Abstract
Cryotherapy has emerged as a primary treatment option for prostate cancer(CaP); however, incomplete ablation in the periphery of the cryogenic lesion can lead to recurrence. Accordingly, we investigated the use of a nontoxic adjunctive agent, Vitamin D3, with cryotherapy to sensitize CaP to low temperature induced, non-ice rupture related cell death. Vitamin D3 (calcitriol) has been identified as a possible adjunct in the treatment of cancer due to its anti-proliferative and anti-tumorigenic properties. This study aimed to identify the cellular responses and molecular pathways activated when vitamin D3 (calcitriol) is combined with cryotherapy in a murine prostate cancer model. Single freeze-thaw events above −15°C had little effect on cancer cell viability; however, pre-treatment with calcitriol in conjunction with cryo significantly increased cell death. The −15°C calcitriol combination increased cell death to 55% following a single freeze, compared to negligible cell loss by freezing or calcitriol alone. Repeat cryo-combination yielded90% cell death, compared to 65% in dual freeze-only cycles. Western blot analysis following calcitriol cryosensitization regimes confirmed the activation of apoptosis. Specifically, pro-apoptotic Bid and pro-caspase–3 were found to decrease at 1h following combination treatment, indicating cleavage to the active forms. A parallel in vivo study confirmed the increased cell death when combining cryotherapy with calcitriol pre-treatment. The development of an adjunctive therapy combining calcitriol and cryotherapy represents a potentially highly effective, less toxic, minimally invasive treatment option. These results suggest a role for calcitriol and cryo as a combinatorial treatment for CaP with the potential for clinical translation.
Keywords: Prostate cancer, Vitamin D, cryosurgery
Introduction
In 2010, an estimated 217,730 men will be diagnosed with prostate cancer(CaP)in the U.S.1 While the current treatment options available offer favorable cure rates, the development of new minimally invasive treatments are vital to improving patient quality of life. In 2008 the AUA issued a Best Practices Statement on cryosurgery for the treatment of CaP, indicating its emergence as a mainstream treatment option.2 A report by Cohen et al. on 10 year patient outcome has shown cryoablation to be equivalent to that of other therapies used to treat CaP.3
Advances in cryosurgery over the last 10–15 years have greatly increased treatment efficacy and decreased negative side effects. These advances include improvements in cryotechnology, the use of urethral warming devices to minimize urethral sloughing, computer-aided ultrasound to guide the ablation process, as well as thermocouples to monitor temperature.2,4 Cryotherapy, as with other thermal techniques, does not create a uniform treatment zone; rather, there is a gradient of temperature, from the center where the temperature is at it lowest, with elevated temperatures toward the periphery.5 In the center cells are typically destroyed due to physical ice rupture and post-thaw necrosis. However, in the periphery it has been shown that other forms of cell death are dominant, including apoptosis.6–8 Local recurrence is thought to be a result of incomplete cell death in the periphery of the freeze zone.9 Chemotherapeutic drugs have been used as combination treatments with cryotherapy to increase the efficacy of tumor cell death.6–9 Many of these drugs, such as Taxotere and 5–Fluorouracil, have been shown to sensitize CaP to freeze-induced apoptosis.5–7 In vivo, however, chemotherapeutic drugs present toxic side effects as well as concern over drug resistance. Accordingly, the need for the identification of novel, non-toxic treatments for CaP is essential. For this reason, researchers continue to investigate possible adjuvants (cryosensitizers)that when combined with low temperature ablation can increase the level of cell death in the periphery of the cryolesion.
One compound which has shown promise as a cryosensitizer is Vitamin D3(VD3).10,11 Vitamin D circulates in the body bound to a Vitamin D binding protein (VDBP)and is converted to a biologically active form through two distinct hydroxylation reactions, forming first the precursor protein 25(OH)D3, followed by the active metabolite, 1,25(OH)2D3 (calcitriol).12 Research has indicated that at least ten organs in the body, including the prostate, have the ability to convert the precursor forms into the active metabolite, extending calcitriol production into the realm of paracrine signaling.13 Calcitriol is a ligand for the Vitamin D Receptor (VDR), which is a member of the nuclear receptor super family and has a direct role in gene transcription through its binding to nuclear vitamin D response elements(VDREs)on the DNA.14 VDRE’s are present on many different genes, and therefore the effects that calcitriol elicits on a cell can be diverse.
VD3 is well known for its role in calcium and bone homeostasis; however there is a wide range of emerging scientific literature documenting additional properties.15 Research has suggested that there are both nuclear and cytosolic VDRs, and that each may play a different role in vitamin D signaling.16 Calcitriol has also been implicated in the reduction of inflammation, which is critical to the initiation and progression of CaP, as tumor tissues can overexpress certain pro-inflammatory molecules leading to cancer progression.17 This is important in the early stages of CaP (i.e. prostatic intraepithelial neoplasia (PIN)), which represent cell transformation between normal epithelial cells and CaPcells.18 More specifically, research has shown that prostate epithelial cells possess both the VDR and the necessary enzymes to convert 25(OH)D3 to 1,25(OH)2D3 and therefore can produce the biologically active metabolite of Vitamin D.19 However, other studies have demonstrated that as CaP progresses, these cells lose 1α-hydroxylase activity, the enzyme necessary for this conversion.20 Therefore, CaP progression and development is associated with the loss of the prostatic epithelial cells to produce calcitriol, and as such calcitriol supplementation may help to inhibit the progression of the disease. Calcitriol has also been found to activate apoptosis in various cancers by increasing calcium levels as well as altering mitochondrial signaling.21–24
A recent parallel study by Kimura et al. described the use of VD3 as a cryosensitizer in an in vivo murine CaP model.10 Their results demonstrated that intratumoral calcitriol injection into orthotopically grown tumors increased the area of necrosis and decreased the number of proliferating cells within the cryolesion after freezing.10 Further, Baust et al. have also reported on the beneficial effects of VD3 in combination with freezing in an in vitro cell model.11 These studies indicate that Vitamin D and its downstream signaling molecules may represent a potential novel approach for prostate cancer treatment. Though there is sufficient evidence to suggest that VD3 and related genes play a role in the tumor progression of many cancers, the molecular mechanisms by which this occurs are not well understood. Given the complex properties of VD3 coupled with reports on the use of VD3 as an adjunctive agent,9,11 we investigated the utilization and mechanisms of action of VD3 as a cryosensitizer in a short term, direct exposure model in conjunction with a mild freezing event, focusing analysis on both ablative capacity and the signaling mechanisms involved. This short term direct exposure model was utilized in both in vitro and in vivo studies to further assess the potential of minimally invasive, non systemic, short term cryosensitization strategies, thereby reducing the potential of clinical complications such as long term dose delivery and toxicity. Data presented herein demonstrate that VD3 is a highly effective cryosensitizer yielding synergistic CaP cell death at −15°C through the up regulation of the apoptotic cascade.
Materials and methods
Cell Culture
The murine prostate cancer cell line, RM-9, was obtained from Dr. Vladimir Mouraviev (Duke University Medical School, Durham NC)and originally derived from the Mouse Prostate Reconstitution (MPR) model system(Baylor College of Medicine).25 Cultures were maintained in Falcon T-75 flasks at 37°C, 5% CO2 in Dulbecco’s Modified Eagle’s Medium (Caisson Labs, North Logan UT) with 10% Fetal Bovine Serum (Caisson Labs), 1% Penicillin/Streptomycin (Mediatech, Manassas VA), and 10mM HEPES (Calbiochem, Gibbstown NJ)with media exchange every 2 days. Experimental subcultures were plated into tissue cultureware at 5×106/cm2 2 days prior to use.
Freezing Protocol
Costar 8-well strips with 100μLmedium/wellor 60mm Falcon dishes with 4mL medium/dish were placed on a pre-cooled block within a circulating temperature controlled bath at a pre-set target temperature. Sample temperature was monitored with a type-T thermocouple (Omega, Stamford CT) and ice nucleation was initiated by liquid nitrogen vapor(crystallized water vapor)when samples reached −2°C(±1°C). Samples were held for ten minutes at target temperatures (−10, −15, or −20°C)and then allowed to thaw at room temperature(RT) (ten minutes)before recovery incubation at 37°C or initiation of a repeat freeze-thaw cycle.
Vitamin D3 treatment
1,25α dihydroxyvitamin D3 (Calcitriol, Calbiochem) was reconstituted in ethanol and diluted to final concentrations in DMEM immediately prior to application. Calcitriol dilutions [50, 75, 100, 150nM]were applied to samples 24h prior to freezing and remained on samples during the first 24h of recovery (37°C).
Cell Viability
Cell viability was assessed using the metabolic activity assay alamarBlue™ (Invitrogen, Carlsbad CA) diluted 1:20 in Hanks Balanced Salt Solution (Mediatech). Media was aspirated and replaced with 100μl/well alamarBlue™ for 1h incubation at 37°Cand subsequently analyzed with a Tecan SPECTRAFluorPlus plate reader(TECAN Austria GmbH) at excitation λ = 530nm and emission λ = 590 nm. Media was returned to samples and incubated under standard conditions. Assessment was repeated for 3 days of recovery.
Microscopy
Samples were treated with calcitriol for 24 hours and then frozen to −15°C as described. Phase contrast images were taken24 hours post-freeze using Zeiss Axiovert imaging software using 10x magnification. To confirm the role of apoptosis, parallel samples were treated as described, allowed to recover for 1, 8, and 24 hours, then were labeled with tri-stain fluorescent probes Hoechst(living cells, 0.06 μg μl−1), propidium iodide(necrotic cells, 0.007 μg μl−1), and YO-PRO-1 (apoptotic cells, 0.8μM) (blue, red, and green fluorescence, respectively) (Molecular Probes, Eugene OR). Probes were incubated for 20 minutes in the dark before imaging with Zeiss Axiovert software at 10x magnification.
Western Blot
Total protein was isolated using RIPA (Radioimmunoprecipitation Assay) buffer with protease inhibitor cocktail and quantified using a BCA (bicinchoninic acid) protein assay kit (Pierce, Thermo Scientific, Rockford IL). Equal amounts of protein [30–50ug]were separated on 12% polyacrylamide gels for 50 minutes at 200Vand then transferred to PVDF membranes using a semi-dry transfer system (Bio-Rad, Hercules CA) for 30 minutes at 15V. Membranes were blocked with NAP (Non-Animal Protein) buffer (G-Biosciences, Maryland Heights MO) for 1 hour at RT. Membranes were exposed to primary antibody overnight at 4°C in a humidity chamber; antibodies were diluted in a 1:2 solution of NAP/TBST (Tris-buffered saline with 0.1% Tween-20). Primary antibodies included Bid (1:250), Caspase-3(1:500), Caspase-9 (1:200), Akt (1:500), and Parp (1:500) (mu reactive, CST, Danvers MA). Membranes were then washed in TBST before 1h RT secondary antibody hybridization in 1:2 NAP/TBST with Goat anti-rabbit-HRP(1:10,000, Pierce) and StrepTactin-HRP (1:40,000, Bio-Rad) for molecular weight marker visualization. Membranes were then washed before detection using the LumiGLO/Peroxidase chemiluminescent detection kit (CST) on a FujiFilm LAS-3000 system. Molecular weight was confirmed by migration of the Western C-HRP protein marker (Bio-Rad).
Data Analysis
Relative fluorescent units were converted to percent survival as compared to 37°C pre-treatment controls. Viability percentages are expressed as percent ± SEM. Experiments were repeated a minimum of three times. One representative experiment was chosen and presented in Figures 1 and 2; each condition had an n of 7. Statistical analysis of significance was performed by single factor ANOVA; statistically significant when p<0.05. Densitometry was performed on western blots using Fuji Film Multi Gauge software V2.3 and is described as percent of control with background values subtracted.
Figure 1. Cell Viability of RM-9 cells in Response to Low Temperature Exposure.
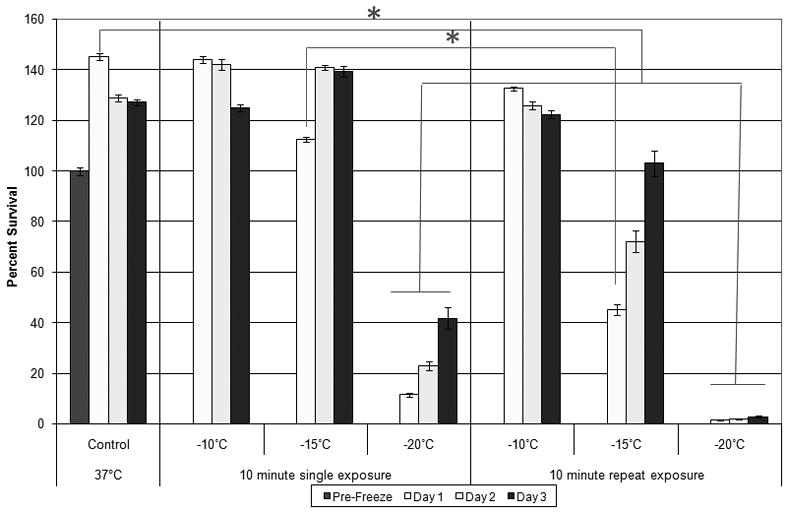
Each freeze injury was imposed for 10 minutes total time, followed by 10 minute passive thawing at RT prior to recovery incubation at 37°C. Repeat freeze exposure was conducted immediately following the 10 minute passive thaw. Viability was assessed 1, 2, and 3 days post-freeze. Percent survival based on pre-freeze controls. Significant cell death did not occur following a single freeze event to −10°C or −15°C. Repeated 10 minute, −15°C freezes results in a loss of over half of the starting cell population. Exposure to cryotreatment at −20°C significantly decreases cell survival (ANOVA; p<0.05).
Figure 2. Cell Viability of RM-9 cells in Response to Calcitriol and Low Temperature Exposure.
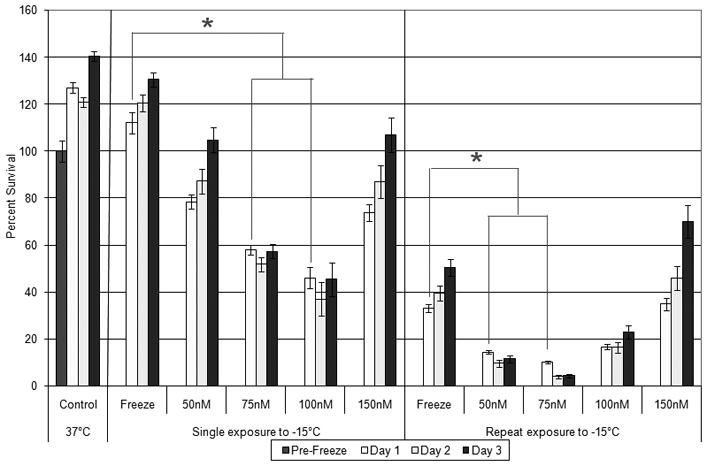
Calcitriol [50 to 150nM] was applied 24 hours prior to subjecting RM-9 cells to single or repeated −15°C, 10 minute cryoexposures. Viability was assessed 1, 2, and 3 days post-freeze. Percent survival based on pre-freeze controls. Combination of low dose calcitriol and freeze exposure increases cryo effectiveness (ANOVA; p<0.05).
In vivo Studies
Murine studies were performed at Duke University under IACUC approval (Dr. T. Polascik). Briefly, hind leg tumors were created in mice by subcutaneous injection of RM-9 (2×105) cells as previously described.10 Animals were subjected to cryo (dual 15s/−40°C, as measured by thermocouple monitoring at the core of the tumor) with 17-gauge argon cryoneedles (SeedNet, Galil, Plymouth Meeting, Pa), with or without calcitriol. Appropriate time was given between freeze cycles to allow for complete tissue thawing (~5 minutes). Calcitriol (4.0 μg/kg) was administered via direct intratumoral injection 18h prior to cryo to mimic a short-term exposure ideal for in vivo treatment while also allowing sufficient time for cells to undergo a full cell cycle. Animals were sacrificed 24h post-treatment and tumors were excised and stored at −80°C.10 Tissue samples were diced and homogenized both manually and via sonication (Fisher Scientific Sonic Dismembrator, Model 550). Protein was then extracted with RIPA buffer and western blot analysis performed as described. Primary antibodies were hybridized overnight at 4°C in 2mL 1:2 NAP/TBST and Caspase 3(1:500) or Caspase 9(1:200)(CST).
Results
Calcitriol pre-treatment to low temperature exposure exerts a synergistic effect on cell viability
Analysis of sample viability indicated no effect on the RM-9CaPcells when exposed to a range of calcitriol concentrations [25–150nM], even in doses as high as 400nM (data not shown). A freeze profile analysis of RM-9cells in response to low temperature exposure indicated that following exposure to−10°C or−15°C viability was not significantly altered from time matched 37°C controls following 1 day of recovery (144 ±1.3% and 113 ±1.0%vs. 145 ±1.5%, respectively) (Figure 1). A dual freeze-thaw cycle at −10°C again yielded no significant reduction in cell number compared to controls(133 ±0.8% ); however, following repeat exposure to −15°C, a 55% loss of viability Day 1 post-treatment was observed resulting in only 45 ±2.2%survival compared to pre-freeze controls (P<0.05). Cryo treatment at−20°C resulted in 11.5±0.6% survival one day post freeze for single applications and 1.5 ±0.1% with no regrowth for repeat exposures, both of which were significantly lower than controls (p<0.05, Figure 1).
Following establishment of RM-9 response to freezing, cryosensitization studies using calcitriol prior to cryo were performed. Combinatorial treatment of calcitriol [50nM] and a single freeze to −15°C resulted in a 34± 3.1% decrease in viability as compared to time-matched freeze alone(p<0.01) (Figure 2). Pre-treatment with [75nM] calcitriol resulted in a decrease in viability to 59±2.1%, representing a 54 ±2.1%decrease from the freeze alone(p<0.01). Pre-treatment with[100nM] calcitriol followed by a single −15°C exposure yielded66±4.7% cell death compared to time-matched −15°C freeze alone (p<0.01). However, when calcitriol was increased to [150nM], the observed level of cell death was reduced to39±3.5% compared with time-matched freeze-alone samples. The 150nM treatment was found to be less effective than the 75 and 100nM treatments but similar to the 50nM pre-treatment condition(p=0.43).
Since prostate cryosurgical procedures often utilize a dual freeze-thaw application to maximize ablation, we investigated the effect of a dual freeze calcitriol combination to determine if cell death was enhanced. Repeat freeze exposure resulted in increased cell death compared to a single freeze for all concentrations of calcitriol examined (Figure 2). Cultures exposed to a dual −15°C freeze alone yielded 33±1.8% viability at 24 hours post-freeze, compared to the 112±4.4% viability following a single freeze application. Pre-treatment with [50, 75, 100, 150nM] calcitriol and dual cryotreatment yielded post-freeze viabilities of 15±0.9%, 10±0.6%, 16±1.1%, and 35±2.7%, respectively. Compared to the single freeze calcitriol conditions, all of the double freeze calcitriol samples yielded significantly lower survival (p<0.01for all conditions). As in the single cryotreatment exposure, [150nM] calcitriol was the least effective in increasing cell death yielding equivalent survival to repeat freeze only samples (P=0.68, Figure 2). Phase microscopy evaluation of the samples exposed to calcitriol [50, 75, 100, 150nM] and a single −15°C freeze confirmed the changes in cell morphology and level of cell death associated with cryosensitization using calcitriol and correlated with the metabolic activity viability assessment (Figure 3).
Figure 3. Micrographs of RM-9 cells 24 hours following calcitriol, cryo, or combination treatment.
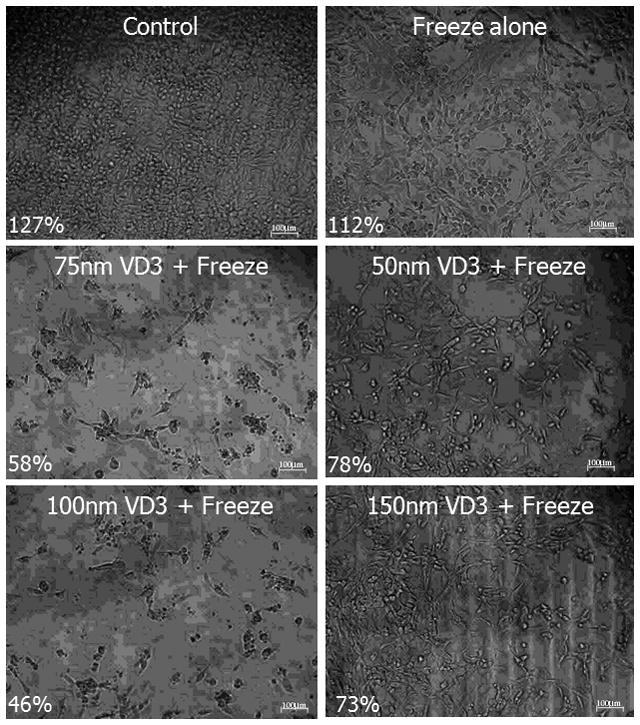
Phase contrast images (10X) were acquired 24 hours post-freeze treatment. Samples were treated with 50, 75, 100, or 150nM calcitriol and exposed to a single, 10 minute, −15°C freeze. Percentages indicate correlating viability data (Fig 2). Calcitriol pretreatment [75nM-100nM] in conjunction with −15°C cryoexposure produces a loss of cells, reduced attachment and morphology alterations.
Fluorescent micrographs confirm apoptotic activation in combination treatments
To confirm the increased cell death determined by viability analysis in combination treatments, fluorescent microscopy was conducted to evaluate viable(Hoechst), apoptotic(Yo-PRO-1), and necrotic (PI) populations. These analyses corroborated the viability studies, with minimal necrosis/apoptosis (as seen by PI/Yo-PRO-1staining) in non-frozen and VD3 controls. There was however a slight increase in necrotic staining cells in the VD3 control after 48h of continual exposure (equivalent to 24h experimental treatment and 24h recovery). There was an increase in cell death in freeze alone over controls, which further increased following combination treatment as indicated by increased red/green staining. Analysis of combination samples revealed an increased level of apoptosis at 8 and 24 hours of recovery which was not seen in the freeze alone (Figure 4).
Figure 4. Fluorescent microscopy of RM-9 cells in response to freezing with or without Vitamin D3 pretreatment.
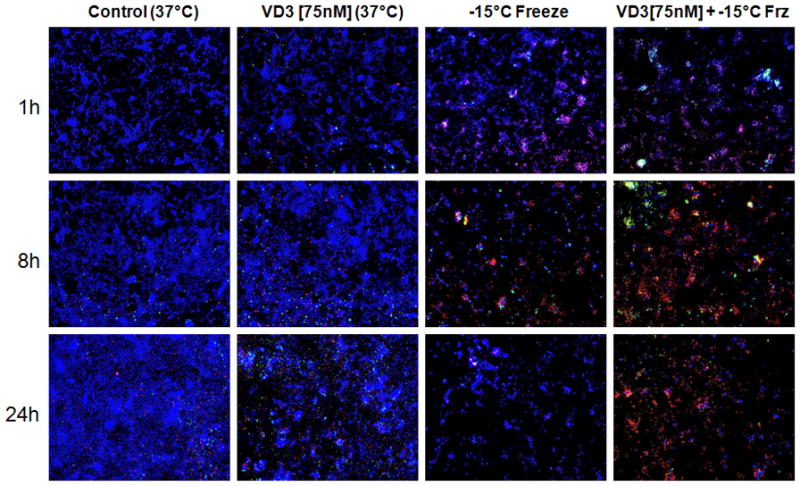
Samples were treated with [75nM] calcitriol for 24 hours prior to a 10 minute -15°C single freeze exposure. Images were acquired 1, 8, and 24 hours post-freeze treatment with Zeiss AxioVision software. Non-frozen and vitamin D3 controls taken in time-matched manner. Combination treatment at all timepoints results in a greater number of cells staining for PI/Yo-Pro 1, corresponding to apoptotic and necrotic cells, respectively.
Apoptotic signaling is increased in calcitriol and cryo combination treatment
To evaluate the mechanics of apoptotic cell death associated with calcitriol cryosensitization, temporal western blot analysis was performed on post-freeze samples (Figure 5). Densitometric analysis of Bid, a pro-apoptotic protein that is cleaved when activated, revealed that cryo alone resulted in a small initial change from controls (10%) after 1h of recovery, with a decrease to 36% of controls 8h after freezing. Following 24h of recovery, Bid levels remained similar at 40%. With calcitriol treatment alone, Bid levels were 76, 58, and 70% of controls at 1, 8, and 24h recovery, respectively. The largest decrease in Bid levels occurred in the combination treatments; at 1h post-freeze, samples pre-treated with calcitriol exhibited a 53% reduction in protein compared to controls. After 8h of recovery, Bid levels remained decreased at 42% of controls, with 24h levels at 50% (Figure 5A).
Figure 5. Western blot analysis of in vitro −15°C cryo, calcitriol and combinatorial treatments.
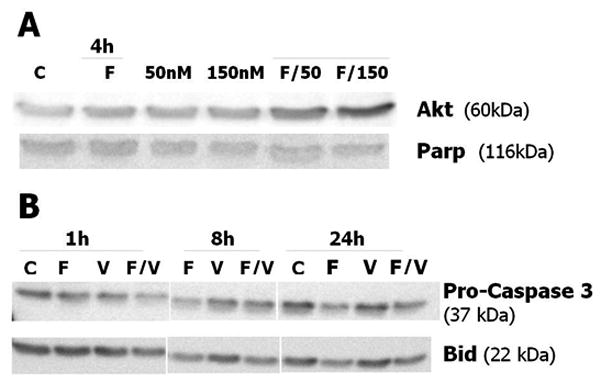
Total protein [30ug/condition] collected 1, 4, 8 or 24 hours post-treatment separated by SDS-PAGE. Conditions: C (37°C control); F (single 10 min/−15°C freeze); V (75nM VD3); F/V (VD3 [75nM] +10min/−15°C freeze); (A) Akt levels are upregulated 4 hrs post-freeze in the combination cryo+VD3 [150nM] condition, whereas Parp exhibits no significant change. (B) Decreases in Bid and pro-caspase-3 at 1hr post-treatment in the combination samples are observed. Signaling proteins for VD3 treatment alone are similar to controls at all timepoints.
Caspase 3, the “executioner caspase,” was also assessed to investigate involvement of the caspase cascade. The pro-caspase 3 precursor decreased slightly to 75% of controls1h post-freeze, decreasing to 21% at 8h. After 24h, samples had recovered slightly to 37% of pro-caspase 3 control levels. Protein levels did not change significantly between the 1h and 8h calcitriol alone controls, at 48% and 55%, though protein levels increased in the 24h calcitriol control sample (87% of original 1h control). In combination samples 1h post-freeze, 74% decrease in pro-caspase 3 over non-frozen controls was observed, indicating cleavage and caspase activation. At 8h recovery, pro-caspase 3 levels remained low in the post-freeze samples (21%), with the 24h post-freeze samples yielding higher levels (59%, Figure 5A).
Along with pro-death signaling, the pro-survival signaling molecule Akt was investigated given the surviving cell populations following freezing and cryosensitization seen in the initial viability studies. Assessment of Akt did not reveal significant variation in protein levels between controls, freeze alone, [50nM] VD3, or [150nM] VD3 (100, 118, 119, 126% respectively). When samples were exposed to a combination of [50nM] VD3 and a single −15°C freeze, Akt levels increased two-fold by 4h recovery (211%, Fig 5B). Levels of Akt were found to increase further to nearly three-fold more than controls following combination treatment of freeze to −15°C and [150nM] VD3 (266%), indicating phosphorylation, downstream signaling, and subsequent upregulation. This observation correlated with the viability studies which showed treatment with high concentrations of calcitriol [150nM] prior to freeze was associated with increased survival. Parp, a DNA repair protein, was also evaluated at 4h post-freeze in these samples. Analysis revealed the freeze alone condition to have 97% of control protein levels, [50nM] and [150nM] calcitriol resulted in 86% and 91% of controls, and combinations of freeze with [50nM] and freeze with [150nM], 96% and 91% respectively. As no significant changes in Parp levels were seen, this indicated that the −15°C freeze with or without 50 or 150nM of VD3 did not cause sufficient cellular damage to cleave Parp into an active form.
In vivo Molecular Analysis
The in vivo murine model also gave us insight into the molecular signaling pathways activated in response to calcitriol and cryotreatment. Pro-caspase 3 and pro-caspase 9 protein levels both decreased following calcitriol treatment as well as combination calcitriol and cryotreatment, but not in cryotreatment alone (Figure 6). This indicated a higher level of apoptosis in the combination condition, supporting the activity of calcitriol as a cryosensitizing agent. The reduction of pro-caspase 9 protein levels following combination treatment may suggest a substantial mitochondrial-mediated pathway in calcitriol sensitization, which was also supported by the in vitro data with the observed Bid cleavage. It is possible that Bid initially translocates to the mitochondrial membrane and assists in opening of mitochondrial permeability pores, allowing the release of cytochrome c from the mitochondria, formation of Apaf-1 and subsequent activation of caspase-9.
Figure 6. Protein levels from RM-9 cells following in vivo cryo, calcitriol, and combinatorial treatment in a murine animal study.
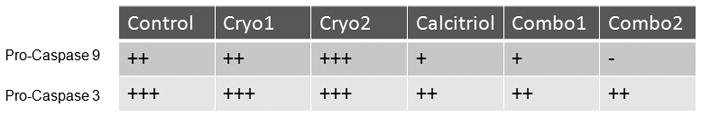
Table constructed based upon the western blot analysis described in Kimura, et. al. 2010. Sections of tumor were excised from the right hind leg 24 hours post-treatment and tissue was homogenized; total protein was isolated and 50 ug per condition was separated by SDS-PAGE and transferred to PVDF. Control (no treatment), Cryo 1 and 2 (two animals that each received cryoablation alone), Cal (calcitriol alone), and Combo 1 and Combo 2 (two animals that received the combination of calcitriol and cryo). Apoptotic signaling indicated by decreasing levels of Pro-caspase-3 and -9 in combination and calcitriol treatments suggests cleavage and activation.
Discussion
Previous studies have identified VD3 as a potential cryosensitizer in human CaP cells in vitro.11 In the current study, we further explored the role of calcitriol in the RM-9 system to evaluate the breadth of application potential across a variety of forms and stages of CaP. The RM-9 line is androgen responsive at low passages and nonresponsive at higher passages, representing the critical stage of CaP progression to a more aggressive form.26
Recently it has been shown that late stage androgen-insensitive prostate cancer is highly resistant to moderate freezing temperatures such as those associated with the periphery of a cryogenic lesion.27 Given this, we focused our study on the periphery in an attempt to achieve complete cell death within the ice ball margin, there by preventing recurrence. We investigated the use of calcitriol as a cryosensitizer in an aggressive, rapidly dividing, late stage in vitro CaP model. We hypothesized that combining calcitriol with the moderate sub-freezing temperatures experienced in the periphery of the cryogenic lesion would result in an increase in apoptotic cell death as compared to either cryotherapy or calcitriol treatment alone. Our results indicate that treatment with calcitriol prior to freezing sensitized CaP cells, thereby promoting an enhanced apoptotic and necrotic response following exposure to temperatures representative of those in the ice-ball margin (−15°C). Moderate concentrations of calcitriol [75–100nM] were found to be most effective, evident by the morphological, cellular and molecular markers of viability investigated herein.
With the confirmation of cryosensitization properties of calcitriol in theRM-9 model, investigation into the apoptotic signaling pathways activated during the freezing event were conducted. Immunoblotting revealed the reduction ofpro-caspase-3, as well as the apoptotic precursor protein Bid, following combination treatment. These observed reductions were associated with protein activation and downstream apoptotic signal transduction. Interestingly, samples pre-treated with calcitriol began to experience higher levels of apoptotic signaling at earlier time points than freeze only samples, supporting the use of calcitriol in cryosensitization. The timing of apoptotic activation between 1 and 8 hours was not unexpected given the short cell cycle time (~12 hours) of RM-9 cells, thus allowing for a rapid response to the cellular stresses experienced during the freeze-thaw cycle.
In addition to the activation of apoptosis, the studies also demonstrated an increase in Akt levels following treatment with elevated calcitriol concentrations [150nM]. Akt upregulation seen at 4h post-freeze suggests pro-survival signaling through the PI3-kinase pathway may occur in response to high levels of VD3, affording cells protection from molecular based freeze injury which correlated with the observed increased post-freeze viability. These data suggest differential, dose dependent molecular pathway activation by calcitriol when utilized as a cryosensitizer at moderate [75–100nM] and high [150nM and above] concentrations. Many transcription factors can differ in their effects on a cell depending on both the concentration and duration of the signal received; it is possible that a high concentration of calcitriol in the cell exerts a different effect entirely by altering its interactions with binding sites on the DNA via nuclear transcriptional coregulators.28
Our results indicate that cryosensitization with calcitriol concentrations of [75–100nM] in combination with −15°C exposure resulted in increased cell death over freezing alone. However, reaching these effective therapeutic concentrations clinically poses difficulties. There have been several clinical trials on calcitriol in CaP studying various concentrations, methods of delivery, and dosage schedules. Depending on the delivery method (oral, intravenous, or subcutaneous), variances in peak serum calcitriol concentrations occurred. In an oral delivery study, the maximum serum concentration of 1,25(OH)2D3 measured was 6nM,29 due to the fact that VD3 metabolism is tightly regulated in vivo. Intratumoral injection, which was utilized in the Kimura et al. in vivo study,10 may be a more effective method of delivery that would allow maximal calcitriol levels while limiting system toxicity. A previous study that investigated the use of intravenous (i.v.) vs. intratumoral (i.t.) drug injection efficacy of tumors in rats also supports the case for i.t. injection, with lower drug accumulation in vital organs and an overall improved anti-tumor treatment outcome.30 Together, these results suggest that intratumoral dosage should be further explored in calcitriol cryosensitization.
Serum concentrations were not measured in the Kimura et al. murine in vivo study, as the active 1,25(OH)2D3 has a very short half life of 15 hours; however, the results indicate that a 4μg/kg dosage was sufficient to induce cryosensitization. This study found significantly greater areas of necrotic tissue, as measured by hematoxylin and eosin staining, within tumors excised 24 hours post-freeze when pre-treated for 18 hours with calcitriol. These results also correlated with fluorescence immunohistochemistry, hypoxia, and proliferation analyses, which suggest combination calcitriol and cryoablation therapy as superior to either alone.10
For patients whose cancer remains localized and does not encompass the entire prostate, focal therapy is an area that may benefit from the use of cryosensitizers. Advancements in imaging are allowing for more accurate cryotherapy dosing, and by combining the use of sensitizing agents, treatments can become more effective with the same cryo dosage. This would allow reduction of the aggressive nature of current CaP cryoablation and subsequently reduce associated side effects. Over-freezing of the tumor often occurs due to the goal of complete ablation, but this can cause destruction of healthy surrounding tissue.4 Due to the close proximity of the prostate to the neurovascular bundle, over freezing often leads to impotence and sexual dysfunction.4 Additionally, the results of our study suggest the possibility that a single freeze-thaw cycle with calcitriol pre-treatment may result in a similar level of cell death to the current dual freeze-thaw cycle standard. The switch to a single freeze event would reduce the risk of over-freezing, recurrence, and procedural time, allowing enhanced CaP ablation in a shorter time.5 Reducing these aforementioned effects would therefore give patients a better quality of life by preserving genitourinary function.
For over fifteen years, researchers have been interested in the involvement of vitamin D and its downstream effects in cancer development and progression. However, the mechanism of action in vivo has not been elucidated, partly due to the fact that calcitriol can elicit varied results in different systems, making interpretation of treatment difficult.31,32 The cross talk between the downstream signaling effects of calcitriol and cryotherapy appear to more effectively activate apoptotic cascades than either alone. The exact role that calcitriol plays in cryotherapy is still under investigation; however, the results obtained from the Kimura et al. in vivo murine study correlate well with our in vitro findings, indicating that calcitriol sensitization may have benefits when applied clinically.10 Issues of calcitriol concentration, dosage timing, and dosage method still need to be addressed. Due to the high risk of hypercalcemia as well as the reported challenges in reaching high serum levels of calcitriol in oral treatments, intratumoral injection of calcitriol may provide a potential path for clinical utilization.
Acknowledgments
The authors would like to acknowledge that this work has been partially funded by CPSI Biotech and the National Institutes of Health, grant numbers R43CA1123993–01A1 and R43CA118537–01A1.
Footnotes
This work is an original research article and has not been previously published or submitted for publication elsewhere.
Conflict of Interest Statement:
In the spirit of full disclosure, the authors declare that K. Santucci receives compensation as a consultant for CPSI Biotech; Dr. Snyder, Dr. JM Baust, and Dr. Van Buskirk are employed by CPSI Biotech; Dr. JG Baust, Dr. Mouraviev, Dr. Polascik, and Dr. Gage declare no conflict of interest.
References
- 1.Horner MJ, Ries LG, Krapcho M, Neyman N, Aminou R, Howlader N, et al. SEER Cancer Statistics Review, 1975–2006. National Cancer Institute; Bethesda, MD: [Google Scholar]
- 2.Babaian RJ, Donnelly B, Bahn D, Baust JG, Dineen M, Ellis D, et al. Best practice statement on cryosurgery for the treatment of localized prostate cancer. J Urol. 2008;180(5):1993–2004. doi: 10.1016/j.juro.2008.07.108. [DOI] [PubMed] [Google Scholar]
- 3.Cohen JK, Miller RJJ, Ahmed S, Lotz MJ, Baust J. Ten-year biochemical disease control for patients with prostate cancer treated with cryosurgery as primary therapy. Urology. 2008;71(3):515–518. doi: 10.1016/j.urology.2007.09.059. [DOI] [PubMed] [Google Scholar]
- 4.Saliken JC, Donnelly BJ, Rewcastle JC. The evolution and state of modern technology for prostate cryosurgery. Urology. 2002;60(2 Suppl 1):26–33. doi: 10.1016/s0090-4295(02)01681-3. [DOI] [PubMed] [Google Scholar]
- 5.Gage AA, Baust JG. Cryosurgery for tumors -a clinical overview. Technol Cancer Res Treat. 2004;3(2):187–199. doi: 10.1177/153303460400300212. [DOI] [PubMed] [Google Scholar]
- 6.Clarke DM, Baust JM, Van Buskirk RG, Baust JG. Addition of anticancer agents enhances freezing-induced prostate cancer cell death: implications of mitochondrial involvement. Cryobiology. 2004;49(1):45–61. doi: 10.1016/j.cryobiol.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 7.Clarke DM, Baust JM, Van Buskirk RG, Baust JG. Chemo-cryo combination therapy: an adjunctive model for the treatment of prostate cancer. Cryobiology. 2001;42(4):274–285. doi: 10.1006/cryo.2001.2333. [DOI] [PubMed] [Google Scholar]
- 8.Clarke DM, Hollister WR, Baust JG, Van Buskirk RG. Cryosurgical Modeling: Sequence of Freezing and Cytotoxic Agent Application Affects Cell Death. Mol Urol. 1999;3(1):25–31. [PubMed] [Google Scholar]
- 9.Baust JG, Gage AA, Clarke D, Baust JM, Van Buskirk R. Cryosurgery--a putative approach to molecular-based optimization. Cryobiology. 2004;48(2):190–204. doi: 10.1016/j.cryobiol.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 10.Kimura M, Rabbani Z, Mouraviev V, Tsivian M, Caso J, Satoh T, et al. Role of Vitamin D(3) as a Sensitizer to Cryoablation in a Murine Prostate Cancer Model: Preliminary In Vivo Study. Urology. 2010 doi: 10.1016/JUrology.2010.03.0011.. (on-line) [DOI] [PubMed] [Google Scholar]
- 11.Baust JM, Klossner DP, Robilotto AT, Van Buskirk RG, Gage AA, Mouraviev V, et al. Vitamin D3 therapy increases cryoablation efficacy: a novel strategy for the treatment of prostate cancer. British Journal of Urology. 2010 submitted. [Google Scholar]
- 12.McCullough ML, Bostick RM, Mayo TL. Vitamin D gene pathway polymorphisms and risk of colorectal, breast, and prostate cancer. Annu Rev Nutr. 2009;29:111–132. doi: 10.1146/annurev-nutr-080508-141248. [DOI] [PubMed] [Google Scholar]
- 13.Norman AW. From vitamin D to hormone D: fundamentals of the vitamin D endocrine system essential for good health. Am J Clin Nutr. 2008;88(2):491S–499S. doi: 10.1093/ajcn/88.2.491S. [DOI] [PubMed] [Google Scholar]
- 14.Peehl DM, Shinghal R, Nonn L, Seto E, Krishnan AV, Brooks JD, et al. Molecular activity of 1,25-dihydroxyvitamin D3 in primary cultures of human prostatic epithelial cells revealed by cDNA microarray analysis. J Steroid Biochem Mol Biol. 2004;92(3):131–141. doi: 10.1016/j.jsbmb.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 15.Feldman D, Malloy PJ, Krishnan AV, Balint E. Vitamin D: biology, action and clinical implications. In: Marcus R, Feldman D, Nelson DA, Rosen CJ, editors. Osteoporosis. Acedemic Press; San Diego: 2007. pp. 317–382. [Google Scholar]
- 16.Osborne JE, Hutchinson PE. Vitamin D and systemic cancer: is this relevant to malignant melanoma? Br J Dermatol. 2002;147(2):197–213. doi: 10.1046/j.1365-2133.2002.04960.x. [DOI] [PubMed] [Google Scholar]
- 17.Krishnan AV, Feldman D. Molecular pathways mediating the anti-inflammatory effects of calcitriol: implications for prostate cancer chemoprevention and treatment. Endocr Relat Cancer. 2010;17(1):R19–38. doi: 10.1677/ERC-09-0139. [DOI] [PubMed] [Google Scholar]
- 18.Montironi R, Thompson D, Scarpelli M, Mazzucchelli R, Peketi P, Hamilton PW, et al. Karyometry detects subvisual differences in chromatin organization state between cribriform and flat high-grade prostatic intraepithelial neoplasia. Mod Pathol. 2004;17(8):928–937. doi: 10.1038/modpathol.3800142. [DOI] [PubMed] [Google Scholar]
- 19.Schwartz GG, Whitlatch LW, Chen TC, Lokeshwar BL, Holick MF. Human prostate cells synthesize 1,25-dihydroxyvitamin D3 from 25-hydroxyvitamin D3. Cancer Epidemiol Biomarkers Prev. 1998;7(5):391–395. [PubMed] [Google Scholar]
- 20.Hsu JY, Feldman D, McNeal JE, Peehl DM. Reduced 1alpha-hydroxylase activity in human prostate cancer cells correlates with decreased susceptibility to 25-hydroxyvitamin D3-induced growth inhibition. Cancer Res. 2001;61(7):2852–2856. [PubMed] [Google Scholar]
- 21.Johnson CS, Hershberger PA, Trump DL. Vitamin D-related therapies in prostate cancer. Cancer Metastasis Rev. 2002;21(2):147–158. doi: 10.1023/a:1020836226594. [DOI] [PubMed] [Google Scholar]
- 22.Park WH, Seol JG, Kim ES, Hyun JM, Jung CW, Lee CC, et al. Induction of apoptosis by vitamin D3 analogue EB1089 in NCI-H929 myeloma cells via activation of caspase 3 and p38 MAP kinase. Br J Haematol. 2000;109(3):576–583. doi: 10.1046/j.1365-2141.2000.02046.x. [DOI] [PubMed] [Google Scholar]
- 23.Welsh J. Vitamin D3 and its receptor in mammary gland: from normal development to breast cancer. Vitamion D Endocrine System. In: Norman AW, Bouillon R, Thomasset M, editors. Structural, Biological, Genetic and Clinical Aspects. University of Califronia; Riverside, CA: 2000. pp. 453–460. [Google Scholar]
- 24.Narvaez CJ, Welsh J. Role of mitochondria and caspases in vitamin D-mediated apoptosis of MCF-7 breast cancer cells. J Biol Chem. 2001;276(12):9101–9107. doi: 10.1074/jbc.M006876200. [DOI] [PubMed] [Google Scholar]
- 25.Thompson TC, Timme TL, Park SH, Yang G, Ren C. Mouse prostate reconstitution model system: A series of in vivo and in vitro models for benign and malignant prostatic disease. Prostate. 2000;43 (4):248–254. doi: 10.1002/1097-0045(20000601)43:4<248::aid-pros3>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 26.Baley PA, Yoshida K, Qian W, Sehgal I, Thompson TC. Progression to androgen insensitivity in a novel in vitro mouse model for prostate cancer. J Steroid Biochem Mol Biol. 1995;52(5):403–413. doi: 10.1016/0960-0760(95)00001-g. [DOI] [PubMed] [Google Scholar]
- 27.Klossner DP, Baust JM, Van Buskirk RG, Gage AA, Baust JG. Cryoablative response of prostate cancer cells is influenced by androgen receptor expression. BJU Int. 2008;101(10):1310–1316. doi: 10.1111/j.1464-410X.2008.07499.x. [DOI] [PubMed] [Google Scholar]
- 28.Thakur MK, Paramanik V. Role of steroid hormone coregulators in health and disease. Horm Res. 2009;71(4):194–200. doi: 10.1159/000201107. [DOI] [PubMed] [Google Scholar]
- 29.Beer TM. Development of weekly high-dose calcitriol based therapy for prostate cancer. Urol Oncol. 2003;21(5):399–405. doi: 10.1016/s1078-1439(03)00170-4. [DOI] [PubMed] [Google Scholar]
- 30.Lammers T, Peschke P, Kuhnlein R, Subr V, Ulbrich K, Huber P, et al. Effect of intratumoral injection on the biodistribution and the therapeutic potential of HPMA copolymer-based drug delivery systems. Neoplasia. 2006;8(10):788–795. doi: 10.1593/neo.06436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee J, Youn JI. The photoprotective effect of 1,25-dihydroxyvitamin D3 on ultraviolet light B-induced damage in keratinocyte and its mechanism of action. J Dermatol Sci. 1998;18(1):11–18. doi: 10.1016/s0923-1811(98)00015-2. [DOI] [PubMed] [Google Scholar]
- 32.Stambolsky P, Tabach Y, Fontemaggi G, Weisz L, Maor-Aloni R, Siegfried Z, et al. Modulation of the vitamin D3 response by cancer-associated mutant p53. Cancer Cell. 2010;17(3):273–285. doi: 10.1016/j.ccr.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]


