Summary
One goal of aging research is to develop interventions that combat age-related illnesses and slow aging. Although numerous mutations have been shown to achieve this in various model organisms, only a handful of chemicals have been identified to slow aging. Here we report that celecoxib, a non-steroidal anti-inflammatory drug (NSAID) widely used to treat pain and inflammation, extends C. elegans lifespan and delays the age-associated physiological changes, such as motor activity declines. Celecoxib also delays the progression of age-related proteotoxicity as well as tumor growth in C. elegans. Celecoxib was originally developed as a potent COX-2 inhibitor. However, the result from a structural-activity analysis demonstrated that the anti-aging effect of celecoxib might be independent of its COX-2 inhibitory activity, as analogs of celecoxib that lack cyclooxygenase-2 (COX-2) inhibitory activity produces a similar effect on lifespan. Furthermore, we found that celecoxib acts directly on 3’-phosphoinositide-dependent kinase-1 (PDK-1), a component of the insulin/IGF-1 signaling (IIS) cascade to increase lifespan.
Keywords: longevity, C. elegans, celecoxib, cyclooxygenase-2 inhibitor, NSAID, insulin-like signaling, PDK-1
Introduction
The identification of chemical interventions that can ameliorate age-related illness and degeneration has been an important aspect of current aging research. A drug that can extend lifespan by slowing down the normal aging process may also delay the progression and the onset of multiple age-related diseases. The nematode C. elegans has recently been recognized as an excellent model system for identifying genetic or pharmacological interventions altering longevity, mainly because of its short lifespan and amenability to genetic manipulation. Many genetic pathways that were identified in C. elegans to regulate longevity turned out to be evolutionarily conserved. For example, mutations inhibiting the insulin/IGF-1-like signaling (IIS) have been shown to extend lifespan and delay various age-related physiological changes (Guarente & Kenyon 2000; Garigan et al. 2002; Herndon et al. 2002; Kenyon 2005). The IIS pathway is highly conserved and has been shown to influence longevity in model organisms ranging from worms to mice (Kenyon 2010). In addition, a number of compounds have been reported to extend lifespan in worms. These include a sirtuin activator resveratrol (Wood et al. 2004; Bass et al. 2007), an antihyperglycemic drug metformin (Onken & Driscoll 2010), a variety of antioxidants (e.g. vitamin E) (Harrington & Harley 1988; Adachi & Ishii 2000; Melov et al. 2000), and several serotonin receptor antagonists (e.g. mianserin) as well as anticonvulsant medicines (e.g. ethosuximide) that affect neuronal activity (Evason et al. 2005; Petrascheck et al. 2007; Evason et al. 2008). Here, we report that the anti-inflammatory drug celecoxib and its derivatives significantly extend C. elegans lifespan and delay the onset of age-associated proteotoxicity and tumor growth.
Since the discovery and introduction of aspirin more than a century ago, non-steroidal anti-inflammatory drugs (NSAIDs) have become the most widely used therapeutic agents in the treatment of conditions such as pain, fever, and inflammation. NSAIDs act primarily by inhibiting cyclooxygenase (COX), thereby blocking the formation of prostaglandins (PGs) in normal and inflamed tissues. COX exists as two distinct isoforms. While COX-1 is constitutively expressed in most tissues, COX-2 is expressed in inflamed tissues in response to proinflammatory stimuli (Diaz et al. 1998; Lipsky 1999; Dannenberg et al. 2001). Celecoxib (Celebrex®) (Fig. 1A) is one of the selective inhibitors of COX-2 that were originally developed as a new class of NSAID to reduce the gastrointestinal toxicities that are associated with COX-1 blockage. In addition to their potent anti-inflammatory and analgesic effects, long-term use of different NSAIDs (including celecoxib) have been reported to reduce the risk and delay the onset of various age-related diseases, including cancers (Thun et al. 1991; Smalley & DuBois 1997; Thompson et al. 1997; Fukutake et al. 1998; Hida et al. 1998; Kismet et al. 2004), Alzheimer’s disease and other neurodegenerative diseases (in t' Veld et al. 2001; Aisen 2002; Etminan et al. 2003; Asanuma et al. 2004). Studies in our laboratory have now further linked the drug to normal aging. Interestingly, while the primary target of celecoxib in clinical uses is COX-2, our results suggest that celecoxib might extend C. elegans lifespan via a mechanism that is independent of COX-2 but share similar phenotypic features as IIS pathway mutants. The lifespan extension resulting from celecoxib treatments requires the activity of DAF-16, the FOXO transcription factor known to regulate development and longevity in response to IIS (Lin et al. 1997). Our data also suggest that celecoxib might extend lifespan by inhibiting the kinase activity of 3-phosphoinositide-dependent kinase-1 (PDK-1), a key component of the IIS cascade.
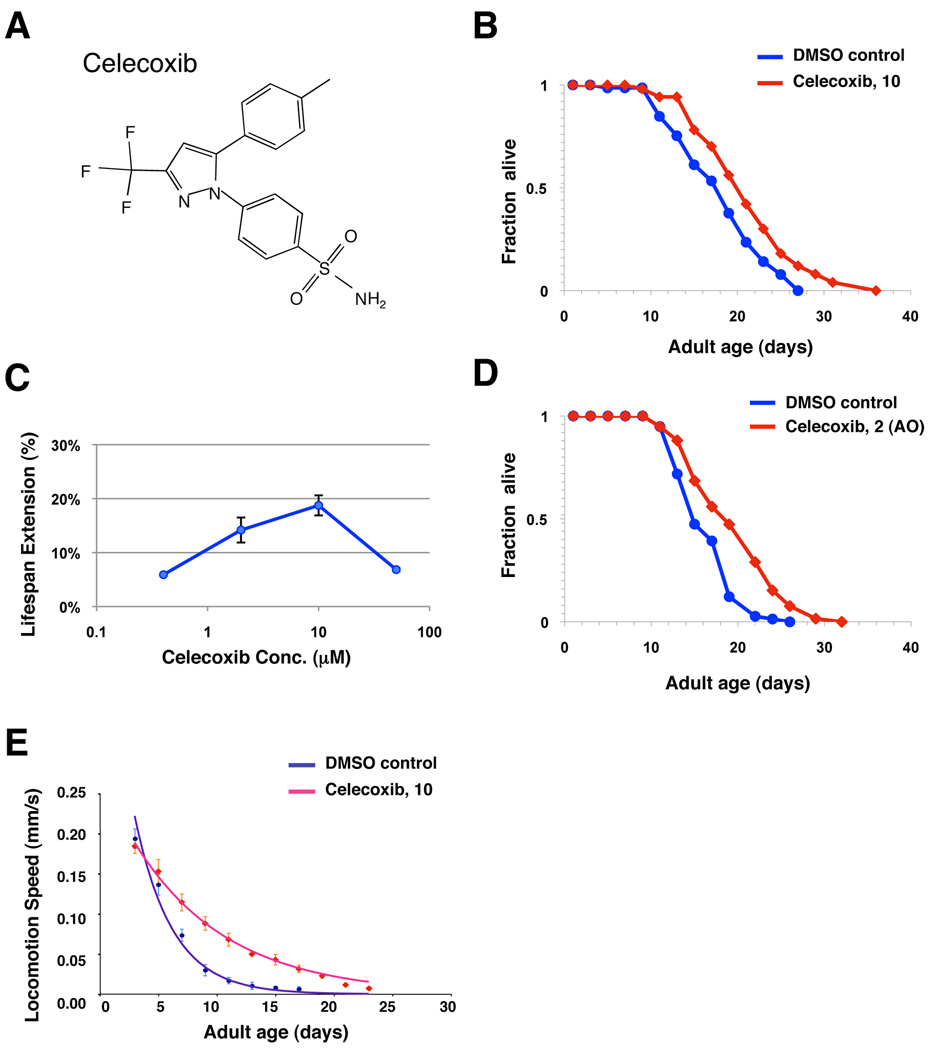
Figure 1. Celecoxib extends adult lifespan and delays age-associated physiological changes.
(A) Chemical structure of celecoxib. (B) Survival curves of wild-type (N2) animals treated with either DMSO control (blue) or 10 µM of celecoxib (red). The treatments were initiated from the time of hatching and continued until death. Statistical details and repetition of this experiment are summarized in Table S1. (C) Dosage-response analysis of celecoxib (Cbx). Wild-type (N2) animals were exposed to DMSO control or 0.5, 2, 10, and 50 µM celecoxib. The average percentage change in lifespan of at least two independent experiments was plotted as a function of dosage. Statistical details and repetition of this experiment are summarized in Table S1. (D) Survival curves of wild-type (N2) animals exposed to an adult-only treatment of either DMSO control (blue) or 2 µM of celecoxib (red). The treatments were initiated from the first day of adulthood and continued until death. (E) The speed of spontaneous locomotion of wild-type (N2) animals treated with either DMSO control (blue) or 10 µM of celecoxib (red). Locomotion speed was quantified every other day until death as previously described (Hsu et al. 2009), and the mean locomotion speed of these worms was plotted as a function of age. Error bars represent SD. Locomotion speed decayed throughout lifespan and can be best fitted by first-order exponential decay, and the rate of the decay (DMSO control, rate = 0.2686, R2 = 0.9623; celecoxib, rate = 0.1179, R2 = 0.9931) was calculated using the method previously described (Hsu et al. 2009).
Results
Celecoxib treatment extends C. elegans lifespan and delays the age-related decline of motor activity
One goal of aging studies is to identify drugs that can slow aging and delay age-related illness and degeneration. To identify compounds that might slow aging and extend lifespan in C. elegans, we assayed a panel of randomly selected compounds that have known effects on human physiology. Animals grown on agarose plates were exposed to each drug at two different concentrations from hatching. To ensure the drugs retain its potency throughout the entire experiment, animals were transferred to fresh plates with drugs every 2–4 days. Among those examined, celecoxib (Fig. 1A) had the greatest effect on longevity, extending adult mean lifespan by up to 20% (Fig. 1B, Table S1). Results from our dose-response analysis indicated that animals treated with external concentrations of 10 µM and 2 µM celecoxib displayed the largest lifespan extension at 20°C (Fig. 1C, Table S1). Animals exposed to either higher or lower concentrations of celecoxib, exhibited a smaller or an insignificant lifespan extension (Fig. 1C, Table S1). Moreover, the longevity effect of celecoxib was not temperature sensitive. Celecoxib caused similar increases on lifespan at 15°C (Table S1). The rate of age-associated motor activity decline has been shown to be a prominent predictor of lifespan as well as a great physiological parameter of animals’ healthiness (Hsu et al. 2009). Thus, we examined the locomotion speed of celecoxib treated animals throughout the entire lifespan. Our result showed that the rate of the motor activity decay (DMSO control, rate = 0.2686; celecoxib, rate = 0.1179) is significantly reduced in celecoxib treated worms (Fig. 1E), indicating that both health-span and lifespan are increased when worms are exposed to long-term celecoxib treatments.
It has been reported that C. elegans lifespan can be extended by feeding the worms with dead bacteria, which would reduce their susceptibility to bacterial infections (Garigan et al. 2002). Therefore, it is possible that celecoxib may exert its longevity effect by killing the bacteria, instead of acting directly on the worms. To test this possibility, we examined the effects of celecoxib on the growth of two commonly used bacteria strains, OP50 and HT115. The results indicate that the growth of OP50 and HT115 were completely unaltered when exposed to celecoxib (Fig. S1).
In C. elegans, signaling pathways that regulate longevity have been suggested to have different temporal requirements to regulate longevity. For instance, the insulin/IGF-1-like signaling (IIS) functions during early adulthood to regulate longevity (Dillin et al. 2002a). Conversely, mitochondrial respiration functions during larval development to influence longevity (Dillin et al. 2002b). We found that the lifespan extending effect of celecoxib treatment initiated at the first day of adulthood is comparable to those initiated from hatching (Fig. 1D, Table S1). This finding suggests that exposure to celecoxib only during adulthood is sufficient to produce the anti-aging effect.
Celecoxib further extends the lifespan of animals with reduced food uptake and mitochondrial respiration
To determine whether celecoxib extends lifespan via biological processes previously known to modulate aging in C. elegans, we next tested the effect of combining celecoxib treatment and various mutations that alter lifespan. Dietary restriction (DR) is known to extend lifespan in a wide range of species (Masoro 2005), and can be mimicked by the mutations of eat-2 gene that is required for pumping food into the pharynx (Lakowski & Hekimi 1998). Therefore, we first examined whether the lifespan of eat-2(ad1116) mutants can be further extended by celecoxib. Treatments with celecoxib significantly extended the lifespan of eat-2 mutants by 17% (Fig. 2A, Table S1). The FoxA transcription factor PHA-4 has been previously shown to be required for eat-2 mutations to extend lifespan in worms (Panowski et al. 2007). Treatments with celecoxib also extended the lifespan of pha-4(zu225) mutants to a similar extent (Table S1). Moreover, the rate of pumping (eating) was not affected in celecoxib treated animals [Day 1 adult pumping rate: control, 282 ± 22 min−1; celecoxib, 276 ± 24 min−1; n=15; p = 0.54], indicating that celecoxib may not exert its effects via changes in appetite or food limitation. Together our results suggest that DR is not the primary mechanism underlying the anti-aging effect of celecoxib.
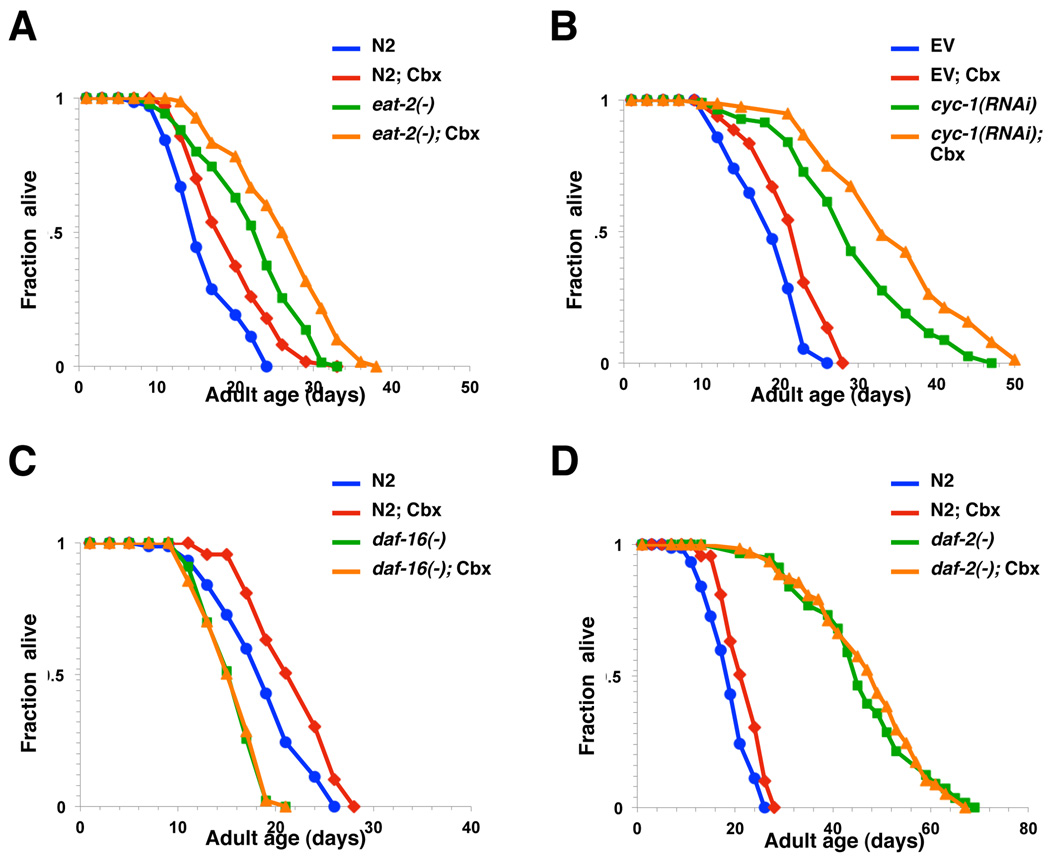
Figure 2. Celecoxib extends adult lifespan in a daf-16-dependent manner.
Survival curves of N2 animals and (A) eat-2(ad1116), (B) cyc-1(RNAi), (C) daf-16(mu86), or (D) daf-2(e1370) mutants grown on plates containing DMSO control (blue or green) or 2 µM of celecoxib (red or orange). All drug treatments were initiated from the first day of adulthood and continued until death, except cyc-1(RNAi). In all cases, these data represent the results of a single trial. Repetition of this experiment and statistical details are summarized in Table S1.
Reduction of the mitochondrial respiration by RNAi also extends lifespan in C. elegans (Dillin et al. 2002b). To investigate whether celecoxib plays a role in the mitochondrial respiration to influence longevity, we treated the worms grown on cyc-1 RNAi bacterial with celecoxib. Exposure to celecoxib further extended the lifespan of cyc-1(RNAi) animals by 17% (Fig. 2B, Table S1). This finding suggests that celecoxib may not influence longevity by reducing mitochondrial respiratory chain activity.
Celecoxib extends lifespan in a daf-16-depedent manner
Mutations affecting the IIS pathway have been shown to influence C. elegans lifespan (Kenyon et al. 1993; Larsen et al. 1995; Morris et al. 1996; Kimura et al. 1997; Lin et al. 1997; Ogg et al. 1997; Ogg & Ruvkun 1998; Paradis & Ruvkun 1998; Paradis et al. 1999). For example, animals carrying reduction-of-function mutations in daf-2, a homolog of human insulin/IGF-1 receptor, or mutations in components of its downstream PI3K/PDK-1/AKT signaling pathway, are significantly long-lived (Panowski & Dillin 2009; Kenyon 2010). The FOXO transcription factor DAF-16 is required for IIS mutations to extend lifespan (Kenyon et al. 1993; Lin et al. 1997; Ogg et al. 1997). Thus, we next examined whether daf-16 is also required for celecoxib to influence longevity. We found that treatment with celecoxib failed to extend the lifespan of animals carrying a null mutation of daf-16 (Fig. 2C, Table S1), suggesting that celecoxib may influence longevity by inhibiting a component of the IIS pathway upstream of DAF-16 or by activating DAF-16 directly. We have also tested the effect of celecoxib on daf-2 mutants and found no significant lifespan extension (Fig. 2D, Table S1). Again, this supports the idea that celecoxib may extends lifespan by modulating the IIS pathway activity.
Celecoxib extends lifespan via a mechanism independent of its COX-2 inhibitory activity
Although, celecoxib was originally developed as a potent COX-2 inhibitor, our finding that celecoxib extends lifespan in a daf-16-dependent manner raises the possibility that the longevity effect of celecoxib may be independent of its COX-2 inhibitory activity. The reason is two-fold. First, in mammalian models, celecoxib is known to have additional cellular targets. For instance, several studies suggested that celecoxib might inhibit tumor growth, at least in part, by acting on a COX-2-independent mechanism, when treated at a higher dosage (e.g. IC50 = 40 nM for COX-2 inhibition; > 20 µM for apoptosis induction) (Hsu et al. 2000; Kismet et al. 2004; Zhu et al. 2004). More importantly, no COX isoforms have been identified in unicellular organisms, the plant kingdom, insects and nematodes, including C. elegans (Simmons et al. 2004).
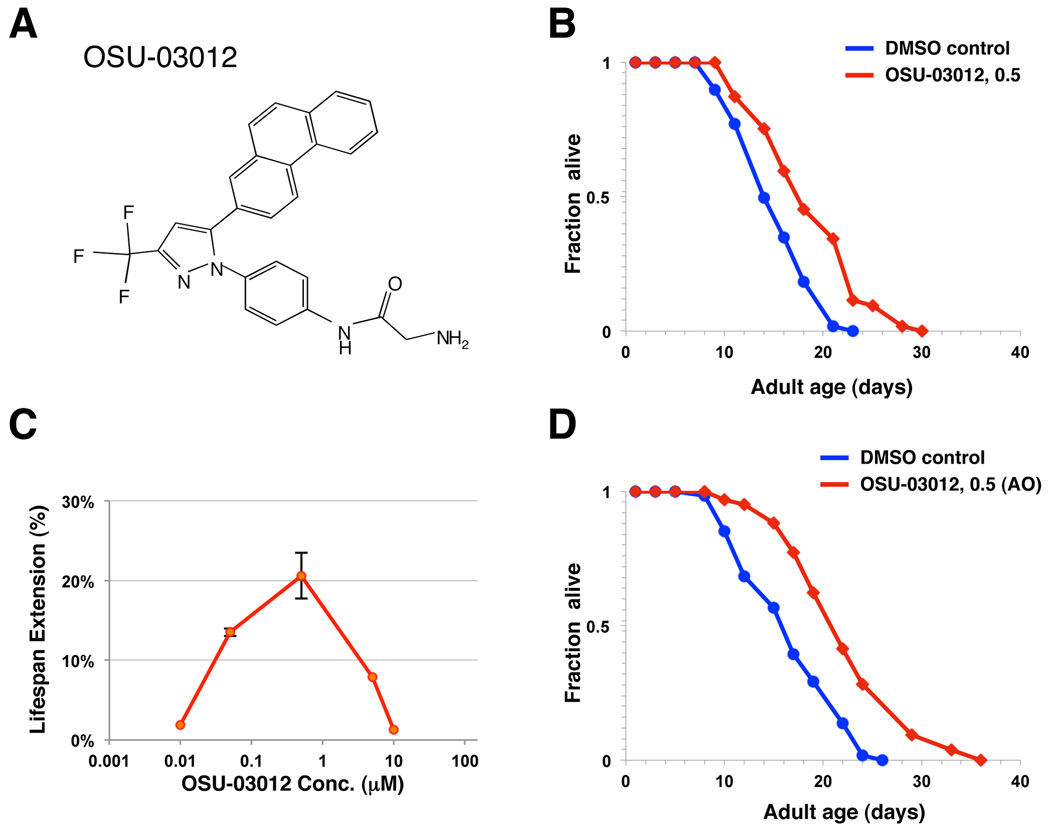
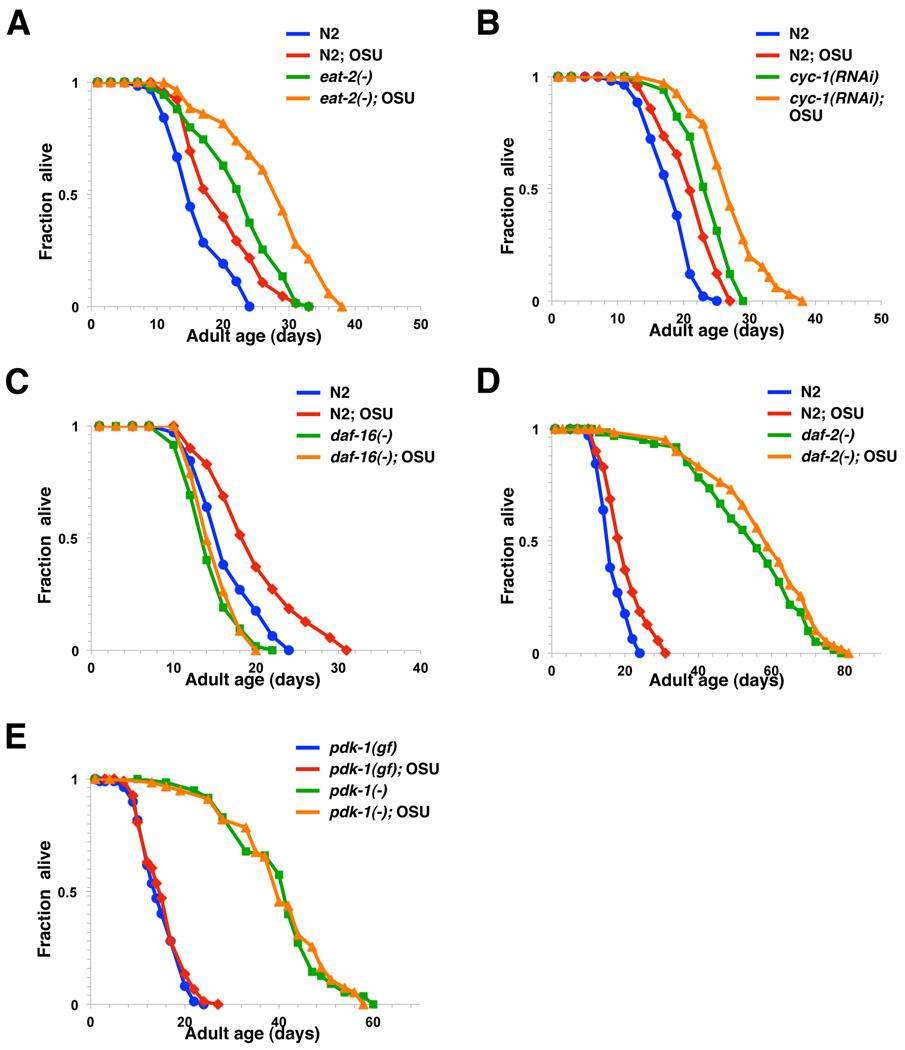
To investigate whether the longevity activity of celecoxib can be dissociated from its COX-2 inhibitory activity, we analyzed the lifespan of animals exposed to OSU-03012, a close structural analog of celecoxib (Fig. 3A) that exhibits no detectable COX-2 inhibitory activity up to 50 µM (Zhu et al. 2004). Treatment with OSU-03012 significantly extends worm lifespan to an extent similar to celecoxib when initiated from hatching (Fig. 3B, Table S2). Wild-type animals treated with 0.5 µM OSU-03012 displayed the largest lifespan extension (Fig. 3C, Table S2). Adult-only treatment of 0.5 µM OSU-03012 displayed an even greater lifespan extension (35%) (Fig. 3D, Table S2). Similar to what we have observed with celecoxib, exposure to OSU-03012 further extends the lifespan of eat-2(ad1116) and cyc-1(RNAi) mutants, but not the lifespan of daf-16(mu86) and daf-2(e1370) mutants (Fig. 4A–D, Table S2). Since OSU-03012 exhibits no detectable COX-2 inhibitory activity, our findings strongly suggest that celecoxib and its derivative OSU-03012 act on a target other than COX-2 to modulate longevity in C. elegans. It should be noted that we couldn’t rule out the possibility that different mutants may exhibit varied sensitivity to the drugs. However, this is unlikely to be the case, as daf-16(mu86) mutants failed to respond to all three different concentrations of OSU-03012 we have examined (Table S2).
Figure 3. OSU-03012, a derivative of celecoxib, also extends lifespan.
(A) Chemical structure of celecoxib derivative, OSU-03012. (B) Survival curves of wild-type (N2) animals treated with either DMSO control (blue) or 0.5 µM of OSU-03012 (red). The treatments were initiated from the time of hatching and continued until death. Statistical details and repetition of this experiment are summarized in Table S2. (C) Dosage-response analysis of OSU-03012 (OSU). Wild-type (N2) animals were treated with DMSO control or 0.01, 0.05, 0.5, 5 and 10 µM OSU-03012. The average percentage change in lifespan was plotted as a function of dosage. Statistical details and repetition of this experiment are summarized in Table S2. (D) Survival curves of wild-type (N2) animals exposed to an adult-only treatment of either DMSO control (blue) or 0.5 µM of OSU-03012 (red). The treatments were initiated from the first day of adulthood and continued until death.
Figure 4. OSU-03012 extends lifespan via a DAF-16 and PDK-1-dependent mechanism.
Survival curves of N2 animals and (A) eat-2(ad1116), (B) cyc-1(RNAi), (C) daf-16(mu86), (D) daf-2(e1370), (E) pdk-1(mg142), or pdk-1(sa680) mutants grown on plates containing DMSO control (blue or green) or 0.5 µM of OSU-03012 (red or orange). pdk-1(mg142) is a gain of function (gf) mutation of pdk-1, whereas pdk-1(sa680) is a loss of function (−) mutation of pdk-1. In all cases, these data represent the results of a single trial. Repetition of this experiment and statistical details are summarized in Table S2.
Celecoxib and OSU-03012 might extend lifespan by inhibiting PDK-1 activity
Among all the potential secondary targets reported to date (e.g. PDK-1, Bcl-2, PPAR-δ, etc. (Kismet et al. 2004)), inhibition of PDK-1, a known IIS pathway component upstream of DAF-16, by celecoxib is particularly intriguing. It has been reported that celecoxib and a number of its derivatives exhibit different degrees of inhibitory activity against human PDK-1 (e.g. celecoxib, IC50 = 48 µM; OSU-03012, IC50 = 5 µM) (Zhu et al. 2004). Given the strong antagonistic activity of OSU-03012 on human PDK-1 both in vitro and in vivo (Zhu et al. 2004), we have also tested the effect of OSU-03012 on pdk-1 mutants’ lifespan. Treatment with OSU-03012 failed to extend the lifespan of either the long-lived loss-of-function pdk-1(sa680) mutants, or the short-lived gain-of-function pdk-1(mg142) mutants (Paradis et al. 1999) (Fig. 4E, Table S2).
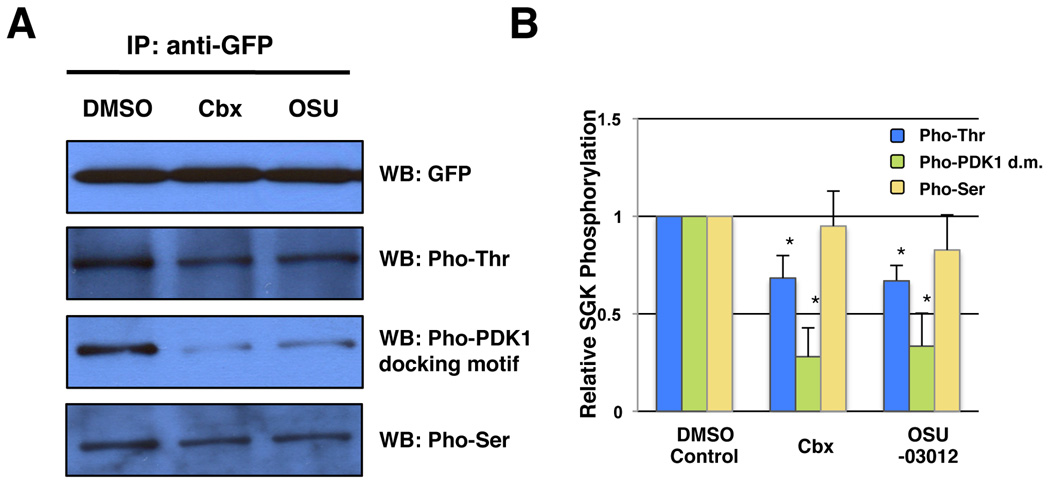
To determine whether the activity of C. elegans PDK-1 could indeed be inhibited by celecoxib and OSU-03012 in vivo, we analyzed the phosphorylation status of SGK-1 (serum- and glucocorticoid-induced kinase 1), a known substrate of PDK-1, in animals exposed to both drugs. It has been reported that the Thr256 residue in the activation loop of human SGK1 is phosphorylated by PDK1 (Tessier & Woodgett 2006), whereas the Ser422 residue in the hydrophobic motif might be phosphorylated by mTOR (Hong et al. 2008). The phosphorylation status of SGK-1 is assessed by immunoprecipitating SGK-1::GFP fusion proteins from drug treated BR2773 animals (i.e. a transgenic strain expressing SGK-1::GFP) and blotting with anti-phospho-Thr, anti-phospho-Ser or anti-phospho-(Ser/Thr)PDK-1 docking motif antibodies. We found that treatments with both drugs significantly reduce Threonine phosphorylation of SGK-1 by PDK-1, while Serine phosphorylation of SGK-1 remains basically unaltered (Fig. 5). Thus, our findings strongly suggest that celecoxib and OSU-03012 might act directly on PDK-1 or a component upstream of PDK-1 in the IIS pathway to increase longevity in worms.
Figure 5. Effect of celecoxib and OSU-03012 on PDK-1 activity.
(A–B) The in vivo kinase activity of PDK-1 was assessed by measuring the phosphorylation status of SGK-1, a known substrate of PDK-1. Whole worm extracts were prepared from transgenic animals expressing SGK-1::GFP fusion proteins (BR2773) treated with DMSO control, 10 µM celecoxib, or 0.5 µM OSU-03012 from hatching. Extracts were made from synchronized Day 1 adult worms and subjected to immnoprecipitation using anti-GFP antibodies. The immunoprecipitates were then immunoblotted using either anti-GFP, anti-phospho-Thr, anti-phospho-Ser or anti-phospho-(Ser/Thr)PDK-1 docking motif antibodies. The blots shown in (A) represent the result of a single experiment. The average of three independent experiments were quantified and presented in (B). The relative phosphorylation status of SGK-1 was analyzed by densitometry using ImageJ 1.37 software (NIH). Asterisks indicate significant changes (P < 0.05). P values were calculated by unpaired Student’s t-test.
Celecoxib and OSU-03012 treatments enhance DAF-16 activity
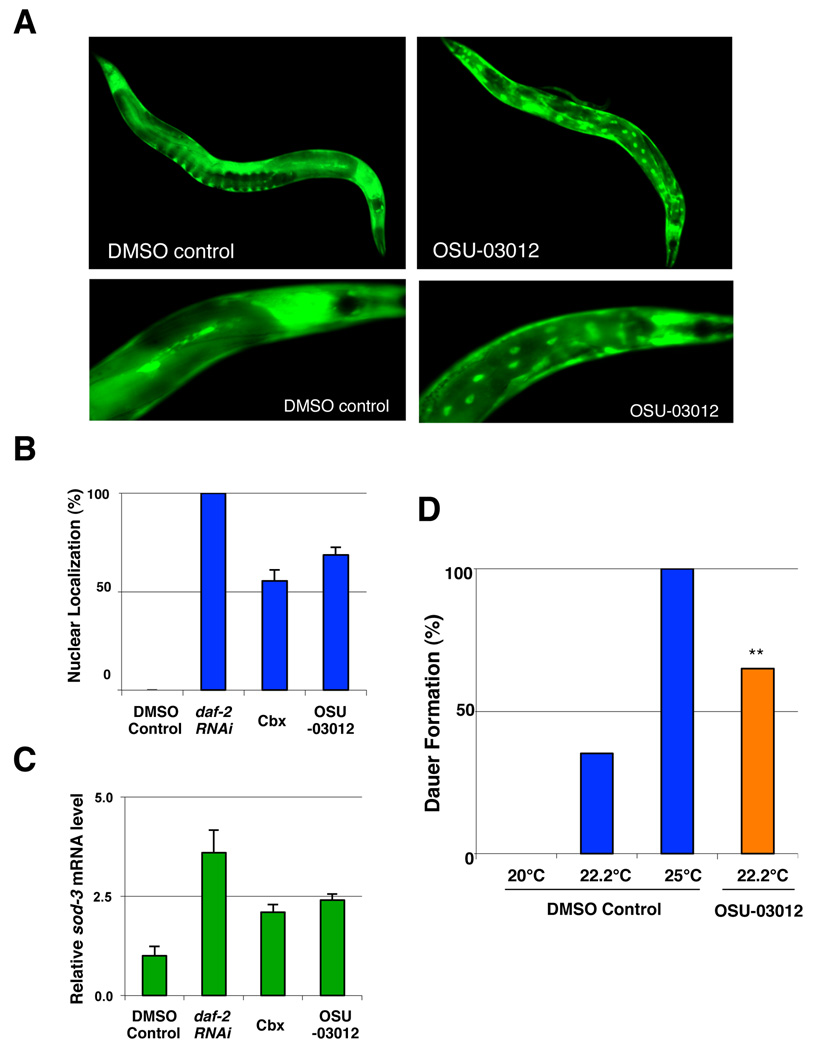
Previous studies have shown that DAF-16 accumulates in the nucleus when the activity of its upstream kinases is reduced (Henderson & Johnson 2001; Lin et al. 2001). To further examine the idea that celecoxib and OSU-03012 might act on a component of the IIS pathway upstream of DAF-16, likely PDK-1, to influence longevity, we examined the nuclear localization of DAF-16 using a GFP reporter strain (TJ356) (Henderson & Johnson 2001). In agreement with our model, we found an increased level of nuclear localized DAF-16::GFP fusions after 72 hr of treatment with celecoxib or OSU-03012 (Fig. 6A–B), indicating that celecoxib and OSU-03012 treatments might promote DAF-16 activation. Interestingly, we have also observed a higher level of nuclear localized DAF-16::GFP in the anterior end compared to the posterior end of the animals (Fig. 6A). This may presumably be due to the way worms absorb the drugs (i.e. pumping via pharynx). Since it is possible that the nuclear localized DAF-16 is not fully activated, we also measured the expression level of sod-3, a known daf-16 target gene involved in stress responses, by qRT-PCR (Honda & Honda 1999). The expression of sod-3 significantly increased in animals exposed to celecoxib or OSU-03012 for 72 hr (Fig. 6C).
Figure 6. OSU-03012 alters the nuclear localization and the activity of DAF-16.
(A) Nuclear accumulation of DAF-16 induced by OSU-03012 treatment. Images of TJ356 animals, which carry an integrated daf-16::gfp array in a wild-type background, exposed to either DMSO control or 0.5 µM of OSU-03012 for 72 hours. (B) Quantification of the nuclear accumulation of DAF-16::GFP in response to 10 µM celecoxib and 0.5 µM OSU-03012 treatments. Worms were scored for the presence or absence of GFP accumulation within the intestinal nuclei at the first day of adulthood (n ≥ 120 for each treatment). An animal was scored as having nuclear DAF-16 if more than one intestinal nucleus contained DAF-16-GFP. RNAi treatment by feeding of daf-2 was also performed as a positive control. Lifespans following each drug treatment were determined to confirm the effectiveness of the drug treatment. (C) Effects of celecoxib and OSU-03012 on DAF-16 transcriptional activity. Wild-type N2 animals were exposed to DMSO control, 10 µM celecoxib, or 0.5 µM OSU-03012 from hatching. Relative mRNA levels of sod-3 of these animals were measured by quantitative RT-PCR and the mean of three different sample sets are shown. The relative mRNA levels were normalized against act-1 (beta-actin) levels. Error bars: ± STD. (D) daf-2(e1370) mutants (P0) were exposed to DMSO control or 0.5 µM OSU-03012 at 20°C. The F1 eggs were then moved to the different temperatures indicated for 72 hours before being scored for dauer formation. Each bar represents combined data of three independent experiments per condition (n ≥ 150 for each treatment). Asterisks indicate significant changes (*, P < 0.005). P values were calculated by Pearson's chi-square test.
In addition to regulating longevity, the IIS and DAF-16 also controls entry into an alternative developmental state, named dauer in C. elegans (Morris et al. 1996; Kimura et al. 1997; Lin et al. 1997). The dauer is a growth-arrested, stress-resistant alternative larval stage that is induced by food limitation, high temperatures, and crowding. Strong daf-2 alleles enter dauer stage without any environmental cues, while weak daf-2 alleles (e.g. e1370) enhance dauer formation only at high temperature (Fig. 6D). To examine the ability of OSU-03012 to promote dauer formation, we exposed the daf-2(e1370) mutants to the drug from hatching and analyzed the dauer formation. We found that OSU-03012 treatment increased dauer formation of daf-2(e1370) mutants at 22.2°C from 35% of untreated animals to 62% (Fig. 6D), suggesting that treatment of OSU-03012 can further lower the IIS pathway activity in a sensitized daf-2(e1370) background. Together, our results are consistent with a model that celecoxib and OSU-03102 act on a component of the IIS pathway, most likely PDK-1, to extend C. elegans lifespan.
Celecoxib and OSU-03012 treatments ameliorate age-associated illness
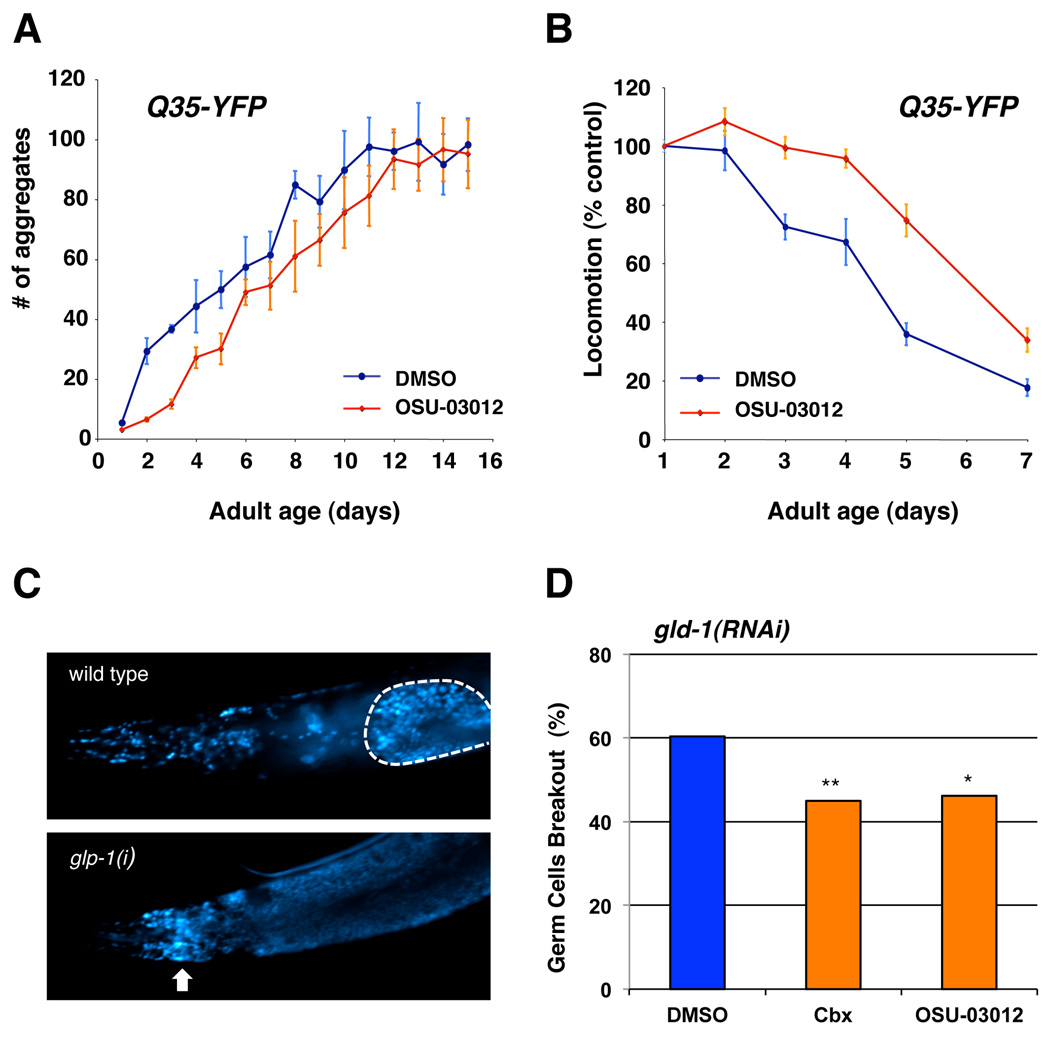
An intriguing question in biology is how the normal aging process is coupled to age-related diseases. Could a drug that extends lifespan also delay the onset of age-related diseases? Reducing IIS pathway activity has been shown to ameliorate the onset and severity of progressive age-related neuronal degeneration associated with aberrant protein aggregations (e.g. Alzheimer’s and Huntinton’s disease) as well as tumor growth in C. elegans disease models (Morley et al. 2002; Hsu et al. 2003; Cohen et al. 2006; Pinkston et al. 2006). For instance, YFP fusions containing 35 glutamine repeats (Q35-YFP) expressed in the body wall muscle cells form aggregates and cause mobility loss as the animals age (Morley et al. 2002). Both the formation of polyQ aggregates and its proteotoxicity are delayed in long-lived IIS pathway mutants and accelerated in short-lived IIS pathway mutants (Morley et al. 2002; Hsu et al. 2003). To test whether OSU-03012 treatment can also ameliorate the aggregation-associated proteotoxicity by inhibiting the IIS pathway in a worm model, we first analyzed the age-dependent formation of polyQ aggregates in drug treated animals. We found that the formation of polyQ aggregates in animals exposed to OSU-03012 is delayed compared to the control animals (Fig. 7A). The aggregation-associated proteotoxicity is also significantly ameliorated in OSU-03012 treated animals (Fig. 7B).
Figure 7. OSU-03012 delays the progression of age-related diseases.
(A) OSU-03012 delays the accumulation of polyQ aggregates. The number of polyQ aggregates in Q35 animals treated with DMSO control (blue) or 0.5 µM of OSU-03012 (red) were scored and plotted as a function of age. 10–15 worms were randomly selected from a pool of > 200 worms under each condition, and counted for aggregates every day. Data are mean ± SD. (B) Relative locomotion speed of Q35 animals treated with DMSO control (blue) or 0.5 µM of OSU-03012 (red). Locomotion speed was quantified every day as previously described, and the mean locomotion speed was plotted as a function of age. Data are mean ± SD as a percentage of day 1 DMSO control. (C) Images of day 6 adult wild types (left panel) or gld-1(RNAi) (right panel) animals stained with the DNA-intercalating dye DAPI (4’,6’-diamidino-2-phenylindole) using standard method. The gonad of wild-type animal was outlined by white dashed line. The white arrows indicate the presence of undifferentiated germ cells outside of the gonad in gld-1(RNAi) mutants. (D) Worms exposed to DMSO control, 10 µM celecoxib, or 0.5 µM OSU-03012 from hatching were scored for the presence or absence of undifferentiated germ cells outside of the gonad as shown in the right panel of (C) at day 6 of adulthood (n ≥ 125 for each treatment). The percentage of animals with undifferentiated germ cells found in the head is plotted here. Each bar represents combined data of 2–4 independent experiments per condition. Mean ± S.D. = 60.4 ± 3.3, 45.0 ± 1.7, and 46.2 ± 2.6 for DMSO, cbx, and OSU-03012, respectively. Asterisks indicate significant changes (**, P < 0.005, *, P < 0.05). P values were calculated by Pearson's chi-square test.
Mutations in gld-1, a tumor suppressor gene required for oocyte development in C. elegans, cause lethal germline tumors (Francis et al. 1995). The growth of these tumors is significantly suppressed in long-lived IIS pathway mutants (Pinkston et al. 2006). To examine whether the progression of germline tumor growth in gld-1 mutants could be delayed by celecoxib and OSU-03012, we monitored the growth of germline tumors in drug-treated mutants. As predicted, the growth of the germline tumors in gld-1 mutants is inhibited by celecoxib and OSU-03012 treatments, presumably by inhibiting PDK-1 activity (Fig. 7C–D). The inhibitory effects of these drugs on the proliferation of germline cells appears to occur only when gld-1 is mutated, since both the brood size and the length of the reproductive period in N2 animals are not altered by celecoxib treatment (Fig. S2). Interestingly, both of these compounds have been proposed as a cancer chemoprevention drug. Our findings have demonstrated that celecoxib, a compound widely used as an anti-inflammatory drug in humans, extends lifespan and delays the progression of age-related proteotoxicity and tumor growth in C. elegans.
Discussion
In this study, we report that celecoxib, a non-steroidal anti-inflammatory drug (NSAID), extends both mean and maximum lifespan in C. elegans. Furthermore, the physical healthiness, as indicated by the age-associated decay rate of motor activity (Hsu et al. 2009), is significantly improved in celecoxib treated animals. The effect of celecoxib on aging is not a result of a change in the nutritional value of the bacteria, since celecoxib has no effect on bacteria growth (Fig. S1). These findings prompt one critical question: What is the mechanism of action by which celecoxib extends lifespan? Celecoxib was originally developed as a selective COX-2 inhibitor for the treatments of pain and inflammation. Therefore, one might naturally predict celecoxib to extend lifespan via a mechanism involving reduced COX activity. However, several lines of evidences suggest that the lifespan-extending effect of celecoxib is independent of its COX-2 inhibitory activity. First, no homolog of mammalian COXs have been identified in unicellular organisms, the plant kingdom, insects and nematodes, including C. elegans (Simmons et al. 2004). We have also performed our own search for a C. elegans homolog of mammalian COXs using bioinformatics approaches based on sequence homology and failed to identify any COX isoforms in C. elegans. Secondly, results from our structural-activity analysis demonstrated that the anti-aging effect of celecoxib is likely to be independent of its COX-2 inhibitory activity, as a structural analog of celecoxib that completely lacks cyclooxygenase-2 (COX-2) inhibitory activity produces a similar effect on lifespan (Fig. 3–4). Finally, celecoxib is known to affect the activity of other proteins at a higher dosage in the mammalian system. For instance, several studies have suggested that celecoxib might induce apoptosis and inhibit tumor growth, at least in part, by acting on a COX-2-independent mechanism (Hsu et al. 2000; Kismet et al. 2004; Zhu et al. 2004). However, the dosage required to induce apoptosis is significantly higher than the dosage required for COX-2 inhibition (i.e. IC50 = 40 nM for COX-2 inhibition; > 20 µM for apoptosis induction). Nevertheless, in the absence of its primary target (i.e. COXs), it is plausible that celecoxib acts on one of the secondary targets to produce the longevity effects in C. elegans.
In C. elegans, a number of environmental and physiological signals have been shown to influence longevity (Kenyon 2010). Reduction of food intake, mitochondrial respiration activity, insulin/IGF-1-like signaling (IIS), and signals from the germline cells have all been reported to extend worm lifespan (Kenyon 2010). The results of our genetic studies shown here have revealed the relationship between celecoxib and these known pathways. First, our results indicate that celecoxib and its derivative OSU-03012 do not influence longevity by acting on the mechanism that mediates DR response (Fig. 2A, 4A). It also appears that celecoxib and its derivatives do not influence longevity by altering the mitochondrial respiratory chain activity (Fig. 2B, 4B). Interestingly, we found that, in modulating C. elegans lifespan, celecoxib and its derivatives are completely dependent on the activity of the FOXO transcription factor DAF-16 (Fig. 2C, 4C). Consistently, we have found that worms exposed to celecoxib or OSU-03012 exhibit increased level of nuclear localized DAF-16, increased expression of DAF-16 target genes, and enhanced dauer formation (Fig. 6). Together, these findings strongly suggest that chronic treatments of celecoxib and its derivatives may extend lifespan by modulating the IIS pathway and DAF-16 activity.
In mammals, it has been shown that celecoxib inhibits mammalian PDK-1 activity, a known IIS pathway component, at higher dosage (Zhu et al. 2004). A number of celecoxib derivatives, including OSU-03012, have also been reported to exhibit different degrees of inhibitory activity against mammalian PDK-1, while lacking COX-2 inhibitory activity (Zhu et al. 2004). In C. elegans, PDK-1 is known to function in the IIS pathway to control longevity, development, and metabolism (Paradis et al. 1999). A reduction-of-function mutation in pdk-1 results in increased lifespan (Paradis et al. 1999). Therefore, given the known role of PDK-1 in IIS and lifespan regulation, it has emerged to be the most likely physiological target of celecoxib and OSU-03012 in influencing worm aging. Indeed, treatments with OSU-03012 failed to extend the lifespan of both pdk-1(sa680) and pdk-1(mg142) mutants (Fig. 4E), suggesting that these drugs may exert their effects by altering PDK-1 activity. Thus, when PDK-1 is mutated, the longevity effects of these compounds are compromised. At the molecular lever, both sa680 and mg142 alleles contain a missense mutation located in the kinase domain of PDK-1, in close proximity to one another [i.e sa680, G255R; mg142, A263V (Paradis et al. 1999)]. The PDK1 kinase domain has at least three ligand-binding sites; the ATP-binding pocket, the peptide substrate-binding site, and a groove in the N-terminal lobe that binds its kinase substrates. Many inhibitors of PDK-1 were designed or screened to target these sites to compete with either the substrates or ATP (Bobkova et al. 2010). In fact, celecoxib and OSU-03012 have been proposed to inhibit mammalian PDK-1 through competition with ATP (Zhu et al. 2004). Thus, mutations such as mg142 or sa680 that would likely change the tertiary structure of the kinase domain may very well alter the PDK-1 inhibitory activity of a compound. Furthermore, we have shown that in vivo PDK-1 activity is significantly reduced in celecoxib or OSU-03012 treated animals (Fig. 5). Together, our findings support the model that celecoxib and OSU-03012 function as PDK-1 inhibitors to increase longevity in worms. Alternatively, celecoxib may act on a component upstream of PDK-1 in the IIS pathway or act on an unknown target that indirectly alters IIS and PDK-1 activity. However, the latter model is less preferable since it has been shown that celecoxib and OSU-03012 can directly inhibit mammalian PDK-1 activity in vitro (Zhu et al. 2004).
In addition to longevity and dauer formation, the IIS pathway has been shown to influence many other aspects of worm physiology, such as developmental timing, reproduction, feeding rate, fat storage, etc (Kenyon et al. 1993; Kimura et al. 1997; Gems et al. 1998). However, we have observed no significant differences in fecundity (Fig. S2) and developmental timing [egg to egg time: control, 70.0 ± 1.4 hr; celecoxib, 70.2 ± 1.5 hr; p = 0.68] between the drug treated and control animals. Furthermore, it has been reported that the lifespan of the hypomorphic daf-2(e1370) mutants can be further extended by daf-2 RNAi (Arantes-Oliveira et al. 2003). However, only a small (7–9%), but not statistically significant, extension of lifespan has been observed in daf-2(e1370) mutants when treated with OSU-03012 (Fig. 4D, Table S2). Similarly, compared to the robust effects of mutations in the IIS pathway (e.g. daf-2 or pdk-1) on longevity (>100%), the effects of celecoxib and OSU-03012 is rather small (~20%). The reason why these drug treatments did not completely phenocopy daf-2 or pdk-1 mutations may be two-fold. First, the maximum level of inhibition of IIS activity that could be achieved by the drug treatments may be limited by other detrimental secondary effects associated with these compounds (e.g. secondary targets), as exposure of the animals to high doses of these drugs do cause lethality (Fig. 1C, 3C). For instance, the external concentration of OSU-03012 that produced the optimal longevity effect (i.e. 0.5 µM) is much lower than the reported IC50 of the compound for PDK-1 inhibition (i.e. 5 µM) (Zhu et al. 2004). The internal concentration of the drug is likely to be even lower. Therefore, the effects of the drugs we observed on worm physiology may be suboptimal compared to other IIS mutants or RNAi. Second, as we mentioned earlier, we have observed a higher level of nuclear localized DAF-16::GFP in the anterior end compared to the posterior end of the animals (Fig. 6A). This may presumably be due to the way worms absorb the drugs (i.e. pumping via pharynx). Therefore, it is possible that the effects we observed with these drugs are limited by the number and the types of cells they can reach. This may explain why certain aspects of worm physiology are not affected by the drug treatments.
It has been proposed that mild stresses early in life may causes a lifespan extension by enhancing the existing damage repair mechanisms (Rattan 2008). This effect, which is often referred to as hormesis, has also been observed in worms, as mild heat-shock and oxidative stress result in a small but significant extension in lifespan (Lithgow et al. 1995; Cypser & Johnson 2002). Therefore, while our results strongly suggest that celecoxib and its derivatives might extend lifespan by inhibiting PDK-1 activity, we cannot rule out the possibility that the increased longevity is the result of a hormetic effect induced by the cytotoxicity of celecoxib, since high doses of celecoxib do cause lethality.
It is noteworthy that the external concentrations of celecoxib that extend lifespan (2–10 µM) is very close to the maximum serum concentration (1.8 ± 0.6 µM) found in osteoarthritis patients who were orally administered 200 mg celecoxib (Itthipanichpong et al. 2005). The internal concentration of celecoxib that extends lifespan in worms, however, is likely to be 10–100 fold lower than external concentrations (Rand & Johnson 1995; Evason et al. 2005).
In addition to its use as an anti-inflammatory drug for the treatment of rheumatoid arthritis and osteoarthritis, celecoxib has been shown to exert potent anti-cancer activities as well. Several epidemiological, preclinical and clinical studies have shown that regular use of celecoxib significantly reduce the risk of multiple cancers, including colorectal, pancreatic, lung, skin, and breast cancers (reviewed in (Kismet et al. 2004)). For instance, it has been shown in recent clinical trials that celecoxib is very effective in preventing colorectal adenomatous polyps (Arber et al. 2006; Bertagnolli et al. 2006). In addition to its role in cancer prevention, celecoxib appears to be effective in treating tumors that have already formed (reviewed in (Gasparini et al. 2003)). Despite these ongoing clinic investigations, the molecular mechanism underlying celecoxib-mediated antitumor effects in vivo remains unclear. While celecoxib can inhibit COX-2 and cause cell cycle arrest and apoptosis in certain cancer cells, accumulating evidence suggest that inhibition of COX-2 may not play a dominant role in this drug’s anticancer effects. For instance, it has been shown that the antitumor effect of celecoxib can be obtained in cancer cells that don’t express COX-2 (Grosch et al. 2001; Chuang et al. 2008). Furthermore, a structure-function analysis of several dozens of celecoxib analogs reveals that the antitumor potency is not dependent on its COX-2 inhibitory activity (Zhu et al. 2002; Zhu et al. 2004). Interestingly, these findings are consistent with our observations in C. elegans, as celecoxib also delays the progression of tumor growth, likely in a COX-2 independent manner. Considering recent studies associating celecoxib use with a higher risk of cardiovascular events (Solomon et al. 2005), celecoxib derivatives such as OSU-03012 that target PDK-1 specifically may be a more suitable candidate for future development of anti-cancer or even anti-aging drugs.
Celecoxib or OSU-03012 treatment in worms also delays the onset of polyQ-mediated protein aggregation and proteotoxicity (Fig. 7A–B). This beneficial effect may be the result of a direct inhibition of a mechanism that normally promotes aging (e.g. IIS pathway) by the drugs. Thus, the age-dependent progression of polyQ proteotoxicity is delayed when the rate of aging is reduced. Alternatively, celecoxib may act on a specific target that independently controls the development of aggregate-mediated proteotoxicity. In humans, epidemiological studies have shown that long-term use of NSAIDs reduces the risk and delays the onset of Alzheimer’s disease as well as other neurodegenerative diseases (Aisen 2002; Asanuma et al. 2004). Recent studies have reported that a subset of NSAIDs can lower the production of amyloidigenic Aβ42 or Aβ40 peptides, potentially independent of its COX-inhibitory activity (Weggen et al. 2001; Gasparini et al. 2004). However, nonselective NSAIDs appear to be more effective than selective COX-2 inhibitors in protection from Aβtoxicity. Moreover, clinical trails so far have failed to show any beneficial effect of celecoxib in AD (Firuzi & Pratico 2006).
Overall, our results support a model that celecoxib and its derivative OSU-03012 act through PDK-1, a conserved component of the IIS pathway, to extend lifespan in C. elegans. These findings may serve as a starting point for developing new therapeutics combating various aging-related diseases
Experimental Procedures
Strains
All strains used were maintained and handled as described previously (Brenner 1974). CF1037: daf-16(mu86)I, DA1116: eat-2(ad1116)II, CF1041: daf-2(e1370)III, JT9609: pdk-1(sa680)X, GR1318: pdk-1(mg142)X; TJ356: zIs356 [Ex(daf-16::gfp + rol-6)], BR2773: byEx[sgk-1::gfp], AM140: rmIs132[unc-54p::Q35::yfp].
Pharmacological compounds
Celecoxib was extracted from Celebrex capsules obtained from Amerisource Health (Malvern, PA) with ethyl acetate followed by recrystallization from a mixture of ethyl acetate and hexane. 2-amino-N-[4-[5-(2-phenanthrenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl]-phenyl]acetamide (OSU-03012) was synthesized by Dr. Chen’s laboratory as described previously (Zhu et al. 2004). These compounds were dissolved in DMSO for storage and diluted in water before use. Compounds at various concentrations were added to the NG plates approximately 8–12 hours before transferring animals onto these drug-containing plates. The final DMSO concentration was kept at 0.1% after adding drugs to the plates.
RNA-interference (RNAi) experiments
HT115 bacteria transformed with RNAi vectors (L4440) expressing dsRNA of the genes indicated were grown at 37°C in LB with 10 µg/mL tetracycline and 50 µg/mL carbenicillin, then seeded onto NG-carbenicillin plates and supplemented with 100 µL of 0.1M IPTG. Eggs were added to plates and transferred to new plates every 3–6 days.
Lifespan analysis
Lifespan analysis were conducted at 15 or 20°C as described previously (Kenyon et al. 1993; Apfeld & Kenyon 1999). Strains were grown at 20°C for at least two generations without starvation before used in lifespan analysis. At least 60 worms were used for each experiment. In all experiments, the pre-fertile period of adulthood was used as t = 0 for lifespan analysis. Statview 5.01 (SAS) software was used for statistical analysis to determine the means and percentiles. In all cases, P values were calculated using the log-rank (Mantel-Cox) method. For a typical drug treatment experiment, unless indicated otherwise, parental worms were cultured in the presence of the drug, and progeny were selected at the L4 stage to start the experiments. Thus, these worms were exposed to the drug from fertilization until death. To ensure the drugs retain its potency throughout the entire experiment, animals were transferred to fresh plates with the same drugs every 2–4 days.
DAF-16 nuclear localization assay
For quantification of DAF-16::GFP localization, synchronized eggs from TJ356 animals (i.e. transgenic animals expressing DAF-l6::GFP) was seeded onto either DMSO control or relevant drug plates. The GFP expression was then analyzed using an Olympus BX61 fluorescent microscope at 40× or 100× magnifications. Using a blind assay, worms were scored for the presence or absence of GFP accumulation within the intestinal nuclei as one day-old adult (n = 120 or greater for all treatments). An animal was scored as having nuclear GFP if more than one intestinal nuclei contained DAF-16-GFP. Lifespans following each treatment were analyzed to confirm the effectiveness of each drug treatment.
Quantitative RT-PCR analysis
To measure the mRNA level of sod-3 in drug-treated animals, total RNA was isolated from approximately 5,000 Day 1 adult worms grown on either control or drug-containing NG plates. cDNA was then prepared from 4 µg of total RNA using Superscript III Reverse Transcriptase (Invitrogen). TaqMan real-time PCR experiments were then performed in using the Chromo 4 system (MJ Research). Relative mRNA level of sod-3 were calculated and normalized against the internal control (act-1, the β-actin). Primer and probe sequences are available upon request.
Immuno-precipitation and western blotting analysis
Worm extracts were prepared from Day1 adult BR2773 (sgk-1::gfp ) worms grown on either control or drug-containing HG plates. Animals were harvested and washed two times with cold M9 buffer. Animals were then washed once with homogenization buffer (HB buffer, 20 mM Hepes, pH 7.6, 100 mM NaCl, 10 mM KCl, 1.5 mM MgCl2, 0.1 mM EDTA, 0.5 mM EGTA, 44 mM Sucrose, 0.5% Trion X-100). The worm pellet was resuspended in 3× volume of HB buffer with 1.5 mM NaF, 2 mM Na2VO4, and protease inhibitors mix (Roche). The worm pellet was then lysed by applying to the freeze-and-thaw cycle twice. The lysate was transferred into a Dounce homogenizer and stroked 30 times with a B pestle. The lysate was collected and spun at 14,000 g for 20 min. The supernatant was collected and protein concentration was measured by Bradford assay (Pierce).
For immunoprecipitation experiments, 2.5 mg of total protein was first incubated with rabbit polyclonal anti-GFP antibody (Abcam, #6556) at 1:500 dilution for 3–5 hrs at 4°C. Forty µl of 50% protein A-agarose slurry was then added to the extract and incubated for another 3–5 hrs at 4°C. The beads were washed three times with TNTG buffer (20 mM Tris, pH 7.4, 150 mM NaCl, 0.1% Triton X-100, 10% glycerol) with protease inhibitors. After the final wash, the beads were boiled with SDS sample buffer for Western blotting analysis using respective antibodies. The mouse monoclonal anti-phospho (Ser/Thr)-PDK-1 docking motif antibody (#9634) and the rabbit polyclonal anti-phosphothreonine antibody (#9381) were purchased from Cell Signaling Technology. The mouse monoclonal anti-phosphoserine antibody was purchased from Sigma (#P3430).
polyQ aggregation quantitation
Approximately 200 synchronized eggs of Q35-yfp expressing animals were placed on plates containing OSU-03012 or DMSO control. Animals were then transferred to fresh plates with the same drugs every 3–4 days. 10–15 worms from each group were randomly selected to be scored for aggregates every day. Animals selected were viewed at 100× magnification with a stereomicroscope equipped for epifluorecence. Images of these animals were taken, and the number of aggregates in each animal was blindly counted by three independent observers after all the images have been collected. Aggregates were defined as discrete structures with clear boundaries on all sides.
Mobility assay
The spontaneous locomotion speed were measured using an automated worm tracking system as previously described (Hsu et al. 2009).
DAPI staining for gld-1(−) tumor formation
Day 6 adult worms grown on gld-1 RNAi bacteria and treated with different drugs were collected and fixed with cold methanol at −20°C for 30 min. The fixed samples were then incubated with 100ng/mL of DNA-intercalating dye DAPI (4',6'-diamidino-2-phenylindole) for 20 min. After washing by M9 buffer, the breaking out of the germ cells from gonads was visualized and scored using fluorescence microscopy.
Dauer formation assay
daf-2 (e1370) animals were grown on plates containing DMSO, celecoxib, or OSU-03012 at 20 °C, F1 eggs were incubated at 22.5°C or 25°C, and animals were scored for dauer arrest 72 h later.
Supplementary Material
Acknowledgments
We thank Carol Mousigan for technical assistance. We also thank the Caenorhabditis Genetic Center (CGC), which is funded by NIH for providing the worm strains required for this work. This work was supported in part by a pilot award from the Nathan Shock Center (P30 AG13283), and a R01 grant (R01 AG028516) from the National Institute on Aging (NIA) to ALH.
Footnotes
Author Contributions
The author(s) have made the following declarations about their contributions: Conceived and designed the experiments: TTC CSC ALH. Performed the experiments: TTC WCC. Analyzed the data: TTC WCC ALH. Contributed reagents/materials/analysis tools: CSC. Wrote the paper: TTC ALH.
References
- Adachi H, Ishii N. Effects of tocotrienols on life span and protein carbonylation in Caenorhabditis elegans. J Gerontol A Biol Sci Med Sci. 2000;55:B280–B285. doi: 10.1093/gerona/55.6.b280. [DOI] [PubMed] [Google Scholar]
- Aisen PS. The potential of anti-inflammatory drugs for the treatment of Alzheimer's disease. Lancet Neurol. 2002;1:279–284. doi: 10.1016/s1474-4422(02)00133-3. [DOI] [PubMed] [Google Scholar]
- Apfeld J, Kenyon C. Regulation of lifespan by sensory perception in Caenorhabditis elegans. Nature. 1999;402:804–809. doi: 10.1038/45544. [DOI] [PubMed] [Google Scholar]
- Arantes-Oliveira N, Berman JR, Kenyon C. Healthy animals with extreme longevity. Science. 2003;302:611. doi: 10.1126/science.1089169. [DOI] [PubMed] [Google Scholar]
- Arber N, Eagle CJ, Spicak J, Racz I, Dite P, Hajer J, Zavoral M, Lechuga MJ, Gerletti P, Tang J, Rosenstein RB, Macdonald K, Bhadra P, Fowler R, Wittes J, Zauber AG, Solomon SD, Levin B. Celecoxib for the prevention of colorectal adenomatous polyps. N Engl J Med. 2006;355:885–895. doi: 10.1056/NEJMoa061652. [DOI] [PubMed] [Google Scholar]
- Asanuma M, Miyazaki I, Ogawa N. Neuroprotective effects of nonsteroidal anti-inflammatory drugs on neurodegenerative diseases. Curr Pharm Des. 2004;10:695–700. doi: 10.2174/1381612043453072. [DOI] [PubMed] [Google Scholar]
- Bass TM, Weinkove D, Houthoofd K, Gems D, Partridge L. Effects of resveratrol on lifespan in Drosophila melanogaster and Caenorhabditis elegans. Mech Ageing Dev. 2007;128:546–552. doi: 10.1016/j.mad.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Bertagnolli MM, Eagle CJ, Zauber AG, Redston M, Solomon SD, Kim K, Tang J, Rosenstein RB, Wittes J, Corle D, Hess TM, Woloj GM, Boisserie F, Anderson WF, Viner JL, Bagheri D, Burn J, Chung DC, Dewar T, Foley TR, Hoffman N, Macrae F, Pruitt RE, Saltzman JR, Salzberg B, Sylwestrowicz T, Gordon GB, Hawk ET. Celecoxib for the prevention of sporadic colorectal adenomas. N Engl J Med. 2006;355:873–884. doi: 10.1056/NEJMoa061355. [DOI] [PubMed] [Google Scholar]
- Bobkova EV, Weber MJ, Xu Z, Zhang YL, Jung J, Blume-Jensen P, Northrup A, Kunapuli P, Andersen JN, Kariv I. Discovery of PDK1 kinase inhibitors with a novel mechanism of action by ultrahigh throughput screening. J Biol Chem. 2010;285:18838–18846. doi: 10.1074/jbc.M109.089946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang HC, Kardosh A, Gaffney KJ, Petasis NA, Schonthal AH. COX-2 inhibition is neither necessary nor sufficient for celecoxib to suppress tumor cell proliferation and focus formation in vitro. Mol Cancer. 2008;7:38. doi: 10.1186/1476-4598-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen E, Bieschke J, Perciavalle RM, Kelly JW, Dillin A. Opposing activities protect against age-onset proteotoxicity. Science. 2006;313:1604–1610. doi: 10.1126/science.1124646. [DOI] [PubMed] [Google Scholar]
- Cypser JR, Johnson TE. Multiple stressors in Caenorhabditis elegans induce stress hormesis and extended longevity. J Gerontol A Biol Sci Med Sci. 2002;57:B109–B114. doi: 10.1093/gerona/57.3.b109. [DOI] [PubMed] [Google Scholar]
- Dannenberg AJ, Altorki NK, Boyle JO, Dang C, Howe LR, Weksler BB, Subbaramaiah K. Cyclo-oxygenase 2: a pharmacological target for the prevention of cancer. Lancet Oncol. 2001;2:544–551. doi: 10.1016/S1470-2045(01)00488-0. [DOI] [PubMed] [Google Scholar]
- Diaz A, Chepenik KP, Korn JH, Reginato AM, Jimenez SA. Differential regulation of cyclooxygenases 1 and 2 by interleukin-1 beta, tumor necrosis factor-alpha, and transforming growth factor-beta 1 in human lung fibroblasts. Exp Cell Res. 1998;241:222–229. doi: 10.1006/excr.1998.4050. [DOI] [PubMed] [Google Scholar]
- Dillin A, Crawford DK, Kenyon C. Timing requirements for insulin/IGF-1 signaling in C. elegans. Science. 2002a;298:830–834. doi: 10.1126/science.1074240. [DOI] [PubMed] [Google Scholar]
- Dillin A, Hsu AL, Arantes-Oliveira N, Lehrer-Graiwer J, Hsin H, Fraser AG, Kamath RS, Ahringer J, Kenyon C. Rates of behavior and aging specified by mitochondrial function during development. Science. 2002b;298:2398–2401. doi: 10.1126/science.1077780. [DOI] [PubMed] [Google Scholar]
- Etminan M, Gill S, Samii A. Effect of non-steroidal anti-inflammatory drugs on risk of Alzheimer's disease: systematic review and meta-analysis of observational studies. Bmj. 2003;327:128. doi: 10.1136/bmj.327.7407.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evason K, Collins JJ, Huang C, Hughes S, Kornfeld K. Valproic acid extends Caenorhabditis elegans lifespan. Aging Cell. 2008;7:305–317. doi: 10.1111/j.1474-9726.2008.00375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evason K, Huang C, Yamben I, Covey DF, Kornfeld K. Anticonvulsant medications extend worm life-span. Science. 2005;307:258–262. doi: 10.1126/science.1105299. [DOI] [PubMed] [Google Scholar]
- Firuzi O, Pratico D. Coxibs and Alzheimer's disease: should they stay or should they go? Ann Neurol. 2006;59:219–228. doi: 10.1002/ana.20774. [DOI] [PubMed] [Google Scholar]
- Francis R, Barton MK, Kimble J, Schedl T. gld-1, a tumor suppressor gene required for oocyte development in Caenorhabditis elegans. Genetics. 1995;139:579–606. doi: 10.1093/genetics/139.2.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukutake M, Nakatsugi S, Isoi T, Takahashi M, Ohta T, Mamiya S, Taniguchi Y, Sato H, Fukuda K, Sugimura T, Wakabayashi K. Suppressive effects of nimesulide, a selective inhibitor of cyclooxygenase-2, on azoxymethane-induced colon carcinogenesis in mice. Carcinogenesis. 1998;19:1939–1942. doi: 10.1093/carcin/19.11.1939. [DOI] [PubMed] [Google Scholar]
- Garigan D, Hsu AL, Fraser AG, Kamath RS, Ahringer J, Kenyon C. Genetic analysis of tissue aging in Caenorhabditis elegans: a role for heat-shock factor and bacterial proliferation. Genetics. 2002;161:1101–1112. doi: 10.1093/genetics/161.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparini G, Longo R, Sarmiento R, Morabito A. Inhibitors of cyclo-oxygenase 2: a new class of anticancer agents? Lancet Oncol. 2003;4:605–615. doi: 10.1016/s1470-2045(03)01220-8. [DOI] [PubMed] [Google Scholar]
- Gasparini L, Rusconi L, Xu H, del Soldato P, Ongini E. Modulation of beta-amyloid metabolism by non-steroidal anti-inflammatory drugs in neuronal cell cultures. J Neurochem. 2004;88:337–348. doi: 10.1111/j.1471-4159.2004.02154.x. [DOI] [PubMed] [Google Scholar]
- Gems D, Sutton AJ, Sundermeyer ML, Albert PS, King KV, Edgley ML, Larsen PL, Riddle DL. Two pleiotropic classes of daf-2 mutation affect larval arrest, adult behavior, reproduction and longevity in Caenorhabditis elegans. Genetics. 1998;150:129–155. doi: 10.1093/genetics/150.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosch S, Tegeder I, Niederberger E, Brautigam L, Geisslinger G. COX-2 independent induction of cell cycle arrest and apoptosis in colon cancer cells by the selective COX-2 inhibitor celecoxib. FASEB J. 2001;15:2742–2744. doi: 10.1096/fj.01-0299fje. [DOI] [PubMed] [Google Scholar]
- Guarente L, Kenyon C. Genetic pathways that regulate ageing in model organisms. Nature. 2000;408:255–262. doi: 10.1038/35041700. [DOI] [PubMed] [Google Scholar]
- Harrington LA, Harley CB. Effect of vitamin E on lifespan and reproduction in Caenorhabditis elegans. Mech Ageing Dev. 1988;43:71–78. doi: 10.1016/0047-6374(88)90098-x. [DOI] [PubMed] [Google Scholar]
- Henderson ST, Johnson TE. daf-16 integrates developmental and environmental inputs to mediate aging in the nematode Caenorhabditis elegans. Curr Biol. 2001;11:1975–1980. doi: 10.1016/s0960-9822(01)00594-2. [DOI] [PubMed] [Google Scholar]
- Herndon LA, Schmeissner PJ, Dudaronek JM, Brown PA, Listner KM, Sakano Y, Paupard MC, Hall DH, Driscoll M. Stochastic and genetic factors influence tissue-specific decline in ageing C. elegans. Nature. 2002;419:808–814. doi: 10.1038/nature01135. [DOI] [PubMed] [Google Scholar]
- Hida T, Leyton J, Makheja AN, Ben-Av P, Hla T, Martinez A, Mulshine J, Malkani S, Chung P, Moody TW. Non-small cell lung cancer cycloxygenase activity and proliferation are inhibited by non-steroidal antiinflammatory drugs. Anticancer Res. 1998;18:775–782. [PubMed] [Google Scholar]
- Honda Y, Honda S. The daf-2 gene network for longevity regulates oxidative stress resistance and Mn-superoxide dismutase gene expression in Caenorhabditis elegans. Faseb J. 1999;13:1385–1393. [PubMed] [Google Scholar]
- Hong F, Larrea MD, Doughty C, Kwiatkowski DJ, Squillace R, Slingerland JM. mTOR-raptor binds and activates SGK1 to regulate p27 phosphorylation. Mol Cell. 2008;30:701–711. doi: 10.1016/j.molcel.2008.04.027. [DOI] [PubMed] [Google Scholar]
- Hsu AL, Ching TT, Wang DS, Song X, Rangnekar VM, Chen CS. The cyclooxygenase-2 inhibitor celecoxib induces apoptosis by blocking Akt activation in human prostate cancer cells independently of Bcl-2. J Biol Chem. 2000;275:11397–11403. doi: 10.1074/jbc.275.15.11397. [DOI] [PubMed] [Google Scholar]
- Hsu AL, Feng Z, Hsieh MY, Xu XZ. Identification by machine vision of the rate of motor activity decline as a lifespan predictor in C. elegans. Neurobiol Aging. 2009;30:1498–1503. doi: 10.1016/j.neurobiolaging.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu AL, Murphy CT, Kenyon C. Regulation of aging and age-related disease by DAF-16 and heat-shock factor. Science. 2003;300:1142–1145. doi: 10.1126/science.1083701. [DOI] [PubMed] [Google Scholar]
- in t' Veld BA, Ruitenberg A, Hofman A, Launer LJ, van Duijn CM, Stijnen T, Breteler MM, Stricker BH. Nonsteroidal antiinflammatory drugs and the risk of Alzheimer's disease. N Engl J Med. 2001;345:1515–1521. doi: 10.1056/NEJMoa010178. [DOI] [PubMed] [Google Scholar]
- Itthipanichpong C, Chompootaweep S, Wittayalertpanya S, Kemsri W, Thaworn N, Lilitkarntrakul P, Parikamsil S. Clinical pharmacokinetic of celecoxib in healthy Thai volunteers. J Med Assoc Thai. 2005;88:632–638. [PubMed] [Google Scholar]
- Kenyon C. The plasticity of aging: insights from long-lived mutants. Cell. 2005;120:449–460. doi: 10.1016/j.cell.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–464. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- Kenyon CJ. The genetics of ageing. Nature. 2010;464:504–512. doi: 10.1038/nature08980. [DOI] [PubMed] [Google Scholar]
- Kimura KD, Tissenbaum HA, Liu Y, Ruvkun G. daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science. 1997;277:942–946. doi: 10.1126/science.277.5328.942. [DOI] [PubMed] [Google Scholar]
- Kismet K, Akay MT, Abbasoglu O, Ercan A. Celecoxib: a potent cyclooxygenase-2 inhibitor in cancer prevention. Cancer Detect Prev. 2004;28:127–142. doi: 10.1016/j.cdp.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Lakowski B, Hekimi S. The genetics of caloric restriction in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 1998;95:13091–13096. doi: 10.1073/pnas.95.22.13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen PL, Albert PS, Riddle DL. Genes that regulate both development and longevity in Caenorhabditis elegans. Genetics. 1995;139:1567–1583. doi: 10.1093/genetics/139.4.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin K, Dorman JB, Rodan A, Kenyon C. daf-16: An HNF-3/forkhead family member that can function to double the life-span of Caenorhabditis elegans. Science. 1997;278:1319–1322. doi: 10.1126/science.278.5341.1319. [DOI] [PubMed] [Google Scholar]
- Lin K, Hsin H, Libina N, Kenyon C. Regulation of the Caenorhabditis elegans longevity protein DAF-16 by insulin/IGF-1 and germline signaling. Nat Genet. 2001;28:139–145. doi: 10.1038/88850. [DOI] [PubMed] [Google Scholar]
- Lipsky PE. Specific COX-2 inhibitors in arthritis, oncology, and beyond: where is the science headed? J Rheumatol Suppl. 1999;56:25–30. [PubMed] [Google Scholar]
- Lithgow GJ, White TM, Melov S, Johnson TE. Thermotolerance and extended life-span conferred by single-gene mutations and induced by thermal stress. Proc Natl Acad Sci U S A. 1995;92:7540–7544. doi: 10.1073/pnas.92.16.7540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masoro EJ. Overview of caloric restriction and ageing. Mech Ageing Dev. 2005;126:913–922. doi: 10.1016/j.mad.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Melov S, Ravenscroft J, Malik S, Gill MS, Walker DW, Clayton PE, Wallace DC, Malfroy B, Doctrow SR, Lithgow GJ. Extension of life-span with superoxide dismutase/catalase mimetics. Science. 2000;289:1567–1569. doi: 10.1126/science.289.5484.1567. [DOI] [PubMed] [Google Scholar]
- Morley JF, Brignull HR, Weyers JJ, Morimoto RI. The threshold for polyglutamine-expansion protein aggregation and cellular toxicity is dynamic and influenced by aging in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2002;99:10417–10422. doi: 10.1073/pnas.152161099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JZ, Tissenbaum HA, Ruvkun G. A phosphatidylinositol-3-OH kinase family member regulating longevity and diapause in Caenorhabditis elegans. Nature. 1996;382:536–539. doi: 10.1038/382536a0. [DOI] [PubMed] [Google Scholar]
- Ogg S, Paradis S, Gottlieb S, Patterson GI, Lee L, Tissenbaum HA, Ruvkun G. The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature. 1997;389:994–999. doi: 10.1038/40194. [DOI] [PubMed] [Google Scholar]
- Ogg S, Ruvkun G. The C. elegans PTEN homolog, DAF-18, acts in the insulin receptor-like metabolic signaling pathway. Mol Cell. 1998;2:887–893. doi: 10.1016/s1097-2765(00)80303-2. [DOI] [PubMed] [Google Scholar]
- Onken B, Driscoll M. Metformin induces a dietary restriction-like state and the oxidative stress response to extend C. elegans Healthspan via AMPK, LKB1, and SKN-1. PLoS One. 2010;5:e8758. doi: 10.1371/journal.pone.0008758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panowski SH, Dillin A. Signals of youth: endocrine regulation of aging in Caenorhabditis elegans. Trends Endocrinol Metab. 2009;20:259–264. doi: 10.1016/j.tem.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Panowski SH, Wolff S, Aguilaniu H, Durieux J, Dillin A. PHA-4/Foxa mediates diet-restriction-induced longevity of C. elegans. Nature. 2007;447:550–555. doi: 10.1038/nature05837. [DOI] [PubMed] [Google Scholar]
- Paradis S, Ailion M, Toker A, Thomas JH, Ruvkun G. A PDK1 homolog is necessary and sufficient to transduce AGE-1 PI3 kinase signals that regulate diapause in Caenorhabditis elegans. Genes Dev. 1999;13:1438–1452. doi: 10.1101/gad.13.11.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradis S, Ruvkun G. Caenorhabditis elegans Akt/PKB transduces insulin receptor-like signals from AGE-1 PI3 kinase to the DAF-16 transcription factor. Genes Dev. 1998;12:2488–2498. doi: 10.1101/gad.12.16.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrascheck M, Ye X, Buck LB. An antidepressant that extends lifespan in adult Caenorhabditis elegans. Nature. 2007;450:553–556. doi: 10.1038/nature05991. [DOI] [PubMed] [Google Scholar]
- Pinkston JM, Garigan D, Hansen M, Kenyon C. Mutations that increase the life span of C. elegans inhibit tumor growth. Science. 2006;313:971–975. doi: 10.1126/science.1121908. [DOI] [PubMed] [Google Scholar]
- Rand JB, Johnson CD. Genetic pharmacology: interactions between drugs and gene products in Caenorhabditis elegans. Methods Cell Biol. 1995;48:187–204. doi: 10.1016/s0091-679x(08)61388-6. [DOI] [PubMed] [Google Scholar]
- Rattan SI. Hormesis in aging. Ageing Res Rev. 2008;7:63–78. doi: 10.1016/j.arr.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Simmons DL, Botting RM, Hla T. Cyclooxygenase isozymes: the biology of prostaglandin synthesis and inhibition. Pharmacol Rev. 2004;56:387–437. doi: 10.1124/pr.56.3.3. [DOI] [PubMed] [Google Scholar]
- Smalley WE, DuBois RN. Colorectal cancer and nonsteroidal anti-inflammatory drugs. Adv Pharmacol. 1997;39:1–20. doi: 10.1016/s1054-3589(08)60067-8. [DOI] [PubMed] [Google Scholar]
- Solomon SD, McMurray JJ, Pfeffer MA, Wittes J, Fowler R, Finn P, Anderson WF, Zauber A, Hawk E, Bertagnolli M. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N Engl J Med. 2005;352:1071–1080. doi: 10.1056/NEJMoa050405. [DOI] [PubMed] [Google Scholar]
- Tessier M, Woodgett JR. Serum and glucocorticoid-regulated protein kinases: variations on a theme. J Cell Biochem. 2006;98:1391–1407. doi: 10.1002/jcb.20894. [DOI] [PubMed] [Google Scholar]
- Thompson HJ, Jiang C, Lu J, Mehta RG, Piazza GA, Paranka NS, Pamukcu R, Ahnen DJ. Sulfone metabolite of sulindac inhibits mammary carcinogenesis. Cancer Res. 1997;57:267–271. [PubMed] [Google Scholar]
- Thun MJ, Namboodiri MM, Heath CW., Jr Aspirin use and reduced risk of fatal colon cancer. N Engl J Med. 1991;325:1593–1596. doi: 10.1056/NEJM199112053252301. [DOI] [PubMed] [Google Scholar]
- Weggen S, Eriksen JL, Das P, Sagi SA, Wang R, Pietrzik CU, Findlay KA, Smith TE, Murphy MP, Bulter T, Kang DE, Marquez-Sterling N, Golde TE, Koo EH. A subset of NSAIDs lower amyloidogenic Abeta42 independently of cyclooxygenase activity. Nature. 2001;414:212–216. doi: 10.1038/35102591. [DOI] [PubMed] [Google Scholar]
- Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M, Sinclair D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430:686–689. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- Zhu J, Huang JW, Tseng PH, Yang YT, Fowble J, Shiau CW, Shaw YJ, Kulp SK, Chen CS. From the cyclooxygenase-2 inhibitor celecoxib to a novel class of 3-phosphoinositide-dependent protein kinase-1 inhibitors. Cancer Res. 2004;64:4309–4318. doi: 10.1158/0008-5472.CAN-03-4063. [DOI] [PubMed] [Google Scholar]
- Zhu J, Song X, Lin HP, Young DC, Yan S, Marquez VE, Chen CS. Using cyclooxygenase-2 inhibitors as molecular platforms to develop a new class of apoptosis-inducing agents. J Natl Cancer Inst. 2002;94:1745–1757. doi: 10.1093/jnci/94.23.1745. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.