Summary
To see if age-related changes in bone could predict subsequent lifespan, we measured multiple aspects of femur size and shape at 4, 15 and 24 months of age in genetically heterogeneous mice. Mice whose cortical bone became thicker from 4 to 15 months, associated with preservation of the endosteal perimeter, survived longer than mice whose endosteal cavity expanded, at the expense of cortical bone, over this age range. Femur size at age 4 months was also associated with a difference in life expectancy: mice with larger bones (measured by length, cortical thickness, or periosteal perimeter) had shorter lifespans. Femur length, midlife change in cortical bone thickness, and midlife values of CD8 T memory cells each added significant power for longevity prediction. Mice in the upper half of the population for each of these three endpoints lived, on average, 103 days (12%) longer than mice with the opposite characteristics. Thus measures of young adult bone dimensions, changes as a result of bone remodeling in middle age, and immunological maturation provide partially independent indices of aging processes that together help to determine lifespan in genetically heterogeneous mice.
Key words for indexing: biomarkers, longevity, femur, mouse, T cells, aging rate
Introduction
Studies in rodents and humans have documented thousands of traits that change with age in adult life, but have been notably less successful (Miller, 2001a; Sprott, 1999; Johnson, 2006) in providing evidence of early and midlife traits that can predict the length of healthy lifespan and the rate of aging. Many studies have documented risk factors for specific late life diseases, and assessment of obesity, glucose tolerance, hypertension, and history of previous infarction, hip fracture, or neoplasia are routinely incorporated into clinical practice on the basis of their predictive power. In contrast, studies of how lifespan can be influenced by early developmental processes, and midlife rates of change, are difficult to conduct in a species with a mean lifespan over 70 years. Although the shorter lifespan of rodents has the potential to speed up such assessments, they depend upon the development of methods that can assess relevant physiological traits, preferably at several points over the life course, without harm to the mouse.
The availability of in-vivo micro-CT instrumentation that can provide accurate assessments of mouse bone dimensions and mineral content provided an opportunity to assess whether early and midlife variation in bone properties could predict longevity. We have conducted a longitudinal study using 519 mice, bred by a four-way cross scheme (Jackson et al., 1999; Volkman et al., 2003) so that the population would be molded by both genetic and non-genetic influences. We chose to use this heterogeneous population, called "UM-HET3," for this study for several reasons. UM-HET3 mice have been used in several previous studies (Reeves et al., 2007; Volkman et al., 2004; Volkman et al., 2003) to map genes related to the morphology and mechanical properties of the femur and the vertebrae, thus providing a good deal of prior information about bone biology on which to base our experimental design and with which to compare our current results. Secondly, we have also used UM-HET3 mice for several previous studies of phenotypes that are predictive of lifespan, and have shown that both body size (Miller et al., 2002) and midlife T cell subset patterns (Harper et al., 2004; Miller and Chrisp, 2002) can predict lifespan of individual animals. Thus we selected the UM-HET3 population for an analysis of whether changes in bone morphology at various stages of adult life have implications for life span. We also hope that observations made using genetically heterogeneous populations may have broader applicability, both for experimental animals and for human biology, than studies based on mice of a single, potentially idiosyncratic genotype (Miller et al., 1999). Lastly, we were aware that the NIA has selected the UM-HET3 population for its Intervention Testing Program (Miller et al., 2007), and therefore believe that information about bone development in aging UM-HET3 mice can serve as a foundation for studies of anti-aging drugs and their possible effects on bones.
We report that measurements of the rate of midlife change in endosteal perimeter and cortical thickness of the femur predict subsequent longevity, and that measurements of femoral size at 4 months provides additional, independent, predictive power. Measures of femur size, endosteal preservation, and an age-sensitive T cell subset can separate mice into groups that differ by 12% in median lifespan.
Results
Among the 519 mice evaluated, 10% died prior to 656 days, median survival was 880 days, and 90% died prior to 1061 days. The oldest mouse survived to 1296 days. Measurements taken at 15 and 24 months correspond to ages at which survival was, respectively, 99% and 80% among the 519 mice with bone data and recorded ages at death. Thus values measured on mice at 24 months of age may not be representative on the entire test population, but only on the 80% that survived as long as two years.
Tests for femur length, cross sectional area, cortical thickness, periosteal perimeter, endosteal perimeter, and bone mineral content were recorded at 4 months of age for 519 mice whose date of death was subsequently recorded. Table 2 presents the mean levels (with sem) for each of these endpoints. Cox regression calculations were used to determine which of these characteristics was associated with differences in survival and mortality risk, and Table 2 shows the hazard ratios (HR) and p-values for each trait. High values of Len, Csa, CThick, Perio, and Bmc were all associated with shorter lifespan, with unadjusted p-values ranging from p = 0.003 for Bmc to p < 0.001 for Len and Csa. Each of these measures is positively correlated with each of the others, with regression coefficients ranging from 0.36 to 0.91, and each is a measure of femur size or robustness. A principal factors calculation (not shown) showed that the first principal factor had positive loadings on each of these endpoints (0.5 for Len, and > 0.8 for all other measures), and an eigenvalue of 3.5. In a Cox regression model, this first principal factor was significantly associated with mortality risk (HR = 1.18, p < 0.001). We conclude that mice with larger femurs have elevated mortality risk after age 4 months.
Table 2.
Mean values for Bone Traits, and Results of Univariate Cox Regression Analyses
| Age = 4 Months (N=519) |
Age = 15 Months (N=505) |
Age = 24 months (N=416) |
||||
|---|---|---|---|---|---|---|
| Variable | Mean ± SEM | HR P-value |
Mean ± SEM | HR P-value |
Mean ± SEM | HR P-value |
| Len | 15.9 ± 0.02 | 1.5 0.001 ↓ |
16.6 ± 0.02 | 1.4 0.005 ↓ |
16.6 ± 0.03 | 1.2 0.13 |
| Csa | 1.11 ± 0.006 | 3.3 0.001 ↓ |
1.31 ± 0.007 | 0.89 0.7 |
1.40 ± 0.01 | 0.65 0.08 ↑ |
| CThick | 0.32 ± 0.002 | 41 0.003 ↓ |
0.34 ± 0.002 | 0.17 0.1 |
0.34 ± 0.003 | 0.06 0.005 ↑ |
| Perio | 4.88 ± 0.013 | 1.5 0.007 ↓ |
5.34 ± 0.02 | 1.2 0.2 |
5.66 ± 0.02 | 1.2 0.15 |
| Endo | 2.94 ± 0.009 | 1.2 0.45 |
3.22 ± 0.01 | 1.7 0.001 ↓ |
3.58 ± 0.02 | 2.2 0.001 ↓ |
| Bmc | 3.22 ± 0.021 | 1.3 0.003 ↓ |
3.93 ± 0.024 | 0.9 0.4 |
4.31 ± 0.03 | 0.9 0.5 |
Notes: SEM = standard error of the mean. HR = hazard ratio from Cox regression. N = number of mice evaluated at the indicated age.
Up arrow ↑ indicates that high values are associated with improved life expectancy.
Down arrow ↓ indicates that low values are associated with improved life expectancy.
P-values are nominal, i.e. they have not been adjusted for multiple comparisons.
The pattern of associations between bone traits and longevity is altered, however, in mice tested at 15 months. Mean levels for each trait are shown in Table 2, for each of the 505 mice that survived to be tested in middle age. Of the five traits that were, at 4 months of age, predictors of survival, only Len is still a significant predictor when measured at 15 months (HR = 1.4, p = 0.005). Endosteal diameter, which was not associated with lifespan when measured in 4 month old mice, is a significant predictor measured in 15 month old animals (HR = 1.7, p < 0.001). High endosteal diameter values identify mice with shorter life expectancy.
Table 2 also shows mean values for each of these traits measured in 24 month old mice; 416 mice survived to be tested at this age. Although high values of CThick were associated with low life expectancy at 4 months (at p = 0.003), high CThick levels were associated with significantly greater life expectancy (p = 0.005) when measured in mice at 24 months of age, a reversal of the direction of the association. A similar reversal was seen for Csa, with high levels associated with shorter lifespan at 4 months (p < 0.001), but with a trend toward association of longer lifespan to lower levels (p = 0.08, not significant) when Csa is measured in 24 month old animals. The association between good survival and low Endo levels, seen at 15 months of age, is even stronger at 24 months of age (Cox regression hazard ratios of 1.7 and 2.2, respectively).
Table 3 shows an alternate approach to evaluating inter-mouse differences in bone aging and mortality risks. For each trait, we calculated a change score by subtracting the 4 month value from the 15 month value for each of the 449 mice that were measured at each age. Mean values of these change scores are shown in Table 3, and each was significantly different from zero (the null hypothesis) at p < 0.0001. We conclude that each of these bone traits is sensitive to age, between 4 and 15 months, in these genetically heterogeneous mice. Four of these change scores are significant predictors of remaining lifespan in Cox regression analyses (Table 3). Lower mortality risks, and thus longest lifespans, are seen in 15 month old mice that exhibit the largest changes in Csa, CThick, and Bmc, and with the smallest change in Endo.
Table 3.
Change Scores for Bone Traits, and Results of Cox Regression Analyses
| Change Score (1 [15 months - 4 months] N = 449 |
Change Score (2 [24 months - 15 months] N = 449 |
|||
|---|---|---|---|---|
| Variable | Mean ± SEM | HR P-value |
Mean ± SEM | HR P-value |
| Len | 0.71 ± 0.01 | 0.99 0.9 |
0.05 ± 0.01 | 0.7 0.2 |
| Csa | 0.21 ± 0.01 | 0.4 0.005 ↑ |
0.09 ± 0.01 | 0.7 0.2 |
| CThick | 0.023 ± 0.002 | 0.02 0.001 ↑ |
−.004 ± 0.002 | 0.2 0.2 |
| Perio | 0.48 ± 0.01 | 0.90 0.5 |
0.33 ± 0.01 | 1.2 0.4 |
| Endo | 0.28 ± 0.01 | 1.9 0.003 ↓ |
0.37 ± 0.01 | 2.2 0.003 ↓ |
| Bmc | 0.73 ± 0.03 | 0.81 0.01 ↑ |
0.39 ± 0.03 | 1.0 0.7 |
Notes: Change score is calculated as the value at the older age, minus the value at the younger age in the same animal. SEM = standard error of the mean. Each change score is significantly different from zero at p < 0.0001. HR = hazard ratio from Cox regression using the change score as independent variable. N = number of mice evaluated at the indicated age.
Up arrow ↑ indicates that high change scores are associated with improved life expectancy.
Down arrow ↓ indicates that low change scores are associated with improved life expectancy.
P-values are nominal, i.e. they have not been adjusted for multiple comparisons.
We calculated a similar set of change scores to evaluate changes between 15 and 24 months of age, for the 369 mice that were evaluated at each age (Table 3). Each of these change scores is significantly different from zero at p < 0.0001, except for CThick (p = 0.08 for change score); this is consistent with the idea that Len, Csa, Perio, Endo, and Bmc continue to increase between 15 and 24 months of age, although interpretation of these values is complicated by the absence of information on the 20% of mice that died prior to 24 months. Among mice that survived to 24 months, high rates of change in Endo are again associated with lower remaining life expectancy, as noted for changes between 4 and 15 months of age. None of the other change scores from 15 to 24 months was significantly associated with subsequent mortality risk. Figure 2 shows scatterplots relating life span to CThick change scores and Endo change scores, from 4 to 15 months, among individual mice. Those mice whose cortical thickness declines from 4 to 15 months (CThick change score less than 0) tend to die at earlier ages than those whose CThick values are above zero. Similarly, mice that have the largest increase in endosteal perimeter in this age range (high Endo change scores) tend to die at early ages compared to mice whose endosteal perimeter does not change or decreases in midlife.
Figure 2.
Longer life span in mice with increase in cortical bone thickness and preservation of endosteal perimeter between 4 and 15 months of age. Each symbol represents an individual mouse. Lines shown are least-squares regression, each p < 0.05.
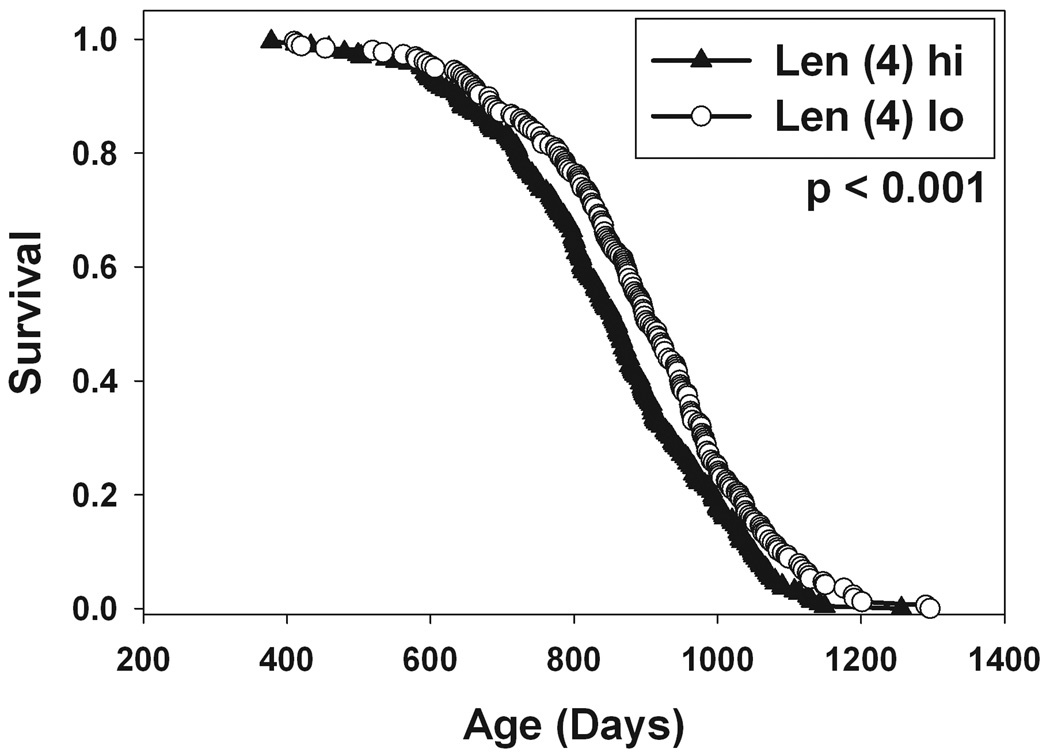
The analyses above suggested that bone traits were associated with survival in two ways: (a) small femurs in 4 month old mice were predictive of better survival, and (b) preservation or increase in femur cortical thickness in midlife was predictive of better survival. To see if these two sets of changes provided independent predictive power, we evaluated a series of bivariate Cox regression models, each combining a measure of bone size at 4 months with an index of change between 4 and 15 months. The strongest association (evaluated by likelihood ratio χ2) was for the combination of Len at 4 months and the CThick change score. Len was associated with a hazard ratio of 1.37 (p = 0.005), and CThick change score with a hazard ratio of 0.03 (p = 0.002). Figure 3 provides an illustration of the survival patterns for subsets of mice distinguished by one or both of these predictors. Mice divided into two groups on the basis of Len at 4 months (left panel) differ by 47 days in median survival (p = 0.001 by log-rank test comparing mice with high or low levels of Len). CThick change score, by itself, divides mice into two groups that differ by 28 days in median lifespan (not shown). The middle panel of Figure 3 compares survival curves for mice that are in the lower half for Len and also in the upper half for CThick change score, to those with the opposite category for both traits. Mice with low levels of Len (4) and high levels of the CThick change score live on average 78 days longer than mice with the opposite phenotype (see Figure 3, middle panel).
Figure 3.
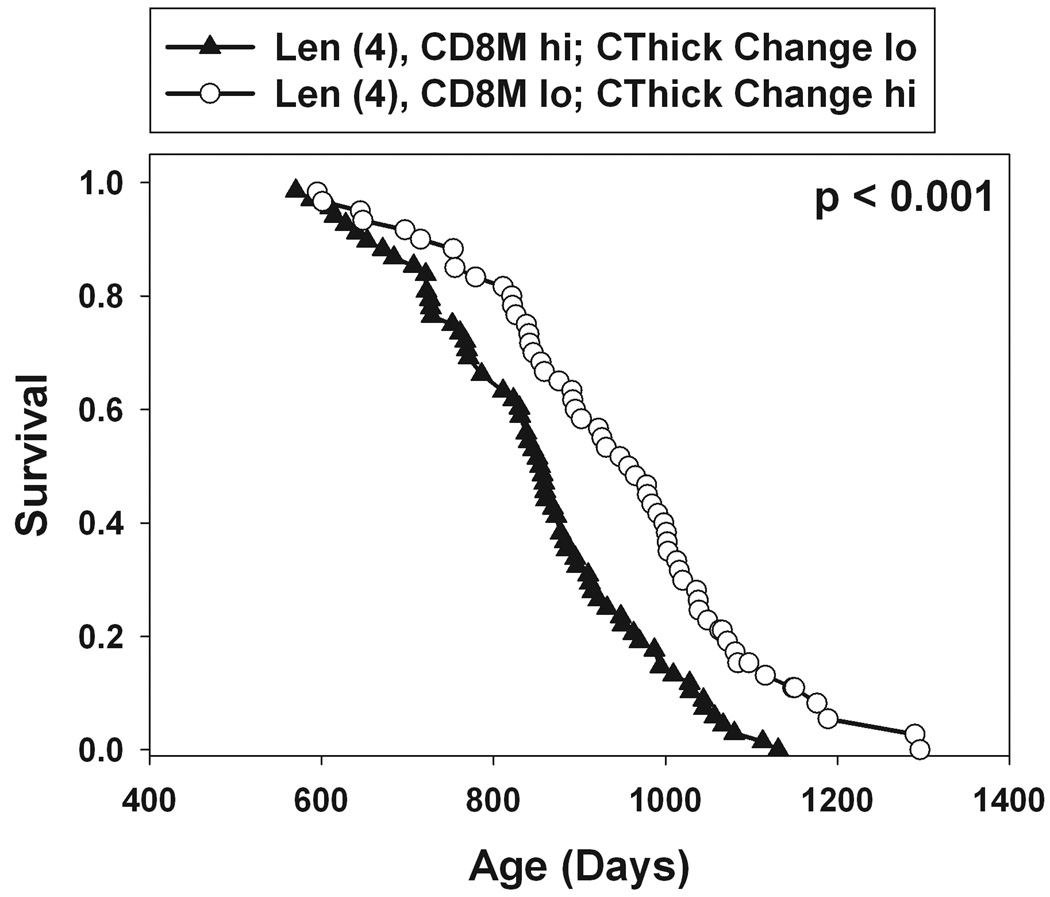
Survival plots for subpopulations of mice differing in combinations of bone and immune traits. Left: populations of mice in the top or bottom half of the distribution for femoral length at 4 months of age. Middle: circles indicate mice that were in the bottom half of the distribution for femur length at 4 mon and also in the top half of the CThick change score distribution (15 mon minus 4 mon). Triangles indicate mice in top half of the femur length distribution and the bottom half of the CThick change score listing. Right: as for the middle panel, with the additional criterion based on the CD8 memory cell median score. p-values calculated by log-rank test comparing the two survival distributions illustrated.
Prediction of lifespan by a combination of bone and immune predictors
Studies of earlier populations of UM-HET3 mice had shown that mice with elevated levels of memory T cells, at 18 months of age, had shorter lifespans than mice with lower memory T cell fractions (Harper et al., 2004). We therefore thought it would be of interest to discover whether the two classes of predictor variables, i.e. immune and bone endpoints, would provide independent predictive power in Cox regression models. In the current group of UM-HET3 mice, the fraction of CD8 cells with the memory phenotype (CD8M), measured at 18 months of age, was again associated with differences in remaining lifespan (HR = 1.01; p = 0.002 by Cox regression). Populations divided by the median level of CD8M differed by 36 days in their median survival (not shown). To see if this measure of age-sensitive immune status provided additional predictive power beyond that available from bone traits alone, we performed a Cox regression with included Len (at 4 months), CThick change score (from 4 to 15 months), and CD8M levels (at 18 months). Table 4 shows the results of the regression calculation. Each of these traits added significant power to the overall regression at 0.01 < p < 0.03. Each individual measurement can divide the population into halves that differ by 47, 28, or 36 days in median lifespan. When mice are classified on all three indices, however, those with the optimal combination (short femur length at 4 months, greatest change in cortical thickness between 4 and 15 months, and lowest number of CD8 memory cells at 18 months) have a median survival that is 103 days longer than the group with the opposite set of traits. This value is 12% of the median lifespan for the population as a whole. The right panel of Figure 3 shows survival curves for these two mouse subpopulations.
Table 4.
Cox regression for a model combining bone and immune predictors of lifespan
| Model | Hazard Ratio | p-values | Effect Sizes (univariate) |
Effect size (days) (overall model) |
|---|---|---|---|---|
| Len (4) | 1.33 | 0.02 | 47 | 103 |
| CThick (Change) | 0.06 | 0.01 | 28 | |
| CD8M (18) | 1.009 | 0.03 | 36 | |
Hazard ratios and associated p-values for a Cox regression model with three predictors: Len at 4 months, CThick change score from 4 to 15 months, and CD8 memory cell value at 18 months. Effect size (univariate) indicates difference in median lifespan between mice in the top or bottom half of the distribution for each predictor variable considered separately. Right-hand column gives difference in median lifespan between mice with above or below the median values for each of the three predictors, and corresponds to the survival curve shown in Figure 3 (right panel).
The Wang-Allison method (Wang et al., 2004) was used to evaluate differences between these subpopulations in extreme survival. The two subpopulations shown in Figure 3 (right panel) had a joint survival distribution for which only 10% of the mice lived more than 1074 days. At this age, 3/68 (4%) mice in the shorter-lived population still survived, compared to 10/60 (17%) of the longer-lived group; this difference is significant at p = 0.022 by Fisher's exact test. We conclude that a combination of bone and immune predictors can divide the population into subsets that differ in maximal lifespan.
Correlation between immune and bone predictors
Lastly, we evaluated the question of whether mice that preserve femoral bone in midlife also preserve youthful immune status, a hypothesis consistent with the idea that each of these indices provides information about inter-mouse differences in rate of aging. Mice with low levels of CD8M were indeed found to have relatively high levels of CThick change score (R = −0.1, p = 0.03) and low levels of Endo (measured at 15 months) (R = 0.12, p = 0.01). Both relationships are in the predicted direction, in that mice with youthful CD8M levels also have values for Endo and CThick change scores that are associated with longer survival. The association is in each case statistically significant, but small in size.
Discussion
Micro-CT methods now permit serial assessment of bone geometry in individual mice as they age. We have shown here that measurements of bone size and shape can predict mouse lifespan at ages well below the median age at death. The spectrum of traits that have predictive value changes with age: for some traits, like CThick, high values can be predictive of longevity at one age and low values at other ages. We found that combinations of bone measures and age-sensitive immune endpoints can divide the population into subsets that differ in median lifespan by as much as 100 days, with significant difference in maximal lifespan. The study was conducted in genetically heterogeneous mice, to provide sources of both genetic and non-genetic differences, and to facilitate a later search for genetic polymorphisms relevant to bone aging and mouse longevity.
Traits that predict life expectancy can, in principle, emerge from at least three categories, each with its own implications for biology of bone development, disease risk, and aging.
Some traits may serve as indicators of risk of specific lethal illnesses; these are the classical "risk factors." Head trauma, for example, is a well-known risk factor for some forms of human neurodegenerative disease, just as are inherited alleles for Huntington's disease. In the area of bone biology, a malformed acetabulum is an important risk factor, in Labrador Retrievers, for late-life hip arthritis, often serious enough to require humane euthanasia of the animal. Disease-specific risk factors of this kind do not provide useful information about the biology of the aging process and its regulation, despite their importance as clues to the risk, and in some cases the pathogenesis, of important and potentially fatal illnesses.
A second class of predictors consists of traits that may indeed be closely related to inter-individual differences in aging, regardless of the effects of aging on the trait itself. Among dogs, for example, smaller purebred dogs tend to live much longer than dogs of larger breeds (Li et al., 1996; Michell, 1999), and small stature is also associated with longer lifespan among non-purebred dogs (Patronek et al., 1997). Similarly, short stature in humans is associated with a dramatic reduction in risk of most forms of neoplasia (Davey et al., 2000; Albanes et al., 1988; Hebert et al., 1993; Tretli, 1989; Petrelli et al., 2002; Tretli and Robsahm, 1999). The association between short stature and increased longevity in dogs is associated with, and presumably reflects, resistance of shorter dogs to many forms of illness, including cataracts (Williams et al., 2004), lethal cardiovascular, neurological, and neoplastic disease (Bonnett et al., 2005), and other geriatric complaints (Hoskins and McCurnin, 1997). Small body size is also associated with higher life expectancy among related breeds of mice (Miller et al., 2000), and among individual mice of the UM-HET3 stock (Miller et al., 2002). It is plausible, though not proven, that some of the differences among mouse stocks, mice, and dog breeds in life expectancy might represent effects of growth hormone and/or IGF-1, in that single gene mutations that inhibit GH and IGF-1 signals often lead to improved longevity in mice (Brown-Borg et al., 1996; Coschigano et al., 2000; Flurkey et al., 2001), as well as to postponement of many of the lethal and non-lethal consequences of aging (Flurkey et al., 1992; Vergara et al., 2004; Ikeno et al., 2003; Kinney et al., 2001). Differences among individuals, or breeds, in body stature or in underlying GH/IGF levels (Eigenmann et al., 1984) , may lead to differences in aging rate and thus modulate incidence and severity of a wide range of age-related traits and diseases, whether or not size and hormone levels change systematically with age. Measurements of bone size and robustness, taken early in adult life, fall into this category.
The third class of predictors, conceptually, might represent variables that are age-sensitive in adults, and whose pace of change in adult life can serve as a surrogate for the pace of the underlying aging process (Miller, 2001a). Such predictors can be considered "biomarkers" of aging, in the same sense that blood alcohol level can be considered a biomarker for recent consumption of alcoholic beverages, i.e. as an easily measurable index of an unobserved process (aging, or drinking). It is easy to construct a set of biomarkers that distinguish long-lived from short-lived dog breeds: an individual dog that reaches age 6 or 8 years without any evidence of cataracts, arthritis, anosmia, presbycusis, or cardiovascular decline can be considered to be aging more slowly than a dog with problems in most of these age-sensitive systems. Biomarkers of aging, like risk factors for disease, may well be associated statistically with altered life expectancy, but are also expected to have predictive power for a wide range of age-sensitive traits unrelated to specific lethal illnesses. It has been remarkably difficult to validate biomarkers of aging that are distinct from disease-specific risk factors (class 1 above) and from surrogates for processes that control aging rate (class 2 above).
We believe that the bone traits listed in Table 2 include representatives of two of these three classes. The measurements taken at age 4 months reflect, we think, different estimators of overall robustness and size; these are all correlated with one another, and are all associated with lifespan in the same direction. Long femur length, large cross-sectional area, thick cortical bone, long periosteal diameter, and high bone mineral content are all seen in mice destined for relatively short lives. The ability of these measures, taken at 4 months of age, to predict life expectancy seems likely to represent the influence of some underlying physiological factor, possibly related to the GH/IGF-1 axis, with effects on both aging and bone morphology.
The predictive factors that attain significance only in midlife, however, may well be of another type, class 3 above, i.e. biomarkers of the pace of aging. In 15 month old mice, those with the largest periosteal diameters have the shortest life expectancy, and by 24 months high values for cortical bone thickness, originally associated with poor survival, are now significantly associated with higher life expectancy. Measures of cross-sectional area show a similar pattern: a negative association with survival when measured at 4 months, and a positive (though not significant at p = 0.08) association at the 24 month time point. All three of these measures provide related information about the size of the marrow cavity, the thickness of the remaining cortical bone, and the relative ability of bone-forming process in the interior of the femur to keep pace with bone resorption. Thus optimal survival is seen in mice that have relatively small femurs when young (low values for CThick, Csa, and Perio as well as Len), but which retain as much bone thickness as possible at oldest ages and thus have relatively high values for Cthick and low values for Endo. The bivariate regression analysis shows that measurements sensitive to bone remodeling between 4 and 15 months of age add predictive power beyond models that rely only on indices of bone size at age 4 months.
Neither variety of bone measurement is a plausible candidate for a risk factor for lethal disease in UM-HET3 mice. Terminal necropsies have been reported for 886 UM-HET3 mice (Lipman et al., 2004), including 344 virgin females. Most of these mice die of neoplasms, including fibrosarcoma, hemangiosarcoma, lymphoma, and pulmonary adenocarcinoma. Non-neoplastic illnesses can account for death in about 6% of the virgin females. It seems very unlikely that inter-animal differences either in bone size, or in midlife bone remodeling, contribute to the risk of these terminal illnesses. We therefore favor the hypothesis that differences in the pace at which cortical bone thickness is lost (and endosteal perimeter increased) are related to differences among the mice in the rate of aging, which in turn affects risks and severity of lethal diseases in these animals.
Our data show a correlation between the rate of bone remodeling and survival in mice. There appear to be two distinct varieties of bone remodeling, likely to differ in their mode of regulation and their functional consequences. The first is targeted remodeling, a mechanism to respond to the need for maintaining mechanical integrity, including the repair of microdamage in humans. The second is stochastic remodeling, associated with metabolic homeostasis, providing access to stored chemical constituents such as calcium (Parfitt, 2002; Han et al., 1997; Burr and Martin, 1993). Stochastic remodeling is regulated by hormones and a variety of growth factors, while targeted remodeling has been reported as a response to osteocyte apoptosis (resulting from microdamage and continued mechanical usage), habitual mechanical demand, and changes in the extracellular matrix with advanced age (Waldorff et al., 2010; Noble, 2003; Heaney, 2003; Burr, 2002). Independent of the remodeling mechanism, however, it has been proposed that the local process of remodeling results in a local net loss in bone volume (Parfitt, 2002). This proposed loss of bone with remodeling, supported by histological observations, occurs despite the tight coupling known to exist between bone resorption and formation. As a result, a substantial increase in remodeling rate has been correlated to an increase in bone loss. This phenomena is particularly apparent in the dramatic increase in rate of bone loss in human females during the peri- and post-menopausal years. Similar loss of bone is observed in mouse models of estrogen deficiency as a result of ovariectomy (Bouxsein et al., 2005; Alexander et al., 2001). Interesting, the rate or extent of bone loss due to decrease in estrogen has been observed to be species specific and is hypothesized to be partly genetically regulated (Iwaniec et al., 2006). Within the context of this paper, the maintenance of cortical thickness and periosteal diameter in longer-lived mice suggests that a slower pace of remodeling is associated with survival, and that the rate of age-related bone change may be controlled by processes that also influence the age of occurrence of other late life illnesses, including those that lead to death in UM-HET3 mice. It is important to clarify this perspective and hypothesis. The observed changes to bone on the endosteal and periosteal surfaces might also be considered a modeling drift, since they may be occurring independently on different surfaces. Considering the advanced age of the mice, however, we suspect that these findings are, in fact, a result of a variation in remodeling occurring on both the endosteal and periosteal surfaces. This proposed bone remodeling is likely to reflect interactions among many regulatory pathways, including changes in hormones and hormonal receptors, damage resistance in the extracellular matrix, osteocyte resistance to apoptosis, and factors that influence signaling for osteoclastogenesis and stem cell differentiation. Clearly, these provide valuable avenues for future investigation, as well as the need to verify whether the observations are a result of remodeling or a modeling drift.
Inter-animal variation in levels of CD8 memory T cells (as a proportion of CD8 T cells) provides additional predictive power when added to regression models that include femur length, retained cortical bone thickness, or both bone variables. We have shown previously that age sensitive T cell subsets (Harper et al., 2004; Miller et al., 1997; Miller, 2001b; Miller and Chrisp, 2002), including memory cells of the CD4 and CD8 lineages, in both spleen and blood, can predict lifespan in genetically heterogeneous mice. In the current population, the CD8M subset has independent predictive value in regression models that already contain both kinds of bone information. When the mice are divided into subsets distinguished by the median values of all three predictors, the 1/8th of the mice with the most favorable combination has a median life expectancy more than 100 days above that of the 1/8th of the mice with the opposite set of predictors, and differs significantly in a surrogate for maximal lifespan (the Wang-Allison test (Wang et al., 2004)). The simplest interpretation is that the factors that modulate age-dependent T cell changes are at least in part distinct from those that influence early and midlife variation in femoral bone.
The challenge of finding mid-life endpoints that can serve as surrogate markers for differences in rate of aging, so easy to meet in studies across species and across breeds of long-lived vs. short-lived dogs, is still a live issue in rodent and human biogerontology. Further developments in this domain would help address the theoretical issue of whether individual mice, or individual people, do in fact age at varying rates, and at the same time provide a practical tool for screening putative anti-aging drugs to find candidates for further detailed and costly analyses.
Experimental Procedures
Mice
Genetically heterogeneous mice were produced by a cross between (C57BL/6J×BALB/cJ)F1 males and (C3H/HeJ×DBA/2J)F1 females. Mice of this variety are called UM-HET3 in previous publications, and have been used to document genetic polymorphisms that control femoral and vertebral dimensions, and mechanical fragility in 18 month old mice (Reeves et al., 2007; Volkman et al., 2004; Volkman et al., 2003), as well as genetic variation relevant to immune aging (Jackson et al., 1999; Miller et al., 2003), and for evaluation of immunological predictors of lifespan (Miller and Chrisp, 2002; Miller et al., 1997). With respect to nuclear genes, each mouse in the population can be considered a full sib of each other mouse, sharing a random 50% of its genetic inheritance. This study used only female mice, bred over a period of 24 months, housed in groups of 4 mice/cage, and given free access to water and mouse chow. The colony was evaluated every three months to document its specific-pathogen-free status as previously described (Miller et al., 2007); all such tests were negative throughout the experiments reported here. In addition to the bone assessments described below, each mouse was subjected to a tail-tip biopsy for genotyping (to be reported elsewhere), and subjected to tail venipuncture at 18 months to provide blood for T cell evaluation. Mice were inspected daily for general health, and age at death recorded for those found dead. Mice found to be extremely ill were euthanized if, in the judgment of an experienced caretaker, they were thought unlikely to survive more than another day; in these cases the date of euthanasia was used to calculate age at death.
The experimental population initially consisted of 600 mice. Fifteen of these died prior to one year of age, and because of technical errors no age at death was recorded for three others. Of the remaining 582 mice, 567 had died by the date of analysis, and 15 (3%) were still alive (right censored in Cox regression analyses). Of these 519 mice had micro-CT scans at 4 months of age, and the analysis is thus based on assessment of these 519 animals.
MicroCT
Mice were placed into an induction chamber of 2% to 5% isoflurane gas (MWI Veterinary Supply). Once the mice were fully anesthetized, they were positioned and secured in the prone position on the scanning bed and maintained on 2% isoflurane gas through a nose cone throughout the imaging. The mice were imaged at 4, 15, and 24 months of age using an eXplore Locus in-vivo micro-computed tomography (microCT) scanner (GE Healthcare Pre-Clinical Imaging, London, Ontario). This system features a high precision gantry system that rotates the x-ray source and detector continuously around the anesthetized animal. Scan protocols are short in duration to limit the radiation dose to the animals, particularly important in longitudinal studies such as this. Images were reconstructed at a 45 micron isotropic voxel size. Histograms were generated to select a global mineralized tissue threshold that delineated bone from all other tissues. Geometric and densitometric analyses were performed on a standardized 3-mm mid-diaphyseal segment in the left femora of each animal at each time point. The selected bone volume of interest was analyzed to determine the transverse properties including, cross-sectional area, cortical thickness, endosteal and perisoteal perimeters, and bone mineral content. The variables are schematically illustrated in Figure 1 and summarized along with their abbreviations in Table 1.
Figure 1.
The overall whole bone femoral length (Len) was calculated from thresholded microCT images. Geometric analyses were performed on a standardized 3-mm mid-diaphyseal segment within the left femora. Cross-sectional area (Csa), cortical thickness (CThick), and periosteal (Perio) and endosteal (Endo) perimeters were measured at 4, 15, and 24 months.
Table 1.
Summary of bone measurements used for this study
| Trait | Abbreviation | Units | Description |
|---|---|---|---|
| Length | Len | mm | Whole bone femoral length |
| Cross-sectional area | Csa | mm2 | Average cortical bone area of a cross-section within the diaphyseal region of interest |
| Cortical thickness | CThick | mm | Average thickness of femoral cortex around circumference of bone within the diaphyseal region of interest |
| Endosteal perimeter | Endo | mm | Perimeter of the inner surface of the cortex (endosteum) within the diaphyseal region of interest |
| Periosteal perimeter | Perio | mm | Perimeter of the outer surface of the cortex (periosteum) within the diaphyseal region of interest |
| Bone mineral content | Bmc | mg/cc | Mineral content of the bone within the diaphyseal region of interest |
T cell subsets
The proportion of peripheral blood CD8 T cells that express high levels of the memory cell marker CD44 was evaluated in each mouse at 18 months of age using flow cytometry as described previously (Jackson et al., 1999; Miller and Chrisp, 2002). This proportion is referred to as the CD8M value in this paper.
Statistical methods
Tabulated average values are shown in tables as mean ± standard error of the mean. Change scores are calculated by subtracting the value at the earlier age from the value for the later age, for all mice for which both measurements were available. Inferences about age effects on each bone measurement, in specific age intervals, were evaluated by testing the hypothesis that the corresponding change score was equal to zero. Associations between predictor values (bone measures, change scores, T cell subsets) and survival were evaluated using Cox proportional hazard regression.
Acknowledgements
We thank Liz Burmeister, Sabrina Friedline, and Meenal Mhasker for animal husbandry assistance, and Kathy Sweet for technical support in the bone measurement program. This work was supported by NIA grants AG011687, AG024824 (Pepper Center) and AG013283 (Shock Center).
Footnotes
Author contributions
Drs. Miller and Goldstein designed the study. Ms. Kreider conducted the bone scans; she and Dr. Goldstein computed measures of femur dimensions from the raw data sets. Dr. Galecki advised on design and analysis procedures. Dr. Miller and Dr. Goldstein wrote the manuscript.
Contributor Information
Jaclynn Kreider, Email: jkriegl@umich.edu.
Andrzej Galecki, Email: agalecki@umich.edu.
Steven A. Goldstein, Email: stevegld@umich.edu.
References
- Albanes D, Jones DY, Schatzkin A, Micozzi MS, Taylor PR. Adult stature and risk of cancer. Cancer Res. 1988;48:1658–1662. [PubMed] [Google Scholar]
- Alexander JM, Bab I, Fish S, Muller R, Uchiyama T, Gronowicz G, Nahounou M, Zhao Q, White DW, Chorev M, Gazit D, Rosenblatt M. Human parathyroid hormone 1–34 reverses bone loss in ovariectomized mice. J Bone Miner. Res. 2001;16:1665–1673. doi: 10.1359/jbmr.2001.16.9.1665. [DOI] [PubMed] [Google Scholar]
- Bonnett BN, Egenvall A, Hedhammar A, Olson P. Mortality in over 350,000 insured Swedish dogs from 1995–2000: I. Breed-, gender-, age- and cause-specific rates. Acta Vet. Scand. 2005;46:105–120. doi: 10.1186/1751-0147-46-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouxsein ML, Myers KS, Shultz KL, Donahue LR, Rosen CJ, Beamer WG. Ovariectomy-induced bone loss varies among inbred strains of mice. J Bone Miner. Res. 2005;20:1085–1092. doi: 10.1359/JBMR.050307. [DOI] [PubMed] [Google Scholar]
- Brown-Borg HM, Borg KE, Meliska CJ, Bartke A. Dwarf mice and the ageing process. Nature. 1996;384:33. doi: 10.1038/384033a0. [DOI] [PubMed] [Google Scholar]
- Burr DB. Targeted and nontargeted remodeling. Bone. 2002;30:2–4. doi: 10.1016/s8756-3282(01)00619-6. [DOI] [PubMed] [Google Scholar]
- Burr DB, Martin RB. Calculating the probability that microcracks initiate resorption spaces. J Biomech. 1993;26:613–616. doi: 10.1016/0021-9290(93)90023-8. [DOI] [PubMed] [Google Scholar]
- Coschigano KT, Clemmons D, Bellush LL, Kopchick JJ. Assessment of growth parameters and life span of GHR/BP gene-disrupted mice. Endocrinology. 2000;141:2608–2613. doi: 10.1210/endo.141.7.7586. [DOI] [PubMed] [Google Scholar]
- Davey SG, Hart C, Upton M, Hole D, Gillis C, Watt G, Hawthorne V. Height and risk of death among men and women: aetiological implications of associations with cardiorespiratory disease and cancer mortality. Journal of Epidemiology & Community Health. 2000;54:97–103. doi: 10.1136/jech.54.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eigenmann JE, Patterson DF, Froesch ER. Body size parallels insulin-like growth factor I levels but not growth hormone secretory capacity. Acta Endocrinologica. 1984;106:448–453. doi: 10.1530/acta.0.1060448. [DOI] [PubMed] [Google Scholar]
- Flurkey K, Miller RA, Harrison DE. Cellular determinants of age-related decrements in the T-cell mitogen response of B6CBAF1 mice. J. Gerontol. Biol. Sci. 1992;47:B115–B120. doi: 10.1093/geronj/47.4.b115. [DOI] [PubMed] [Google Scholar]
- Flurkey K, Papaconstantinou J, Miller RA, Harrison DE. Lifespan extension and delayed immune and collagen aging in mutant mice with defects in growth hormone production. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:6736–6741. doi: 10.1073/pnas.111158898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han ZH, Palnitkar S, Rao DS, Nelson D, Parfitt AM. Effects of ethnicity and age or menopause on the remodeling and turnover of iliac bone: implications for mechanisms of bone loss. J Bone Miner. Res. 1997;12:498–508. doi: 10.1359/jbmr.1997.12.4.498. [DOI] [PubMed] [Google Scholar]
- Harper JM, Galecki AT, Burke DT, Miller RA. Body weight, hormones and T-cell subsets as predictors of lifespan in genetically heterogeneous mice. Mechanisms of Ageing & Development. 2004;125:381–390. doi: 10.1016/j.mad.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Heaney RP. Is the paradigm shifting? Bone. 2003;33:457–465. doi: 10.1016/s8756-3282(03)00236-9. [DOI] [PubMed] [Google Scholar]
- Hebert PR, Rich-Edwards JW, Manson JE, Ridker PM, Cook NR, O'Connor GT, Buring JE, Hennekens CH. Height and incidence of cardiovascular disease in male physicians. Circulation. 1993;88:1437–1443. doi: 10.1161/01.cir.88.4.1437. [DOI] [PubMed] [Google Scholar]
- Hoskins JD, McCurnin DM. Geriatric care in the late 1990s. Veterinary. Clinics. of North America. - Small Animal. Practice. 1997;27:1273–1284. doi: 10.1016/s0195-5616(97)50126-4. [DOI] [PubMed] [Google Scholar]
- Ikeno Y, Bronson RT, Hubbard GB, Lee S, Bartke A. Delayed occurrence of fatal neoplastic diseases in ames dwarf mice: correlation to extended longevity. Journals. of. Gerontology. Series. A. -Biological. Sciences. & Medical. Sciences. 2003;58:291–296. doi: 10.1093/gerona/58.4.b291. [DOI] [PubMed] [Google Scholar]
- Iwaniec UT, Yuan D, Power RA, Wronski TJ. Strain-dependent variations in the response of cancellous bone to ovariectomy in mice. J Bone Miner. Res. 2006;21:1068–1074. doi: 10.1359/jbmr.060402. [DOI] [PubMed] [Google Scholar]
- Jackson AU, Fornes A, Galecki A, Miller RA, Burke DT. Multiple-trait quantitative trait loci analysis using a large mouse sibship. Genetics. 1999;151:785–795. doi: 10.1093/genetics/151.2.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TE. Recent results: biomarkers of aging. Exp. Gerontol. 2006;41:1243–1246. doi: 10.1016/j.exger.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Kinney BA, Meliska CJ, Steger RW, Bartke A. Evidence that Ames dwarf mice age differently from their normal siblings in behavioral and learning and memory parameters. Hormones & Behavior. 2001;39:277–284. doi: 10.1006/hbeh.2001.1654. [DOI] [PubMed] [Google Scholar]
- Li Y, Deeb B, Pendergrass W, Wolf N. Cellular proliferative capacity and life span in small and large dogs. J. Gerontol. A. Biol. Sci. Med. Sci. 1996;51:B403–B408. doi: 10.1093/gerona/51a.6.b403. [DOI] [PubMed] [Google Scholar]
- Lipman R, Galecki A, Burke DT, Miller RA. Genetic loci that influence cause of death in a heterogeneous mouse stock. J. Gerontol. Biol. Sci. 2004;59A:977–983. doi: 10.1093/gerona/59.10.B977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michell AR. Longevity of British breeds of dog and its relationships with sex, size, cardiovascular variables and disease. Veterinary Record. 1999;145:625–629. doi: 10.1136/vr.145.22.625. [DOI] [PubMed] [Google Scholar]
- Miller RA. Biomarkers of aging. Sci. Aging Knowledge. Environ. 2001a:e2. doi: 10.1126/sageke.2001.1.pe2. 2001. [DOI] [PubMed] [Google Scholar]
- Miller RA. Biomarkers of aging: prediction of longevity by using age-sensitive T-cell subset determinations in a middle-aged, genetically heterogeneous mouse population. Journals of Gerontology Series A, Biological Sciences & Medical Sciences. 2001b;56:B180–B186. doi: 10.1093/gerona/56.4.B180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RA, Austad S, Burke D, Chrisp C, Dysko R, Galecki A, Monnier V. Exotic mice as models for aging research: polemic and prospectus. Neurobiol. Aging. 1999;20:217–231. doi: 10.1016/s0197-4580(99)00038-x. [DOI] [PubMed] [Google Scholar]
- Miller RA, Chrisp C. T cell subset patterns that predict resistance to spontaneous lymphoma, mammary adenocarcinoma, and fibrosarcoma in mice. J. Immunol. 2002;169:1619–1625. doi: 10.4049/jimmunol.169.3.1619. [DOI] [PubMed] [Google Scholar]
- Miller RA, Chrisp C, Atchley WR. Differential longevity in mouse stocks selected for early life growth trajectory. J. Gerontol. Biol. Sci. 2000;55A:B455–B461. doi: 10.1093/gerona/55.9.b455. [DOI] [PubMed] [Google Scholar]
- Miller RA, Chrisp C, Galecki A. CD4 memory T cell levels predict lifespan in genetically heterogeneous mice. FASEB J. 1997;11:775–783. doi: 10.1096/fasebj.11.10.9271362. [DOI] [PubMed] [Google Scholar]
- Miller RA, Harper JM, Galecki A, Burke DT. Big mice die young: early-life body weight predicts longevity in genetically heterogeneous mice. Aging Cell. 2002;1:22–29. doi: 10.1046/j.1474-9728.2002.00006.x. [DOI] [PubMed] [Google Scholar]
- Miller RA, Harrison DE, Astle CM, Floyd RA, Flurkey K, Hensley KL, Javors MA, Leeuwenburgh C, Nelson JF, Ongini E, Nadon NL, Warner HR, Strong R. An aging interventions testing program: study design and interim report. Aging Cell. 2007;6:565–575. doi: 10.1111/j.1474-9726.2007.00311.x. [DOI] [PubMed] [Google Scholar]
- Miller RA, Jackson AU, Galecki AT, Burke DT. Genetic polymorphisms in mouse genes regulating age-sensitive and age-stable T cell subsets in mice. Genes and Immunity. 2003;4:30–39. doi: 10.1038/sj.gene.6363895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble B. Bone microdamage and cell apoptosis. Eur. Cell Mater. 2003;6:46–55. doi: 10.22203/ecm.v006a05. [DOI] [PubMed] [Google Scholar]
- Parfitt AM. Targeted and nontargeted bone remodeling: relationship to basic multicellular unit origination and progression. Bone. 2002;30:5–7. doi: 10.1016/s8756-3282(01)00642-1. [DOI] [PubMed] [Google Scholar]
- Patronek GJ, Waters DJ, Glickman LT. Comparative longevity of pet dogs and humans: implications for gerontology research. J Gerontol A Biol Sci Med Sci. 1997;52:B171–B178. doi: 10.1093/gerona/52a.3.b171. [DOI] [PubMed] [Google Scholar]
- Petrelli JM, Calle EE, Rodriguez C, Thun MJ. Body mass index, height, and postmenopausal breast cancer mortality in a prospective cohort of US women. Cancer Causes Control. 2002;13:325–332. doi: 10.1023/a:1015288615472. [DOI] [PubMed] [Google Scholar]
- Reeves GM, McCreadie BR, Chen S, Galecki AT, Burke DT, Miller RA, Goldstein SA. Quantitative trait loci modulate vertebral morphology and mechanical properties in a population of 18-month-old genetically heterogeneous mice. Bone. 2007;40:433–443. doi: 10.1016/j.bone.2006.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprott RL. Biomarkers of aging. J. Gerontol. Biol. Sci. 1999;54A:B464–B465. doi: 10.1093/gerona/54.11.b464. [DOI] [PubMed] [Google Scholar]
- Tretli S. Height and weight in relation to breast cancer morbidity and mortality. A prospective study of 570,000 women in Norway. Int. J Cancer. 1989;44:23–30. doi: 10.1002/ijc.2910440105. [DOI] [PubMed] [Google Scholar]
- Tretli S, Robsahm TE. Height, weight and cancer of the oesophagus and stomach: a follow-up study in Norway. Eur. J Cancer Prev. 1999;8:115–122. doi: 10.1097/00008469-199904000-00005. [DOI] [PubMed] [Google Scholar]
- Vergara M, Smith-Wheelock M, Harper JM, Sigler R, Miller RA. Hormone-treated Snell dwarf mice regain fertility but remain long-lived and disease resistant. J. Gerontol. Biol. Sci. 2004;59:1244–1250. doi: 10.1093/gerona/59.12.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkman SK, Galecki AT, Burke DT, Miller RA, Goldstein SA. Quantitative trait loci that modulate femoral mechanical properties in a genetically heterogeneous mouse population. Journal of Bone and Mineral Research. 2004;19:1497–1505. doi: 10.1359/JBMR.040506. [DOI] [PubMed] [Google Scholar]
- Volkman SK, Galecki AT, Burke DT, Paczas MR, Moalli MR, Miller RA, Goldstein SA. Quantitative trait loci for femoral size and shape in a genetically heterogeneous mouse population. Journal of Bone and Mineral Research. 2003;18:1497–1505. doi: 10.1359/jbmr.2003.18.8.1497. [DOI] [PubMed] [Google Scholar]
- Waldorff EI, Christenson KB, Cooney LA, Goldstein SA. Microdamage Repair and Remodeling Requires Mechanical Loading. J Bone Miner. Res. 2010;25:734–745. doi: 10.1359/jbmr.091016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Li Q, Redden DT, Weindruch R, Allison DB. Statistical methods for testing effects on "maximum lifespan". Mech Ageing Dev. 2004;125:629–632. doi: 10.1016/j.mad.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Williams DL, Heath MF, Wallis C. Prevalence of canine cataract: preliminary results of a cross-sectional study. Veterinary Ophthalmology. 2004;7:29–35. doi: 10.1111/j.1463-5224.2004.00317.x. [DOI] [PubMed] [Google Scholar]