Summary
Progress in unraveling the genetic origins of healthy aging is tempered, in part, by a lack of replication of effects, which is often considered a signature of false positive findings. We convincingly demonstrate that the lack of genetic effects on an aging-related trait can be due to trade-offs in the gene action. We focus on the well-studied apolipoproetin E (APOE) e2/3/4 polymorphism and on lifespan and ages at onset of cardiovascular diseases (CVD) and cancer, using data on 3,924 participants of the Framingham Heart Study Offspring cohort. Kaplan-Meier estimates show that the e4 allele carriers live shorter lives than the non-e4 allele carriers (log rank=0.016). The adverse effect was attributed to the poor survival of the e4 homozygotes, whereas the effect of the common e3/4 genotype was insignificant. The e3/4 genotype, however, was antagonistically associated with onsets of those diseases predisposing to an earlier onset of CVD and a later onset of cancer compared to the non-e4 allele genotypes. This trade-off explains the lack of a significant effect of the e3/4 genotype on survival; adjustment for it in the Cox regression model makes the detrimental effect of the e4 allele highly significant (p=0.002). This trade-off is likely caused by the lipid-metabolism-related (for CVD) and non-related (for cancer) mechanisms. An evolutionary rationale suggests that genetic trade-offs should not be an exception in studies of aging-related traits. Deeper insights into biological mechanisms mediating gene action are critical for understanding the genetic regulation of a healthy lifespan and for personalizing medical care.
Keywords: Aging, Longevity regulation, mortality, trade-offs, disease, lifespan, genetics
Introduction
The rapidly growing elderly population worldwide raises serious concerns about increasing the healthy lifespan (Olshansky et al. 2007; Sierra et al. 2008). Because longevity and various aging-related traits are heritable (Vijg & Suh 2005; Martin et al. 2007), understanding the role of genes in regulating such traits could be a basis for major breakthroughs in addressing this problem.
A currently prevailing hypothesis in genetic association studies of complex traits, including aging-related diseases and phenotypes, is that they can be caused by common genetic variants (Christensen et al. 2006; Cutler & Mattson 2006; Gibson 2009). However, decades of candidate-gene studies and recent substantial investments in genome-wide association studies (GWAS) following the “common disease – common variant” hypothesis have not yet provided major breakthroughs. Although GWAS have revealed about 2000 genome-wide associations at p<5×10−8 for multiple complex traits (http://www.genome.gov/gwastudies), these genetic effects are very modest (Gorlov et al. 2008; Gibson 2009; Goldstein 2009; Plomin et al. 2009), explaining only a small fraction of genetic variability in susceptibility to such traits (Frazer et al. 2009). GWAS have also failed to identify common risk alleles that substantially affect human longevity (Lunetta et al. 2007; Beekman et al. 2010; Newman et al. 2010).
Candidate-gene studies have been more successful in that they identified several common allelic variants (e.g., of APOE (Christensen et al. 2006; Salvioli et al. 2006), CETP (Barzilai et al. 2003; Koropatnick et al. 2008), FOXO3A (Willcox et al. 2008; Flachsbart et al. 2009) genes) which could explain modest susceptibility to the aging-related traits and predisposition to long life in humans. Such modest progress in finding genes involved in regulation of human aging is surprising because numerous aging-related genes have been identified in lower organisms (Kenyon 2005; Cutler & Mattson 2006; Greer & Brunet 2008). Possible explanations of such a modest progress include a high complexity of biological functions of such genes in the human organism (Goh et al. 2007; Martin et al. 2007) and the lack of an evolutionary program directly working against aging-related traits with post-reproductive manifestation (Di Rienzo & Hudson 2005; Vijg & Suh 2005).
A vivid example is the apolipoprotein E (APOE) common polymorphism (e2, e3, and e4), which is one of the best studied polymorphisms in humans. The APOE polymorphism is shown to be involved in regulation of numerous geriatric diseases including cognitive impairment, Alzheimer's disease (AD), atherosclerosis, stroke, diabetes, cancer, etc. (Smith 2002; Farlow et al. 2004; Moore et al. 2004; Jofre-Monseny et al. 2008). Studies show that this polymorphism can be also associated with longevity because the proportion of carriers of the e4 allele in the oldest-old population is found to be smaller compared to that in adult controls (Christensen et al. 2006; Jacobsen et al. 2010). One cross-sectional study (Gerdes et al. 2000) explained large differences in frequencies of the e2 and e4 alleles in adults and centenarians by minor differences in age-specific risks of death suggesting the role of the APOE as a frailty gene rather than longevity gene. Another study of a large sample of elderly Canadians did not reveal, however, significant association between the APOE polymorphism and frailty (Rockwood et al. 2008).
The APOE gene has been extensively studied for its associations with various reproductive-age-related traits including lipid metabolism, oxidative stress, and inflammation (Finch 2007; Jofre-Monseny et al. 2008). Given the broad biological role of the APOE gene (Mahley 1988; Jofre-Monseny et al. 2008), it can be involved in regulation of various aspects of aging (Finch & Morgan 2007; Bonomini et al. 2010; Finch 2010). Specifically, the e4 allele is associated with elevated plasma cholesterol and low density lipoprotein levels (Mahley 1988); it typically promotes inflammation and is associated with higher oxidative stress, which can cause CVD and neurodegenerative diseases later in life (Eichner et al. 2002; Jofre-Monseny et al. 2008). Furthermore, the role of the e4 allele in human development is increasingly recognized. For instance, it has been shown that healthy juveniles carrying the e4 allele have a thinner entorhinal cortex (a major component of brain memory network) which might predispose to Alzheimer’s disease (Shaw et al. 2007).
The APOE polymorphism has unique evolutionary-developed human-specific functions (Finch 2010). Specifically, the e4 allele is considered as an ancestral variant with the e3 allele spread uniquely in humans (Fullerton et al. 2000). Despite its broad adverse effects on various traits, the e4 allele is still common in human population (Drenos & Kirkwood 2010). Therefore, this allele might also be beneficial. This is the essence of the Charlesworth’s (Charlesworth 1996) and Martin’s (Martin 1999) hypothesis, who suggested that the e4 allele had been subject to balancing evolutionary selection because of its potential protective role on other traits. For instance, several studies have shown that the e4 allele could be protective against some infectious diseases (Oria et al. 2005; Oria et al. 2007) and a lipophilic virus (Charlesworth 1996). The e4 allele can be also protective against liver damage caused by the hepatitis C virus (Wozniak et al. 2002; Fabris et al. 2005). Epidemiological studies also suggest a protective role of the e4 allele on such post-reproductive trait as macular degeneration (Klaver et al. 1998; Souied et al. 1998; Thakkinstian et al. 2006).
Nevertheless, the effect of even such a well-studied gene on survival remains controversial (see, e.g., (Little et al. 2009; Panza et al. 2009) and references therein). Survival is the most important phenotype, because it summarizes the entire life course of individuals. If, for instance, a study documents an effect of a certain genetic variant on a life-threatening aging-related trait but its effect on survival is uncertain (as in the case of the APOE), it might well be because such a genetic variant can exhibit pleiotropy with trade-off in the effects on different causes of death. More generally, a lack of replication of a genetic effect on a complex (non-Mendelian) aging-related phenotype in various populations and settings could be due to trade-off in the gene action on different risk factors for such a phenotype rather than due to false positive findings.
The aim of this work is to test the above hypothesis by comparing the effects of the APOE e2/3/4 polymorphism on survival, lifespan, and ages at onsets of major causes of death in developed countries, i.e., cardiovascular diseases (CVD) and cancer (all sites but skin), using data from the Framingham Heart Study Offspring (FHSO) cohort followed up for about 36 years.
Materials and Methods
Study Design and Population
The FHSO cohort was launched in 1971, i.e., 22 years after the original Framingham Heart Study (FHS) cohort (launched in 1948). The study design has been described by Dawber et al. (1951), Feinleib et al. (1975), and Splansky et al. (2007). Briefly, the FHSO respondents (N=5,124) aged 5–70 years were biological descendants (N=3,514), their spouses (N=1,576), and adopted offspring (N=34) of the FHS participants. The publicly-released limited-access FHSO data available for this study have phenotypic information assessed at six FHSO examinations performed in 1971–1975, 1979–1982, 1984–1987, 1987–1990, 1991–1995, and 1996–1997. The FHSO participants have been followed for the onset of CVD, cancer, and death through regular examinations at the FHS clinic, surveillance of hospital admissions, and death registries (Splansky et al. 2007; Govindaraju et al. 2008) for up to about 36 years, currently through 2007. Biospecimens were collected in the late 1980s and through 1990s (Cupples et al. 2007). The procedure used for the APOE genotyping in the FHSO is described in Lahoz et al. (2001). Information on the APOE e2/3/4 polymorphism in the FHSO was available for 3,924 subjects.
Analysis
Associations of the APOE polymorphism with survival, longevity, and ages at onset of CVD (diseases of heart and stroke) and cancer (all sites but skin) were primarily characterized by Kaplan-Meier empirical age patterns. Kaplan-Meier estimates were complemented by evaluation of the relative risks (RRs) of death using the proportional hazard Cox regression model. An individual’s age, e.g., age at baseline plus time elapsed since the baseline examination through 2007, was used as a time variable in these analyses. We estimated unadjusted Cox regression models and the models adjusted for age, sex, prevalence of CVD and cancer, relatedness, body mass index (BMI, kg/m2), total cholesterol (mg/100 ml), high density lipoprotein (HDL) cholesterol (mg/100 ml), smoking (ever smoke), and drinking (currently drink or not). Relatedness was categorized as singleton and one individual per extended FHSO family vs. other family members. In these analyses, cholesterol levels were divided by 10 for better visibility. Because BMI, cholesterol, smoking, and drinking are age-sensitive covariates, we first evaluated whether the results are consistent across the FHSO examinations. For this, we estimated the Cox regression models for each FHSO examination with covariates measured at the respective examinations. The results of these analyses showed that the age at measurement of the covariates makes no qualitative difference to our major conclusions. Accordingly, for further analyses we used measurements of BMI, cholesterol, smoking, and drinking at the FHSO baseline in order to maximize sample size and follow up time. Statistical analyses of the data were performed using SPSS software (release 17.0, Chicago, Illinois, USA). Corrections for sampling type I error were disregarded in these analyses due to findings from numerous prior studies on the associations of the APOE polymorphism with various aging-related traits and the limited number of independent tests.
Results
Basic characteristics of the 3,924 genotyped FHSO participants are listed in Table 1 for each APOE genotype. Table 1 indicates selective survival of individuals carrying genotypes with the risk (i.e., e4) allele. Therefore, non-risk allele carriers were taken as a reference group, with subjects carrying the risk allele categorized according to genotypes and presence of the e4 allele.
Table 1.
Basic characteristics of the 3,924 genotyped FHSO participants
| Factor | e2/2 | e2/3 | e2/4 | e3/3 | e3/4 | e4/4 |
|---|---|---|---|---|---|---|
| N (%*) | 17 (0.4) | 505 (12.9) | 67 (1.7) | 2518 (64.2) | 753 (19.2) | 64 (1.6) |
| Mean age (SD), yrs | 40.6 (9.1) | 35.8 (10.0) | 36.1 (8.6) | 35.8 (10.2) | 35.9 (10.0) | 35.8 (10.2) |
| Dead (%**) | 2 (11.8) | 75 (14.9) | 12 (17.9) | 370 (14.7) | 125 (16.6) | 17 (27.0) |
| CVD (%**) | 4 (23.5) | 111 (22.0) | 15 (22.4) | 551 (21.9) | 198 (26.3) | 16 (25.0) |
| Cancer (%**) | 3 (17.6) | 92 (18.2) | 16 (23.9) | 462 (18.3) | 116 (15.4) | 12 (18.8) |
Percentage is within the entire sample;
percentage is within the APOE genotypes.
The cardiovascular disease (CVD) group includes the prevalence of diseases of heart and stroke; the prevalence of cancer includes all sites but skin; mean age is measured at baseline.
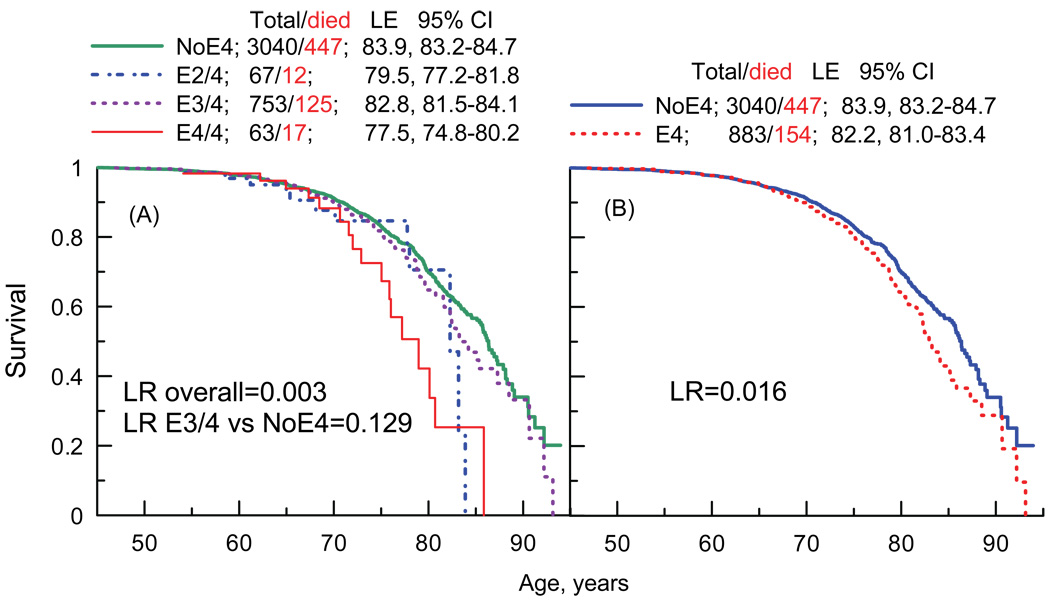
Figure 1 documents the overall significance of the association between the APOE polymorphism and survival. This association is more pronounced when the risk allele carriers are not aggregated (Figure 1A). The poor survival of the e4/4 carriers is a major contributor to the adverse effect of the e4 allele in the aggregated sample (Figure 1B), because the effect of the most frequent risk-allele genotype (e3/4) is insignificant (Figure 1A). Life expectancy (LE) estimates show that the e4-allele homozygotes and the e2/4 heterozygotes live shorter lives on average than the non-risk allele carriers and the e3/4 heterozygotes. The difference in LE can be as much as 6.4 years. Figure 1 also shows that the effect of the risk allele is limited to older ages. At those ages, however, the risk-allele carriers are at consistently higher risk of premature death compared to the non-e4-allele carriers in any old-age group. Thus, Figure 1 provides evidence of connections of the APOE polymorphism to aging, because the e4 carriers die younger than the non-e4 age-peers.
Figure 1.
Kaplan-Meier survival age patterns for carriers of different APOE genotypes. (A) Survival patterns for carriers of the non-e4 (NoE4), e2/4, e3/4, and e4/4 genotypes. (B) Survival patterns for carriers of the non-e4 (NoE4) and any risk allele (E4) genotypes. Log rank (LR) measures equality of the survival distributions. Total/died denotes the total number of carriers and the number of deaths among them. LE=life expectancy; CI=confidence interval.
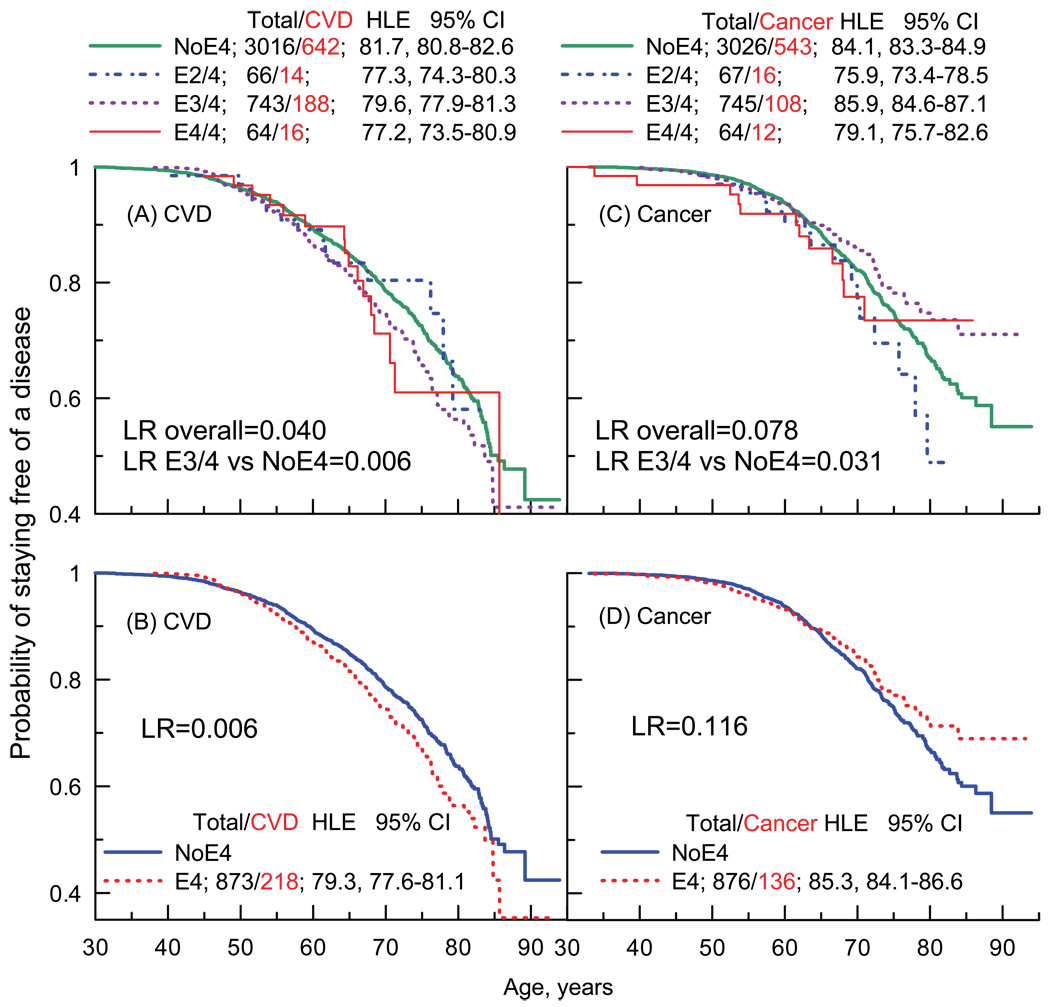
Given connections of the APOE polymorphism with various geriatric diseases (see the Introduction), a next logical step is to test association of this polymorphism with age at onset of major causes of death in developed societies, i.e., CVD and cancer. Figure 2 shows the results of Kaplan-Meier estimates and empirical estimates of healthy life expectancy (HLE) defined as life without CVD or cancer. Figures 2A and 2B (log rank [LR]) document an overall association of the APOE polymorphism with age at onset of CVD. An overall negative effect of the e4 allele is highly significant (LR=0.006). This effect is predominantly due to the adverse effect of the e3/4 genotype. Figure 2A also shows that after about 50 years of age, the e3/4 heterozygotes consistently contract CVD at younger ages than the non-risk allele carriers.
Figure 2.
Kaplan-Meier age patterns of probability of staying free of a disease for carriers of different APOE genotypes. (A) Probability patterns for onsets of CVDs for carriers of the non-e4 (NoE4), e2/4, e3/4, and e4/4 genotypes. (B) Probability patterns for onsets of CVDs for carriers of the non-e4 (NoE4) and any risk allele (E4) genotypes. (C) and (D) The same as (A) and (B) but for cancer. Log rank (LR) measures equality of the probability distributions. Total/CVD (or cancer) denotes the total number of carriers and the number of CVD (or cancer) onsets among them. Healthy life expectancy (HLE) is defined as life without CVD (diseases of heart and stroke) or cancer (all sites but skin). CI=confidence interval.
An overall association of the APOE with age at onset of cancer (Figures 2C and 2D) is at best marginally significant when we consider individual genotypes (LR=0.078). Although carriers of the e2/4 and e4/4 genotypes tend to live shorter lives than the non-e4 carriers, this is definitively not the case for the e3/4 heterozygotes who, contrarily, enjoy the longest life without cancer (HLE=85.9 years), i.e., longer than carriers of the most favorable genotypes (HLE=84.1). The protective effect for the e3/4 heterozygotes is significant but limited to older (about 65+years) ages (Figure 2C, LR=0.031). The protective effect of the e3/4 genotype appears to be cancer specific, i.e., this genotype is unlikely to protect against prostate or breast cancers (LR=0.537) but it likely protects against other cancers (LR=0.016).
Thus, Figure 2 documents a genetic trade-off such that the same genotype (i.e., e3/4) can favor CVD but be protective of cancer. This result is an example of how the failure to find significant effects in genetic association studies can be due to an inherent complexity of biological functions of the same gene in an aging human organism. Specifically, this genetic trade-off can explain the lack of consistency in the associations of the e3/4 genotype with survival. To elucidate whether this can indeed be the case, we evaluated relative risks (RRs) of death using the Cox regression model with several adjustments.
First, we evaluated the RRs of death using the model with no adjustments (Table 2) to ensure that the model and empirical estimates are consistent. Significance of the RR estimates for the aggregated and non-aggregated groups of the e4 carriers as well as for the e3/4 carriers resemble the LR estimates shown in Figure 1. Adjustment by age and sex neither changes the RRs nor their significance. Adjustment by the prevalence of CVD and cancer is, however, highly effective and considerably improves significance of the estimates. Furthermore, the RR of death for the e3/4 carriers compared to the non-e4 carriers doubles rising from 13% to 26% and becomes significant after adjustment. This implies that the association of the e3/4 genotype with overall survival is confounded by cancer- and CVD-related deaths in opposite fashion. The latter results in no significant association of this genotype with survival in unadjusted estimates (see also Figure 1A). We also verified that relatedness, body mass index (BMI), smoking, and drinking did not affect the associations (Table 2).
Table 2.
Relative risks of death for carriers of different APOE genotypes.
| Models* | Allele | Genotypes | |||
|---|---|---|---|---|---|
| e4, any | e2/4 | e3/4 | e4/4 | overall p-value |
|
| M0 | 1.25 (0.018) | 1.38 (0.270) | 1.17 (0.127) | 2.26 (9.7×10−4) | 4.2×10−3 |
| M1 | 1.21 (0.040) | 1.31 (0.357) | 1.13 (0.225) | 2.30 (7.5×10−4) | 5.2×10−3 |
| M2 | 1.34 (1.9×10−3) | 1.27 (0.408) | 1.26 (0.023) | 2.70 (6.6×10−5) | 1.8×10−4 |
| M3 | 1.34 (1.9×10−3) | 1.28 (0.405) | 1.26 (0.023) | 2.68 (7.0×10−5) | 1.9×10−4 |
| M4 | 1.35 (1.3×10−3) | 1.32 (0.350) | 1.27 (0.019) | 2.71 (6.1×10−5) | 1.4×10−4 |
M0: no adjustments; M1: adjustments by age and sex; M2: M1 + prevalence of CVD and cancer; M3: M2 + Relatedness; M4: M3 + body mass index (kg/m2), smoking, and drinking.
Numbers show relative risks (p-values) for carriers of the risk allele (e4, any) and for carriers of each genotype with the e4 allele (“Genotypes”). Column “overall p-value” shows significance of the overall model in the latter case, i.e., this is the result of the test of the null hypothesis that all the effect coefficients for this categorical variable are zero. The non-e4-allele carriers were the reference group.
Sample size is given in Figure 1.
Because the APOE gene is known to be associated with lipid metabolism (see the Introduction) cholesterol levels can mediate the effect of the e3/4 genotype on diseases and, consequently, on survival. A key question is whether an abnormal lipid profile can be a common risk factor for CVD and cancer or the observed genetic trade-off is due to different mechanisms. To answer this question, we evaluated RRs of onsets of CVD and cancer using the Cox regression model.
Again, we first validated that the Cox model and empirical estimates are consistent (Table 3, M0). As in the case of survival, adjustment of the associations with age at onset of CVD by age, sex, and relatedness, did not affect the estimates (not shown). Additional adjustment by total and high density lipoprotein (HDL) cholesterol explains the significant reduction in the RRs for contracting CVD early in life. Adjustment by cancer did not change the estimates. Adjustment by major potential behavioral risk factors for CVD including BMI, smoking, and drinking did not change the estimates either despite the highly significant effect of BMI (p=8.1×10−7) and smoking (p=6.0×10−8) on risk of CVD. Adjustment of the associations with age at onset of cancer (Table 3) by the same risk factors as in the case of CVD (except substituting cancer by CVD) did not alter the estimates. These results imply that association of the APOE polymorphism with cancer is unlikely mediated by lipid metabolism.
Table 3.
Relative risks of onsets of CVD and cancer for carriers of different APOE genotypes.
| Trait | Model | Allele | Genotypes | |||
|---|---|---|---|---|---|---|
| e4, any | e2/4 | e3/4 | e4/4 | overall p-value |
||
| CVD | M0* | 1.24 (0.007) | 0.99 (0.968) | 1.26 (0.006) | 1.29 (0.310) | 0.040 |
| M1** | 1.14 (0.113) | 1.04 (0.892) | 1.17 (0.068) | 0.90 (0.692) | 0.306 | |
| M1**+cancer | 1.13 (0.127) | 1.04 (0.883) | 1.16 (0.078) | 0.90 (0.682) | 0.334 | |
| M2**+cancer | 1.14 (0.099) | 1.05 (0.851) | 1.17 (0.060) | 0.90 (0.686) | 0.277 | |
| M3**+cancer | 1.14 (0.112) | 0.98 (0.994) | 1.17 (0.057) | 0.88 (0.629) | 0.258 | |
| Cancer | M0* | 0.86 (0.116) | 1.38 (0.205) | 0.80 (0.032) | 1.08 (0.788) | 0.080 |
| M1**+CVD | 0.84 (0.080) | 1.38 (0.208) | 0.78 (0.019) | 1.08 (0.800) | 0.054 | |
| M2**+CVD | 0.84 (0.071) | 1.36 (0.228) | 0.78 (0.017) | 1.07 (0.815) | 0.052 | |
| M3**+CVD | 0.84 (0.065) | 1.33 (0.266) | 0.77 (0.016) | 1.06 (0.854) | 0.058 | |
M0: no adjustments; M1: adjustments by age, sex, relatedness, total cholesterol (mg/1000 ml), and high density lipoprotein cholesterol (mg/1000 ml); M2: M1 + body mass index (kg/m2); M3: M2 + smoking and drinking. Cholesterol levels were divided by 10 for better visibility.
Numbers show relative risks (p-values) for carriers of the risk allele (e4, any) and for carriers of each genotype with the e4 allele (“Genotypes”). Column “overall p-value” shows significance of the overall model in the latter case. The non-e4-allele carriers were the reference group.
Sample size is given in Figure 2.
Sample sizes are slightly smaller due to about 2% of missing values for the total and high density lipoprotein cholesterol measurements.
Discussion
Our analysis explicitly shows that carriers of the risk (e4) allele live consistently shorter lives than the non-risk allele age-peers, when they reach older ages. A major contributor to this adverse effect of the e4 allele is the e4/4 homozygous genotype, e.g., e4/4 carriers lived 6.4 years shorter lives than the non-e4 carriers whereas the lifespan of the e3/4 heterozygotes was about the same as that of the non-e4 allele carriers. A striking result of this study is that the e4 risk-allele can show a pleiotropic effect with a trade-off, i.e., it can predispose to early onset of CVD but postpone cancers to older ages. This trade-off does explain the insignificance of the association of the e3/4 genotype with survival. Furthermore, adjustment for this trade-off makes the detrimental effect of the e4 allele more apparent. Importantly, a possibility of trade-offs between CVD and cancer in the FHS population has also been documented at the phenotypic level (Ukraintseva et al. 2010), which reinforces validity of the observed trade-off in the effects of the APOE polymorphism on the respective diseases. Thus, genetic trade-offs appear to be an important source of confounding in studies of genetic effects on aging-related traits, which can at least partly explain inconsistencies of genetic associations in different populations and settings.
The lack of sensitivity of the association of the APOE with survival to BMI, smoking, and drinking, suggests a mediating role of other causes of death. These can be neurodegenerative diseases which are well-known to be associated with the e4 allele (Corder et al. 1994) and are highly common at advanced ages (Bishop et al. 2010). Although the FHSO data available for this study do not provide information on onsets of these diseases, implicitly this conclusion is supported by increased risks of overall deaths at older ages. The latter is in line with recent findings on the increased effect of the APOE gene on survival at older ages in healthy Danes (Jacobsen et al. 2010).
The detrimental effect of the e4 allele on age at onset of CVD was mostly explained in our study by impaired lipid metabolism (high total and/or low HDL cholesterol levels). This effect was not modulated by such important behavioral risk factors for CVD as BMI and smoking. By comparison, the protective effect of the e3/4 genotype on age at onset of cancer was not modulated either by lipid metabolism or by these behavioral risk factors. These results suggest that the trade-off in the effects of the e4 allele involves different biological mechanisms affecting onsets of cancer and CVD. This finding implicates the need for a deeper understanding of mechanisms mediating genetic effects on complex (non-Mendelian) phenotypes in order to properly translate genetic findings into personalized medical care. Our findings also suggest that the protective effect of the e3/4 genotype on cancer is site specific, being largely attributed to non-prostate and non-breast cancer sites.
Is there any bio-evolutionary rationale for genetic trade-offs? Cancer and CVD are diseases with largely post-reproductive manifestations. Therefore, it is unlikely that evolution directly worked against them because evolution primarily maximizes fitness of individuals of reproductive age. It might well be the case that a certain risk factor for a late life disease can be relevant to reproductive age and fitness. Then, evolution can be relevant to late-life diseases (Di Rienzo & Hudson 2005; Drenos & Kirkwood 2010). The problem, however, is that such reproductive-age-related factors might well be relevant to fitness but: (i) not relevant to post-reproductive age diseases at all, (ii) predispose to them, (iii) be protective of them, or (iv) predispose to some diseases but be protective of the others. For instance, reproductive-age-related factors might be evolutionary advantageous but, simultaneously, they can predispose to late-life diseases in humans and non-human species (Williams & Day 2003; Martin 2007; Kulminski et al. 2010). Then genetic trade-offs in which the same genetic variant can be protective against one disease but be predisposing to another one would not be an exception in studies of complex aging-related traits (Frazer et al. 2009). This conclusion is in line with the hypothesis of balancing evolution (see the Introduction).
In terms of balancing evolution of the APOE polymorphism, there is a broad consensus on the mechanism of detrimental action of the e4 allele described in the Introduction which is in line with our findings. The mechanism of its protective role is, however, unclear (de Jong 2006). APOE was proposed as a potent inhibitor of angiogenesis and tumor cell growth (Vogel et al. 1994). One study reported on protection of the e4 allele against colorectal cancer (Kervinen et al. 1996) with a similar trend for colon adenomas in another study (Shinomiya et al. 2001). The protective role of the e2/3 genotype for these cancers was seen in a study by Watson et al. (Watson et al. 2003). APOE may influence colorectal cancers through lipid metabolism, insulin regulation, and inflammation (Slattery et al. 2005). Carriers of the e4 allele might also develop CNS neoplasms at later ages, though the mechanism of this association is unclear (Zunarelli et al. 2000). Yet, despite these findings, there is no consensus either on the effect of the APOE gene on neoplasia or on its mechanism (Finch 2007). This prevents concluding that the trade-off observed in this study can explain the maintenance of the e4 allele in humans.
Overall, this study explicitly demonstrates that unraveling the genetic origin of aging-related traits requires deeper insights into biological mechanisms mediating gene action, than those currently offered by mechanistic bio-statistical approaches. Identifying causes of genetic trade-offs is critical for understanding the relationships among factors influencing total survival and onsets of aging-related disorders and for finding optimal combinations of such factors for individual genotypes.
Acknowledgments
The research reported in this paper was supported by Award Number R01AG030612 from the National Institute on Aging. The Framingham Heart Study (FHS) is conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with the FHS Investigators. This manuscript was prepared using a limited access dataset obtained from the NHLBI. This manuscript was not prepared in collaboration with investigators of the FHS and does not necessarily reflect the opinions or views of the FHS, Boston University, or the NHLBI.
Footnotes
Authors Contributions: AMK contributed to the study conception, design, statistical analysis, interpretation of the results, and writing the manuscript. IC contributed to the study design and interpretation of the results. SVU contributed to writing the final version and discussion of genetic trade-off. KGA contributed to statistical analyses. LA, DW, and IA contributed to preparation of the data. KCL contributed to assessing the logic of the analysis and to writing the final version. AIY contributed to discussion and interpretation of the results and drafting the manuscript.
References
- Barzilai N, Atzmon G, Schechter C, Schaefer EJ, Cupples AL, Lipton R, Cheng S, Shuldiner AR. Unique lipoprotein phenotype and genotype associated with exceptional longevity. JAMA. 2003;290:2030–2040. doi: 10.1001/jama.290.15.2030. [DOI] [PubMed] [Google Scholar]
- Beekman M, Nederstigt C, Suchiman HE, Kremer D, van der Breggen R, Lakenberg N, Alemayehu WG, de Craen AJ, Westendorp RG, Boomsma DI, de Geus EJ, Houwing-Duistermaat JJ, Heijmans BT, Slagboom PE. Genome-wide association study (GWAS)-identified disease risk alleles do not compromise human longevity. Proc Natl Acad Sci U S A. 2010 doi: 10.1073/pnas.1003540107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop NA, Lu T, Yankner BA. Neural mechanisms of ageing and cognitive decline. Nature. 2010;464:529–535. doi: 10.1038/nature08983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonomini F, Filippini F, Hayek T, Aviram M, Keidar S, Rodella LF, Coleman R, Rezzani R. Apolipoprotein E and its role in aging and survival. Exp Gerontol. 2010;45:149–157. doi: 10.1016/j.exger.2009.11.006. [DOI] [PubMed] [Google Scholar]
- Charlesworth B. Evolution of senescence: Alzheimer's disease and evolution. Curr Biol. 1996;6:20–22. doi: 10.1016/s0960-9822(02)00411-6. [DOI] [PubMed] [Google Scholar]
- Christensen K, Johnson TE, Vaupel JW. The quest for genetic determinants of human longevity: challenges and insights. Nat Rev Genet. 2006;7:436–448. doi: 10.1038/nrg1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Risch NJ, Strittmatter WJ, Schmechel DE, Gaskell PC, Jr., Rimmler JB, Locke PA, Conneally PM, Schmader KE, et al. Protective effect of apolipoprotein E type 2 allele for late onset Alzheimer disease. Nat Genet. 1994;7:180–184. doi: 10.1038/ng0694-180. [DOI] [PubMed] [Google Scholar]
- Cupples LA, Arruda HT, Benjamin EJ, D'Agostino RB, Sr., Demissie S, DeStefano AL, Dupuis J, Falls KM, Fox CS, Gottlieb DJ, Govindaraju DR, Guo CY, Heard-Costa NL, Hwang SJ, Kathiresan S, Kiel DP, Laramie JM, Larson MG, Levy D, Liu CY, Lunetta KL, Mailman MD, Manning AK, Meigs JB, Murabito JM, Newton-Cheh C, O'Connor GT, O'Donnell CJ, Pandey M, Seshadri S, Vasan RS, Wang ZY, Wilk JB, Wolf PA, Yang Q, Atwood LD. The Framingham Heart Study 100K SNP genome-wide association study resource: overview of 17 phenotype working group reports. BMC Med Genet. 2007;8 Suppl 1:S1. doi: 10.1186/1471-2350-8-S1-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler RG, Mattson MP. The adversities of aging. Ageing Res Rev. 2006;5:221–238. doi: 10.1016/j.arr.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Dawber TR, Meadors GF, Moore FE., Jr. Epidemiological approaches to heart disease: the Framingham Study. Am J Public Health Nations Health. 1951;41:279–281. doi: 10.2105/ajph.41.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong PT. Age-related macular degeneration. N Engl J Med. 2006;355:1474–1485. doi: 10.1056/NEJMra062326. [DOI] [PubMed] [Google Scholar]
- Di Rienzo A, Hudson RR. An evolutionary framework for common diseases: the ancestral-susceptibility model. Trends Genet. 2005;21:596–601. doi: 10.1016/j.tig.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Drenos F, Kirkwood TB. Selection on alleles affecting human longevity and late-life disease: the example of apolipoprotein E. PLoS One. 2010;5:e10022. doi: 10.1371/journal.pone.0010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichner JE, Dunn ST, Perveen G, Thompson DM, Stewart KE, Stroehla BC. Apolipoprotein E polymorphism and cardiovascular disease: a HuGE review. Am J Epidemiol. 2002;155:487–495. doi: 10.1093/aje/155.6.487. [DOI] [PubMed] [Google Scholar]
- Fabris C, Toniutto P, Bitetto D, Minisini R, Smirne C, Caldato M, Pirisi M. Low fibrosis progression of recurrent hepatitis C in apolipoprotein E epsilon4 carriers: relationship with the blood lipid profile. Liver Int. 2005;25:1128–1135. doi: 10.1111/j.1478-3231.2005.01156.x. [DOI] [PubMed] [Google Scholar]
- Farlow MR, He Y, Tekin S, Xu J, Lane R, Charles HC. Impact of APOE in mild cognitive impairment. Neurology. 2004;63:1898–1901. doi: 10.1212/01.wnl.0000144279.21502.b7. [DOI] [PubMed] [Google Scholar]
- Feinleib M, Kannel WB, Garrison RJ, McNamara PM, Castelli WP. The Framingham Offspring Study. Design and preliminary data. Prev Med. 1975;4:518–525. doi: 10.1016/0091-7435(75)90037-7. [DOI] [PubMed] [Google Scholar]
- Finch CE. The biology of human longevity : inflammation, nutrition, and aging in the evolution of lifespans. Amsterdam; Boston: Academic Press; 2007. [Google Scholar]
- Finch CE. Evolution in health and medicine Sackler colloquium: Evolution of the human lifespan and diseases of aging: roles of infection, inflammation, and nutrition. Proc Natl Acad Sci U S A. 2010;107 Suppl 1:1718–1724. doi: 10.1073/pnas.0909606106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch CE, Morgan TE. Systemic inflammation, infection, ApoE alleles, and Alzheimer disease: a position paper. Curr Alzheimer Res. 2007;4:185–189. doi: 10.2174/156720507780362254. [DOI] [PubMed] [Google Scholar]
- Flachsbart F, Caliebe A, Kleindorp R, Blanche H, von Eller-Eberstein H, Nikolaus S, Schreiber S, Nebel A. Association of FOXO3A variation with human longevity confirmed in German centenarians. Proc Natl Acad Sci U S A. 2009;106:2700–2705. doi: 10.1073/pnas.0809594106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazer KA, Murray SS, Schork NJ, Topol EJ. Human genetic variation and its contribution to complex traits. Nat Rev Genet. 2009;10:241–251. doi: 10.1038/nrg2554. [DOI] [PubMed] [Google Scholar]
- Fullerton SM, Clark AG, Weiss KM, Nickerson DA, Taylor SL, Stengard JH, Salomaa V, Vartiainen E, Perola M, Boerwinkle E, Sing CF. Apolipoprotein E variation at the sequence haplotype level: implications for the origin and maintenance of a major human polymorphism. Am J Hum Genet. 2000;67:881–900. doi: 10.1086/303070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes LU, Jeune B, Ranberg KA, Nybo H, Vaupel JW. Estimation of apolipoprotein E genotype-specific relative mortality risks from the distribution of genotypes in centenarians and middle-aged men: apolipoprotein E gene is a "frailty gene," not a "longevity gene". Genet Epidemiol. 2000;19:202–210. doi: 10.1002/1098-2272(200010)19:3<202::AID-GEPI2>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Gibson G. Decanalization and the origin of complex disease. Nat Rev Genet. 2009;10:134–140. doi: 10.1038/nrg2502. [DOI] [PubMed] [Google Scholar]
- Goh KI, Cusick ME, Valle D, Childs B, Vidal M, Barabasi AL. The human disease network. Proc Natl Acad Sci U S A. 2007;104:8685–8690. doi: 10.1073/pnas.0701361104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DB. Common genetic variation and human traits. N Engl J Med. 2009;360:1696–1698. doi: 10.1056/NEJMp0806284. [DOI] [PubMed] [Google Scholar]
- Gorlov IP, Gorlova OY, Sunyaev SR, Spitz MR, Amos CI. Shifting paradigm of association studies: value of rare single-nucleotide polymorphisms. Am J Hum Genet. 2008;82:100–112. doi: 10.1016/j.ajhg.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindaraju DR, Cupples LA, Kannel WB, O'Donnell CJ, Atwood LD, D'Agostino RB, Sr., Fox CS, Larson M, Levy D, Murabito J, Vasan RS, Splansky GL, Wolf PA, Benjamin EJ. Genetics of the Framingham Heart Study population. Adv Genet. 2008;62:33–65. doi: 10.1016/S0065-2660(08)00602-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer EL, Brunet A. Signaling networks in aging. J Cell Sci. 2008;121:407–412. doi: 10.1242/jcs.021519. [DOI] [PubMed] [Google Scholar]
- Jacobsen R, Martinussen T, Christiansen L, Jeune B, Andersen-Ranberg K, Vaupel JW, Christensen K. Increased effect of the ApoE gene on survival at advanced age in healthy and long-lived Danes: two nationwide cohort studies. Aging Cell. 2010 doi: 10.1111/j.1474-9726.2010.00626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jofre-Monseny L, Minihane AM, Rimbach G. Impact of apoE genotype on oxidative stress, inflammation and disease risk. Mol Nutr Food Res. 2008;52:131–145. doi: 10.1002/mnfr.200700322. [DOI] [PubMed] [Google Scholar]
- Kenyon C. The plasticity of aging: insights from long-lived mutants. Cell. 2005;120:449–460. doi: 10.1016/j.cell.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Kervinen K, Sodervik H, Makela J, Lehtola J, Niemi M, Kairaluoma MI, Kesaniemi YA. Is the development of adenoma and carcinoma in proximal colon related to apolipoprotein E phenotype? Gastroenterology. 1996;110:1785–1790. doi: 10.1053/gast.1996.v110.pm8964404. [DOI] [PubMed] [Google Scholar]
- Klaver CC, Kliffen M, van Duijn CM, Hofman A, Cruts M, Grobbee DE, van Broeckhoven C, de Jong PT. Genetic association of apolipoprotein E with age-related macular degeneration. Am J Hum Genet. 1998;63:200–206. doi: 10.1086/301901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koropatnick TA, Kimbell J, Chen R, Grove JS, Donlon TA, Masaki KH, Rodriguez BL, Willcox BJ, Yano K, Curb JD. A prospective study of high-density lipoprotein cholesterol, cholesteryl ester transfer protein gene variants, and healthy aging in very old Japanese-american men. J Gerontol A Biol Sci Med Sci. 2008;63:1235–1240. doi: 10.1093/gerona/63.11.1235. [DOI] [PubMed] [Google Scholar]
- Kulminski AM, Culminskaya I, Ukraintseva SV, Arbeev KG, Land KC, Yashin AI. Beta2-adrenergic receptor gene polymorphisms as systemic determinants of healthy aging in an evolutionary context. Mech Ageing Dev. 2010;131:338–345. doi: 10.1016/j.mad.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahoz C, Schaefer EJ, Cupples LA, Wilson PW, Levy D, Osgood D, Parpos S, Pedro-Botet J, Daly JA, Ordovas JM. Apolipoprotein E genotype and cardiovascular disease in the Framingham Heart Study. Atherosclerosis. 2001;154:529–537. doi: 10.1016/s0021-9150(00)00570-0. [DOI] [PubMed] [Google Scholar]
- Little DM, Crooks VC, Petitti DB, Chiu V, Schellenberg GD, Slezak JM, Jacobsen SJ. Mortality, dementia, and apolipoprotein E genotype in elderly white women in the United States. J Am Geriatr Soc. 2009;57:231–236. doi: 10.1111/j.1532-5415.2008.02113.x. [DOI] [PubMed] [Google Scholar]
- Lunetta KL, D'Agostino RB, Sr., Karasik D, Benjamin EJ, Guo CY, Govindaraju R, Kiel DP, Kelly-Hayes M, Massaro JM, Pencina MJ, Seshadri S, Murabito JM. Genetic correlates of longevity and selected age-related phenotypes: a genome-wide association study in the Framingham Study. BMC Med Genet. 2007;8 Suppl 1:S13. doi: 10.1186/1471-2350-8-S1-S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahley RW. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science. 1988;240:622–630. doi: 10.1126/science.3283935. [DOI] [PubMed] [Google Scholar]
- Martin GM. APOE alleles and lipophylic pathogens. Neurobiol Aging. 1999;20:441–443. doi: 10.1016/s0197-4580(99)00078-0. [DOI] [PubMed] [Google Scholar]
- Martin GM. Modalities of gene action predicted by the classical evolutionary biological theory of aging. Ann N Y Acad Sci. 2007;1100:14–20. doi: 10.1196/annals.1395.002. [DOI] [PubMed] [Google Scholar]
- Martin GM, Bergman A, Barzilai N. Genetic determinants of human health span and life span: progress and new opportunities. PLoS Genet. 2007;3:e125. doi: 10.1371/journal.pgen.0030125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RJ, Chamberlain RM, Khuri FR. Apolipoprotein E and the risk of breast cancer in African-American and non-Hispanic white women. A review. Oncology. 2004;66:79–93. doi: 10.1159/000077433. [DOI] [PubMed] [Google Scholar]
- Newman AB, Walter S, Lunetta KL, Garcia ME, Slagboom PE, Christensen K, Arnold AM, Aspelund T, Aulchenko YS, Benjamin EJ, Christiansen L, D'Agostino RB, Sr., Fitzpatrick AL, Franceschini N, Glazer NL, Gudnason V, Hofman A, Kaplan R, Karasik D, Kelly-Hayes M, Kiel DP, Launer LJ, Marciante KD, Massaro JM, Miljkovic I, Nalls MA, Hernandez D, Psaty BM, Rivadeneira F, Rotter J, Seshadri S, Smith AV, Taylor KD, Tiemeier H, Uh HW, Uitterlinden AG, Vaupel JW, Walston J, Westendorp RG, Harris TB, Lumley T, van Duijn CM, Murabito JM. A meta-analysis of four genome-wide association studies of survival to age 90 years or older: the cohorts for heart and aging research in genomic epidemiology consortium. J Gerontol A Biol Sci Med Sci. 2010;65:478–487. doi: 10.1093/gerona/glq028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olshansky SJ, Perry D, Miller RA, Butler RN. Pursuing the longevity dividend: scientific goals for an aging world. Ann N Y Acad Sci. 2007;1114:11–13. doi: 10.1196/annals.1396.050. [DOI] [PubMed] [Google Scholar]
- Oria RB, Patrick PD, Blackman JA, Lima AA, Guerrant RL. Role of apolipoprotein E4 in protecting children against early childhood diarrhea outcomes and implications for later development. Med Hypotheses. 2007;68:1099–1107. doi: 10.1016/j.mehy.2006.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oria RB, Patrick PD, Zhang H, Lorntz B, de Castro Costa CM, Brito GA, Barrett LJ, Lima AA, Guerrant RL. APOE4 protects the cognitive development in children with heavy diarrhea burdens in Northeast Brazil. Pediatr Res. 2005;57:310–316. doi: 10.1203/01.PDR.0000148719.82468.CA. [DOI] [PubMed] [Google Scholar]
- Panza F, Frisardi V, Capurso C, D'Introno A, Colacicco AM, Seripa D, Pilotto A, Vendemiale G, Capurso A, Solfrizzi V. Apolipoprotein E, dementia, and human longevity. J Am Geriatr Soc. 2009;57:740–742. doi: 10.1111/j.1532-5415.2009.02211.x. [DOI] [PubMed] [Google Scholar]
- Plomin R, Haworth CM, Davis OS. Common disorders are quantitative traits. Nat Rev Genet. 2009;10:872–878. doi: 10.1038/nrg2670. [DOI] [PubMed] [Google Scholar]
- Rockwood K, Nassar B, Mitnitski A. Apolipoprotein E-polymorphism, frailty and mortality in older adults. J Cell Mol Med. 2008;12:2754–2761. doi: 10.1111/j.1582-4934.2008.00270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvioli S, Olivieri F, Marchegiani F, Cardelli M, Santoro A, Bellavista E, Mishto M, Invidia L, Capri M, Valensin S, Sevini F, Cevenini E, Celani L, Lescai F, Gonos E, Caruso C, Paolisso G, De Benedictis G, Monti D, Franceschi C. Genes, ageing and longevity in humans: problems, advantages and perspectives. Free Radic Res. 2006;40:1303–1323. doi: 10.1080/10715760600917136. [DOI] [PubMed] [Google Scholar]
- Shaw P, Lerch JP, Pruessner JC, Taylor KN, Rose AB, Greenstein D, Clasen L, Evans A, Rapoport JL, Giedd JN. Cortical morphology in children and adolescents with different apolipoprotein E gene polymorphisms: an observational study. Lancet Neurol. 2007;6:494–500. doi: 10.1016/S1474-4422(07)70106-0. [DOI] [PubMed] [Google Scholar]
- Shinomiya S, Sasaki J, Kiyohara C, Tsuji E, Inoue H, Marugame T, Handa K, Hayabuchi H, Hamada H, Eguchi H, Fukushima Y, Kono S. Apolipoprotein E genotype, serum lipids, and colorectal adenomas in Japanese men. Cancer Lett. 2001;164:33–40. doi: 10.1016/s0304-3835(00)00724-2. [DOI] [PubMed] [Google Scholar]
- Sierra F, Hadley E, Suzman R, Hodes R. Prospects for Life Span Extension. Annu Rev Med. 2008 doi: 10.1146/annurev.med.60.061607.220533. [DOI] [PubMed] [Google Scholar]
- Slattery ML, Sweeney C, Murtaugh M, Ma KN, Potter JD, Levin TR, Samowitz W, Wolff R. Associations between apoE genotype and colon and rectal cancer. Carcinogenesis. 2005;26:1422–1429. doi: 10.1093/carcin/bgi088. [DOI] [PubMed] [Google Scholar]
- Smith JD. Apolipoproteins and aging: emerging mechanisms. Ageing Res Rev. 2002;1:345–365. doi: 10.1016/s1568-1637(02)00005-3. [DOI] [PubMed] [Google Scholar]
- Souied EH, Benlian P, Amouyel P, Feingold J, Lagarde JP, Munnich A, Kaplan J, Coscas G, Soubrane G. The epsilon4 allele of the apolipoprotein E gene as a potential protective factor for exudative age-related macular degeneration. Am J Ophthalmol. 1998;125:353–359. doi: 10.1016/s0002-9394(99)80146-9. [DOI] [PubMed] [Google Scholar]
- Splansky GL, Corey D, Yang Q, Atwood LD, Cupples LA, Benjamin EJ, D'Agostino RB, Sr., Fox CS, Larson MG, Murabito JM, O'Donnell CJ, Vasan RS, Wolf PA, Levy D. The Third Generation Cohort of the National Heart, Lung, and Blood Institute's Framingham Heart Study: design, recruitment, and initial examination. Am J Epidemiol. 2007;165:1328–1335. doi: 10.1093/aje/kwm021. [DOI] [PubMed] [Google Scholar]
- Thakkinstian A, Bowe S, McEvoy M, Smith W, Attia J. Association between apolipoprotein E polymorphisms and age-related macular degeneration: A HuGE review and meta-analysis. Am J Epidemiol. 2006;164:813–822. doi: 10.1093/aje/kwj279. [DOI] [PubMed] [Google Scholar]
- Ukraintseva SV, Arbeev KG, Akushevich I, Kulminski A, Arbeeva L, Culminskaya I, Akushevich L, Yashin AI. Trade-offs between cancer and other diseases: do they exist and influence longevity? Rejuvenation Res. 2010;13:387–396. doi: 10.1089/rej.2009.0941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijg J, Suh Y. Genetics of longevity and aging. Annu Rev Med. 2005;56:193–212. doi: 10.1146/annurev.med.56.082103.104617. [DOI] [PubMed] [Google Scholar]
- Vogel T, Guo NH, Guy R, Drezlich N, Krutzsch HC, Blake DA, Panet A, Roberts DD. Apolipoprotein E: a potent inhibitor of endothelial and tumor cell proliferation. J Cell Biochem. 1994;54:299–308. doi: 10.1002/jcb.240540306. [DOI] [PubMed] [Google Scholar]
- Watson MA, Gay L, Stebbings WS, Speakman CT, Bingham SA, Loktionov A. Apolipoprotein E gene polymorphism and colorectal cancer: gender-specific modulation of risk and prognosis. Clin Sci (Lond) 2003;104:537–545. doi: 10.1042/CS20020329. [DOI] [PubMed] [Google Scholar]
- Willcox BJ, Donlon TA, He Q, Chen R, Grove JS, Yano K, Masaki KH, Willcox DC, Rodriguez B, Curb JD. FOXO3A genotype is strongly associated with human longevity. Proc Natl Acad Sci U S A. 2008;105:13987–13992. doi: 10.1073/pnas.0801030105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams PD, Day T. Antagonistic pleiotropy, mortality source interactions, and the evolutionary theory of senescence. Evolution. 2003;57:1478–1488. doi: 10.1111/j.0014-3820.2003.tb00356.x. [DOI] [PubMed] [Google Scholar]
- Wozniak MA, Itzhaki RF, Faragher EB, James MW, Ryder SD, Irving WL. Apolipoprotein E-epsilon 4 protects against severe liver disease caused by hepatitis C virus. Hepatology. 2002;36:456–463. doi: 10.1053/jhep.2002.34745. [DOI] [PubMed] [Google Scholar]
- Zunarelli E, Nicoll JA, Trentini GP. Apolipoprotein E polymorphism and central nervous system tumors: correlation with cell proliferation indices and clinical outcome. Clin Neuropathol. 2000;19:1–6. [PubMed] [Google Scholar]