Abstract
A highly stereoselective synthesis of a model C(18)–C(35) spiroketal unit (7) of integramycin has been accomplished via an enantioselective stannyl-crotylboration reaction and an N-iodosuccinimide-mediated spiroketalization of 19a.
Integramycin (1, Figure 1) is an HIV-1 integrase inhibitor that was isolated from an Actinoplanes species by Singh and coworkers in 2002.1 Integrase is a critical enzyme for the process by which reverse-transcribed viral DNA is inserted into the host chromosomal DNA, an essential step for HIV replication.2 Integramycin inhibits the third step of the integration process, specifically strand transfer of the proviral DNA into the host cell DNA, with an IC50 value of 4.0 µM. Integramycin (1) contains a cis-octahydronaphthalene core unit with spiroketal and acyl tetramic acid containing side chains.
Figure 1.
Retrosynthetic Analysis of Integramycin (1) and the Model C(18)–(35) Spiroketal Unit (7).
In spite of several reported synthetic studies,3 the total synthesis of integramycin has not been accomplished. As part of our ongoing studies on the synthesis of integramycin, we report herein a highly stereoselective synthesis of a model C(18)–C(35) spiroketal unit 7. Key steps of this synthesis include an N-iodosuccinimide (NIS)-mediated spiroketalization of the 1-hydroxy-9-en-5-one 19a and an application of the stannyl-crotylboration reaction recently developed in our laboratory.4
Our retrosynthetic analysis of integramycin (1) is outlined in Figure 1. We envisioned that the natural product should be accessible by coupling of tetramic acid intermediate 2, cis-octahydronaphthalene carboxaldehyde 3, and Wittig reagent 4. Retrosynthetic disconnection of the C(22)–C(23) bond in 4 gives aldehyde 5 and vinyliodide 6, substrates for a Nozaki-Hiyama-Kishi coupling reaction.
Our synthesis commenced with the Horner-Wadsworth-Emmons reaction5 of the known β-keto phosphonate 86 and aldehyde 9,7 followed by Pd-catalyzed hydrogenation of the resulting olefin to give imide 10 (Scheme 1). Diastereoeslective α-methylation of 10 was performed by using LiHMDS as the base under Evans’ asymmetric alkylation conditions.8 Reductive removal of the oxazolidinone unit by treatment of the alkylated product with NaBH4 afforded primary alcohol 11. Finally, protection of the primary hydroxyl group of 11 by treatment with p-methoxybenzyl tricholoroacetimidate, followed by removal of the TBDPS group with TBAF and oxidation of the resulting primary alcohol under Parihk-Doering reaction conditions9 provided aldehyde 5 in excellent overall yield.
Scheme 1.
Synthesis of Aldehyde 5
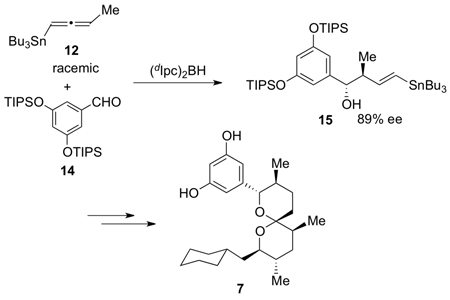
The enantioconvergent, enantioselective stannylcrotylboration reaction4 recently developed in our laboratory can be used to synthesize a variety of highly enantiomerically enriched γ-stannyl homoallylic alcohols by treating racemic allenylstannane 1210 (Scheme 2) with (Ipc)2BH followed by addition of an aldehyde. For the present synthesis, we employed TIPS-protected 3,5-dihydroxybenzaldehyde 14 (1.0 equiv) in the reaction with the highly enantiomerically enriched crotylborane 13, formed in situ by treating 12 (2.0 equiv) with (dIpc)2BH (1.9 equiv) at −20 °C.11 This afforded γ-stannyl homoallylic alcohol 15 in 81% yield and 89% ee with 7.9:1 anti:syn ratio (Scheme 2). Protection of the newly formed hydroxyl group as a TES ether and subsequent treatment with I2 to effect iodine-tin exchange gave vinyliodide 6. Coupling of 5 and 6 by using the Nozaki-Hiyama-Kishi reaction12 provided an inseparable mixture of the allylic alcohols and unreacted aldehyde 5. Treatment of this mixture with TESOTf enabled 16 (as a mixture of diastereomers) to be isolated in 83% yield over the two steps. Removal of the PMB ether with DDQ generated 17, which was subsequently converted into the primary iodide by treatment with I2 and PPh3. Finally, treatment of the iodide with excess of PPh3 in benzene at reflux furnished the phosphonium salt 4.
Scheme 2.
Synthesis of the C(18)–C(35) Fragment 4
We next turned to the task of forming the spiroketal unit stereoselectively. Cyclohexane carboxaldehyde was use as a model substrate in the Wittig reaction with 4, which provided the (Z)-olefinic product in 86% yield (Scheme 3). Removal of both TES protecting groups by treatment with PPTS in MeOH then afforded diol 18. A ruthenium-mediated olefin isomerization13 selectively converted the allylic alcohol 18 to the saturated ketone 19a without scrambling the Z olefin in the substrate. Ketone 19a was obtained as a 1 : 1.3 mixture with the corresponding hemiacetal isomer 19b, along with 10–20% of unidentified inseparable impurities. This impure product mixture was used in the following spiroketalization experiments. Among several reagents14 that have been reported to mediate the oxidative spiroketalization of hemiacetal-alkene (or keto-alcohol-alkene) substrates, we elected to use NIS in our experiments. We hypothesized that hemiacetal isomer 19b would undergo cyclization through iodonium ion intermediate 23 (Path b, Scheme 4), with stereochemical control governed by minimization of 1,3-allylic steric interactions.15 Attack of the hemiacetal hydroxy group in 23 from the top face of the iodonium ion, as indicated in Scheme 4, leads to the doubly anomeric effect stabilized spiroketal 20. Ketone isomer 19a could similarly undergo spirocyclization by Path a via oxonium intermediate 22 with the same stereochemical control as in Path b. In the event, treatment of the 19a/19b mixture with NIS in CH2Cl2 at 23 °C for 24 h delivered the desired spiroketal 20 as the predominant product in 32% overal yield from 18. Some side products were also observed, but could not be isolated in pure form for structural characterization. The iodine substituent was readily cleaved reductively under radical conditions utilizing Bu3SnH and AIBN. Subsequent treatment of the de-iodinated spiroketal with TBAF in THF provided the model spiroketal 7 in 98% yield.
Scheme 3.
First Generation Synthesis of Spiroketal 7
Scheme 4.
Stereoselective NIS-Mediated Spiroketalization
The efficiency of the NIS-mediated spiroketalization of the 19a/19b mixture in Scheme 3 was compromised by the presence of impurities deriving from the preceding olefin isomerization step that could not be separated. In order to investigate the NIS-mediated spiroketalization with a pure substrate, an alternative route to the 19a/19b mixture was developed (Scheme 5). After coupling of 5 with 6 via the Nozaki-Hiyama-Kishi reaction, the secondary alcohol of the allylic alcohol product was protected as a pivaloyl ester, thereby providing 24 in 81% yield. The E olefin was reduced by using diimide generated from NBSH (o-nitrobenzenesulfonyl hydrazide)16 in the presence of Et3N. Reductive cleavage of the PMB ether then provided primary alcohol 25. Iodination of 25, followed by treatment with PPh3, furnished the new C(18)–C(35) fragment 26 in 86% yield. Wittig olefination of cyclohexanecarboxaldehyde and the ylide generated from 26 provided the Z olefin product in 89% yield. The pivaloyl group was removed by treatment with DIBAL-H, and then the resulting secondary alcohol was oxidized to afford ketone 27. Upon removal of the TES protecting group with HOAc and H2O in THF, the pure 19a/b mixture (1:1.3 ratio, as before) was obtained in 87% yield. Treatment of pure 19a/b to NIS in CH2Cl2 at 23 °C for 24 h yielded the spiroketal 20 in 68% yield, along with four unidentified minor isomers (10:7:3:3 ratio) that were obtained in 17% combined yield.
Scheme 5.
Second Genertion Synthesis of Spiroketal 20
aNBSH = o-nitrobenzenesulfonylhydrazide
In summary, we have developed a synthesis of a model spiroketal fragment 7 of integramycin. This synthesis was facilitated by use of the highly stereoselective stannyl-crotylboration reaction recently developed in our laboratory,4 that was used to synthesize the vinylstannane intermediate 15. The spiroketal was established by a stereoselective NIS-mediated spirocyclization of 19a/b that provided the C(18)–C(35) fragment 20. Further progress on the synthesis of integramycin will be reported in due course.
Supplementary Material
Acknowledgment
This work was supported by a grant from the National Institutes of Health (GM026782).
Footnotes
Supporting Information Available: Experimental procedures and full spectroscopic data for all new compounds. This material is available free of charge via Internet at http://pubs.acs.org.
References
- 1.Singh SB, Zink DL, Heimbach B, Genilloud O, Teran A, Silverman KC, Lingham RB, Felock P, Hazuda DC. Org. Lett. 2002;4:1123. doi: 10.1021/ol025539b. [DOI] [PubMed] [Google Scholar]
- 2.(a) Craigie R. J. Biol. Chem. 2001;276:23213. doi: 10.1074/jbc.R100027200. [DOI] [PubMed] [Google Scholar]; (b) Esposito D, Craigie R. AdV. Virus Res. 1999;52:319. doi: 10.1016/s0065-3527(08)60304-8. [DOI] [PubMed] [Google Scholar]
- 3.(a) Dineen TA, Roush WR. Org. Lett. 2005;7:1355. doi: 10.1021/ol050191g. [DOI] [PubMed] [Google Scholar]; (b) Wang L, Floreancig PE. Org. Lett. 2004;6:569. doi: 10.1021/ol036339i. [DOI] [PubMed] [Google Scholar]
- 4.Chen M, Roush WR. J. Am. Chem. Soc. 2011;133 doi: 10.1021/ja2010187. in press (ASAP); .doi.org/10.1021/ja2010187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blanchette MA, Choy W, Davis JT, Essenfeld AP, Masamune S, Roush WR, Sakai T. Tetrahedron Lett. 1984;25:2183. [Google Scholar]
- 6.Roush WR, Brown BB. J. Org. Chem. 1993;58:2162. [Google Scholar]
- 7.Johns BA, Grant CM, Marshall JA. Org. Synth. 2002;79:59. [Google Scholar]
- 8.Evans DA, Ennis MD, Mathre DJ. J. Am. Chem. Soc. 1982;104:1737. [Google Scholar]
- 9.Evans PA, Murthy VS, Roseman JD, Rheingold AL. Angew. Chem. Inter. Ed. 1999;38:3175. [PubMed] [Google Scholar]
- 10.Marshall JA, Chobanion H. Org. Synth. 2005;82:43. [Google Scholar]
- 11.Brown HC, Singaram B. J. Org. Chem. 1984;49:945. [Google Scholar]
- 12.(a) Jin H, Uenishi J, Christ WJ, Kishi Y. J. Am. Chem. Soc. 1986;108:5644. [Google Scholar]; (b) Takai K, Tagashira M, Kuroda T, Oshima K, Utimoto K, Nozaki H. J. Am. Chem. Soc. 1986;108:6048. doi: 10.1021/ja00279a068. [DOI] [PubMed] [Google Scholar]
- 13.Uma R, Davies MK, Crevisy C, Gree R. Eur. J. Org. Chem. 2001:3141. [Google Scholar]
- 14.(a) Bartlett PA, Mori I, Bose JA. J. Org. Chem. 1989;54:3236. [Google Scholar]; (b) Kitching W, Lewis JA, Fletcher MT, De Voss JJ, Drew RAI, Moore CJ. J. Chem. Soc. Chem. Commun. 1986;11:855. [Google Scholar]; (c) Negri DP, Kishi Y. Tetrahedron Lett. 1987;28:1063. [Google Scholar]; (d) Perkins MV, Jacobs MF, Kitching W, Cassidy PJ, Lewis JA, Drew RAI. J. Org. Chem. 1992;57:3365. [Google Scholar]
- 15.Hoffmann RW. Chem. Rev. 1989;89:1841. [Google Scholar]
- 16.(a) Waetzig JD, Hanson PR. Org. Lett. 2008;10:109. doi: 10.1021/ol7025944. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Myers AG, Zheng B, Movassaghi M. J. Org. Chem. 1997;62:7507. doi: 10.1021/jo9710137. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.