Abstract
Objective
Power spectral analysis of heart rate variability is used clinically to assess cardiac autonomic function. High frequency power is related to respiratory sinus arrhythmia and therefore to parasympathetic cardiovagal tone. The relationship of low frequency (LF) power to cardiac sympathetic tone is less clear. We reported previously that LF power may reflect baroreflex function; however, in the previous study LF power was not adjusted for possible influences of respiration. In this study we assessed relationships of LF power, including respiration-adjusted LF power (LFa) using the ANSAR ANX 3.0 device, with cardiac sympathetic innervation and baroreflex function in chronic autonomic failure patients who either had or did not have neuroimaging evidence of cardiac sympathetic denervation.
Methods
Values for LF power with patients seated at baseline and during the Valsalva maneuver were compared between groups with low or normal myocardial concentrations of 6-[18F]fluorodopamine-derived radioactivity. Baroreflex-cardiovagal gain (BRS) was calculated from the slope of cardiac interbeat interval vs. systolic pressure during the Valsalva maneuver with subjects supine.
Results
Individual values for LF and LFa were unrelated to myocardial 6-[18F]fluorodopamine-derived radioactivity. During both sitting rest and the Valsalva maneuver the logs of LF and LFa correlated positively with the log of BRS (r=0.61, p=0.0005; r=0.47, p=0.009; r=0.69, p<0.0001; r=0.60, p=0.0006). Patients with low BRS (≤3 msec/mm Hg) had low LF and LFa regardless of the status of cardiac innervation.
Conclusion
LF and LFa reflect baroreflex function independently of cardiac sympathetic innervation.
Keywords: Power spectral analysis, Heart rate variability, Sympathetic, Parasympathetic, Autonomic
The autonomic nervous system plays major roles in maintaining cardiovascular homeostasis and in the pathophysiology of a variety of disease states [9]. The system includes parasympathetic cholinergic and sympathetic noradrenergic components. Clinicians and researchers have long sought valid, non-invasive, quantitative means to identify pathophysiologically relevant abnormalities of “sympathovagal balance” [17].
One commonly used non-invasive mode of autonomic function testing is based on power spectral analysis of heart rate variability and quantification of low frequency (LF) and high frequency (HF) power. It is generally accepted that HF power reflects respiratory sinus arrhythmia, which is mediated by the parasympathetic cholinergic system; however, the origins and clinical significance of LF power have aroused persistent controversy. Although it was suggested initially that LF power provides an index of cardiac sympathetic outflow [1, 2, 20], more recent studies have cast doubt on this view and support the notion that both sympathetic and parasympathetic outflows determine LF power [4, 21].
We and others have reported that LF power of heart rate variability may not provide a measure of cardiac sympathetic “tone,” because individual values for LF power, with or without adjustment for HF power or total power, are not correlated with the rate of appearance of the sympathetic neurotransmitter, norepinephrine, in coronary sinus plasma [3, 19, 21] and are not correlated with left ventricular myocardial concentrations of the sympathetic imaging agent 6-[18F] fluorodopamine [21]. Cardiac sympathetic denervation detected by 123I-metaiodobenzylguanidine scanning also is unrelated to the LF:HF ratio, a putative index of sympathovagal balance, in patients with Parkinson disease [17]. Moreover, drugs that increase release of norepinephrine from cardiac sympathetic nerves can increase LF power even in patients with neuroimaging evidence of cardiac sympathetic denervation [21].
Sleight et al. [23] reported evidence that LF power may instead be related to baroreflex modulation of cardiac autonomic outflows. They demonstrated that carotid sinus stimulation increased LF power only in individuals with normal baroreflex function and not in those with impaired baroreflex sensitivity. In support of this hypothesis, whereas individual values for the log of LF power do not correlate with cardiac sympathetic outflow, they do correlate strongly positively with the log of baroreflex-cardiovagal gain [21]. Moreover, patients with baroreflex failure have attenuated responses of LF power to drugs that increase norepinephrine release from sympathetic nerves, independently of cardiac sympathetic innervation [21].
It has been suggested that accounting for respiratory influences on LF power improves the accuracy of power spectral analysis of heart rate variability in assessment of cardiac sympathetic outflow (2–5). The ANSAR ANX 3.0 system (ANSAR Medical Technologies Inc., Philadelphia, PA) is the only commercially available device that makes this adjustment. The ANX 3.0 uses a proprietary algorithm that yields a variable termed LFa. In conjunction with a testing protocol for beat-to-beat heart rate, respiration, and blood pressure during baseline sitting, the Valsalva maneuver, and standing, the ANX 3.0 calculates values for LFa and HF and interprets those values in terms of sympathetic and parasympathetic modulation and sympathovagal balance. The results are used to generate reports that include diagnostic implications and recommendations about possible therapy associated with autonomic dysfunction.
Our previous report did not include adjustment of LF for respiratory influences. In addition, patients were tested during supine rest, whereas the ANSAR protocol for clinical autonomic evaluation involves assessments while patients are sitting and then standing.
Chronic autonomic failure syndromes vary greatly in terms of cardiac sympathetic innervation. Three well-studied forms are pure autonomic failure, multiple system atrophy, and Parkinson disease with orthostatic hypotension (OH). Pure autonomic failure and Parkinson disease with OH consistently entail neuroimaging, neurochemical, and post-mortem neuropathologic evidence of cardiac sympathetic denervation [12, 14], whereas most patients with multiple system atrophy have intact cardiac sympathetic innervation [22]. All three diseases are associated with baroreflex-cardiovagal failure [15]. Studying groups of chronic autonomic failure patients provided a powerful means to determine if LFa (LF power adjusted for respiration) is related to cardiac sympathetic innervation, baroreflex function, or both.
METHODS
Subjects
A total of 33 subjects were evaluated at the NIH Clinical Center after giving informed written consent to participate in clinical research protocols approved by the Institutional Review Board of the National Institute of Neurological Disorders and Stroke.
The population was divided into four groups based on normal or decreased cardiac sympathetic innervation assessed by interventricular septal myocardial 6-[18F] fluorodopamine-derived radioactivity and normal or decreased baroreflex-cardiovagal gain assessed by the relationship between cardiac interbeat interval and systolic blood pressure during the Valsalva maneuver. Patient groups included pure autonomic failure (N=7), multiple system atrophy (N=10), and Parkinson disease with (N=3) or without (N=5) orthostatic hypotension. Controls included referred patients without autonomic failure or evidence of central neurodegeneration (N=4) and normal volunteers (N=4).
ANSAR Device and Testing Protocol
Under a Clinical Trial Agreement ANSAR supplied an ANX 3.0 device for clinical evaluation. ANSAR did not participate in any way in the design or conduct of the study or in the interpretation of the results. The testing procedure was conducted according to the company’s instructions and done by or under the supervision of personnel certified by ANSAR to carry out the testing protocol.
Electrocardiographic leads were attached and an automated arm blood pressure cuff applied. The electrocardiogram was used for power spectral analysis of heart rate variability and trans-thoracic electrical impedance for analysis of respiratory rate variability [6]. The ANX 3.0 gives instructions to subjects for the stages of the testing—baseline relaxed breathing for 5 minutes while sitting, deep breathing for 1 minute, return to baseline breathing for 1 minute, 5 Valsalva maneuvers, return to baseline for 2 minutes, and standing for 5 minutes.
A parameter termed RFa was calculated as the area within a 0.12 Hz bin of the spectrum centered at the fundamental respiratory frequency. The fundamental respiratory frequency, in breaths per second, is the frequency of the highest peak of the respiratory rate variability spectrum. In a healthy individual this frequency corresponds to the inverse of the respiratory rate during resting breathing. LFa was calculated from the LF component of the heart rate variability after taking into account RFa [6]. The exact calculations made by the ANSAR device were not available to us.
Based on the obtained LFa and RFa values the ANSAR device reports diagnostic implications and recommendations about possible treatments. “Sympathetic withdrawal” is reported if the standing:baseline ratio of LFa is less than 0.9. OH is diagnosed if a decrease of more than 20 mm Hg in systolic blood pressure or more than 10 mm Hg in diastolic pressure is found after 2 minutes of standing. If there is OH and the heart rate increases during standing, then the OH is reported as non-neurogenic; otherwise the OH is reported as neurogenic. A drop in blood pressure that is insufficient to satisfy criteria for OH is reported as “orthostatic intolerance.” If the blood pressure decreases by less than 5 mm Hg or the heart rate increases by less than 30 bpm, the diagnosis is possible “pre-clinical orthostatic intolerance.”
Cardiac Sympathetic Neuroimaging
Cardiac sympathetic innervation was assessed by positron emission tomographic scanning after i.v. injection of the sympathetic neuroimaging agent 6-[18F]fluorodopamine [11]. The subject was positioned supine feet-first in a General Electric Advance scanner (General Electric, Milwaukee, WI), and approximately 1 mCi of 6-[18F] fluorodopamine was injected at a constant rate over 3 minutes. Thoracic dynamic scanning data were obtained for 30 minutes. The image corresponding to the midpoint of the scanning interval at 7.5 minutes after initiation of the injection was used as described previously [16]. Radioactivity in the interventricular septum that was less than 5000 nCi-kg/cc-mCi defined cardiac sympathetic denervation.
Baroreflex-Cardiovagal Function
For assessment of baroreflex-cardiovagal function each subject was studied while supine with head on pillow. Beat-to-beat blood pressure was measured non-invasively using a Finometer (Finapres Medical Systems, Amsterdam, The Netherlands) or Nexfin (bmeye, Amsterdam, The Netherlands) device. Hemodynamic data were digitized continuously and recorded using a PowerLab data acquisition system (AD Instruments, Castle Hill, Australia) and stored for later analysis on an Apple PowerBook G4 computer (Apple, Cupertino, CA).
After about a 15 minute baseline period, each subject performed at least three Valsalva maneuvers. The patient blew into a plastic tube connected to a sphygmomanometer, keeping a pressure of 30 mm Hg for 12 seconds as described previously [16]. Baroreflex-cardiovagal gain was calculated as the slope of the relationship between the cardiac interbeat interval and the beat-to-beat systolic blood pressure (with 1 beat delay) during the descent of pressure in Phase II of the maneuver [13]. A slope of 3 ms/mm Hg or less, about one-half normal [10, 21], defined baroreflex-cardiovagal failure
Supine LF Power
LF power (0.04 to 0.15 Hz) during supine testing was calculated using Chart 5.4.2 and the HRV module version 1.03 (PowerLab, AD Instruments, Castle Hill, Australia), as described previously [21].
Data Analysis and Statistics
Mean values for HRV parameters during rest sitting and the Valsalva maneuver were compared using one-way analyses of variance (Kaleidagraph 4.0.1, Synergy Software, Reading, PA). We used Fisher’s least significant difference method for post-hoc comparisons between groups. Because of skewed distributions statistical testing was conducted on log-transformed data. Relationships of individual values for the log of LF or log of LFa as the dependent variable and the log of baroreflex slope and the septal concentration of 6-[18F] fluorodopamine-derived radioactivity as independent variables were evaluated by linear regression analysis and calculation of Pearson correlation coefficients. Mean values were expressed ± 1 standard error of the mean. A p-value less than 0.05 was considered to be statistically significant.
RESULTS
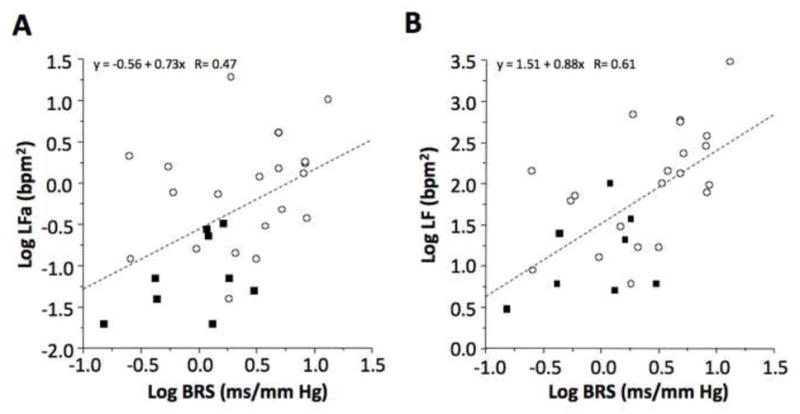
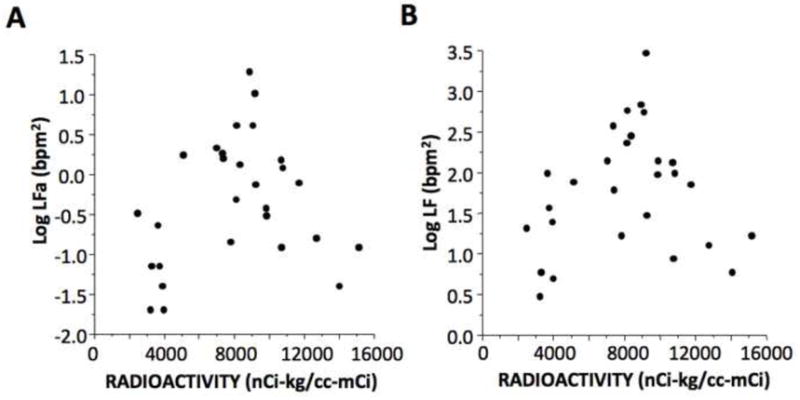
Individual values for the log of LFa and log of LF power were unrelated to cardiac 6-[18F]-fluorodopamine-derived radioactivity (Fig. 1). Values for log LFa were positively correlated with the log of baroreflex-cardiovagal slope (r=0.47, p=0.009; Fig. 2). The log of LF power unadjusted for respiratory influences was also correlated with the log of baroreflex slope (r=0.61, p=0.0005). Values for the log of LF power with subjects sitting, calculated by the ANX 3.0 device, were strongly positively correlated with values for the log of LF power with the same subjects supine, used the Chart HRV module (r=0.77, p<0.0001).
Fig. 1.
Individual values for (A) the log of respiration-adjusted low frequency power (Log LFa) and (B) the log of low frequency power without respiration adjustment (Log LF) as a function of septal myocardial 6-[18F]fluorodopamine-derived radioactivity
Fig. 2.
Individual values for (A) the log of respiration-adjusted low frequency power (Log LFa) and (B) the log of low frequency power without respiration adjustment (Log LF) as a function of the log of baroreflex slope (Log BRS) in patients with neuroimaging evidence of cardiac sympathetic denervation (black squares) and in control subjects (open circles). Dashed line is the line of best fit from the linear regression equation
Although subjects were divided into four groups based on cardiac 6-[18F] fluorodopamine-derived radioactivity and on baroreflex-cardiovagal function, there were too few patients in the group with cardiac denervation and normal baroreflex slope to include in the statistical analyses. Across the groups with normal innervation and normal baroreflex slope (Innerv Nl BRS group), normal innervation and low baroreflex slope (Innerv Low BRS group), and cardiac denervation and low baroreflex slope (Denerv Low BRS group), both log LFa and log LF varied highly significantly as a function of patient group (F=9.6, p=0.0007; F=10.9, p=0.0003). The Denerv Low BRS group had lower mean log LFa than did the Innerv Nl BRS group (p=0.0002; Fig. 3). Mean LF power was lower in the Denerv Low BRS and Innerv Low BRS groups than in the Innerv Nl BRS group (p=0.0001, p=0.005; Fig. 3).
Fig. 3.
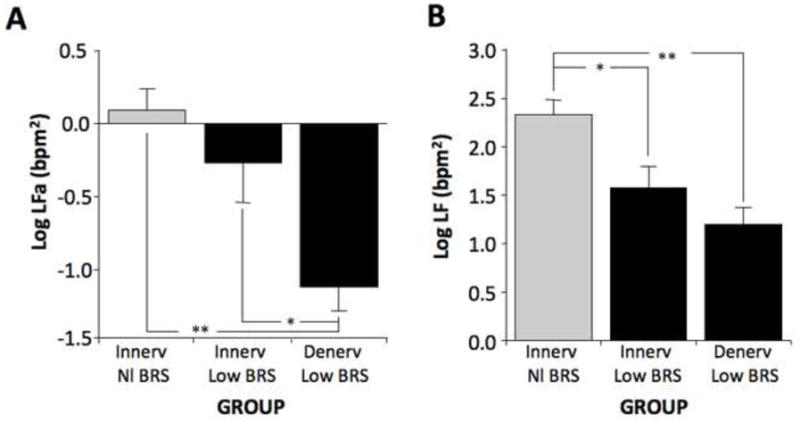
Mean (±SEM) values for (A) the log of respiration-adjusted low frequency power (Log LFa) and (B) the log of low frequency power without respiration adjustment (Log LF) in subject groups with neuroimaging evidence of intact cardiac sympathetic innervation and normal baroreflex slope (Innerv Nl BRS), intact innervation and low baroreflex slope (Innerv Low BRS), and cardiac sympathetic denervation and low baroreflex slope (Denerv Low BRS). (*) significant group difference, p<0.05; (**) p<0.001
Individual values for log LFa and log LF during the Valsalva maneuver were also positively correlated with the log of baroreflex slope (r=0.60, p=0.0006; r=0.69, p<0.0001; Fig. 4) but not with cardiac 6-[18F] fluorodopamine-derived radioactivity. Values for log LFa during the Valsalva maneuver varied with subject group (F=19, p<0.0001), with mean log LFa in the Innervated-Normal Slope group higher than in the Innervated-Low Slope group (p=0.0004) and Denervated-Low Slope group (p< 0.0001; Fig. 4).
Fig. 4.
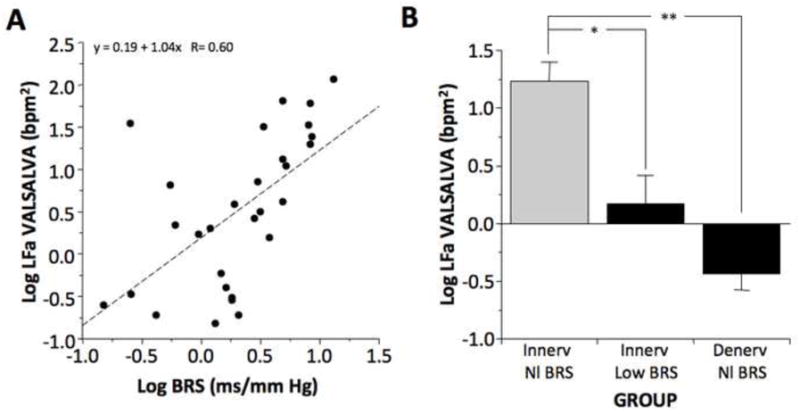
(A) Individual values for the log of respiration-adjusted low frequency power (Log LFa) during the Valsalva maneuver expressed as a function of the log of baroreflex slope (Log BRS); (B) Mean (±SEM) values for Log LFa during the Valsalva maneuver in subject groups with neuroimaging evidence of intact cardiac sympathetic innervation and normal baroreflex slope (Innerv Nl BRS), intact innervation and low baroreflex slope (Innerv Low BRS), and cardiac sympathetic denervation and low baroreflex slope (Denerv Low BRS). (*) significant group difference, p<0.05; (**) p<0.001
All of 13 patients with neurogenic OH in the setting of Parkinson disease or multiple system atrophy had an increase in heart rate during orthostasis, as did 3 of 6 patients with pure autonomic failure. None of the patients with neurogenic OH was diagnosed correctly in the ANSAR reports. Six of the 19 were reported to have possible non-neurogenic OH, 2 were reported to have orthostatic intolerance, 3 could not tolerate the standing stage of the ANSAR protocol, and 8 were not diagnosed with OH. The ANSAR device also reported that 3 normal volunteers, 1 patient with pure autonomic failure, and 1 patient with Parkinson disease and no OH had possible mild or moderate “autonomic nervous system dysfunction” (e.g., due to low RFa or LFa values at baseline). A possible diagnosis of “chronic autonomic nervous system dysfunction” was given to all 10 patients with multiple system atrophy patients, all 3 patients with Parkinson disease and OH, 1 of 5 patients with Parkinson disease and no OH, 5 of 7 patients with pure autonomic failure, and 1 control patient. For 29 of the 33 subjects, including for 3 of the 4 normal volunteers, alpha lipoic acid was recommended as treatment.
DISCUSSION
The present results relate to the ongoing controversy about the sources and clinical diagnostic meaning of LF power of heart rate variability. LF power was unrelated to cardiac sympathetic innervation as indicated by interventricular septal myocardial 6-[18F] fluorodopamine-derived radioactivity. Analogously, a recent study found no relationship between heart rate variability assessed in the frequency domain and myocardial uptake of 123I-metaiodobenzylguanidine, another neuroimaging measure of cardiac sympathetic innervation, in patients with Parkinson disease [17]. Other studies have noted no correlation between LF power and the rate of entry of norepinephrine into cardiac venous plasma, a neurochemical index of cardiac sympathetic tone [3, 5, 19, 21].
In this study we tested whether adjustment of LF power for respiratory influences does provide an index of cardiac sympathetic outflow. The ANSAR device applies the concept that LF power is composed of sympathetic and parasympathetic contributions and that the latter is determined importantly by respiratory sinus arrhythmia [1, 2, 4, 20]. ANSAR reports include LFa as a parameter for respiration-adjusted LF power. Our findings that patients with low baroreflex-cardiovagal slopes had decreased values for LFa compared to subjects with normal baroreflex slopes and that these decreases were independent of cardiac sympathetic innervation cast doubt on the notion that even with respiratory adjustment LF power provides a measure of cardiac sympathetic tone.
On the other hand, LF power both during rest sitting and during the Valsalva maneuver was related to baroreflex-cardiovagal slope. The results therefore replicate and extend on our previous findings from a different cohort of subjects studied during supine rest [21], and they fit with the proposal by Sleight et al. that LF power is a measure of baroreflex function [23]. LF power supine and sitting correlated strongly positively, indicating that our results apply irrespective of posture.
In patients with congestive heart failure, cardiac sympathetic outflow is known to be markedly increased [8], while baroreflex-cardiovagal gain is reduced [7]. Such patients have low LF power [7], in agreement with the concept that LF power tracks baroreflex function rather than cardiac sympathetic outflow.
The ANSAR ANX 3.0 generates reports that include possible diagnoses and treatment options. Although the device correctly identified chronic autonomic nervous system dysfunction in most (but not all) patients with neurogenic OH, it is clear from our results that ANSAR reports often include erroneous diagnoses and consequently inappropriate treatment recommendations. By defining OH in terms of blood pressure changes between the seated position and 2 minutes upright, the ANSAR device failed to detect OH in patients who had unequivocal OH by generally accepted criteria [18]. The ANSAR device also failed to identify OH as neurogenic in all patients who actually had neurogenic OH, due to the assumption that an increase in heart rate during orthostasis excludes neurogenic OH, whereas virtually all patients with neurogenic OH had at least some orthostatic increase in heart rate. Because of these errors the ANSAR device inappropriately recommended treatment with alpha lipoic acid for most of the patients in this study who had neurogenic OH.
In conclusion, with or without adjustment for respiration, LF power evaluated during rest sitting, standing, or during the Valsalva maneuver seems to provide an index not of cardiac sympathetic tone but of baroreflex function. The ANSAR device fails to identify neurogenic OH in patients with chronic autonomic failure.
Acknowledgments
Financial support: Division of Intramural Research, NINDS, NIH.
This research was supported by the Intramural Research Program of the NIH, National Institute of Neurological Disorders and Stroke.
Ms. Tereza Jenkins coordinated patient travel.
Abbreviations
- HF
high frequency
- HFa
adjusted high frequency power
- LF
low frequency
- LFa
adjusted low frequency power
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.Akselrod S. Spectral analysis of fluctuations in cardiovascular parameters: a quantitative tool for the investigation of autonomic control. Trends Pharmacol Sci. 1988;9:6–9. doi: 10.1016/0165-6147(88)90230-1. [DOI] [PubMed] [Google Scholar]
- 2.Akselrod S, Gordon D, Ubel FA, Shannon DC, Berger AC, Cohen RJ. Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control. Science. 1981;213:220–222. doi: 10.1126/science.6166045. [DOI] [PubMed] [Google Scholar]
- 3.Alvarenga ME, Richards JC, Lambert G, Esler MD. Psychophysiological mechanisms in panic disorder: a correlative analysis of noradrenaline spillover, neuronal noradrenaline reuptake, power spectral analysis of heart rate variability, and psychological variables. Psychosom Med. 2006;68:8–16. doi: 10.1097/01.psy.0000195872.00987.db. [DOI] [PubMed] [Google Scholar]
- 4.Aysin B, Aysin E. Effect of respiration in heart rate variability (HRV) analysis. Conf Proc IEEE Eng Med Biol Soc. 2006;1:1776–1779. doi: 10.1109/IEMBS.2006.260773. [DOI] [PubMed] [Google Scholar]
- 5.Baumert M, Lambert GW, Dawood T, Lambert EA, Esler MD, McGrane M, Barton D, Sanders P, Nalivaiko E. Short-term heart rate variability and cardiac norepinephrine spillover in patients with depression and panic disorder. Am J Physiol Heart Circ Physiol. 2009;297:H674–679. doi: 10.1152/ajpheart.00236.2009. [DOI] [PubMed] [Google Scholar]
- 6.Colombo J, Shoemaker WC, Belzberg H, Hatzakis G, Fathizadeh P, Demetriades D. Noninvasive monitoring of the autonomic nervous system and hemodynamics of patients with blunt and penetrating trauma. J Trauma. 2008;65:1364–1373. doi: 10.1097/TA.0b013e31818cc307. [DOI] [PubMed] [Google Scholar]
- 7.Creager MA. Baroreceptor reflex function in congestive heart failure. Am J Cardiol. 1992;69:10G–15G. doi: 10.1016/0002-9149(92)91250-8. discussion 15G–16G. [DOI] [PubMed] [Google Scholar]
- 8.Eisenhofer G, Friberg P, Rundqvist B, Quyyumi AA, Lambert G, Kaye DM, Kopin IJ, Goldstein DS, Esler MD. Cardiac sympathetic nerve function in congestive heart failure. Circulation. 1996;93:1667–1676. doi: 10.1161/01.cir.93.9.1667. [DOI] [PubMed] [Google Scholar]
- 9.Goldstein DS. The Autonomic Nervous System in Health and Disease. Marcel Dekker, Inc; New York, NY: 2001. [Google Scholar]
- 10.Goldstein DS. Dysautonomia in Parkinson’s disease: neurocardiological abnormalities. Lancet Neurol. 2003;2:669–676. doi: 10.1016/s1474-4422(03)00555-6. [DOI] [PubMed] [Google Scholar]
- 11.Goldstein DS, Eisenhofer G, Dunn BB, Armando I, Lenders J, Grossman E, Holmes C, Kirk KL, Bacharach S, Adams R, et al. Positron emission tomographic imaging of cardiac sympathetic innervation using 6-[18F]fluorodopamine: initial findings in humans. J Am Coll Cardiol. 1993;22:1961–1971. doi: 10.1016/0735-1097(93)90786-z. [DOI] [PubMed] [Google Scholar]
- 12.Goldstein DS, Holmes C, Li ST, Bruce S, Metman LV, Cannon RO., 3rd Cardiac sympathetic denervation in Parkinson disease. Ann Intern Med. 2000;133:338–347. doi: 10.7326/0003-4819-133-5-200009050-00009. [DOI] [PubMed] [Google Scholar]
- 13.Goldstein DS, Horwitz D, Keiser HR. Comparison of techniques for measuring baroreflex sensitivity in man. Circulation. 1982;66:432–439. doi: 10.1161/01.cir.66.2.432. [DOI] [PubMed] [Google Scholar]
- 14.Goldstein DS, Orimo S. Cardiac sympathetic neuroimaging: summary of the First International Symposium. Clin Auton Res. 2009;19:133–136. doi: 10.1007/s10286-009-0002-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldstein DS, Pechnik S, Holmes C, Eldadah B, Sharabi Y. Association between supine hypertension and orthostatic hypotension in autonomic failure. Hypertension. 2003;42:136–142. doi: 10.1161/01.HYP.0000081216.11623.C3. [DOI] [PubMed] [Google Scholar]
- 16.Goldstein DS, Tack C. Non-invasive detection of sympathetic neurocirculatory failure. Clin Auton Res. 2000;10:285–291. doi: 10.1007/BF02281111. [DOI] [PubMed] [Google Scholar]
- 17.Haensch CA, Lerch H, Jorg J, Isenmann S. Cardiac denervation occurs independent of orthostatic hypotension and impaired heart rate variability in Parkinson’s disease. Parkinsonism Relat Disord. 2009;15:134–137. doi: 10.1016/j.parkreldis.2008.04.031. [DOI] [PubMed] [Google Scholar]
- 18.Kaufmann H. Consensus statement on the definition of orthostatic hypotension pure autonomic failure and multiple system atrophy. Clin Auton Res. 1996;6:125–126. doi: 10.1007/BF02291236. [DOI] [PubMed] [Google Scholar]
- 19.Kingwell BA, Thompson JM, Kaye DM, McPherson GA, Jennings GL, Esler MD. Heart rate spectral analysis, cardiac norepinephrine spillover, and muscle sympathetic nerve activity during human sympathetic nervous activation and failure. Circulation. 1994;90:234–240. doi: 10.1161/01.cir.90.1.234. [DOI] [PubMed] [Google Scholar]
- 20.Lishner M, Akselrod S, Avi VM, Oz O, Divon M, Ravid M. Spectral analysis of heart rate fluctuations. A non-invasive, sensitive method for the early diagnosis of autonomic neuropathy in diabetes mellitus. J Auton Nerv Syst. 1987;19:119–125. doi: 10.1016/0165-1838(87)90005-1. [DOI] [PubMed] [Google Scholar]
- 21.Moak JP, Goldstein DS, Eldadah BA, Saleem A, Holmes C, Pechnik S, Sharabi Y. Supine low-frequency power of heart rate variability reflects baroreflex function, not cardiac sympathetic innervation. Heart Rhythm. 2007;4:1523–1529. doi: 10.1016/j.hrthm.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Orimo S, Oka T, Miura H, Tsuchiya K, Mori F, Wakabayashi K, Nagao T, Yokochi M. Sympathetic cardiac denervation in Parkinson’s disease and pure autonomic failure but not in multiple system atrophy. J Neurol Neurosurg Psychiatry. 2002;73:776–777. doi: 10.1136/jnnp.73.6.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sleight P, La Rovere MT, Mortara A, Pinna G, Maestri R, Leuzzi S, Bianchini B, Tavazzi L, Bernardi L. Physiology and pathophysiology of heart rate and blood pressure variability in humans: is power spectral analysis largely an index of baroreflex gain? Clin Sci (Lond) 1995;88:103–109. doi: 10.1042/cs0880103. [DOI] [PubMed] [Google Scholar]


