Summary
Astrocytes secrete growth factors that are both neuroprotective and supportive for the local environment. Identified by glial fibrillary acidic protein (GFAP) expression, astrocytes exhibit heterogeneity in morphology and in expression of phenotypic markers and growth factors throughout different adult brain regions. In adult neurogenic niches, astrocytes secrete vascular endothelial growth factor (VEGF) and fibroblast growth factor-2 (FGF-2) within the neurogenic niche, and are also a source of special GFAP-positive multipotent neural stem cells (NSCs). Normal aging is accompanied by a decline in CNS function and reduced neurogenesis. We asked if a decreased availability of astrocyte-derived factors may contribute to the age-related decline in neurogenesis. Determining alterations of astrocytic activity in the aging brain is crucial for understanding CNS homeostasis in aging and for assessing appropriate therapeutic targets for an aging population. We found region-specific alterations in gene expression of GFAP, VEGF and FGF-2 and their receptors in the aged brain corresponding to changes in astrocytic reactivity, supporting astrocytic heterogeneity and demonstrating a differential aging effect. We found that GFAP-positive NSCs uniquely coexpress both VEGF and its key mitotic receptor Flk-1 in both young and aged hippocampus, indicating a possible autocrine/paracrine signaling mechanism. VEGF expression is lost once NSCs commit to a neuronal fate, but Flk-1-mediated sensitivity to VEGF signaling is maintained. We propose that age-related astrocytic changes result in reduced VEGF and FGF-2 signaling, which in turn limits neural stem cell and progenitor cell maintenance and contributes to decreased neurogenesis.
Keywords: Astrogliosis, VEGF, FGF-2, Neurogenesis, Growth factors, Homeostasis
Introduction
Glial cells provide support to maintain neuronal function in the mature nervous system. Astrocytes, in particular, are key modulators of neuronal function and maintain homeostasis of the microenvironment through regulating water flow and pH levels, sustaining proper levels of ions and neurotransmitters, and preserving the blood-brain barrier (Wolburg-Buchholz et al., 2009). Astrocytes are also intimately involved in directly regulating various aspects of neuronal activity. For example, they are reported to modulate spine density and LTP via bidirectional communication with neurons utilizing ATP (Pascual et al., 2005) and Eph4/ephrin-3A (Filosa et al., 2009). Astrocytes also mediate the vascular responses to neuronal activity by propagating neuronal signals and isolating and regulating vascular contributions to the neural parenchyma by means of their astrocytic end-feet processes (Giaume, 2010; Giaume et al., 2010). Furthermore, astrocytes supplement their local microenvironment with a variety of growth factors such as LIF, CNTF, GDNF, FGF-2 and VEGF (Alvarez-Buylla and Lim, 2004; Mani et al., 2005; Shetty et al., 2005; Costantini et al., 2010), most of which are key to modulating neuronal activity. Growth factors expressed by astrocytes are also understood to modulate neurogenesis within the small, spatially-restricted neurogenic niches in the adult brain (Emsley and Macklis, 2006). Most notably, VEGF and FGF-2 are mitotic factors that contribute to neurogenesis by enhancing the proliferation of neural stem cells (NSCs) and their progeny in the subventricular zone (SVZ) and the dentate gyrus (DG) of the adult brain (Cheng et al., 2002; Jin et al., 2002).
Given the crucial involvement of astrocytes in homeostasis and neuronal activity, an alteration in astrocytic function will have a direct effect on neurons. For example, CNS injury induces astrocytic reactivity, defined by cellular hypertrophy (also known as gliosis) and elevated expression of the structural protein glial fibrillary acidic protein (GFAP) (Wu et al., 2005). Reactive astrocytes change their expression of growth factors and cytokines, and may lead to modifications of the local cytoarchitectural organization that may be detrimental to neurons and the surrounding penumbra. Interestingly, a balance between beneficial and harmful effects of astrocytic reactivity may depend on the degree of astrogliosis. Astrocytes with mild-to-moderate reactivity, as defined by spatial restriction of reactive hypertrophy (Wilhelmsson et al., 2006), have the potential to revert back to a non-reactive state. A return to a non-reactive state can be observed with mild inflammation (Sofroniew, 2009; Sofroniew and Vinters, 2010), although the functional relevance of this reduced reactivity remains poorly understood. Conversely, fully expressed astrogliosis following trauma or extensive inflammation is known to change the microenvironmental architecture through interaction of adjacent glia, including NG2-positive cells (Magnus et al., 2008), resulting in scar formation to prevent further tissue damage (Faulkner et al., 2004; Sofroniew, 2005; Voskuhl et al., 2009). However, scar formation is also reported to hinder tissue repair (Wilhelmsson et al., 2006; Drogemuller et al., 2008).
In the aged CNS, increased astrocytic reactivity is observed, but little is known about the effects of aging on individual astrocytes or the extent to which their expression of environmental signals is altered. Age-related impairment in CNS function can result from a decline in the efficacy of the synaptic connections required for proper memory formation, and involve a marked loss of synapses (Maffei and Turrigiano, 2008), a decrease in NMDA-receptor mediated responses (Simón et al., 2009), and overall deficits in long-term potentiation (LTP) (Gooney et al., 2004). Coincidentally, these changes can also be produced by modulating astrocyte function in the young brain (Wolosker et al., 2008; Lalo et al., 2009; Ben Menachem-Zidon et al., 2010; Faissner et al., 2010; Halassa and Haydon, 2010). Therefore, understanding age-related changes in astrocytic function may provide insight into altered function within the hippocampus and the entorhinal cortex, as these areas are associated with a considerable decline in age-related cognitive function, including working, episodic, and odor memory (Murphy et al., 1997; Cansino, 2009). In this study, we investigate age-related modification of astrocytic function in relation to their expression of two important astrocyte-derived growth factors required for neuroprotection, VEGF and FGF-2, and their receptors.
VEGF is a potent angiogenic factor that has been extensively investigated due to its involvement in the formation of blood vessels (angiogenesis) required for cancerous tumor survival (Ferrara et al., 2003; Maione et al., 2004). VEGF plays a central role in the development of the CNS, but its expression is substantially reduced in the postnatal CNS (Gerber et al., 1999). VEGF is expressed exclusive by GFAP-positive cells in the adult brain, in contrast to its expression by neurons and endothelial cells during prenatal development (Yang et al., 2003). FGF-2 is also produced by astrocytes within the adult brain, apart from a select neuronal population in hippocampal area CA2 (Gomez-Pinilla et al., 1992). VEGF and FGF-2 are of key interest, as they are known to be both neuroprotective following an insult to the nervous system (Peterson et al., 1996; Nicoletti et al., 2008), and to have potent mitotic properties by inducing cellular proliferation, including NSCs and their progeny in neurogenic niches of the adult brain (Jin et al., 2002; Rai et al., 2007). Furthermore, the decline in neurogenesis and associated cognitive function seen in aging parallels the decline in VEGF and FGF-2 protein (Shetty et al., 2005). However, it remains to be determined if the age-related decline reflects reduced availability or reduced production of these growth factors. To address this, we analyzed the distribution of VEGF and its receptor Flk-1, as well as FGF-2, in both the young and aged brain. Additionally, we investigated age-related changes in protein and gene expression of VEGF and Flk-1, and FGF-2 and its receptors in the dentate gyrus and entorhinal cortex to better elucidate the role of these factors in two target areas affected by cognitive decline in the aging brain. Finally, we analyzed the expression of VEGF and Flk-1 within NSCs and their neuronal-committed progeny to help us understand how a decline in growth factor availability may directly influence the function of NSCs and their progeny in the aged brain.
Results
GFAP-immunopositive cytoarchitecture and gene expression are altered with aging
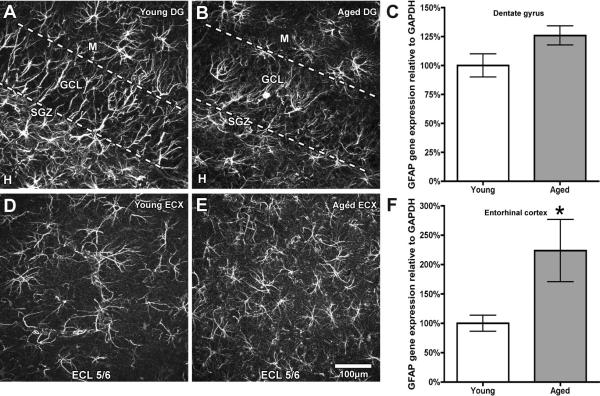
A dense population of GFAP-immunopositive cells is observed in the neurogenic hippocampal dentate subgranular zone (SGZ) that extend distinct radial processes through the granule cell layer (GCL; Fig. 1A). There are relatively few GFAP-positive cell bodies within the SGZ. Similarly, GFAP remains clearly detectable in the aged DG, though these cells appear more compact with shorter processes and the loss of distinct radial extensions across the GCL (Fig. 1B). To determine if the observed changes in process morphology correlate to astrocyte activation in the aged dentate gyrus, GFAP gene expression was analyzed and no significant difference was found (Fig. 1C).
Figure 1. Age-related expression of GFAP varies within the brain.
GFAP immunopositivity is robust in all regions of the young DG (A), with GFAP-positive processes extending across the granular cell layer and within the hilus. In contrast, the extensive branching of GFAP-positive processes is diminished in the aged DG (B). GFAP gene expression shows no significant difference between the young and aged DG (C). In contrast, GFAP-positive cells and processes are less dense in the young ECX than in the young DG (D), but demonstrate substantial increase in staining density in the aged ECX (E). Gene expression measurement reveals a doubling of GFAP gene expression in the aged ECX (F). * indicates p ≤ 0.05 DG = Dentate Gyrus; SGZ = Subgranular Cell Zone; GCL = Granular Cell Layer; M = Molecular Layer; H = Hilus; ; ECX = Entorhinal Cortex; ECL = Entorhinal Cell Layer; Scale bar = 100 μm. Dotted lines delineate GCL boundaries, with bottom line representing the SGZ.
In contrast to the hippocampus, GFAP immunoreactive cells are more dispersed within both the young (Fig. 1D) and aged (Fig. 1E) entorhinal cortex (ECX). GFAP-positive processes are elaborate in the young ECX (Fig. 1D), but appear shorter in the aged ECX, contributing to a more compact GFAP cytoarchitecture with aging (Fig. 1E). Nevertheless, GFAP staining density is more intense in the aged ECX, corresponding to a significant enhancement of GFAP gene expression with aging (Fig. 1F).
Cellular localization of VEGF within neurogenic niches of the young and aged brain
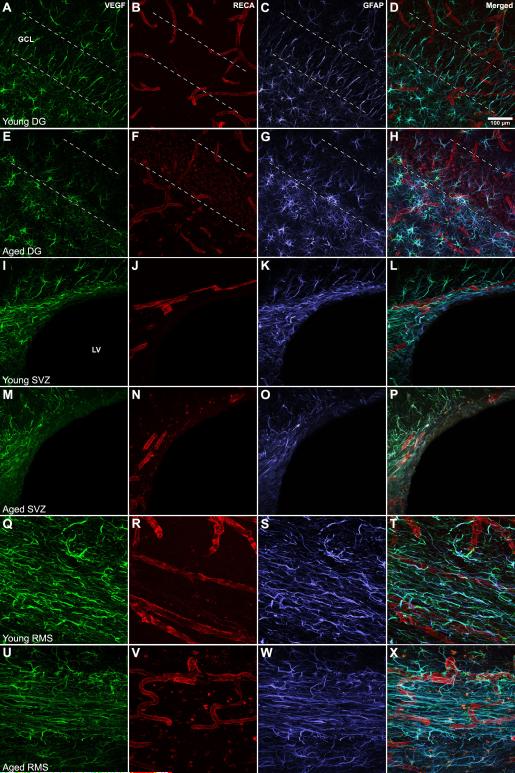
GFAP-positive cells contain VEGF in all regions and ages examined. VEGF is also detected in the end-feet of GFAP-positive processes associated with blood vessel profiles, but not with RECA-positive endothelial cells themselves (Fig. 2 and Fig. 3). In the young DG, VEGF detection is co-localized throughout the full extent of GFAP-positive processes (Fig. 2 A-D). This co-localization is maintained in the aged DG (Fig. 2 E-H). However, VEGF-positive/GFAP-positive processes ramify less in aging as noted above (Fig. 2H). The complete cellular co-localization of VEGF with GFAP is also found in the SVZ (Fig. 2 I-P) and RMS (Fig. 2 Q-X). In contrast to the DG, GFAP-positive cell cytoarchitecture was not attenuated in either the SVZ or RMS with aging.
Figure 2. Cellular localization of VEGF mirrors changes in astrocytic cytoarchitecture within neurogenic niches.
In all regions examined, VEGF-immunopositivity (green) was coexpressed with GFAP-immunopositive cells (blue). These VEGF/GFAP colabeled processes envelop blood vessels (RECA-positive; red), but endothelial cells themselves do not express VEGF. In the aged DG, VEGF-positive/GFAP-positive process ramifications are less elaborate, especially those expanding across the GCL (E-H) when compared to the young (A-D). VEGF is also present in both the SVZ (I-L) and RMS (Q-T), where the extent of their ramifications appears unchanged with age (M-P and U-X respectively). SVZ = Subventricular Zone; RMS = Rostral Migratory Stream; Scale bar = 100 μm. Dotted lines delineate GCL boundaries, with bottom line representing the SGZ.
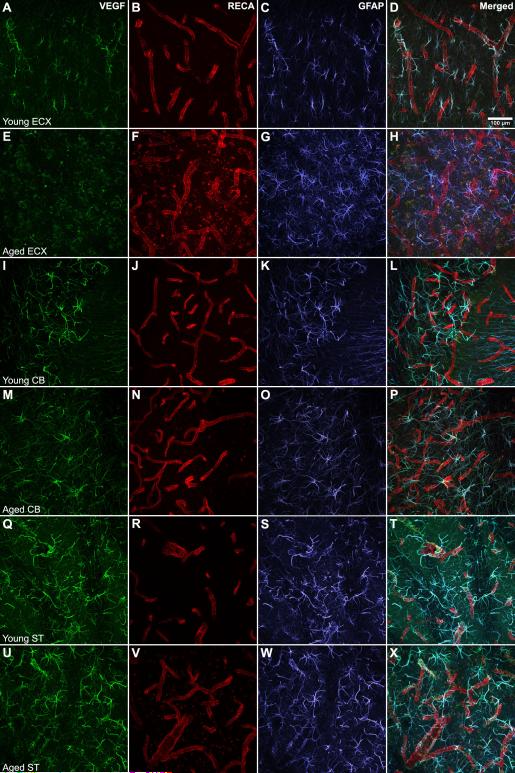
Figure 3. Astrocytic localization of VEGF protein varies with aging.
In the young ECX, GFAP-positive processes (C) coexpress VEGF (A) and envelop RECA-positive blood vessels (B, D). However, substantial differences are observed in the aged ECX (E-H), where VEGF immunoreactivity is nearly undetectable (E) when GFAP-positive cells are still present (G). Moderate differences are observed between the young (I-L) and aged (M-P) CB, with possible attenuation of VEGF and GFAP detection with aging as a results of less elaborate ramifications (M,O,P). Finally, in the ST, no obvious age-related differences are observed in the colocalization of VEGF with GFAP-positive processes between young (Q-T) and aged (U - X) samples. CB = cerebellum; ST = striatum; Scale bar = 100 μm
VEGF distribution varies between non-neurogenic brain regions and with aging
Co-expression of GFAP and VEGF is observed in the young ECX (Fig. 3 A-D). However, while GFAP-positive cells remain clearly detectable within the aged ECX (Fig. 3G), VEGF immunopositivity is sparse (Fig. 3E). When detected, the intracellular distribution of VEGF within GFAP-positive cells is restricted and does not extend into all processes (Fig. 3H). VEGF-positive/GFAP-positive cells are also observed in the cerebellum (Fig. 3I-L), with an age-related reduction in VEGF detection that parallels the reduction in GFAP immunoreactivity (Fig. 3 M-P). Nevertheless, VEGF can be detected throughout all GFAP-positive processes. The age-related reduction in VEGF detection is not observed in other brain regions. For example, the striatum shows no age-related loss of VEGF or GFAP detection (Fig. 3 Q-X).
Neuronal distribution of Flk-1 immunopositivity is maintained with aging
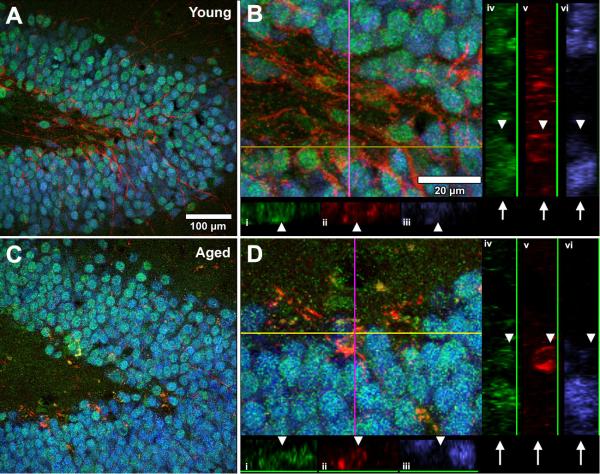
We find no discernable change in Flk-1 detection between young and aged neurogenic DG (Fig. 4), where Flk-1 colocalizes with both the mature neuron marker NeuN, and the immature neuronal marker DCX (Fig. 4 A-B). An equivalent expression pattern is seen in the aged brain, with Flk-1 detected within NeuN- and DCX-positive cells (Fig. 4 C-D). While there are fewer DCX-positive cells in the aged brain, those that are present continue to express Flk-1 (Fig. 4D). We find that weak Flk-1 staining is observed at the limit of detection in NeuN-positive neurons in all layers of the ECX, including layer 2, with no obvious age-related difference (data not shown).
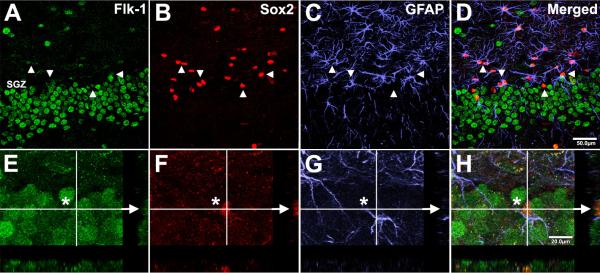
Figure 4. Expression of Flk-1 by immature and mature granule neurons is maintained with aging.
Flk-1 immunopositivity (green) is localized to immature (DCX-positive, red) and mature (NeuN-positive, blue) neurons in both young (A) and aged (C) DG. Coexpression was validated by three-dimensional analysis using orthogonal colocalization of image planes (B,D) through the x-axis (i-iii) and y-axis (iv-vi). Arrowheads denote DCX-positive cells that are NeuN-negative, and arrows show NeuN-positive cells that are DCX-negative. In both cases, Flk-1 expression is present. Scale bar for panels A and C = 100 μm; panels B and D = 20 μm
Expression of VEGF and its receptor, Flk-1, are reduced with aging
To determine if the observed age-related changes in VEGF detection are related to growth factor production, VEGF gene expression was analyzed and found significantly reduced in the aged DG (Fig. 5A). In contrast, no change is observed in the ECX for VEGF expression (Fig. 5C). To evaluate the potential of cells to respond to VEGF signaling, expression of the Flk-1 receptor was also measured. The pattern of expression for the receptor is the opposite of the ligand, with no change observed in the DG with age (Fig. 5B) but a significant age-related reduction of Flk-1 expression in the ECX (Fig. 5D).
Figure 5. Age-related changes in gene expression of VEGF and Flk-1 are region-specific.
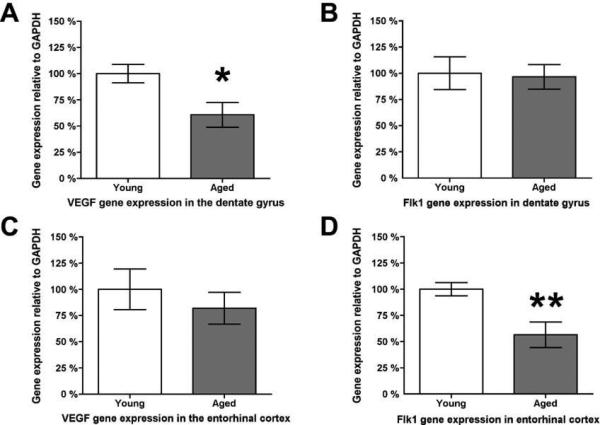
VEGF gene expression is significantly reduced in the dentate gyrus by approximately one-third as a function of age (A), while no age-related change is detected in the gene expression of its receptor Flk-1 (B). In contrast, the entorhinal cortex has no age-related change in gene expression of VEGF (C) but significantly reduces its expression of the receptor Flk-1 by almost one-half as a function of age (D). * indicates p ≤ 0.05; ** indicates p ≤ 0.01
Expression of FGF-2 remains stable with age, while receptor expression is altered
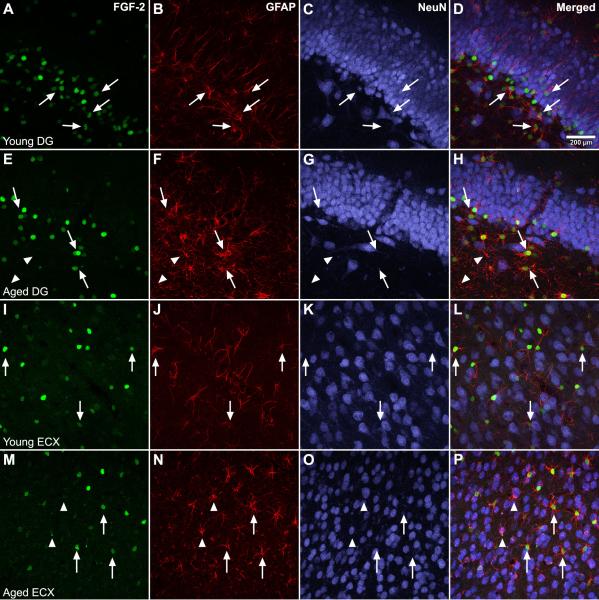
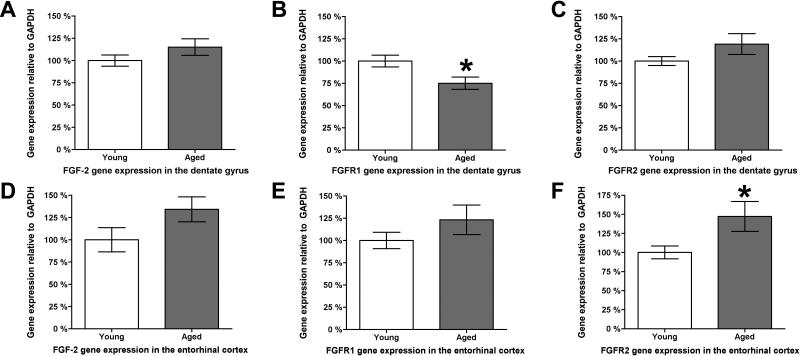
To determine if age-related changes in detection of FGF-2 paralleled those observed with VEGF, the histological detection of FGF-2 was assessed in the DG (Fig. 6 A-H) and ECX (Fig. 6 I-P). In agreement with previous reports, FGF-2 is coexpressed within GFAP-positive astrocytes (Fig. 6 D, H, L, P). There is no detectable difference in the intensity of FGF-2 staining within individual cells with aging in either region, but there are visibly fewer FGF-2-positive cells in the aged DG (Fig. 6 E-H). Furthermore, aging resulted in the emergence in both the aged DG and ECX of a phenotype of GFAP-positive astrocytes that did not coexpress FGF-2 (Fig. 6, arrowheads). In contrast to VEGF, FGF-2 gene expression remains stable with age in both DG and ECX (Fig. 7 A and B). However, an age-related alteration in the expression of its receptors FGFR1 and FGFR2 was detected. Expression of the neuron-expressed FGFR1 receptor declines in the aged DG (Fig. 7 B), but remains stable in the aged ECX (Fig. 7 E). Expression of the glial-expressed FGFR2 receptor, on the other hand, remains stable with aging in the DG (Fig. 7 C), but increases in the aged ECX (Fig. 7 F).
Figure 6. Aging produces a subpopulation of astrocytes without detectable FGF-2.
FGF-2 (green, arrows) is detected within astrocytes (GFAP, red), but not neurons (NeuN, blue) in both the dentate gyrus (A-D) and the entorhinal cortex. With aging, fewer FGF-2-positive cells are observed in the dentate gyrus (E), while there is no apparent change in the frequency of FGF-2-positive cells is seen in aged ECX (M). However, with aging, there is the emergence in both regions of a phenotype of GFAP-positive astrocytes that do not contain detectable FGF-2 (arrowheads; H and P). Scale bar = 200 μm
Figure 7. FGF-2 expression is stable with age, while receptor expression varies.
Gene expression analysis for both FGF-2 and its glial receptor FGFR2 shows that in the dentate gyrus, gene expression remain stable with age (A and C) while expression of neuronal FGFR1 is significantly reduced by approximately 25% (B). In the ECX, slight but non-significant elevations in both FGF-2 and neuronal FGFR1 gene expression are observed as a function of age (D and E), while expression of glial FGFR2 significantly increases by approximately 50% (F). * indicates p ≤ 0.05
VEGF is expressed both by mature astrocytes and Type 1 neural stem cells
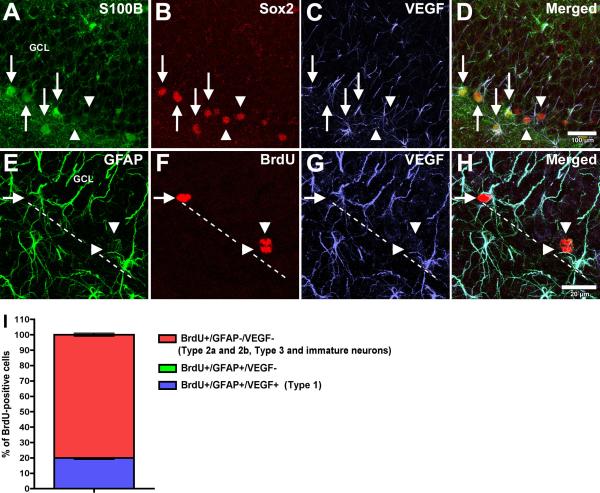
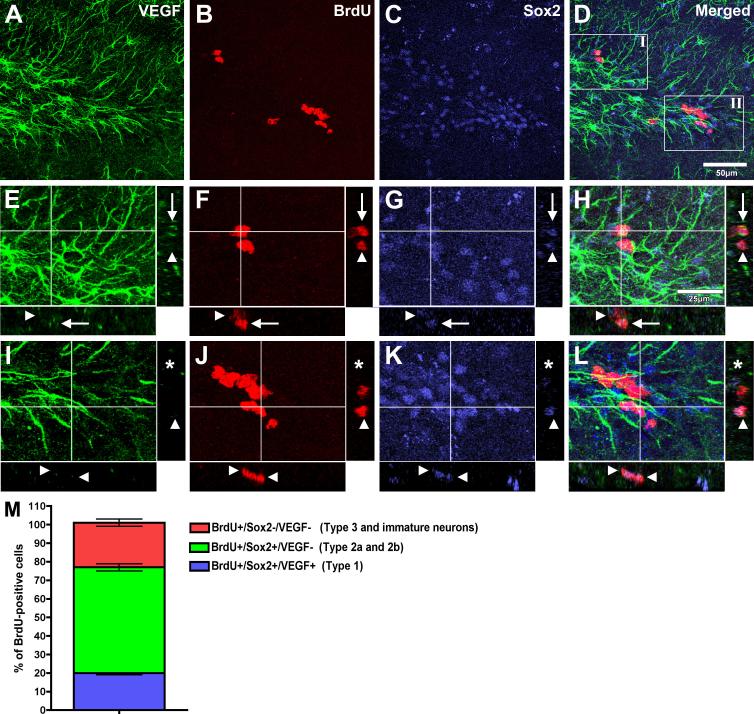
As both mature astrocytes and Type 1 neural stem cells (NSCs) express GFAP (Doetsch et al., 1999; Seri et al., 2001), we asked if NSCs also express VEGF and potentially participate in VEGF signaling in the neurogenic niche. To identify mature astrocytes in the young DG, we used the calcium binding protein S100beta, found only in mature astrocytes (Fig. 8A), in combination with the transcription factor Sox2 (Fig. 8B). We observe VEGF (Fig. 8C) colocalization with these targets (Fig. 8D); however, not all VEGF-positive/Sox2-positive cells express S100beta (Fig. 8 A-D; arrowheads). To further discriminate astrocytes from Type 1 NSCs, we examined the expression of GFAP and VEGF in BrdU-positive proliferating cells (Fig. 8 E-H), as Type 1 NSCs slowly proliferate, while mature astrocytes in the SGZ seldom do (Garcia et al., 2004). Only about 20% of these cycling cells are GFAP+/VEGF+ (Fig. 8I). We found the phenotype of proliferating cells in the young and aged SGZ did not differ, aside from there being fewer proliferating cells in the aged SGZ. As a result, phenotypic quantitation was performed in young subjects. Sox2 is also expressed in cells that exit their NSC stage, rapidly proliferate, and commit to a neuronal fate. Therefore, we analyzed VEGF expression in these cells by determining the frequency of VEGF, Sox2, and BrdU coexpression (Fig. 9 A-D) and found that only 20% of all proliferating cells are VEGF-positive (Fig. 9 E-H, and M), while the majority of Sox2-positive cells lack VEGF expression (Fig. 9 I-L and M).
Figure 8. VEGF is expressed by both mature astrocytes and NSCs.
The mature astrocytic marker S100beta (green, A, D) colocalizes with both Sox2 (B, red) and VEGF (C, blue), indicated by arrows (A-D). However, not all Sox2-positive/VEGF-positive cells are S100beta-positive (arrowheads, A-D), indicating a population of (S100beta-negative) Type-1 NSCs expressing VEGF. To further establish this relationship, proliferating NSCs are identified by their incorporation of BrdU (F) and expression of GFAP (E). Arrowheads in panels E-H indicate that 80% of proliferating (BrdU-positive) cells express neither VEGF (G, H) nor GFAP (H). Arrows (E-H) point to an exclusive co-localization of VEGF with GFAP, indicating that about 20% of proliferating cells in the SGZ are Type I NSCs (I) and not mature astrocytes, as the latter rarely proliferate under baseline conditions. Images and quantification from young dentate gyrus. Scale bar for panels A-D = 100 μm; panels E-H = 20 μm
Figure 9. VEGF is not expressed by transit amplifying neuroblast progenitor cells.
Frequency of colocalization of VEGF (A, green) in proliferating BrdU-positive cells (B, red) that also express Sox2 (C, blue) in the young SGZ. Insets in panel D illustrate regions shown in three-dimensional orthogonal analysis in panels E-H (inset I) and panels I-L (inset II). Arrows represent cells that are GFAP, BrdU, and Sox2-positive. Arrowheads represent cells that are BrdU and Sox2- positive, but GFAP-negative. Asterisks represents cells that are Sox2-positive but lack GFAP and BrdU coexpression. Quantification of proliferating cells in the SGZ (BrdU-positive, B, F, J, M) revealed that approximately 80% are Sox2-positive (C, M). Analysis of this BrdU-positive/Sox2-positive population revealed that 20% of these cells express VEGF (blue, arrows in orthogonal views, H and M). The remaining BrdU-positive/Sox2-positive cells lack VEGF-positive processes (arrowheads in orthogonal views, E-L and M). Scale bar for panels A-D = 50 μm; panels E-H = 25 μm
VEGF and Flk-1 are coexpressed in Type I NSCs prior to neuroblast production
To determine if the rare Type 1 NSCs in the DG also express Flk-1, we histologically analyzed the young DG for the coexpression of Sox2-positive/GFAP-positive cells. We found the majority of Sox2-positive/GFAP-positive cells were Flk-1-negative (Fig. 10 A-D), with coexpression of all three markers confined to a small population (Fig. 10 E-H).
Figure 10. Flk-1 is expressed by NSCs but not mature astrocytes.
Flk-1 is expressed by neuroblasts and mature neurons (Fig. 4), so we asked if Flk-1 could also be expressed by Type I NSCs in the young SGZ. Flk-1 (A, green) does not colocalize with the large fraction of Sox2-positive cells (B, red) that express GFAP (C, blue) found within the hilus and around the SGZ (arrowheads, A-D). However, colocalization with Flk-1 is observed in a small population of Sox2-positive/GFAP-positive cells (asterisk, E-H). Scale bar for panels A-D = 50 μm; panels E-H = 20 μm
Discussion
Astrocytes are crucial for the proper maintenance of the central nervous system milieu and the modulation of neuronal activity, and their altered state may influence neuronal function. Here we analyzed age-related alterations in the morphology and reactivity of astrocytes and their expression of growth factors and receptors. The study was conducted in normal aged brain to establish changes associated with the aging process itself, and to provide a baseline for interpreting the effects of injury or disease. We analyzed the hippocampal formation, including the entorhinal cortex, primarily to understand alterations in the circuitry underlying learning and memory, and assessed both neurogenic and non-neurogenic regions of the adult brain. We examined astrocytic VEGF and FGF-2 and the expression of their receptors (Flk-1, FGFR1 and FGFR2) and found age-related alterations of cellular phenotype and/or gene expression for both growth factors and all receptors. Preventing individual VEGF or FGF-2 signaling leads to a decline in cognition (Zhao et al., 2007; Pati et al., 2009), suggesting their involvement in memory consolidation and possible contribution to the age-related decline in cognition that parallels reduced VEGF and FGF-2 protein (Shetty et al., 2005). Our data is consistent with previous results describing the expression of VEGF and FGF-2 by GFAP-positive cells (Segi-Nishida et al., 2008) and Flk-1 by DCX-positive and NeuN-positive neurons (Palmer et al., 2000). Here, we demonstrate coexpression of VEGF and Flk-1 in Type 1 NSCs within both young and aged SGZ, and find that VEGF expression ceases upon commitment to a neuronal lineage. In contrast, Flk-1 expression continues throughout neuronal differentiation, but not gliogenesis. Thus, endogenous Type I NSCs are unique within the neurogenic niche in their cellular coexpression of both VEGF and its receptor, Flk-1 in agreement with their cultured phenotype (Xiao et al., 2007). The continuous presence of Flk-1 in cells transitioning through the various stages of neurogenesis indicates that a wide population of immature neuroblasts may be influenced by VEGF signals presented by neighboring astrocytes.
Functional diversity of GFAP-positive cell populations in the aged brain
Astrocytes are readily identified by their stellar morphology and the expression of a standard set of markers, most notably, the intermediate filament GFAP. Phenotypic classification by GFAP expression alone is limiting as this groups astrocytes as a homogenous population despite the presence of region-specific morphology and the presence of alternative markers (Kimelberg, 2004; Emsley and Macklis, 2006; Sofroniew, 2009; Sofroniew and Vinters, 2010). While physiological differences between these populations remain unclear, astrocytes from different regions show variable efficacy in supporting neuronal differentiation (Song et al., 2002). We identified region-specific differences in growth factor expression by astrocytes within the aged adult brain. Age-related changes included the emergence of a phenotype in both aged DG and ECX where some GFAP-positive cells lack detectable FGF-2. This is in contrast to the young brain. where all GFAP-positive astrocytes colocalize FGF-2. These changes coincide with decreased availability of astrocyte-derived growth factor proteins, including FGF-2 and VEGF protein in the aged brain (Shetty et al., 2005). Thus, aging produces additional phenotypic variation in astrocyte populations beyond that noted for young brain (Emsley and Macklis, 2006). In agreement with previous reports, we find that the neurogenic regions in the adult mammalian brain supporting NSCs and the process of neurogenesis, as well as the non-neurogenic cerebellum, have more robust VEGF immunoreactivity than other regions of the young adult brain (Wittko et al., 2009). The age-related decline in hippocampal neurogenesis may be particularly sensitive to VEGF downregulation, as both the production and availability of VEGF protein by GFAP-positive cells declines with aging.
Reactivity of astrocytes as an indicator of activation
GFAP defines morphology and indicates the state of cell reactivity, as its expression increases and cell morphology is altered upon stimulation (Eng et al., 2000). We observe age-related mild astrocytic hypertrophy, consistent with induction of mild inflammation, such as with LPS administration, and defined by the lack of interaction with neighboring glia. This is unlike severe astrocytic reactivity observed when glial interaction results in scar formation (Sofroniew, 2009; Sofroniew and Vinters, 2010). While we find no significant change in GFAP expression despite evident astrocytic hypertrophy in the DG, GFAP expression did increase in the ECX. This may be due to induction of GFAP expression in cells that normally do not express it (Kimelberg, 2004; Emsley and Macklis, 2006). GFAP expression may be triggered by age-related signals, such as increased cytokine levels and other microglial-derived factors (Kaul and Lipton, 2006; Cevenini et al., 2010; von Bernhardi R, 2010). Induction of GFAP expression may account for conflicting data regarding the number of GFAP-positive astrocytes within the aged hippocampus (Long et al., 1998; Mouton et al., 2002).
Our data indicate that mild stimulation of astrocytes during non-pathological aging results in a decline of astrocytic growth factor expression rather than the elevation of expression seen in the young brain following injury or ischemia. The aging brain environment may require a different role for astrocyte signaling. For example, astrocytes may decrease their expression of classical angiogenic growth factors and vascular leakage inducing factors, notably VEGF, to prevent further breakdown of the blood brain barrier as aging is accompanied by vascular dysfunction (Farrall and Wardlaw, 2009; Vasilevko et al., 2010). The age-related astrocytic hypertrophy observed did not mimic the astrogliotic response seen in the young brain following injury (Faulkner et al., 2004; Sofroniew, 2005; Voskuhl et al., 2009) raising the alternative possibility that aged astrocytes may be limited in their capacity to activate. Stimulation of the young brain to produce mild astrocytic reactivity devoid of glial scarring can result in subsequent reversion to normal, baseline levels of astrocytic activity (Sofroniew and Vinters, 2010). If this alternative interpretation is correct, reducing activation of aging astrocytes may be a potential therapeutic strategy to restore expression levels to that of the young brain.
Production and availability of astrocyte-derived growth factors may be differentially regulated with aging
We find age-related alteration in detection of immunoreactivity does not always correlate with changes in gene expression. Age-related changes in VEGF protein and gene expression levels are also observed outside the CNS. A decline in VEGF is observed in multiple systems, including the inner ear (Picciotti et al., 2004), muscle (Croley et al., 2005), intervertebral disc tissue (Ohba et al., 2009), and reproductive organs (Neves et al., 2006; Yeh et al., 2008). Discrepancies between measured gene expression (indicating production) and the qualitative assessment of cellular protein staining intensity (indicating availability) are not restricted to VEGF. Protein levels of FGF-2 are known to decrease in the aged dentate gyrus (Shetty et al., 2005), which could be a direct result of GFAP-positive cells not presenting FGF2 protein (Shetty et al., 2005). Interestingly, FGF2 gene expression results show no significant differences between young and aged DG and ECX. This discrepancy between gene expression and protein levels may be due to age-related changes in post-transcriptional processing: alternate splicing, posttranslational protein modifications, protein-protein interactions, protein turnover or degradation, and possibly protein trafficking, none of which are detected by methods looking at gene expression alone (Lindner and Demarez, 2009; Naidoo, 2009; Sinclair and Oberdoerffer, 2009).
Growth factor receptor expression is also modulated in the aging brain. The tyrosine kinase receptor Flk-1 is the major VEGF receptor through which VEGF transmits its mitogenic signaling during neurogenesis (Jin et al., 2002; Warner-Schmidt and Duman, 2007). Similar changes are observed in the gene expression of the VEGF receptor Flk-1, though this is not evident in all systems (Picciotti et al., 2004; Croley et al., 2005; Yeh et al., 2008). FGF-2 signals through FGFR1 and FGFR2. FGFR1 is expressed mainly by neurons and NSCs (Gomez-Pinilla et al., 1992; Frinchi et al., 2008) and appears to be important for the effects of FGF-2 signaling in neurogenesis (Zhao et al., 2007). FGFR2 is detected only within GFAP-positive cells in the adult brain, including GFAP-expressing NSCs (Reuss et al., 2003; Chadashvili and Peterson, 2006; Frinchi et al., 2008). Type 1 NSCs coexpress both FGF-2 and its receptors, suggesting a possible autocrine/paracrine mechanism through which the growth factor may regulate normal function (Reuss et al., 2003; Chadashvili and Peterson, 2006). Furthermore, the concurrent expression of FGF-2 and VEGF by both astrocytes and NSCs indicate a further level of interaction between these growth factors as observed by in vitro studies and most notably, outside the CNS, where FGF-2 and VEGF act synergistically to induce angiogenesis (Asahara et al., 1995; Stavri et al., 1995; Seghezzi et al., 1998; Hata et al., 1999; Liu et al., 2000; Xiao et al., 2007). Our data do not address how aging affects the function of VEGF and FGF-2 receptors. Although we show that Flk-1 expression is maintained in the aged DG, it is possible that functional properties of these receptors are altered. For example, protein expression of Flk-1 in the aging ovary remains stable, but proper function is diminished due to a decline in Flk-1 phosphorylation (Yeh et al., 2008).
Growth factor signaling and responsive cells in the aged neurogenic niche
Our data show that GFAP-positive cells in the DG, including mature astrocytes and NSCs, produce VEGF while its receptor Flk-1 is expressed by both Type I NSCs and their progeny (neural progenitor cells) as they commit to a neuronal lineage. This expression pattern is in contrast to development of the vascular and nervous systems, when VEGF is expressed by neuronal and endothelial cells while Flk-1 is reciprocally expressed by glial cells (Yang et al., 2003). Glial Flk-1 expression in the postnatal brain can be induced following injury or ischemia when VEGF expression is elevated (Islamov et al., 2004; Skold et al., 2005; Wang et al., 2005a; Wang et al., 2005b; Choi et al., 2007; Wittko et al., 2009), suggesting this response may be recapitulating development for repair as VEGF signaling via Flk-1 promotes neuroprotection (Ding et al., 2005; Kilic et al., 2006; Kim et al., 2009; Ma et al., 2009). However, under normal conditions, the developmental “switch” of VEGF and Flk-1 expression in the adult prevails and is maintained in the aged brain.
VEGF signaling promotes neurogenesis, as demonstrated following electroconvulsive shock (Segi-Nishida et al., 2008), exercise (Fabel et al., 2003), anti-depressant treatment (Warner-Schmidt and Duman, 2007, 2008), and exposure to an enriched environment (During and Cao, 2006). However, little is know about the role of VEGF and Flk-1 under basal conditions in the young and aged brain. We asked whether age-related changes in VEGF and Flk-1 expression may contribute to the decline of neurogenesis, and examined the cellular expression patterns of VEGF and Flk-1 in the neurogenic niche to address this. In the absence of a single phenotypic marker, different marker combinations are necessary to define each stage of lineage progression.
We describe the expression of VEGF by GFAP-positive cells in the adult SGZ, in agreement with previous reports (Segi-Nishida et al., 2008). Furthermore, we show that VEGF-expressing cells in the adult DG are both mature astrocytes (GFAP-positive/S100beta-positive) and quiescent Type 1 NSCs (GFAP-positive/Sox2-positive and GFAP-positive/BrdU-positive). In addition, we show that VEGF expression ceases once Type 1 cells give rise to rapidly amplifying Type 2a cells, identified by their expression of Sox2 (Steiner et al., 2006), their rapid cycling properties, and their ability to give rise to neurons (Seri et al., 2001; Kempermann et al., 2004), and remains off through neuronal maturation. We also confirm the expression of Flk-1 by Type 1 cells, Type 2a cells, immature (DCX-positive) and mature (NeuN-positive) neurons (Palmer et al., 2000; Segi-Nishida et al., 2008). We report here that Flk-1 expression continues in Type 2b and Type 3 cells (intermediate progenitor cells), suggesting that VEGF may act on neuroblasts at any stage of neurogenesis.
There is now substantial evidence that both VEGF and FGF-2 signaling stimulates proliferation in the dentate gyrus following certain injury conditions, experimental stimulation, or delivery of exogenous ligand. However, FGF-2 signaling is unnecessary for maintaining baseline levels of neurogenic proliferation in the young DG (Yoshimura et al., 2001; Yoshimura et al., 2003). Though our data do not address the role of VEGF signaling on neurogenic proliferation under basal conditions, the coexpression of Flk-1 by slow-cycling NSCs suggests a possible autocrine/paracrine mechanism of cell maintenance. Furthermore, the expression of Flk-1 by rapidly amplifying progenitor cells indicates that the well-documented mitotic effects of VEGF in the adult brain may modulate cell cycling of this population under basal conditions (Jin et al., 2002; Warner-Schmidt and Duman, 2007). Our data show a decline in VEGF in parallel with the age-related decline in neurogenesis, suggesting sustained VEGF signaling may be necessary to maintain neurogenic proliferation.
Conclusion
We conclude that an age-related, mild reactivity of astrocytes leads to alterations in gene and protein expression of VEGF and FGF-2 and contributes to increased diversity in astrocyte phenotype with aging. The reduced availability of these factors may contribute to both a decline in cognitive function with age and diminished ability of the CNS to respond to injury. One population of GFAP-positive cells, the Type 1 NSCs, may have the ability to regulate their own activity via expression of VEGF, as these cells coexpress both VEGF and its receptor Flk-1. This coexpression within NSCs is maintained into aging. These data emphasize that the aged brain environment is substantially different from its younger counterpart, and suggest there may be a resetting of environmental homeostasis with aging. This is an important consideration for modeling therapies to the aging brain, particularly in regard to neurodegenerative diseases. For example, many animal studies investigating Alzheimer's disease study a younger brain environment, which may not faithfully replicate conditions within the aging brain. We propose that the mild stimulation of astrocytic reactivity in the aging brain may contribute to impaired brain function by reducing both proper neuronal function and growth factors needed to support neurogenesis in the neurogenic niche.
Experimental Procedures
Experimental Animals
Young adult female Fischer 344 rats (8-10 weeks old, n=17) were obtained from Harlan Sprague-Dawley, Inc. (Indianapolis, IN). Aged (22 months old, n=17) female Fischer rats were acquired from the National Institute of Aging Colony at Harlan Sprague-Dawley. All animals were housed on site in an environmentally controlled room, maintained at 12-hour light/dark cycle, and fed with commercial rat chow and water ad libitum. All procedures were approved by an internal Institutional Animal Care and Use Committee (IACUC) and were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Tissue Collection
Molecular analysis
Eleven young and eleven aged adult rats were anaesthetized with an overdose of a 0.9% saline cocktail containing ketamine (5.6 mg/kg), acepromazine (0.75 mg/kg), and xylazine (4.0 mg/kg) and decapitated according to IACUC approved protocols. Whole brains were quickly removed and placed in cold, sterile phosphate buffer saline (PBS, pH 7.4) to reduce RNA degradation. The tissue was quickly divided into 1mm thick sagittal sections using a brain matrix and single punches (2 mm in diameter, Harris Uni Core puncher; Electron Microscopy Sciences, Hatfield, PA) were collected from each section per region (dentate gyrus and entorhinal cortex) and per hemisphere to standardize the amount of tissue used. Two punches from a single region with a combined weight of roughly 4-5 mg were placed in 1.5 mL tubes and immediately flash-frozen in liquid nitrogen and stored at -80°C until further molecular analysis.
For immunohistochemical analysis
Six young and six aged female rats were terminally anaesthetized as described above and transcardially perfused first with 0.9% saline buffer followed by 4% paraformadehyde/0.1% glutaraldehyde. Whole brains were removed and fixed overnight in the paraformadehyde/glutaraldehyde solution and equilibrated in 30% sucrose for at least 72 hrs at 4°C. Each hemisphere was sectioned sagittally with a sliding microtome to obtain 50 μm-thick sections and stored at -20°C in a cryoprotectant solution.
Molecular Analysis
Young and aged dentate gyrus and entorhinal cortex samples were analyzed by quantitative polymerase chain reaction (qPCR) for amplification and quantification of the following genes: GFAP, VEGF-A, Flk-1, FGF-2, FGFR-1, FGFR-2 and GAPDH (Table 1 for forward and reverse primers).
Table 1.
SYBRgreen primers designed with Beacon Designer software for qPCR runs using the BioRad iCycler iQ
| Primer Name | Oligonucleotide Sequence |
|---|---|
| GFAP | 5’-GAGGCTCCATCCCACAACCAC-3’ |
| 3’-CGTGTCAGTTCCAACTTCAAACAGG-5’ | |
| VEGFA | 5’-CCGTCCTGTGTGCCCCTAATG-3’ |
| 3’-GTGCTGGCTTTGGTGAGGTTTG-5’ | |
| Flk-1 | 5’-CCGTCCTCAAAGCATCAGCATAAG-3’ |
| 3’-TCCTTGGTCACTCTTGGTCACAC-5’ | |
| FGF-2 | 5’-TGAACGCCTGGAGTCCAATAA-3’ |
| 3’-CGTTTCAGTGCCACATACCA-5’ | |
| FGFR1 | 5’-TGGATGGCACCTGAGGCATTG-3’ |
| 3’-AGCACCCCAAAAGACCACACAT-5’ | |
| FGFR2 | 5’-CTTCAGGGGACGATTCTGTGTTTTC-3’ |
| 3’-TGAGGCAGGCAGGGGTCATAA-5’ | |
| GAPDH | 5’-CTACAGCAACAGGGTGGTGGAC-3’ |
| 3’-GGGATGGAATTGTGAGGGAGATGC-5’ |
Gene expression
Total RNA was extracted from the flash-frozen tissue punches using the MELT™ Total Nucleic Acid Isolation System (Ambion; Austin, TX) for samples smaller than 10 mg following manufacturer's instructions. The final product was quantified and the quality checked using spectrophotometry. The isolated RNA was reverse transcribed to produce double stranded cDNA via reverse transcription polymerase chain reaction (RT-PCR) using the Promega Im-Prom II Reverse Transcription system kit as per manufacturer's instructions (Promega; Madison, WI). The cDNA end-product was quantified using the Quant-iT PicoGreen dsDNA kit (Invitrogen; Carlsbad, CA) as per manufacturer's instructions and measured using a Tecan Microplate Reader to standardize the amount of cDNA to be used during quantitative polymerase chain reaction (qPCR). Finally, qPCR was used to quantify expression of specific genes using primers optimized for use with SYBRgreen (Table 1) and the iCycler iQ (BioRad; Hercules, CA). Primers were designed in house with the Beacon Designer software and synthesized by Operon Biotechnologies. In experiments comparing GAPDH and beta-Actin, we determined GAPDH to be the best choice for a housekeeping gene based on lower variability between samples. Therefore, all qPCR results were standardized to GAPDH. Samples per run consisted of both young and aged tissue from a single region (either dentate gyrus or entorhinal cortex), as well as appropriate controls from each step: RNA isolation (sample underwent entire procedure but lacked tissue), RT-PCR (RNA sample underwent full procedure without reverse transcriptase), and qPCR (no cDNA template present during run).
Data Analysis
cDNA expression was quantified by comparison of samples to a standard curve from a 1:5 dilution series of standard cDNA derived from rat brain total RNA (Ambion; Austin, TX) included with each run. All standards, controls and samples were plated in triplicate and an outlier from the trio was eliminated if the other two replicates were the same or similar; if the average was in the middle of all three triplicates, none of them was eliminated.
Statistical Analysis
Quantitative data from qPCR was analyzed in GraphPad Prism version 4. qPCR experiments were designed to compare the expression of specific genes between only two different age groups within a single region. Two-tailed unpaired Student t-test was therefore performed to determine if there was a significant difference between the two groups where α was set at 0.05 and population variance was expressed as standard error of the mean (±SEM).
Tissue Processing
Immunofluorescence Staining
Net inserts (Costar) were used to transfer free-floating sections between solutions and minimize handling. To remove cryoprotectant, all sections were rinsed in Tris-buffered saline (TBS) prior to a 3-hour block in 5% donkey serum/0.25% TritonX-100/TBS solution (TBS++). When visualization of rat anti-BrdU was necessary, sections underwent an additional pre-treatment, where they were incubated in 2 N HCl for 10 minutes followed by 0.1 M borate buffer and rinses in TBS prior to the TBS++ block. For multiple immunostaining, sections were incubated for 72 hrs (at 4°C on a shaker table) with the appropriate cocktail of primary antibodies raised in different species to prevent cross-reactivity and diluted in TBS with 0.25% TritonX-100 (TBS+): guinea pig anti-GFAP (1:2500; Advanced Immunochemicals, Long Beach, CA), rabbit anti-VEGF (1:1000; Santa Cruz Biotechnology, Inc., Santa Cruz, CA), rabbit anti-Flk-1 (1:200; Santa Cruz Biotechnology, Inc., Santa Cruz, CA), mouse anti-RECA (1:1000; Accurate, Westbury, NY), mouse anti-NeuN (1:5000; Millipore, Billerica, MA), mouse anti-doublecortin (DCX; Advanced Immunochemicals, Long Beach, CA), rabbit anti-FGF-2 (1:2500; Millipore, Billerica, MA), rat anti-BrdU (1:500; Accurate, Westbury, NY), goat anti-Sox2 (1:500; Santa Cruz Biotechnology, Inc., Santa Cruz, CA), and mouse anti-S100B (1:2500; Abcam, Cambridge, MA). Following two, 1-hour blocks with TBS++, sections were incubated for 1hr with biotinylated donkey anti-rabbit or anti-rat secondary antibodies (1:500; Jackson Immunoresearch, West Grove, PA) to enhance VEGF, Flk-1, and BrdU signal. After two 15 minute rinses with TBS+, sections were incubated for 2 hrs with Alexa Fluor 488- or Cy3-conjugated streptavidin (1:500; Molecular Probes) for biotin visualization, mixed with secondary antibodies (raised in donkey) bound to the fluorophores Alexa488, Cy3 or Cy5 (1:500; Jackson Immunoresearch, West Grove, PA) diluted in TBS+ (1:500) for 2 hours at room temperature. Following two 15 minute rinses with TBS, all sections were mounted onto slides, coverslipped with a glycerol-polyvinyl alcohol plus DABCO (1,4 diazabicyclo [2.2.2] octane; Sigma D2522, Sigma-Aldrich, Inc.) solution to self-seal and prevent as fading, and stored at 4°C in the dark. Negative controls lacking primary antibodies were run along samples.
Qualitative Histological Studies and Determination of Cell Phenotype Distribution
Confocal images were acquired using full signal distribution with sequential channel acquisition of each laser excitation on an Olympus Fluoview 500 Scanning Laser Confocal Microscope (Olympus America, Inc.). Three-dimensional image stacks of at least 25 μm height were collected at 1 μm intervals and interactively viewed using orthogonal projection software (Olympus America, Inc.) to ensure colocalization of signal was identified within a cell and not mistaken for superimposition of spatially distinct cells. For qualitative studies describing VEGF expression in young and aged samples (n = 6 per age group), about 13 sections were analyzed at a 1:6 interval. For studies identifying the phenotype of BrdU-positive cells, at least 150 of these cells were identified per animal (n = 5) and carefully visualized in three-dimensional stacks to determine if they colocalized with the markers of interest. Results are reported as the mean percentage of observed colocalization. Images were composed into multipanel figures using Adobe Photoshop with minimal adjustment to balance signal distribution levels and text annotations as the only alterations.
Acknowledgements
This work was supported by NIH grants AG20047 and AG22555 to D.A.P. We thank Emily Reisenbigler for primer design and technical support of the gene expression studies.
Bibliography
- Alvarez-Buylla A, Lim DA. For the long run: maintaining germinal niches in the adult brain. Neuron. 2004;41:683–686. doi: 10.1016/s0896-6273(04)00111-4. [DOI] [PubMed] [Google Scholar]
- Alzheimer C, Werner S. Fibroblast growth factors and neuroprotection. Adv Exp Med Biol. 2002;513:335–351. doi: 10.1007/978-1-4615-0123-7_12. [DOI] [PubMed] [Google Scholar]
- Asahara T, Bauters C, Zheng LP, Takeshita S, Bunting S, Ferrara N, Symes JF, Isner JM. Synergistic effect of vascular endothelial growth factor and basic fibroblast growth factor on angiogenesis in vivo. Circulation. 1995;92:II365–371. doi: 10.1161/01.cir.92.9.365. [DOI] [PubMed] [Google Scholar]
- Ben Menachem-Zidon O, Avital A, Ben-Menahem Y, Goshen I, Kreisel T, Shmueli EM, Segal M, Ben Hur T, Yirmiya R. Astrocytes support hippocampal-dependent memory and long-term potentiation via interleukin-1 signaling. Brain Behav Immun. 2010 doi: 10.1016/j.bbi.2010.11.007. [DOI] [PubMed] [Google Scholar]
- Cansino S. Episodic memory decay along the adult lifespan: a review of behavioral and neurophysiological evidence. Int J Psychophysiol. 2009;71:64–69. doi: 10.1016/j.ijpsycho.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Cevenini E, Caruso C, Candore G, Capri M, Nuzzo D, Duro G, Rizzo C, Colonna-Romano G, Lio D, Di Carlo D, Palmas MG, Scurti M, Pini E, Franceschi C, Vasto S. Age-related inflammation: the contribution of different organs, tissues and systems. How to face it for therapeutic approaches. Curr Pharm Des. 2010;16:609–618. doi: 10.2174/138161210790883840. [DOI] [PubMed] [Google Scholar]
- Chadashvili T, Peterson DA. Cytoarchitecture of fibroblast growth factor receptor 2 (FGFR-2) immunoreactivity in astrocytes of neurogenic and non-neurogenic regions of the young adult and aged rat brain. J Comp Neurol. 2006;498:1–15. doi: 10.1002/cne.21009. [DOI] [PubMed] [Google Scholar]
- Cheng N, Brantley DM, Chen J. The ephrins and Eph receptors in angiogenesis. Cytokine Growth Factor Rev. 2002;13:75–85. doi: 10.1016/s1359-6101(01)00031-4. [DOI] [PubMed] [Google Scholar]
- Choi JS, Kim HY, Cha JH, Choi JY, Park SI, Jeong CH, Jeun SS, Lee MY. Upregulation of vascular endothelial growth factor receptors Flt-1 and Flk-1 following acute spinal cord contusion in rats. J Histochem Cytochem. 2007;55:821–830. doi: 10.1369/jhc.6A7139.2007. [DOI] [PubMed] [Google Scholar]
- Costantini C, Lorenzetto E, Cellini B, Buffelli M, Rossi F, Della-Bianca V. Astrocytes Regulate the Expression of Insulin-Like Growth Factor 1 Receptor (IGF1-R) in Primary Cortical Neurons During In Vitro Senescence. J Mol Neurosci. 2010;40:342–352. doi: 10.1007/s12031-009-9305-5. [DOI] [PubMed] [Google Scholar]
- Croley AN, Zwetsloot KA, Westerkamp LM, Ryan NA, Pendergast AM, Hickner RC, Pofahl WE, Gavin TP. Lower capillarization, VEGF protein, and VEGF mRNA response to acute exercise in the vastus lateralis muscle of aged vs. young women. J Appl Physiol. 2005;99:1872–1879. doi: 10.1152/japplphysiol.00498.2005. [DOI] [PubMed] [Google Scholar]
- Ding XM, Mao BY, Jiang S, Li SF, Deng YL. Neuroprotective effect of exogenous vascular endothelial growth factor on rat spinal cord neurons in vitro hypoxia. Chin Med J (Engl) 2005;118:1644–1650. [PubMed] [Google Scholar]
- Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- Drogemuller K, Helmuth U, Brunn A, Sakowicz-Burkiewicz M, Gutmann DH, Mueller W, Deckert M, Schluter D. Astrocyte gp130 expression is critical for the control of Toxoplasma encephalitis. J Immunol. 2008;181:2683–2693. doi: 10.4049/jimmunol.181.4.2683. [DOI] [PubMed] [Google Scholar]
- During MJ, Cao L. VEGF, a mediator of the effect of experience on hippocampal neurogenesis. Curr Alzheimer Res. 2006;3:29–33. doi: 10.2174/156720506775697133. [DOI] [PubMed] [Google Scholar]
- Emsley J, Macklis J. Astroglial heterogeneity closely reflects the neuronal-defined anatomy of the adult murine CNS. Neuron Glia Biol. 2006;2:175–186. doi: 10.1017/S1740925X06000202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng LF, Ghirnikar RS, Lee YL. Glial fibrillary acidic protein: GFAP-thirty-one years (1969-2000). Neurochem Res. 2000;25:1439–1451. doi: 10.1023/a:1007677003387. [DOI] [PubMed] [Google Scholar]
- Fabel K, Tam B, Kaufer D, Baiker A, Simmons N, Kuo CJ, Palmer TD. VEGF is necessary for exercise-induced adult hippocampal neurogenesis. Eur J Neurosci. 2003;18:2803–2812. doi: 10.1111/j.1460-9568.2003.03041.x. [DOI] [PubMed] [Google Scholar]
- Faissner A, Pyka M, Geissler M, Sobik T, Frischknecht R, Gundelfinger ED, Seidenbecher C. Contributions of astrocytes to synapse formation and maturation - Potential functions of the perisynaptic extracellular matrix. Brain Res Rev. 2010;63:26–38. doi: 10.1016/j.brainresrev.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Farrall AJ, Wardlaw JM. Blood-brain barrier: ageing and microvascular disease--systematic review and meta-analysis. Neurobiol Aging. 2009;30:337–352. doi: 10.1016/j.neurobiolaging.2007.07.015. [DOI] [PubMed] [Google Scholar]
- Faulkner JR, Herrmann JE, Woo MJ, Tansey KE, Doan NB, Sofroniew MV. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J Neurosci. 2004;24:2143–2155. doi: 10.1523/JNEUROSCI.3547-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- Filosa A, Paixão S, Honsek SD, Carmona MA, Becker L, Feddersen B, Gaitanos L, Rudhard Y, Schoepfer R, Klopstock T, Kullander K, Rose CR, Pasquale EB, Klein R. Neuronglia communication via EphA4/ephrin-A3 modulates LTP through glial glutamate transport. Nat Neurosci. 2009;12:1285–1292. doi: 10.1038/nn.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frinchi M, Bonomo A, Trovato-Salinaro A, Condorelli DF, Fuxe K, Spampinato MG, Mudo G. Fibroblast growth factor-2 and its receptor expression in proliferating precursor cells of the subventricular zone in the adult rat brain. Neurosci Lett. 2008;447:20–25. doi: 10.1016/j.neulet.2008.09.059. [DOI] [PubMed] [Google Scholar]
- Garcia AD, Doan NB, Imura T, Bush TG, Sofroniew MV. GFAP-expressing progenitors are the principal source of constitutive neurogenesis in adult mouse forebrain. Nat Neurosci. 2004;7:1233–1241. doi: 10.1038/nn1340. [DOI] [PubMed] [Google Scholar]
- Gerber HP, Hillan KJ, Ryan AM, Kowalski J, Keller GA, Rangell L, Wright BD, Radtke F, Aguet M, Ferrara N. VEGF is required for growth and survival in neonatal mice. Development. 1999;126:1149–1159. doi: 10.1242/dev.126.6.1149. [DOI] [PubMed] [Google Scholar]
- Giaume C. Astroglial Wiring is Adding Complexity to Neuroglial Networking. Front Neuroenergetics. 2010:2. doi: 10.3389/fnene.2010.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaume C, Koulakoff A, Roux L, Holcman D, Rouach N. Astroglial networks: a step further in neuroglial and gliovascular interactions. Nat Rev Neurosci. 2010;11:87–99. doi: 10.1038/nrn2757. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F, Lee JW, Cotman CW. Basic FGF in adult rat brain: cellular distribution and response to entorhinal lesion and fimbria-fornix transection. J Neurosci. 1992;12:345–355. doi: 10.1523/JNEUROSCI.12-01-00345.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez AM, Berry M, Maher PA, Logan A, Baird A. A comprehensive analysis of the distribution of FGF-2 and FGFR1 in the rat brain. Brain Res. 1995;701:201–226. doi: 10.1016/0006-8993(95)01002-x. [DOI] [PubMed] [Google Scholar]
- Gooney M, Messaoudi E, Maher FO, Bramham CR, Lynch MA. BDNF-induced LTP in dentate gyrus is impaired with age: analysis of changes in cell signaling events. Neurobiology of Aging. 2004;25:1323–1331. doi: 10.1016/j.neurobiolaging.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Halassa MM, Haydon PG. Integrated brain circuits: astrocytic networks modulate neuronal activity and behavior. Annu Rev Physiol. 2010;72:335–355. doi: 10.1146/annurev-physiol-021909-135843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata Y, Rook SL, Aiello LP. Basic fibroblast growth factor induces expression of VEGF receptor KDR through a protein kinase C and p44/p42 mitogen-activated protein kinase-dependent pathway. Diabetes. 1999;48:1145–1155. doi: 10.2337/diabetes.48.5.1145. [DOI] [PubMed] [Google Scholar]
- Islamov RR, Chintalgattu V, Pak ES, Katwa LC, Murashov AK. Induction of VEGF and its Flt-1 receptor after sciatic nerve crush injury. Neuroreport. 2004;15:2117–2121. doi: 10.1097/00001756-200409150-00024. [DOI] [PubMed] [Google Scholar]
- Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci U S A. 2002;99:11946–11950. doi: 10.1073/pnas.182296499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul M, Lipton SA. Mechanisms of neuroimmunity and neurodegeneration associated with HIV-1 infection and AIDS. J Neuroimmune Pharmacol. 2006;1:138–151. doi: 10.1007/s11481-006-9011-9. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Jessberger S, Steiner B, Kronenberg G. Milestones of neuronal development in the adult hippocampus. Trends Neurosci. 2004;27:447–452. doi: 10.1016/j.tins.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Kilic E, Kilic U, Wang Y, Bassetti CL, Marti HH, Hermann DM. The phosphatidylinositol-3 kinase/Akt pathway mediates VEGF's neuroprotective activity and induces blood brain barrier permeability after focal cerebral ischemia. FASEB J. 2006;20:1185–1187. doi: 10.1096/fj.05-4829fje. [DOI] [PubMed] [Google Scholar]
- Kim HM, Hwang DH, Lee JE, Kim SU, Kim BG. Ex vivo VEGF delivery by neural stem cells enhances proliferation of glial progenitors, angiogenesis, and tissue sparing after spinal cord injury. PLoS One. 2009;4:e4987. doi: 10.1371/journal.pone.0004987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimelberg HK. The problem of astrocyte identity. Neurochem Int. 2004;45:191–202. doi: 10.1016/j.neuint.2003.08.015. [DOI] [PubMed] [Google Scholar]
- Lalo U, Andrew J, Palygin O, Pankratov Y. Ca2+-dependent modulation of GABAA and NMDA receptors by extracellular ATP: implication for function of tripartite synapse. Biochem Soc Trans. 2009;37:1407–1411. doi: 10.1042/BST0371407. [DOI] [PubMed] [Google Scholar]
- Lindner AB, Demarez A. Protein aggregation as a paradigm of aging. Biochim Biophys Acta. 2009;1790:980–996. doi: 10.1016/j.bbagen.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Liu X, Zheng SX, Zhou LJ, Zhu XZ. Basic fibroblast growth factor up-regulates the expression of vascular endothelial growth factor in primary cultured rat astrocytes. Acta Pharmacol Sin. 2000;21:19–22. [PubMed] [Google Scholar]
- Long JM, Kalehua AN, Muth NJ, Calhoun ME, Jucker M, Hengemihle JM, Ingram DK, Mouton PR. Stereological analysis of astrocyte and microglia in aging mouse hippocampus. Neurobiol Aging. 1998;19:497–503. doi: 10.1016/s0197-4580(98)00088-8. [DOI] [PubMed] [Google Scholar]
- Ma YY, Li KY, Wang JJ, Huang YL, Huang Y, Sun FY. Vascular endothelial growth factor acutely reduces calcium influx via inhibition of the Ca2+ channels in rat hippocampal neurons. J Neurosci Res. 2009;87:393–402. doi: 10.1002/jnr.21859. [DOI] [PubMed] [Google Scholar]
- Maffei A, Turrigiano G. The age of plasticity: developmental regulation of synaptic plasticity in neocortical microcircuits. Prog Brain Res. 2008;169:211–223. doi: 10.1016/S0079-6123(07)00012-X. [DOI] [PubMed] [Google Scholar]
- Magnus T, Carmen J, Deleon J, Xue H, Pardo AC, Lepore AC, Mattson MP, Rao MS, Maragakis NJ. Adult glial precursor proliferation in mutant SOD1G93A mice. Glia. 2008;56:200–208. doi: 10.1002/glia.20604. [DOI] [PubMed] [Google Scholar]
- Maione P, Rossi A, Airoma G, Ferrara C, Castaldo V, Gridelli C. The role of targeted therapy in non-small cell lung cancer. Crit Rev Oncol Hematol. 2004;51:29–44. doi: 10.1016/j.critrevonc.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Mani N, Khaibullina A, Krum JM, Rosenstein JM. Astrocyte growth effects of vascular endothelial growth factor (VEGF) application to perinatal neocortical explants: receptor mediation and signal transduction pathways. Experimental Neurology. 2005;192:394–406. doi: 10.1016/j.expneurol.2004.12.022. [DOI] [PubMed] [Google Scholar]
- Mologni L, Sala E, Cazzaniga S, Rostagno R, Kuoni T, Puttini M, Bain J, Cleris L, Redaelli S, Riva B, Formelli F, Scapozza L, Gambacorti-Passerini C. Inhibition of RET tyrosine kinase by SU5416. J Mol Endocrinol. 2006;37:199–212. doi: 10.1677/jme.1.01999. [DOI] [PubMed] [Google Scholar]
- Mouton PR, Long JM, Lei DL, Howard V, Jucker M, Calhoun ME, Ingram DK. Age and gender effects on microglia and astrocyte numbers in brains of mice. Brain Res. 2002;956:30–35. doi: 10.1016/s0006-8993(02)03475-3. [DOI] [PubMed] [Google Scholar]
- Murphy C, Nordin S, Acosta L. Odor learning, recall, and recognition memory in young and elderly adults. Neuropsychology. 1997;11:126–137. doi: 10.1037//0894-4105.11.1.126. [DOI] [PubMed] [Google Scholar]
- Naidoo N. ER and aging-Protein folding and the ER stress response. Ageing Res Rev. 2009;8:150–159. doi: 10.1016/j.arr.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Neves D, Santos J, Tomada N, Almeida H, Vendeira P. Aging and orchidectomy modulate expression of VEGF receptors (Flt-1 and Flk-1) on corpus cavernosum of the rat. Ann N Y Acad Sci. 2006;1067:164–172. doi: 10.1196/annals.1354.020. [DOI] [PubMed] [Google Scholar]
- Nicoletti JN, Shah SK, McCloskey DP, Goodman JH, Elkady A, Atassi H, Hylton D, Rudge JS, Scharfman HE, Croll SD. Vascular endothelial growth factor is up-regulated after status epilepticus and protects against seizure-induced neuronal loss in hippocampus. Neuroscience. 2008;151:232–241. doi: 10.1061/j.neuroscience.2007.09.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohba T, Haro H, Ando T, Wako M, Suenaga F, Aso Y, Koyama K, Hamada Y, Nakao A. TNF-alpha-induced NF-kappaB signaling reverses age-related declines in VEGF induction and angiogenic activity in intervertebral disc tissues. J Orthop Res. 2009;27:229–235. doi: 10.1002/jor.20727. [DOI] [PubMed] [Google Scholar]
- Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Pascual O, Casper KB, Kubera C, Zhang J, Revilla-Sanchez R, Sul JY, Takano H, Moss SJ, McCarthy K, Haydon PG. Astrocytic purinergic signaling coordinates synaptic networks. Science. 2005;310:113–116. doi: 10.1126/science.1116916. [DOI] [PubMed] [Google Scholar]
- Pati S, Orsi SA, Moore AN, Dash PK. Intra-hippocampal administration of the VEGF receptor blocker PTK787/ZK222584 impairs long-term memory. Brain Res. 2009;1256:85–91. doi: 10.1016/j.brainres.2008.11.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson DA, Lucidi-Phillipi CA, Murphy DP, Ray J, Gage FH. Fibroblast growth factor-2 protects entorhinal layer II glutamatergic neurons from axotomy-induced death. J Neurosci. 1996;16:886–898. doi: 10.1523/JNEUROSCI.16-03-00886.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotti P, Torsello A, Wolf FI, Paludetti G, Gaetani E, Pola R. Age-dependent modifications of expression level of VEGF and its receptors in the inner ear. Exp Gerontol. 2004;39:1253–1258. doi: 10.1016/j.exger.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Rai KS, Hattiangady B, Shetty AK. Enhanced production and dendritic growth of new dentate granule cells in the middle-aged hippocampus following intracerebroventricular FGF-2 infusions. Eur J Neurosci. 2007;26:1765–1779. doi: 10.1111/j.1460-9568.2007.05820.x. [DOI] [PubMed] [Google Scholar]
- Reuss B, Dono R, Unsicker K. Functions of fibroblast growth factor (FGF)-2 and FGF-5 in astroglial differentiation and blood-brain barrier permeability: evidence from mouse mutants. J Neurosci. 2003;23:6404–6412. doi: 10.1523/JNEUROSCI.23-16-06404.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghezzi G, Patel S, Ren CJ, Gualandris A, Pintucci G, Robbins ES, Shapiro RL, Galloway AC, Rifkin DB, Mignatti P. Fibroblast growth factor-2 (FGF-2) induces vascular endothelial growth factor (VEGF) expression in the endothelial cells of forming capillaries: an autocrine mechanism contributing to angiogenesis. The Journal of Cell Biology. 1998;141:1659–1673. doi: 10.1083/jcb.141.7.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segi-Nishida E, Warner-Schmidt JL, Duman RS. Electroconvulsive seizure and VEGF increase the proliferation of neural stem-like cells in rat hippocampus. Proc Natl Acad Sci USA. 2008;105:11352–11357. doi: 10.1073/pnas.0710858105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seri B, Garcia-Verdugo JM, McEwen BS, Alvarez-Buylla A. Astrocytes give rise to new neurons in the adult mammalian hippocampus. J Neurosci. 2001;21:7153–7160. doi: 10.1523/JNEUROSCI.21-18-07153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty AK, Hattiangady B, Shetty GA. Stem/progenitor cell proliferation factors FGF-2, IGF-1, and VEGF exhibit early decline during the course of aging in the hippocampus: role of astrocytes. Glia. 2005;51:173–186. doi: 10.1002/glia.20187. [DOI] [PubMed] [Google Scholar]
- Simón AM, de Maturana RL, Ricobaraza A, Escribano L, Schiapparelli L, Cuadrado-Tejedor M, Pérez-Mediavilla A, Avila J, Del Río J, Frechilla D. Early Changes in Hippocampal Eph Receptors Precede the Onset of Memory Decline in Mouse Models of Alzheimer's Disease. J Alzheimers Dis. 2009 doi: 10.3233/JAD-2009-1096. [DOI] [PubMed] [Google Scholar]
- Sinclair DA, Oberdoerffer P. The ageing epigenome: damaged beyond repair? Ageing Res Rev. 2009;8:189–198. doi: 10.1016/j.arr.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skold MK, von Gertten C, Sandberg-Nordqvist AC, Mathiesen T, Holmin S. VEGF and VEGF receptor expression after experimental brain contusion in rat. J Neurotrauma. 2005;22:353–367. doi: 10.1089/neu.2005.22.353. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV. Reactive astrocytes in neural repair and protection. Neuroscientist. 2005;11:400–407. doi: 10.1177/1073858405278321. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009;32:638–647. doi: 10.1016/j.tins.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H, Stevens CF, Gage FH. Astroglia induce neurogenesis from adult neural stem cells. Nature. 2002;417:39–44. doi: 10.1038/417039a. [DOI] [PubMed] [Google Scholar]
- Stavri GT, Zachary IC, Baskerville PA, Martin JF, Erusalimsky JD. Basic fibroblast growth factor upregulates the expression of vascular endothelial growth factor in vascular smooth muscle cells. Synergistic interaction with hypoxia. Circulation. 1995;92:11–14. doi: 10.1161/01.cir.92.1.11. [DOI] [PubMed] [Google Scholar]
- Steiner B, Klempin F, Wang L, Kott M, Kettenmann H, Kempermann G. Type-2 cells as link between glial and neuronal lineage in adult hippocampal neurogenesis. Glia. 2006;54:805–814. doi: 10.1002/glia.20407. [DOI] [PubMed] [Google Scholar]
- Sun FY, Guo X. Molecular and cellular mechanisms of neuroprotection by vascular endothelial growth factor. J Neurosci Res. 2005;79:180–184. doi: 10.1002/jnr.20321. [DOI] [PubMed] [Google Scholar]
- Vasilevko V, Passos GF, Quiring D, Head E, Kim RC, Fisher M, Cribbs DH. Aging and cerebrovascular dysfunction: contribution of hypertension, cerebral amyloid angiopathy, and immunotherapy. Ann N Y Acad Sci. 2010;1207:58–70. doi: 10.1111/j.1749-6632.2010.05786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bernhardi R TJ, Eugenín J. Aging-dependent changes of microglial cells and their relevance for neurodegenerative disorders. J Neurochem. 2010;112:1099–1114. doi: 10.1111/j.1471-4159.2009.06537.x. [DOI] [PubMed] [Google Scholar]
- Voskuhl RR, Peterson RS, Song B, Ao Y, Morales LB, Tiwari-Woodruff S, Sofroniew MV. Reactive astrocytes form scar-like perivascular barriers to leukocytes during adaptive immune inflammation of the CNS. J Neurosci. 2009;29:11511–11522. doi: 10.1523/JNEUROSCI.1514-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WY, Dong JH, Liu X, Wang Y, Ying GX, Ni ZM, Zhou CF. Vascular endothelial growth factor and its receptor Flk-1 are expressed in the hippocampus following entorhinal deafferentation. Neuroscience. 2005a;134:1167–1178. doi: 10.1016/j.neuroscience.2005.04.064. [DOI] [PubMed] [Google Scholar]
- Wang Y, Kilic E, Kilic U, Weber B, Bassetti CL, Marti HH, Hermann DM. VEGF overexpression induces post-ischaemic neuroprotection, but facilitates haemodynamic steal phenomena. Brain. 2005b;128:52–63. doi: 10.1093/brain/awh325. [DOI] [PubMed] [Google Scholar]
- Warner-Schmidt JL, Duman RS. VEGF is an essential mediator of the neurogenic and behavioral actions of antidepressants. Proc Natl Acad Sci U S A. 2007;104:4647–4652. doi: 10.1073/pnas.0610282104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner-Schmidt JL, Duman RS. VEGF as a potential target for therapeutic intervention in depression. Curr Opin Pharmacol. 2008;8:14–19. doi: 10.1016/j.coph.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelmsson U, Bushong EA, Price DL, Smarr BL, Phung V, Terada M, Ellisman MH, Pekny M. Redefining the concept of reactive astrocytes as cells that remain within their unique domains upon reaction to injury. Proc Natl Acad Sci U S A. 2006;103:17513–17518. doi: 10.1073/pnas.0602841103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittko IM, Schanzer A, Kuzmichev A, Schneider FT, Shibuya M, Raab S, Plate KH. VEGFR-1 regulates adult olfactory bulb neurogenesis and migration of neural progenitors in the rostral migratory stream in vivo. J Neurosci. 2009;29:8704–8714. doi: 10.1523/JNEUROSCI.5527-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolburg-Buchholz K, Mack AF, Steiner E, Pfeiffer F, Engelhardt B, Wolburg H. Loss of astrocyte polarity marks blood-brain barrier impairment during experimental autoimmune encephalomyelitis. Acta Neuropathol. 2009;118:219–233. doi: 10.1007/s00401-009-0558-4. [DOI] [PubMed] [Google Scholar]
- Wolosker H, Dumin E, Balan L, Foltyn VN. D-amino acids in the brain: D-serine in neurotransmission and neurodegeneration. FEBS J. 2008;275:3514–3526. doi: 10.1111/j.1742-4658.2008.06515.x. [DOI] [PubMed] [Google Scholar]
- Wu Y, Zhang A- Q, Yew DT. Age related changes of various markers of astrocytes in senescence-accelerated mice hippocampus. Neurochemistry International. 2005;46:565–574. doi: 10.1016/j.neuint.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Xiao Z, Kong Y, Yang S, Li M, Wen J, Li L. Upregulation of Flk-1 by bFGF via the ERK pathway is essential for VEGF-mediated promotion of neural stem cell proliferation. Cell Res. 2007;17:73–79. doi: 10.1038/sj.cr.7310126. [DOI] [PubMed] [Google Scholar]
- Yang SZ, Zhang LM, Huang YL, Sun FY. Distribution of Flk-1 and Flt-1 receptors in neonatal and adult rat brains. Anat Rec A Discov Mol Cell Evol Biol. 2003;274:851–856. doi: 10.1002/ar.a.10103. [DOI] [PubMed] [Google Scholar]
- Yeh J, Kim BS, Peresie J. Ovarian vascular endothelial growth factor and vascular endothelial growth factor receptor patterns in reproductive aging. Fertil Steril. 2008;89:1546–1556. doi: 10.1016/j.fertnstert.2007.06.032. [DOI] [PubMed] [Google Scholar]
- Yoshimura S, Takagi Y, Harada J, Teramoto T, Thomas SS, Waeber C, Bakowska JC, Breakefield XO, Moskowitz MA. FGF-2 regulation of neurogenesis in adult hippocampus after brain injury. Proc Natl Acad Sci U S A. 2001;98:5874–5879. doi: 10.1073/pnas.101034998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura S, Teramoto T, Whalen MJ, Irizarry MC, Takagi Y, Qiu J, Harada J, Waeber C, Breakefield XO, Moskowitz MA. FGF-2 regulates neurogenesis and degeneration in the dentate gyrus after traumatic brain injury in mice. J Clin Invest. 2003;112:1202–1210. doi: 10.1172/JCI16618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M, Li D, Shimazu K, Zhou Y- X, Lu B, Deng C- X. Fibroblast growth factor receptor-1 is required for long-term potentiation, memory consolidation, and neurogenesis. Biological Psychiatry. 2007;62:381–390. doi: 10.1016/j.biopsych.2006.10.019. [DOI] [PubMed] [Google Scholar]