Abstract
In the peripheral nervous system, Schwann cells make myelin, a specialized sheath that is essential for rapid axonal conduction of action potentials. Immature Schwann cells initially interact with many axons, but, through a process termed radial sorting, eventually interact with one segment of a single axon as promyelinating Schwann cells. Previous studies have identified genes that are required for Schwann cell process extension and proliferation during radial sorting. Previous analyses also show that ErbB signaling is required for Schwann cell proliferation, myelination, radial sorting, and the proper formation of unmyelinated Remak bundles. Because ErbB signaling and Schwann cell proliferation are both required during radial sorting, we sought to determine if the primary function of ErbB signaling in this process is to regulate Schwann cell proliferation or if ErbB signaling also controls other aspects of radial sorting. To address this question, we applied small molecule inhibitors in vivo in zebrafish to independently block ErbB signaling and proliferation. Ultrastructural analysis of treated animals revealed that both ErbB signaling and Schwann cell proliferation are required for radial sorting in vivo. ErbB signaling, however, is required for Schwann cell process extension, while Schwann cell proliferation is not. These results provide in vivo evidence that ErbB signaling plays a direct role in process extension during radial sorting, in addition to its role in regulating Schwann cell proliferation.
Introduction
Myelin is an essential component of vertebrate nerves, insulating axons to allow for the rapid conduction of action potentials. In the peripheral nervous system, Schwann cell precursors migrate along axons and then differentiate into immature Schwann cells that are faced with a fate choice (Jessen and Mirsky 2005). Immature Schwann cells associated with large caliber axons will become promyelinating and then myelinating Schwann cells, while those that continue to associate with several small caliber axons will become non-myelinating (“Remak”) Schwann cells (Jessen and Mirsky 2005; Nave and Salzer 2006).
Prior to myelination, Schwann cells must transition from interacting with many axons as immature Schwann cells to just one segment of one axon as promyelinating Schwann cells. This occurs by a process termed radial sorting, during which immature Schwann cells progressively “sort” large caliber axons to the periphery of an axon bundle and then myelinate them (Webster et al. 1973). Initially, immature Schwann cells are arrayed around the edges of axon bundles. Next, Schwann cells insert processes into the axon bundle, and a premyelinating Schwann cell will pick just one axon and sort it to the periphery of the axon bundle. At this point, the Schwann cell will differentiate into a promyelinating Schwann cell, express stage-specific transcription factors including Oct6/Pou3f1 and Krox20/Egr2, and begin to wrap the axon with a myelin sheath (Svaren and Meijer, 2008).
To ensure that all axons are either myelinated or ensheathed by the end of radial sorting, Schwann cells must coordinate the extension and stabilization of their processes, in addition to regulating the number of Schwann cells present (Jessen and Mirsky 2005; Webster et al. 1973). The latter involves extensive proliferation of Schwann cells during the period of radial sorting (Webster et al. 1973). Over the past several years, several factors that regulate radial sorting have emerged, including molecules that effect Schwann cell process extension such as Rac1, β1-integrin, integrin linked kinase (ILK), and laminin-γ1 (Benninger et al. 2007; Chen and Strickland 2003; Feltri et al. 2002; Nodari et al. 2007; Pereira et al. 2009; Yu et al. 2009; Yu et al. 2005). Additional molecules required for Schwann cell proliferation during radial sorting include Cdc42, focal adhesion kinase (FAK), laminin-2, laminin-8, and laminin-γ1 (Benninger et al. 2007; Chen and Strickland 2003; Grove et al. 2007; Yang et al. 2005; Yu et al. 2009; Yu et al. 2005). An important goal of current work is to understand how these and other factors interact to coordinate the events of radial sorting.
A key signal during Schwann cell development is Neuregulin1 (Nrg1), which is expressed in axons and signals through ErbB receptors on Schwann cells (Nave and Salzer, 2006). Nrg1/ErbB signaling controls many steps of Schwann cell development, including migration, proliferation, survival, radial sorting, Remak bundle formation, and myelination (Newbern and Birchmeier 2010).
Because previous studies have revealed the importance of Nrg1 signaling in Schwann cell proliferation and because Schwann cells proliferate extensively during radial sorting, we wondered if Nrg1/ErbB signaling is solely required to regulate Schwann cell proliferation during radial sorting or whether it plays a more direct role (e.g. in process extension). A previous in vitro study found that the addition of excess Schwann cells to a co-culture containing neurons deficient in the Nrg1 Type III isoform was not sufficient to rescue myelination (Taveggia et al. 2005). This suggested that Nrg1/ErbB signaling may regulate something other than Schwann cell number during the initiation of myelination, possibly radial sorting. To test this in vivo, we independently blocked ErbB signaling and proliferation using small molecule inhibitors just prior to the onset of myelination in zebrafish. This allowed us to temporally control inhibition of ErbB signaling and proliferation, so that early events in Schwann cell development proceeded normally. Ultrastructural analysis of drug treated nerves revealed that both ErbB signaling and Schwann cell proliferation are required for radial sorting. In both cases, there is an arrest of radial sorting, but further analysis revealed that inhibiting ErbB signaling or Schwann cell proliferation affects radial sorting in different ways. In the absence of ErbB signaling, no Schwann cell processes are present within the axon bundle, and fewer axons are contacted by a Schwann cell. In contrast, Schwann cells were able to extend processes into axon bundles in control larvae and those treated with cell cycle inhibitors, despite the sorting defect in the latter. Our analysis reveals that ErbB signaling regulates Schwann cell process extension during radial sorting, in addition to its role in regulating proliferation to control Schwann cell numbers.
Materials and Methods
Fish care
Wildtype embryos (TL and WIK) and Tg(FoxD3:GFP) embryos (Gilmour et al. 2002) were raised at 28.5°C and were staged as described previously (Kimmel et al. 1995).
Small molecule inhibitors
Small molecule inhibitors were diluted in dimethyl sulfoxide (DMSO) and used at the given concentrations: ErbB inhibitor AG1478, 2 μM (EMD; Lyons et al., 2005); S-phase inhibitor aphidicolin, 150 μM (Sigma; Spadari et al., 1985); and G2/M inhibitor camptothecin, 500 nM (EMD; Jones et al., 1997). Small molecule inhibitors were added directly to the embryo medium at 48 hours postfertilization (h) and extra DMSO was added to bring its final concentration to 1%. Control embryos were treated with 1% DMSO from 48 h. Embryo media and inhibitors were changed daily.
Immunofluorescence and in situ hybridization
Immunofluorescence and in situ hybridization staining were carried out using standard techniques. The following primary antibodies were used: Anti-acetylated tubulin, 1:1000 (Sigma); anti-MBP, 1:50 (Lyons et al. 2005); and anti-phosphohistone H3, 1:200 (Upstate). Alexa 488 or 568 secondary antibodies (Molecular Probes) were used at a concentration of 1:2000. Fluorescent images were collected on a Zeiss Pascal LSM5 confocal microscope. oct6 and krox20 in situ probes were previously described (Lyons et al. 2005; Monk et al. 2009).
Live imaging
Embryos were anesthetized with 0.016% Tricaine (w/v) and mounted in 1.5% low melting point agarose for imaging.
Schwann cell counts
Tg(FoxD3:GFP) embryos were treated with various inhibitors as described from 48h to 3d. In addition, embryos were treated after gastrulation with 0.2 mM 1-phenyl-2-thiourea to prevent pigment melanization. Five embryos from each condition were live-mounted as described; Schwann cells at the level of the yolk extension were counted.
Acridine Orange Staining
Apoptotic corpses were visualized with acridine orange (Sigma) as previously described (Lyons et al., 2005).
Transmission electron microscopy
Transmission electron microscopy (TEM) was performed as previously described (Lyons et al. 2008). Images were collected with a JEOL1230 and pseudocolored using Adobe Photoshop software.
Statistical analysis
Kruskal-Wallis tests were performed on each data set followed by the Mann-Whitney-U test using R software. Standard deviations were calculated using Microsoft Excel software.
Results
ErbB signaling and Schwann cell proliferation are required for the expression of promyelin genes and normal Schwann cell number
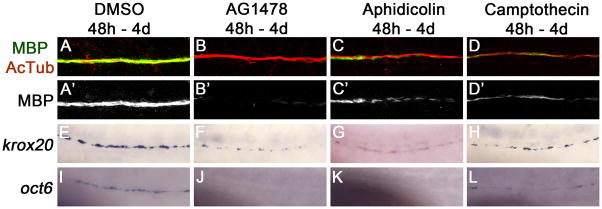
We previously found that inhibiting either ErbB signaling using the small molecule inhibitor AG1478 or proliferation with aphidicolin and hydroxyurea was sufficient to reduce the expression of myelin basic protein (MBP) in the zebrafish posterior lateral line nerve (PLLn; Lyons et al., 2005). By 48 hours postfertilization (h), Schwann cells have completed their migration along the lateral line nerve (Lyons et al. 2005). To allow the analysis of ErbB signaling and proliferation during radial sorting without confounding effects on Schwann cell migration, we began treating zebrafish embryos with small molecule inhibitors at 48 h. Similar to previous studies (Lyons et al., 2005), AG1478 treatment (at a reduced concentration of 2 μM) and alternative methods for blocking the cell cycle (aphidicolin alone or camptothecin alone) yielded a reduction in MBP staining (Fig. 1A–D). In the larvae treated with the cell cycle inhibitors, there was a large decrease in the number of cells in M phase, marked by phosphohistone H3 expression (data not shown).
Figure 1. ErbB signaling and Schwann cell proliferation are required for the normal expression of myelinating and promyelinating markers.
Myelin basic protein (MBP) expression, which marks myelinating Schwann cells, is reduced along the posterior lateral line nerve in animals treated with AG1478 (B), aphidicolin (C), and camptothecin (D) as compared to DMSO-treated controls (A). MBP is in green, acetylated tubulin (AcTub) marking axons is shown in red. MBP is shown alone in A′–D′. krox20 and oct6 expression is also reduced in AG1478 (F, J), aphidicolin (G, K), and camptothecin (H, L) treated nerves as compared to DMSO control nerves (E, I). For all conditions, at least 10 larvae were examined in at least two different experiments.
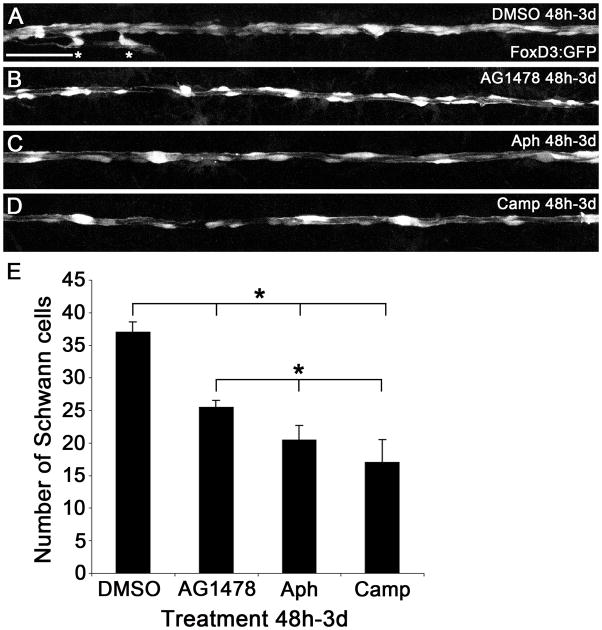
Inhibition of ErbB signaling reduces, but does not eliminate, Schwann cell proliferation (Lyons et al., 2005). To determine how the number of Schwann cells compared among our various treatments, we examined zebrafish carrying the FoxD3:GFP transgene, which expresses GFP in Schwann cells, to count Schwann cells in a region of the PLLn after 24 hours of treatment (Fig. 2). The number of Schwann cells was significantly reduced in AG1478-, aphidicolin-, and camptothecin-treated embryos as compared to the DMSO control embryos (Fig. 2). There were, however, more Schwann cells in AG1478-treated embryos (25.4 ± 1.1 s.d.) than aphidicolin- and camptothecin-treated embryos (20.4 ± 2.3 and 17 ± 3.5; Fig. 2B–E). These results are consistent with our previous analysis showing that inhibiting ErbB signaling results in a partial block of Schwann cell proliferation (Lyons et al., 2005). Using acridine orange staining, we found that there was no significant increase in cell death between the DMSO control and AG1478-treated embryos (data not shown). There was an increase in cell death in the cell cycle inhibitor-treated embryos, which is in line with previous reports showing that these drugs can lead to apoptosis (data not shown; Cotter 1992).
Figure 2. Number of Schwann cells in embryos treated with DMSO, AG1478, Aphidicolin, and Camptothecin.
Embryos carrying the FoxD3:GFP transgene were treated from 48h to 3d with DMSO (A), AG1478 (B), aphidicolin (Aph, C), or camptothecin (Camp, D) and imaged at the level of the yolk extension. Asterisks in (A) indicate Schwann cells migrating along axon branches that innervate sensory organs; these cells were not included in the totals shown. Scale bar is 50 μm. (E) Quantification of Schwann cell number (n = 5 embryos in each treatment). The Kruskal-Wallis test yielded a p value of p = 0.00088 and subsequent Mann-Whitney-U tests comparing the DMSO control to either AG1478-, aphidicolin-, or camptothecin-treated embryos gave significant p values (large bracket). Additionally, a Kruskal-Wallis test performed among all treatments except the DMSO control gave a p value of p = 0.0061 and Mann-Whitney-U tests comparing the AG1478-treated embryos to aphidicolin- or camptothecin-treated embryos yielded significant p values (small bracket). Error bars show plus one standard deviation. * = p < 0.05
Schwann cells that have sorted an individual axon express oct6 and krox20, which are required for progression beyond the promyelinating stage (Svaren and Meijer 2008). Expression of both transcripts was reduced after treatment with AG1478 or cell cycle inhibitors (Fig. 1E–L; Lyons et al., 2005), suggesting that ErbB signaling and post-migratory cell division are required for Schwann cells to progress to the promyelinating stage.
Radial sorting has begun in the lateral line nerve by 48 h
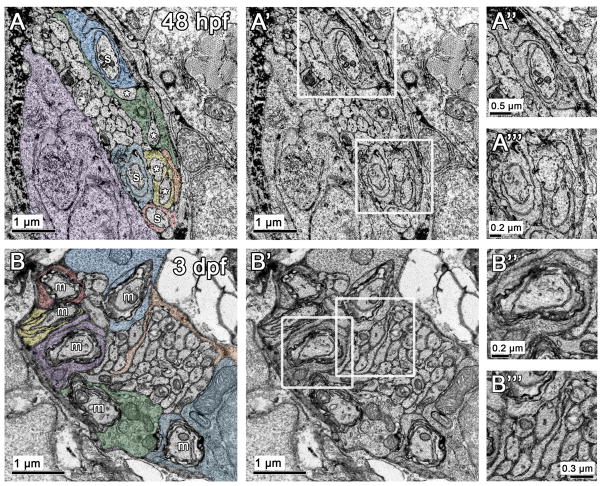
Previous studies suggest that radial sorting is underway in the lateral line nerve by 48 h, as Schwann cells express oct6 by this time (Monk et al. 2009), but the ultrastructure of the nerve at this stage has not been extensively investigated. Our ultrastructural analysis reveals that radial sorting is already underway at 48 h, and proceeds in a process similar to that described in mammals. Schwann cell processes are evident within the axon bundle of the lateral line nerve, and axons can be found at several stages – unsorted, contacted by a Schwann cell process, encircled by a Schwann cell process, or with several wraps of uncompacted myelin (Fig. 3A). For our analysis, we defined “sorted” as an axon that was completely separated from other axons by Schwann cell cytoplasm that is not obviously contacting other axons. For example, in figure 3A, the axons labeled “S” are sorted but those axons labeled “*” are not because the Schwann cell processes they are associated with (yellow or green) are surrounding two axons each. In mammals, immature and mature Schwann cells secrete a basal lamina (Jessen and Mirsky 2005; Webster et al. 1973). At these early stages in zebrafish, however, the nerve and surrounding tissue were too tightly apposed to allow us to definitively define a basal lamina.
Figure 3. Radial sorting is underway at 48 h and continues over time in the posterior lateral line nerve.
(A) Ultrastructural analysis reveals that radial sorting is underway in the lateral line nerve at 48 h. In this example, three axons are already sorted (s, Schwann cells pseudocolored blue or red). Two sorted axons already have two wraps of Schwann cell cytoplasm around them (pseudocolored blue). Other Schwann cell processes (pseudocolored yellow and green) enter the axon bundle and interact with more than one axon (*). A Schwann cell body is pseudocolored purple. (A′) Same image as in (A) without pseudocoloring. (A″–A‴) Magnification of regions boxed in A′. (B) By 3 d, several myelinated axons (m) are present within the posterior lateral line nerve; different pseudocolors indicate that each myelinated axon is associated with its own Schwann cell. A Schwann cell process pseudocolored in orange is present within the unsorted axon bundle. (B′) Same image as in (B) without pseudocoloring. (B″–B‴) Magnification of regions boxed in B′.
By 24 hours later, at 3 day postfertilization (d), there were several myelinated axons with compacted Schwann cell cytoplasm wrapped around the axon (Fig. 3B). The process of radial sorting was still ongoing, as evidenced by the presence of a large cluster of unsorted axons and Schwann cell processes within the axon bundle (Fig. 3B, orange process). At 3 d, unsorted axons are of a similar size, and the sorted axons are generally larger than unsorted axons (Fig. 3). In mature mammalian nerves, axons over 1 μm2 are myelinated, while smaller axons are in Remak bundles (Nave and Salzer 2006). At this stage, we did not observe mature Remak bundles in the region of the posterior lateral line nerve that we examined (at the level of the sixth somite; Fig. 3). There were large bundles of unmyelinated axons, but these were not individually ensheathed, as evidenced by the lack of Schwann cell membrane between axons (Fig. 3). Additionally, at this early developmental stage axons smaller than 1 μm2 were being sorted and myelinated.
ErbB signaling and Schwann cell proliferation are required for radial sorting
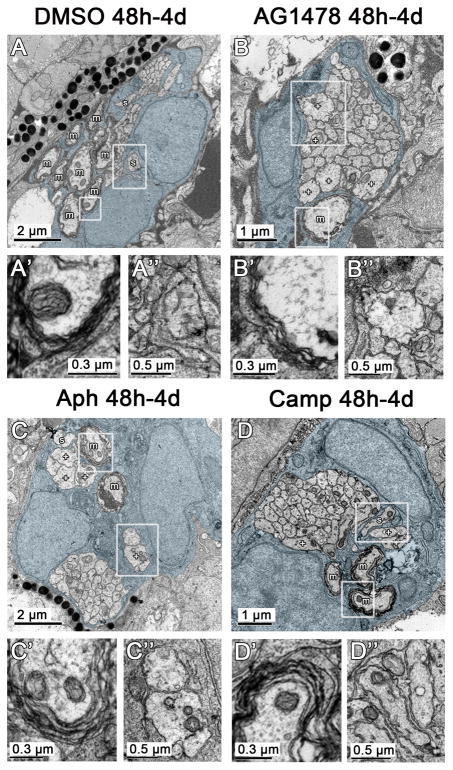
In order to analyze the roles of ErbB signaling and proliferation during radial sorting, we conducted ultrastructural analysis of lateral line nerves in larvae treated with AG1478, aphidicolin, or camptothecin from 48 h to 4 d. In all cases, there was a reduction in the number of sorted and myelinated axons (Fig. 4,5A). It is important to note that large caliber axons were present but not myelinated in the experimental conditions, which shows that these treatments do not generally disrupt axonal growth (Fig. 4). In addition, immature Schwann cells were present but unable to continue radial sorting in all treatment conditions. These results indicate that ErbB signaling and Schwann cell proliferation are required for radial sorting in zebrafish.
Figure 4. ErbB signaling and Schwann cell proliferation are required for radial sorting.
(A) Cross section through the posterior lateral line nerve in a control treated with DMSO from 48 h to 4 d reveals several myelinated axons (m) and two sorted axons (s), and Schwann cell processes within the axon bundle (Schwann cell cytoplasm pseudocolored blue throughout figure). Magnified image of boxed myelinated axon (A′) or boxed sorted axon (A″). (B) In the nerve treated with AG1478 from 48 h to 4 d there is a significant reduction in the number of myelinated and sorted axons. However, several large caliber axons (+) that are similar in size to myelinated axons in control nerves (A) remain in the large bundle of unsorted axons and no Schwann cell processes are present within the axon bundle; however, Schwann cell processes still surround the exterior of the bundle. Magnified boxed regions shown in B′ and B″. Fewer wraps of myelin are present around the one myelinated axon as compared to the DMSO control (A′ versus B′); at these early stages the individual wraps of myelin can be traced around the axon but a regular periodicity is not observed until later in development (Monk et al., 2009). (C, D) Similar to nerves treated with AG1478, nerves treated with aphidicolin (aph) or camptothecin (camp) from 48 h to 4 d also have a reduction in the number of myelinated axons. Sorted axons are still present, however, and Schwann cell processes have invaded the main axon bundle. Like AG1478 treated nerves, several unsorted, large caliber axons are present. Boxed regions magnified in C′, C″, D′, and D″.
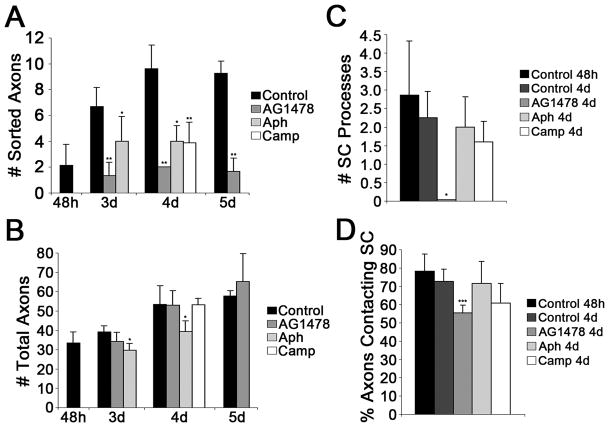
Figure 5. ErbB signaling is required for Schwann cell process extension while Schwann cell proliferation is not.
(A) The number of sorted axons was quantified for the treatment conditions at several time points. Control nerves are treated from 48 h with DMSO. Kruskal-Wallis tests performed on the data sets from 3 and 4 d yielded significant p values (p = 0.00218 and p = 0.00013, respectively). There is a significant decrease in the number of sorted axons in AG1478-treated nerves at 3, 4, and 5 d, in aphidicolin-treated (Aph) nerves at 3 and 4 days, and in camptothecin-treated (Camp) nerves at 4 d. (B) The total number of axons per nerve was quantified for all of the treatment conditions. AG1478- and camptothecin-treated nerves had similar axon numbers to control, while aphidicolin-treated nerves had slightly reduced axon numbers. (C) The number of Schwann cell processes extended into the axon bundle was counted for each condition at 4 d and at 48 h in control nerves; the Kruskal-Wallis test gave a significant p-value of 0.0057. No Schwann cell processes were found in AG1478 treated nerves, while all other treatments had processes extended. (D) The number of axons in contact with a Schwann cell, including myelinated and sorted axons, was counted for each of the treatments at 4 d and for the control at 48 h and shown as a percentage of the total axon number. The Kruskal-Wallis test gave a significant p-value of 0.0052. AG1478 treated nerves had significantly fewer axons being contacted by a Schwann cell as compared to all other treatments. Error bars show + 1 standard deviation. Four to seven nerves from three different embryos were examined for each treatment. AG1478 and aphidicolin experiments were performed twice with similar results; results from one experiment shown. All p values are from Mann-Whitney-U tests versus the age-matched control. * = p < 0.05, ** = p < 0.005.
Closer examination revealed that nerves from ErbB inhibited and cell cycle inhibited zebrafish have distinct radial sorting phenotypes. In AG1478-treated nerves, no Schwann cell processes were inserted into the axon bundle, whereas there were processes present within axon bundles from control, aphidicolin-, or camptothecin-treated animals (Fig. 4,5C). Because Schwann cells did not extend processes into axon bundles in AG1478-treated fish at 4 d, there were significantly fewer axons in contact with Schwann cells in these animals than in control and cell cycle inhibited larvae (Fig. 5D). These results indicate that ErbB signaling, but not cell division, is required for the insertion of Schwann cells processes within axon bundles during radial sorting.
To determine if the defect we observe after inhibition of ErbB signaling or cell proliferation is due to a delay in Schwann cell development, we looked at earlier and later time points. A radial sorting defect is also apparent in nerves that had been treated with either AG1478 or aphidicolin from 48 h to 3 d (Fig. 5A; 6A–C). In nerves from larvae treated with AG1478 from 48 h to 5 d, the radial sorting defect persisted and no new axons were sorted (Fig. 5A, 6D,E). By 5 d, aphidicolin-treated larvae displayed severe developmental defects, and we were not able to examine the nerves at this stage (data not shown, treated embryos at 4 d shown in Fig. S1). There was no significant difference between the number of sorted axons at 48 h in control embryos and the number of sorted axons in AG1478-, aphidicolin-, or camptothecin-treated nerves at the later time points examined (Fig. 5A). In addition, the number of axons in AG1478-or camptothecin-treated nerves was similar to control (Fig. 5B). There was a reduction in the number of axons in embryos treated with aphidicolin at 3 and 4 dpf compared to the DMSO control (Fig. 5B). These results suggest that inhibiting ErbB signaling or the cell cycle from 48 h completely arrests radial sorting, because no new axons were sorted at 3–5 d in treated animals, despite the presence of immature Schwann cells.
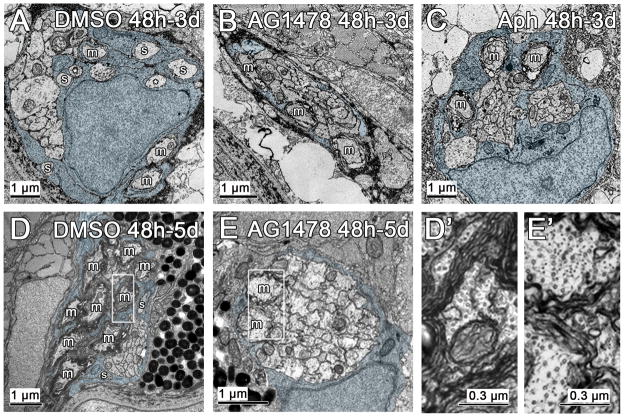
Figure 6. Inhibiting ErbB signaling or proliferation arrests radial sorting.
(A) In control nerves that have been treated with DMSO from 48 h to 3 d (DMSO 48h-3d), a few myelinated axons (m) are present, as well as several sorted axons (s), and two axons (*) that are being touched by the same Schwann cell process (pseudocolored blue throughout figure). In contrast, in nerves treated with AG1478 from 48 h to 3 d (AG1478 48h-3d), there are myelinated axons present, but no additional sorted axons (B). (C) In nerves treated with aphidicolin from 48 h to 3 d (Aph 48h-3d), again myelinated axons are present and a Schwann cell process is bisecting the main bundle of unsorted axons. (D) In nerves treated with DMSO from 48 h to 5 d (DMSO 48h-5d), there are many myelinated axons (boxed region in D′) and a few sorted axons. (E) Nerves treated with AG1478 from 48 h to 5 d have a similar number of myelinated axons (boxed region magnified in E′) as at 3 d (B), suggesting that radial sorting has been arrested. Myelinated axons in nerves treated with AG1478 have fewer wraps of myelin than those in the DMSO control (E′ versus D′).
We previously found that Schwann cell migration recovers after temporary inhibition of ErbB signaling (Lyons et al., 2005). To determine if the same is true for myelination, we blocked ErbB signaling with AG1478 from 48h to 4d and then removed the drug. Immunostaining of embryos treated with AG1478 from 48h to 5d reveals an almost complete loss of MBP signal as compared to the DMSO control (Fig. S2A,B). In contrast, some MBP is present in embryos that were allowed to recover for one or two days following removal of AG1478 at 4d (Fig. S2C–F). This result suggests that AG1478 treatment does not irreversibly arrest Schwann cells at an immature developmental state, so that they recover and progress to the myelinating stage after restoration of ErbB signaling.
ErbB signaling is required for the elaboration of the myelin sheath
Nrg1/ErbB signaling regulates the thickness of the myelin sheath in mammals (Michailov et al. 2004; Taveggia et al. 2005). Analysis of the axons sorted (likely prior to treatment) in nerves from AG1478-treated larvae revealed that ErbB signaling is also required for elaboration of the myelin sheath in zebrafish. Myelinated axons in AG1478-treated larvae consistently had fewer wraps of Schwann cell cytoplasm than those in the control and cell cycle inhibited larvae (Fig. 4A′–D′, 6D′, E′; 4–6 wraps in controls at 5 d vs. 2–3 wraps in AG1478-treated larvae). Schwann cells lacking ErbB signaling, however, were able to extrude the cytoplasm from the wraps of myelin that they did form, indicating that ErbB signaling is not required for the compaction of the myelin sheath, similar to the situation in mammals (Garratt et al. 2000; Michailov et al. 2004; Taveggia et al. 2005).
Discussion
To examine the requirements for ErbB signaling and cell division during radial sorting in vivo, we applied small molecule inhibitors after Schwann cells completed their initial migration along the lateral line nerve in zebrafish. By applying the inhibitors after migration is complete, we can focus on the specific function of ErbB signaling and cell proliferation during radial sorting without confounding effects resulting from defects at earlier steps in Schwann cell development. In these experiments, the entire animal is bathed in the inhibitors, but it is likely that the defects in radial sorting result from direct effects on Schwann cells. ErbB2 and ErbB3 receptors are expressed in Schwann cells, and these ErbB receptors act autonomously in Schwann cells in other contexts in zebrafish and mammals (Garratt et al. 2000; Grant et al. 2005; Lyons et al. 2005; Nave and Salzer 2006; Riethmacher et al. 1997). Additionally, Schwann cells proliferate extensively before reaching the promyelinating stage in zebrafish and mammals, consistent with a requirement for Schwann cell proliferation during radial sorting (Lyons et al. 2005; Webster et al. 1973). Blockade of either ErbB signaling or cell division causes significant defects in radial sorting, but there is an important difference between these two treatments. ErbB signaling was required for the extension of Schwann cell processes into the axon bundle, whereas Schwann cell proliferation was not. Supporting and extending a previous in vitro study (Taveggia et al. 2005), our experiments provide in vivo evidence that ErbB signaling plays a role in regulating Schwann cell process extension during radial sorting, in addition to its more extensively characterized role in stimulating Schwann cell proliferation.
Previous work supports the idea that the levels of Nrg1 expressed on an axon determine the differentiated fates of the associated Schwann cells. High levels of Nrg1 signal coming from a large axon instruct a Schwann cell to differentiate as a myelinating cell, while low levels of Nrg1 from several small caliber axons lead to the formation of a Remak bundle (Michailov et al. 2004; Nave and Salzer 2006; Taveggia et al. 2005). Schwann cell process extension is required for both radial sorting leading to myelination and ensheathment of axons in a Remak bundle. Therefore it is possible that the defects observed in radial sorting and Remak bundle formation in the absence of Nrg1/ErbB signaling (Taveggia et al. 2005) are caused by the failure of Schwann cells to properly extend processes into an axon bundle. Additionally, the amount of Nrg1 produced in a large caliber axon determines the thickness of the myelin sheath (Michailov et al. 2004; Taveggia et al. 2005). Similar to these mammalian studies, inhibition of ErbB signaling in zebrafish blocks the thickening of the myelin sheath (Fig. 4, 6; Lyons et al., 2005).
Radial sorting requires the coordination of Schwann cell process extension and Schwann cell proliferation (Jessen and Mirsky 2005; Webster et al. 1973). Schwann cell process extension and stabilization is required for Schwann cells to be able to subdivide the axon bundle and sort axons to the periphery, while Schwann cell proliferation is critical for properly matching the number of Schwann cells to the number of axons that will be myelinated. Accordingly, many of the genes involved in radial sorting seem to primarily regulate one or the other of these Schwann cell functions (Benninger et al. 2007; Feltri et al. 2002; Grove et al. 2007; Nodari et al. 2007; Pereira et al. 2009; Yang et al. 2005). The group of genes that regulate Schwann cell process extension but not proliferation includes rac1, β1-integrin, and ILK (Benninger et al. 2007; Feltri et al. 2002; Nodari et al. 2007; Pereira et al. 2009). A second group of genes is required for proliferation but not process extension and includes cdc42, FAK, laminin-2, and laminin-8 (Benninger et al. 2007; Grove et al. 2007; Yang et al. 2005). While it may be useful to classify radial sorting genes into two complementary groups, the two classes are not mutually exclusive, likely reflecting close coordination between Schwann cell proliferation and division. For example, laminin-γ1 is required for both process extension and Schwann cell proliferation (Chen and Strickland 2003; Yu et al. 2009; Yu et al. 2005), and our analysis provides evidence that Nrg1/ErbB signaling is also required for both process extension and proliferation during radial sorting.
In conclusion, we find that ErbB signaling is required for Schwann cell process extension in addition to regulating Schwann cell proliferation during radial sorting. It is important to note that other signals must also activate Schwann cell division, because these cells do divide—although in reduced numbers—when ErbB signaling is blocked (Lyons et al. 2005). Further studies will be needed to understand how Schwann cell proliferation and process extension are coordinated during radial sorting. Additionally, it will be important to address whether Schwann cell proliferation is required during radial sorting solely to increase Schwann cell numbers, or whether there is a role for a special terminal or asymmetric division before a Schwann cell can commit to myelination. Finally, it will be interesting to investigate how Nrg1 and other signals instruct a Schwann cell to maintain one process around the axon it will later myelinate while withdrawing other processes, and control the decision between Remak bundle formation and the continuation of radial sorting.
Supplementary Material
(A–D) Zebrafish larvae treated with DMSO, AG1478, aphidicolin, or camptothecin from 48 h – 4 d were imaged. (C–D) Aphidicolin and camptothecin treated larvae have slight heart edema.
(A) Embryos treated with DMSO from 48h-5d have MBP in the ganglion of the PLLn (to the left) and along the axons, whereas those treated with AG1478 (B) have a severe reduction in MBP expression. In (B) MBP is also labeling a neuromast, marked by an asterisk. (C, E) Embryos were treated with DMSO from 48h-4d and then were washed into clean embryo medium and have robust MBP expression along the PLLn. (D, F) Embryos were treated with AG1478 from 48h-4d and then were washed into clean embryo medium. One day after removal of AG1478, at 5d, MBP expression can be observed along the PLLn (D). This expression increases at 6d, and MBP is also expressed in the PLL ganglion (F). (A′–F′) Same samples as in A–F with MBP shown in green and acetylated tubulin (AcTub) marking axons in red. Ten larvae were examined for each condition. Scale bar is 50 μm.
Acknowledgments
We thank members of our laboratory, especially Julie Perlin, for helpful discussion, Tuky Reyes for excellent fish care, John Perrino for TEM support, and David Goode for help with statistics. A.R.R. was supported by a Stanford Graduate Fellowship and by an NIH training grant. D.A.L. was supported by a postdoctoral fellowship from the Muscular Dystrophy Association (MDA4061) and a David Phillips Fellowship from the Biotechnology and Biological Sciences Research Council, UK. This work was supported by NIH grant NS050223 to W.S.T.
References
- Benninger Y, Thurnherr T, Pereira JA, Krause S, Wu X, Chrostek-Grashoff A, Herzog D, Nave K-A, Franklin RJM, Meijer D, et al. Essential and distinct roles for cdc42 and rac1 in the regulation of Schwann cell biology during peripheral nervous system development. J Cell Biol. 2007;177(6):1051–61. doi: 10.1083/jcb.200610108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z-L, Strickland S. Laminin gamma1 is critical for Schwann cell differentiation, axon myelination, and regeneration in the peripheral nerve. J Cell Biol. 2003;163(4):889–99. doi: 10.1083/jcb.200307068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter TG. Induction of apoptosis in cells of the immune system by cytotoxic stimuli. Semin Immunol. 1992;4(6):399–405. [PubMed] [Google Scholar]
- Feltri ML, Graus Porta D, Previtali SC, Nodari A, Migliavacca B, Cassetti A, Littlewood-Evans A, Reichardt LF, Messing A, Quattrini A, et al. Conditional disruption of beta 1 integrin in Schwann cells impedes interactions with axons. J Cell Biol. 2002;156(1):199–209. doi: 10.1083/jcb.200109021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garratt AN, Voiculescu O, Topilko P, Charnay P, Birchmeier C. A dual role of erbB2 in myelination and in expansion of the schwann cell precursor pool. J Cell Biol. 2000;148(5):1035–46. doi: 10.1083/jcb.148.5.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmour DT, Maischein HM, Nusslein-Volhard C. Migration and function of a glial subtype in the vertebrate peripheral nervous system. Neuron. 2002;34(4):577–88. doi: 10.1016/s0896-6273(02)00683-9. [DOI] [PubMed] [Google Scholar]
- Grant KA, Raible DW, Piotrowski T. Regulation of latent sensory hair cell precursors by glia in the zebrafish lateral line. Neuron. 2005;45(1):69–80. doi: 10.1016/j.neuron.2004.12.020. [DOI] [PubMed] [Google Scholar]
- Grove M, Komiyama NH, Nave K-A, Grant SG, Sherman DL, Brophy PJ. FAK is required for axonal sorting by Schwann cells. J Cell Biol. 2007;176(3):277–82. doi: 10.1083/jcb.200609021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen KR, Mirsky R. The origin and development of glial cells in peripheral nerves. Nat Rev Neurosci. 2005;6(9):671–82. doi: 10.1038/nrn1746. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203(3):253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Lyons DA, Naylor SG, Mercurio S, Dominguez C, Talbot WS. KBP is essential for axonal structure, outgrowth and maintenance in zebrafish, providing insight into the cellular basis of Goldberg-Shprintzen syndrome. Development. 2008;135(3):599–608. doi: 10.1242/dev.012377. [DOI] [PubMed] [Google Scholar]
- Lyons DA, Pogoda H-M, Voas MG, Woods IG, Diamond B, Nix R, Arana N, Jacobs J, Talbot WS. erbb3 and erbb2 are essential for schwann cell migration and myelination in zebrafish. Curr Biol. 2005;15(6):513–24. doi: 10.1016/j.cub.2005.02.030. [DOI] [PubMed] [Google Scholar]
- Michailov GV, Sereda MW, Brinkmann BG, Fischer TM, Haug B, Birchmeier C, Role L, Lai C, Schwab MH, Nave K-A. Axonal neuregulin-1 regulates myelin sheath thickness. Science. 2004;304(5671):700–3. doi: 10.1126/science.1095862. [DOI] [PubMed] [Google Scholar]
- Monk KR, Naylor SG, Glenn TD, Mercurio S, Perlin JR, Dominguez C, Moens CB, Talbot WS. A G protein-coupled receptor is essential for Schwann cells to initiate myelination. Science. 2009;325(5946):1402–5. doi: 10.1126/science.1173474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nave K-A, Salzer JL. Axonal regulation of myelination by neuregulin 1. Curr Opin Neurobiol. 2006;16(5):492–500. doi: 10.1016/j.conb.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Newbern J, Birchmeier C. Nrg1/ErbB signaling networks in Schwann cell development and myelination. Semin Cell Dev Biol. 2010;21(9):922–8. doi: 10.1016/j.semcdb.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nodari A, Zambroni D, Quattrini A, Court FA, D’Urso A, Recchia A, Tybulewicz VLJ, Wrabetz L, Feltri ML. Beta1 integrin activates Rac1 in Schwann cells to generate radial lamellae during axonal sorting and myelination. J Cell Biol. 2007;177(6):1063–75. doi: 10.1083/jcb.200610014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira JA, Benninger Y, Baumann R, Gonçalves AF, Ozçelik M, Thurnherr T, Tricaud N, Meijer D, Fässler R, Suter U, et al. Integrin-linked kinase is required for radial sorting of axons and Schwann cell remyelination in the peripheral nervous system. J Cell Biol. 2009;185(1):147–61. doi: 10.1083/jcb.200809008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riethmacher D, Sonnenberg-Riethmacher E, Brinkmann V, Yamaai T, Lewin GR, Birchmeier C. Severe neuropathies in mice with targeted mutations in the ErbB3 receptor. Nature. 1997;389(6652):725–30. doi: 10.1038/39593. [DOI] [PubMed] [Google Scholar]
- Svaren J, Meijer D. The molecular machinery of myelin gene transcription in Schwann cells. Glia. 2008;56(14):1541–51. doi: 10.1002/glia.20767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taveggia C, Zanazzi G, Petrylak A, Yano H, Rosenbluth J, Einheber S, Xu X, Esper RM, Loeb JA, Shrager P, et al. Neuregulin-1 type III determines the ensheathment fate of axons. Neuron. 2005;47(5):681–94. doi: 10.1016/j.neuron.2005.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster HD, Martin R, O’Connell MF. The relationships between interphase Schwann cells and axons before myelination: a quantitative electron microscopic study. Dev Biol. 1973;32(2):401–16. doi: 10.1016/0012-1606(73)90250-9. [DOI] [PubMed] [Google Scholar]
- Yang D, Bierman J, Tarumi YS, Zhong Y-P, Rangwala R, Proctor TM, Miyagoe-Suzuki Y, Takeda Si, Miner JH, Sherman LS, et al. Coordinate control of axon defasciculation and myelination by laminin-2 and -8. J Cell Biol. 2005;168(4):655–66. doi: 10.1083/jcb.200411158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W-M, Chen Z-L, North AJ, Strickland S. Laminin is required for Schwann cell morphogenesis. J Cell Sci. 2009;122(Pt 7):929–36. doi: 10.1242/jcs.033928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu WM, Feltri ML, Wrabetz L, Strickland S, Chen ZL. Schwann cell-specific ablation of laminin gamma1 causes apoptosis and prevents proliferation. J Neurosci. 2005;25(18):4463–72. doi: 10.1523/JNEUROSCI.5032-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A–D) Zebrafish larvae treated with DMSO, AG1478, aphidicolin, or camptothecin from 48 h – 4 d were imaged. (C–D) Aphidicolin and camptothecin treated larvae have slight heart edema.
(A) Embryos treated with DMSO from 48h-5d have MBP in the ganglion of the PLLn (to the left) and along the axons, whereas those treated with AG1478 (B) have a severe reduction in MBP expression. In (B) MBP is also labeling a neuromast, marked by an asterisk. (C, E) Embryos were treated with DMSO from 48h-4d and then were washed into clean embryo medium and have robust MBP expression along the PLLn. (D, F) Embryos were treated with AG1478 from 48h-4d and then were washed into clean embryo medium. One day after removal of AG1478, at 5d, MBP expression can be observed along the PLLn (D). This expression increases at 6d, and MBP is also expressed in the PLL ganglion (F). (A′–F′) Same samples as in A–F with MBP shown in green and acetylated tubulin (AcTub) marking axons in red. Ten larvae were examined for each condition. Scale bar is 50 μm.