymorphic allele.
We previously identified two functionally significant IGF1R mutations (A37T and R407H) that are rare but enriched in Ashkenazi Jewish centenarians as compared to younger controls and are associated with reduced activity of the IGF1 receptor as measured in immortalized lymphocytes (Suh et al. 2008). Since we do not have complete genotype information of the IGF1R mutation carriers, functional information obtained from immortalized lymphocytes from these subjects is correlational and cannot establish cause-effect relationship between the mutations and the associated phenotypes, e.g. longevity. Indeed, it is formally possible that the association of these IGF1R mutants and centenarian status might reflect variations of linked alleles, in this or another locus, that were not evaluated in our study.
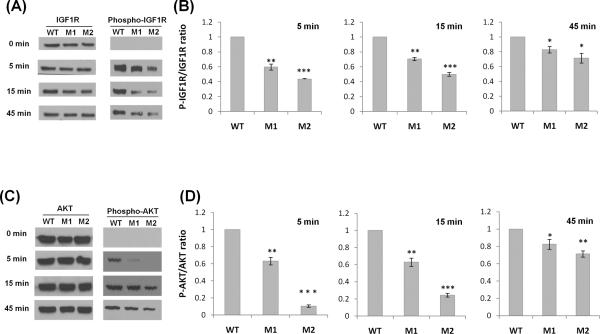
To determine whether these human longevity-associated IGF1R mutations affect IGF1 signaling, we engineered mouse embryonic fibroblasts (MEFs) expressing the different human IGF1R variants in a mouse Igf1r null background. After lentiviral transfection of Igf1r−/− MEFs (Sell et al. 1994), we tested the differences in IGF1 signaling. The results show that, compared to MEFs expressing the wild type (WT) IGF1R, MEFs expressing the human longevity-associated IGF1R variants, A37T (M1) and R407H (M2), showed attenuation of IGF1 signaling, as demonstrated by significant reduction in the phosphorylation of both IGF1R and AKT after IGF1 treatment (Fig. 1). Of note, IGF1R expression at the protein (Fig. 1A) and RNA (data not shown) levels was similar in the engineered MEFs for all variants.
Fig. 1.
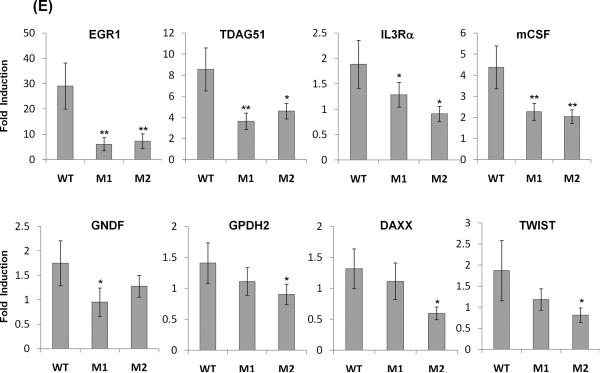
The longevity-associated IGF1R mutations cause attenuated IIS signaling and reduced expression of IGF1-activated genes in Igf1r−/− MEFs. Modeling of human IGF1R variants expressed from lentivirus in Igf1r−/− MEFs; WT: wild type, M1: A37T, M2: R407H. Cells were serum-starved for 12 h and treated with 100 nM IGF1 for 5, 15, or 45 min interval. (A and C) Immunoblot analysis using antibodies against IGF1R, phospho-IGF1R, AKT and phospho-AKT at 0, 5, 15, and 45 min after IGF1 treatment in representative immunoblots. (B and D) Phosphorylation levels were assessed in three independent experiments and differences were tested by a 2-tailed unequal-variance t test: *P<0.05, **P<0.01, *** P<0.001. Error bars represent SD. (E) The engineered MEFs expressing different human IGF1R variants were serum-starved for 12 hrs and treated with 100 nM IGF1 for 15 min. Cells were returned to serum free media for 75 min prior to RNA extraction. Transcript levels were measured by quantitative real-time PCR and normalized against GAPDH with the mean expression level as calibrator (ΔΔCT). Fold change was based on three independent experiments performed in triplicate and differences were tested by a t test: *P<0.05, **P<0.01. Error bars represent SD.
Once activated, IGF1R elicits the activation of a cascade of intracellular proteins leading to the regulation of gene expression, cell proliferation or cell death (Kenyon 2010). To test if the longevity-associated IGF1R mutations affect gene expression, we measured the transcript levels of 8 genes involved in mitogenesis, apoptosis and differentiation processes (Cao et al. 1990), which are known to be up-regulated by IGF1 treatment (Dupont et al. 2001). We found that as compared to the WT, both A37T (M1) and R407H (M2) IGF1R mutations significantly reduced expression of early growth response 1 (EGR1), T cell death-associated gene 51 (TDAG51), interleukin 3 receptor alpha (IL3RA) and macrophage colony stimulating factor 1 (mCSF) in response to IGF1 treatment (Fig. 1E). Similarly the expression of glial cell-derived neurotropic factor (GDNF) is attenuated in A37T (M1) cells, and expression of glycerol phosphate dehydrogenase 2 (GPDH2), the death domain-associated protein (DAXX), and TWIST are all attenuated in cells expressing the R407H (M2) mutation (Fig. 1E).
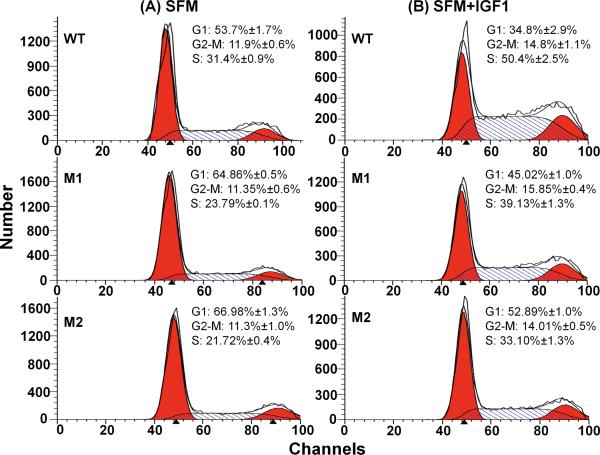
To investigate the functional consequences of differential IGF1 signaling and downstream gene expression caused by the longevity-associated IGF1R mutations, we measured cell cycle profiles. Fig. 2 shows the results of FACS analysis of MEFs expressing different human IGF1R alleles either in serum free media (SFM) or in SFM supplemented with IGFI. The cells expressing the longevity-associated IGF1R mutations clearly accumulated in the G1 phase of the cell cycle in SFM; 64.9% for M1 and 67% for M2 as compared to 53.7% for WT (both P<0.05, Fig. 2A). After the cells were stimulated to proliferate by IGFI (Fig. 2B), 50.4% of WT cells were in S phase vs. 39.1% of M1 cells and 33.9% of M2 cells (both P<0.05), showing that the longevity-associated IGF1R mutations cause a defect in cell cycle progression.
Fig. 2.
Cell cycle profiles of MEFs expressing the different human IGF1R in Igf1r− MEFs; WT: wild type, M1: A37T, M2: R407H. (A) Control incubation in serum free medium (SFM). (B) Cells cultured in serum free medium for 24 h followed by 24 h incubation with 10nM IGF1 (SFM+IGF1). Cells were fixed, permeablized, and stained with propidium iodide to measure DNA content by fluorescence-activated cell sorting (FACS) analysis. The mean percentages of cells ±SEM in each phase of the cell cycle are indicated with representative profiles from WT, M1, and M2 mutations, n=3.
In summary, we have demonstrated that the human longevity-associated IGF1R mutations cause functional impairments in IGF1 signaling, regulated gene expression, and cell cycle progression. The mechanistic parallels between the effects of IIS mutations in model organisms and those produced by longevity-associated IGF1R mutations in humans suggest that evolutionary conservation of IIS reduction and unusual longevity includes humans.
Supplementary Material
Acknowledgments
We thank Dr. Renato Baserga (Kimmel Cancer Center, PA, USA) for the full length human IGF1R cDNA and mouse Igf1r−mouse embryonic fibroblasts. This work was funded by NIH grant AG024391, AG027734, and AG17242. CT is a recipient of Ellison/AFAR postdoctoral fellowship.
References
- Cao X, Koski R, Gashler A, McKiernan M, Morris C, Gaffney R, Hay R, Sukhatme V. Identification and characterization of the Egr-1 gene product, a DNA-binding zinc finger protein induced by differentiation and growth signals. Mol Cell Biol. 1990;10:1931–1939. doi: 10.1128/mcb.10.5.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont J, Khan J, Qu B, Metzler P, Helman L, LeRoith D. Insulin and IGF-1 induce different patterns of gene expression in mouse fibroblast NIH-3T3 cells: identification by cDNA microarray analysis. Endocrinology. 2001;142:4969–4975. doi: 10.1210/endo.142.11.8476. [DOI] [PubMed] [Google Scholar]
- Flachsbart F, Caliebe A, Kleindorp R, Blanche H, von Eller-Eberstein H, Nikolaus S, Schreiber S, Nebel A. Association of FOXO3A variation with human longevity confirmed in German centenarians. Proc Natl Acad Sci U S A. 2009;106:2700–2705. doi: 10.1073/pnas.0809594106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon CJ. The genetics of ageing. Nature. 2010;464:504–512. doi: 10.1038/nature08980. [DOI] [PubMed] [Google Scholar]
- Pawlikowska L, Hu D, Huntsman S, Sung A, Chu C, Chen J, Joyner AH, Schork NJ, Hsueh WC, Reiner AP, Psaty BM, Atzmon G, Barzilai N, Cummings SR, Browner WS, Kwok PY, Ziv E. Association of common genetic variation in the insulin/IGF1 signaling pathway with human longevity. Aging Cell. 2009;8:460–472. doi: 10.1111/j.1474-9726.2009.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sell C, Dumenil G, Deveaud C, Miura M, Coppola D, DeAngelis T, Rubin R, Efstratiadis A, Baserga R. Effect of a null mutation of the insulin-like growth factor I receptor gene on growth and transformation of mouse embryo fibroblasts. Mol Cell Biol. 1994;14:3604–3612. doi: 10.1128/mcb.14.6.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soerensen M, Dato S, Christensen K, McGue M, Stevnsner T, Bohr VA, Christiansen L. Replication of an association of variation in the FOXO3A gene with human longevity using both case-control and longitudinal data. Aging Cell. 2010;9:1010–1017. doi: 10.1111/j.1474-9726.2010.00627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh Y, Atzmon G, Cho M, Hwang D, Liu B, Leahy D, Barzilai N, Cohen P. Functionally significant insulin-like growth factor I receptor mutations in centenarians. Proc Natl Acad Sci U S A. 2008;105:3438–3442. doi: 10.1073/pnas.0705467105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcox B, Donlon T, He Q, Chen R, Grove J, Yano K, Masaki K, Willcox D, Rodriguez B, Curb J. FOXO3A genotype is strongly associated with human longevity. Proc Natl Acad Sci U S A. 2008;105:13987–13992. doi: 10.1073/pnas.0801030105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.