Introduction
Cardiovascular diseases (CVD) remain the leading cause of death in modern societies (Lloyd-Jones et al.). Advancing age is the major risk factor for CVD (Lakatta 2002; Lakatta & Levy 2003). Most CVD are linked to disorders of arteries (Lloyd-Jones et al.) and it is now recognized that aging increases risk of CVD in large part by causing arterial dysfunction, which then leads to clinical vascular and cardiac diseases (Lakatta & Levy 2003).
Many changes to arteries likely contribute to the increased risk of CVD with aging. One of these is the development of vascular endothelial dysfunction, as most commonly indicated by impaired endothelium-dependent dilation (EDD) (Celermajer et al. 1994; Taddei et al. 1995; Lakatta & Levy 2003). Impaired EDD with aging is mediated by excessive superoxide, at least in part as a result of increases in the oxidant enzyme nicotinamide adenine dinucleotide phosphate-oxidase (NADPH oxidase) (Zalba et al. 2000; Bedard & Krause 2007; Donato et al. 2007; Durrant et al. 2009). Increased superoxide reduces bioavailability of nitric oxide (NO) both by reacting with NO to form peroxynitrite and by oxidizing tetrahydrobiopterin (BH4), an essential co-factor for NO production by endothelial nitric oxide synthase (eNOS) (Cosentino et al. 1998; Taddei et al. 2001; Landmesser et al. 2003; Blackwell et al. 2004; Shi et al. 2004). Another key vascular change with aging is the stiffening of large elastic arteries (Lakatta & Levy 2003), possibly linked in part to development of vascular endothelial dysfunction (Fitch et al. 2001; Wilkinson et al. 2004). Large elastic artery stiffness has emerged as a major independent risk factor for age-associated CVD and a key therapeutic target (Lakatta & Levy 2003; Nilsson et al. 2009; Mitchell et al.; Vlachopoulos et al. 2010).
Oxidative stress and inflammation are believed to be important mechanisms contributing to these effects of aging on arteries (Brandes et al. 2005; Donato et al. 2007; Csiszar et al. 2008; Donato et al. 2008; Ungvari et al. 2010). Arterial oxidative stress develops with aging as a consequence of excessive production of superoxide by NADPH oxidase or other mechanisms (eNOS uncoupling, mitochondrial dysfunction) (Vasquez-Vivar et al. 1998; Ungvari et al. 2008; Durrant et al. 2009; Rippe et al. 2010) and possibly via selective reductions in antioxidant enzymes, including superoxide dismutase (SOD) (Sun et al. 2004; Rippe et al.). As a result of oxidative stress, injury or other causes, vascular inflammation also develops with aging, as indicated by increases in expression of pro-inflammatory cytokines such as interleukins 1 and 6 (IL-1, IL6), interferon γ (INFγ) and tumor necrosis factor α (TNFα) (Csiszar et al. 2008; Donato et al. 2008; Rippe et al. 2010; Ungvari et al. 2010).
Given the above, treatments that improve or reverse vascular endothelial dysfunction and large elastic artery stiffness in middle/older age may prevent much of the increase in CVD risk with aging (Lakatta & Levy 2003; Lonn et al. 2010; Seals 2010). In this context, the nitrite anion (inorganic nitrite) would seem to hold promise. Once considered an inert byproduct of NO metabolism, nitrite now is recognized as a physiologically important storage form of NO (Lundberg & Weitzberg 2008). Nitrite is a cytoprotective molecule considered to have broad therapeutic potential for the prevention and treatment of CVD (Calvert & Lefer; Lundberg & Weitzberg 2008). Sodium nitrite administration protects against ischemia/reperfusion injury (Bryan et al. 2007; Bryan et al. 2008; Gonzalez et al. 2008) and improves vascular endothelial dysfunction in hypercholesterolemic mice (Stokes et al. 2009). However, the efficacy of sodium nitrite for treating arterial aging is entirely unknown.
In the present study, we hypothesized that short-term oral sodium nitrite treatment would improve or reverse vascular endothelial dysfunction and large elastic artery stiffness in old mice while having no effects in young mice. As a secondary aim, we sought to gain initial insight into the mechanisms by which nitrite-induced improvements in function in the old mice might be mediated, particularly those related to reduced oxidative stress and inflammation.
Results
Animal characteristics
Characteristics of the groups are shown in Table 1. Body mass and heart mass were greater and gastrocnemius muscle mass was smaller in the old compared with the young groups (p<0.05). Carotid artery baseline diameter was greater in the old animals (p<0.05) and sodium nitrite treatment had no effect on these characteristics. Carotid artery preconstriction to phenylephrine was not different between groups.
Table 1.
Animal characteristics
| YC | OC | YN | ON | |
|---|---|---|---|---|
| Body mass (g) | 23 ± 1 | 35 ± 1* | 23 ± 1 | 34 ± 1* |
| Heart mass (mg) | 136 ± 9 | 194 ± 8* | 137 ± 1 | 189 ± 9* |
| Gastrocnemius mass (mg) | 195 ± 9 | 156 ± 14* | 191 ± 13 | 146 ± 7* |
| Carotid artery lumen diameter (μm) | 389 ± 11 | 421 ± 8* | 387 ± 10 | 410 ± 13* |
Values are mean ± SEM.
p < 0.05 vs. YC
Sodium nitrite treatment restores NO-mediated EDD in old mice
EDD to acetylcholine (ACh) was lower in old compared with young control mice (p<0.05) as a result of a smaller NO dilatory influence, as indicated by a smaller reduction in EDD in the presence vs. absence of the NO inhibitor N-G-nitro-L-arginine methyl ester (L-NAME) (Figure 1 A and B). Nitrite treatment restored EDD to ACh in old mice by restoring NO-mediated dilation, but had no effect in the young animals (Figure 1 A and B). There were no differences in NO-mediated EDD among the young control and young and old nitrite treated animals. Endothelium-independent dilation to sodium nitroprusside did not differ among the groups (Figure 1C). TEMPOL, a superoxide dismutase mimetic (Figure 2A), apocynin, a NADPH oxidase inhibitor (Figure 2B), and sepiapterin, a precursor of BH4 (Figure 2C) each restored EDD to ACh in old control animals, while not affecting responses in young control or old sodium nitrite treated animals. eNOS protein in the aorta was lower in the old vs. young control animals (Table 2), and this was not influenced by sodium nitrite treatment. Sodium nitrite supplementation increased eNOS expression in young mice. These data demonstrate that short-term sodium nitrite treatment restores NO-mediated EDD in old mice via a superoxide/NADPH oxidase/BH4-related mechanism and not by affecting vascular smooth muscle sensitivity to NO or eNOS protein.
Figure 1. Endothelium-dependent, nitric oxide-dependent and endothelium-independent dilation.
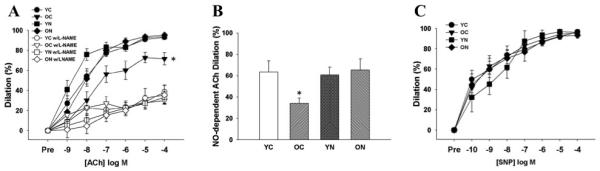
(A) Dose-responses to the endothelium-dependent dilator acetylcholine (ACh) in the absence and presence of the endothelial nitric oxide (NO) synthase inhibitor N-G-nitro-L-arginine methyl ester (L-NAME) in young and old control (YC and OC) and nitrite-supplemented (YN and ON) mice. (B) NO-dependent dilation (Max DilationACh – Max DilationACh+L-NAME). (C) Dose-responses to the endothelium-independent dilator sodium nitroprusside (SNP). Values are mean ± SEM. (n = 7 per group). * p < 0.05 vs. YC.
Figure 2. Superoxide-, NADPH oxidase- and tetrahydrobiopterin-dependent modulation of endothelial-dependent dilation.
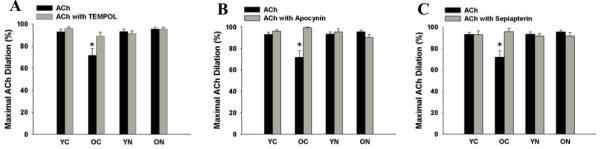
(A) Maximal dilation of carotid arteries to acetylcholine (ACh) and ACh + TEMPOL, a superoxide dismutase mimetic. (B) Maximal dilation of carotid arteries to ACh and to ACh + apocynin, a NADPH oxidase inhibitor. (C) Maximal dilation of carotid arteries to ACh and to ACh + sepiapterin, an exogenous tetrahydrobiopterin donor. Values are mean ± SEM. (n = 6 – 9 per group) * p < 0.05 vs. YC.
Table 2.
Protein expression in aorta
| YC | OC | YN | ON | |
|---|---|---|---|---|
| MnSOD | 1.0 ± 0.1 | 0.7 ± 0.1* | 0.2 ± 0.05* | 0.7 ± 0.1* |
| CuZnSOD | 1.0 ± 0.3 | 1.2 ± 0.1 | 1.2 ± 0.2 | 1.1 ± 0.2 |
| ecSOD | 1.0 ± 0.1 | 1.1 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.2 |
| eNOS | 1.0 ± 0.1 | 0.7 ± 0.1* | 1.5 ± 0.1* | 0.6 ± 0.1* |
Values are mean ± SEM.
p < 0.05 vs. YC.
MnSOD, manganese superoxide disumtase; CuZnSOD, Copper Zinc superoxide dismutase; ecSOD, extracelluar superoxide dismutase; eNOS, endothelial nitric oxide synthase.
Sodium nitrite treatment normalizes aortic pulse wave velocity in old mice
Large elastic artery stiffness, as determined by aortic pulse wave velocity, was ~70% greater in old compared with young control mice (p<0.05) (Figure 3). Sodium nitrite treatment reversed the age-associated increase in aortic pulse wave velocity in old mice. These observations indicate that short-term treatment with sodium nitrite ameliorates age-associated stiffening of the aorta.
Figure 3. Large elastic artery stiffness.
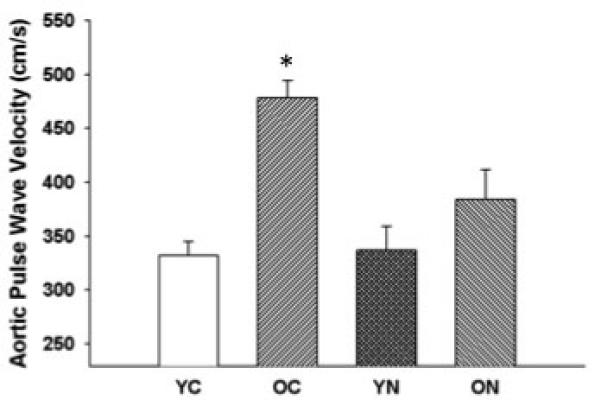
Aortic pulse wave velocity in young and old control (YC and OC) and young and old nitrite supplemented (YN and ON) mice. Values are mean ± SEM. (n = 5 per group) * P < 0.05 vs. YC.
Sodium nitrite treatment reduces vascular oxidative stress and inflammation in old mice
Nitrotyrosine, a cellular marker of oxidative stress, was ~100% greater in the aorta of old compared with young control mice (p<0.05) (Figure 4A), and was associated with similarly greater aortic superoxide production, and an ~50% reduction in aortic SOD activity (p<0.05) (Figure 4 B and C). NADPH oxidase subunit p67 protein expression was ~300% greater in the aorta of old compared with young control mice (p<0.05) (Figure 4D). Nitrite treatment in old animals reduced aortic nitrotyrosine to levels observed in young control mice, and this was associated with complete reversal of the age-associated changes in superoxide production, SOD activity and aortic p67 expression. Protein expression of manganese SOD (MnSOD) in aorta was decreased in old control mice (p<0.05) and this was unaltered by nitrite supplementation; young nitrite supplemented mice had reduced MnSOD (p<0.05) (Table 2). Protein expression of the inflammatory cytokines IL-1β, IL6, INFγ and TNFα was increased in aorta of old compared with young mice. Short-term nitrite treatment reduced aortic inflammatory cytokines selectively in old mice, normalizing expression to levels observed in young controls (p<0.05, Figure 5). These observations indicate that short-term sodium nitrite treatment ameliorates arterial oxidative stress (by normalizing superoxide production and SOD activity) and inflammation with aging.
Figure 4. Aortic oxidative stress, superoxide production and oxidant/antioxidant enzymes.
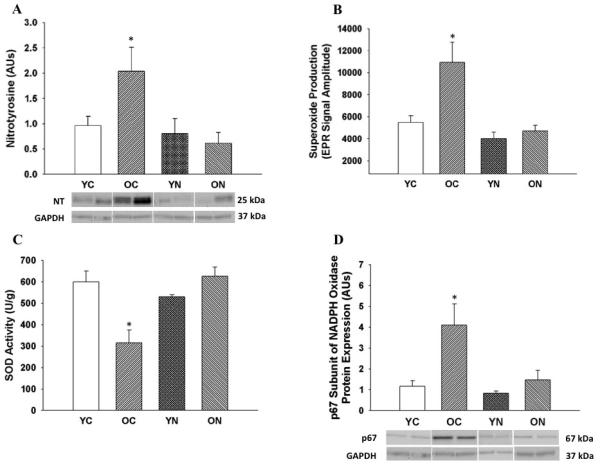
(A) Nitrotyrosine in aorta of young and old control (YC and OC) and young and old nitrite supplemented (YN and ON) mice. Data are expressed relative to GAPDH and normalized to YC mean value. Representative western blot images below. (B) Mean electron paramagnetic resonance (EPR) signal for superoxide from aortic rings. (C) Aortic superoxide dismutase (SOD) enzymatic activity. (D) Protein expression of p67 subunit of NADPH oxidase in aorta of young and old control (YC and OC) and young and old nitrite supplemented (YN and ON) mice. Data are expressed relative to GAPDH and normalized to YC mean value. Representative western blot images below. Values are mean ± SEM. (n = 6 – 9 per group) * p < 0.05 vs. YC.
Figure 5. Aortic inflammatory cytokines.
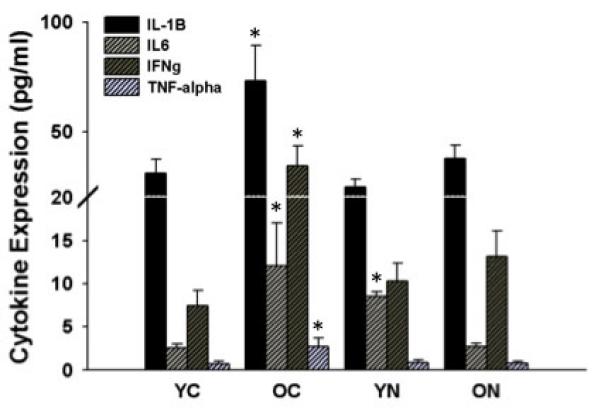
Expression of inflammatory cytokines IL-1β, IL-6, IFNγ and TNFα in aorta from young and old control (YC and OC) and young and old nitrite supplemented (YN and ON) mice. Values are mean ± SEM. (n = 5 – 8 per group) * p < 0.05 vs. YC.
Sodium nitrite treatment restores the reductions in nitrite concentrations in plasma, large elastic arteries and heart aging
To obtain sufficient tissue to determine nitrite concentrations in large elastic arteries, carotid arteries and aorta were pooled from each group of mice (Figure 6). Nitrite concentrations in the large elastic arteries, heart and plasma were lower in old compared with young control animals (p<0.05). In the old mice, nitrite treatment restored nitrite concentrations to levels either not different from (elastic arteries and heart) or well above (plasma) those observed in young control mice (p<0.05). Nitrite supplementation also increased nitrite levels in young mice (p<0.05). These results show that nitrite concentrations in large elastic arteries, heart and plasma decrease with aging, and that short-term sodium nitrite treatment restores nitrite concentrations in old mice.
Figure 6. Circulating and tissue nitrite concentrations from plasma, pooled large elastic arteries and heart.
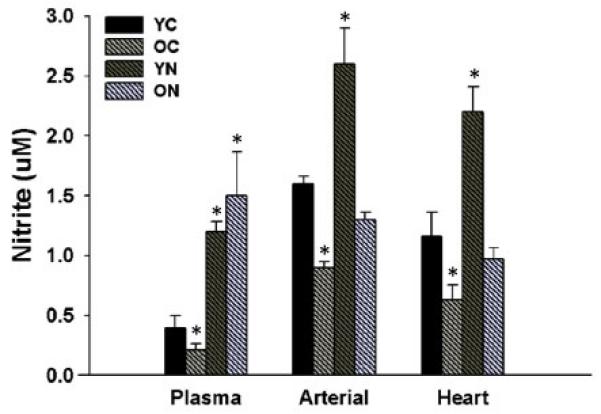
Nitrite concentrations in plasma, large elastic arteries (pooled aortic and carotid arteries) and heart in young and old control (YC and OC) and young and old nitrite supplemented (YN and ON) mice. Values are mean ± SEM. (n = 8 animals per group) * p < 0.05 vs. YC.
Discussion
The key finding of the present study was that 3 weeks of sodium nitrite treatment in old C57BL6 mice ameliorated carotid artery endothelial dysfunction and reduced large elastic artery stiffness to a level not different from young mice. Age is the major risk factor for CVD and vascular endothelial dysfunction and large elastic stiffening are believed to explain much of the increase in CVD risk with aging (Lakatta 2002; Vita & Keaney 2002; Mitchell et al.). Our preclinical findings, therefore, suggest that sodium nitrite has intriguing translational potential as a treatment for the prevention of age-associated CVD in humans. The present results also provide novel insight into the mechanisms by which sodium nitrite reverses arterial aging, which include restoring NO and BH4 bioavailability, suppressing superoxide-dependent oxidative stress by reducing NADPH oxidase and increasing SOD activity, and reducing inflammation. Finally, our results indicate that aging is associated with reduced concentrations of nitrite in large elastic arteries, the heart and plasma, providing additional rationale for therapies that enhance circulating and tissue nitrite stores.
Vascular endothelial dysfunction
The present results are consistent with previous reports from our laboratory (Durrant et al. 2009; Lesniewski et al. 2009; Rippe et al.) and others (Muller-Delp et al. 2002; Soucy et al. 2006) that vascular endothelial dysfunction develops with aging, as indicated by impaired EDD. The latter is mediated by reduced NO bioavailability (Luscher & Barton 1997; Spier et al. 2004; Sindler et al. 2009) as a result of NADPH oxidase-associated increases in superoxide (Hamilton et al. 2002; Rippe et al. 2010) and inadequate BH4 bioactivity (Cosentino et al. 1998; Blackwell et al. 2004; Eskurza et al. 2005; Delp et al. 2008).
Recently it was shown that 3 weeks of sodium nitrite treatment reverses impaired EDD to ACh in cremaster muscle arterioles of hypercholesterolemic mice (Stokes et al. 2009). In the present study, we show that in a model of primary arterial aging, short-term treatment with sodium nitrite completely restores EDD in old mice to levels observed in young animals. Importantly, our findings show for the first time that sodium nitrite treatment restores EDD in a setting of impaired baseline function by restoring NO bioavailability (i.e., the NO-component of EDD) to normal control levels. eNOS protein in the aorta was reduced with aging, but was not influenced by sodium nitrite treatment in the older mice, consistent with earlier findings in myocardial tissue (Bryan et al. 2007).
The fact that administration of TEMPOL, a SOD mimetic, restored EDD in old control mice, while having no effect in young animals or old sodium nitrite treated mice, supports the idea that a reduction in superoxide was responsible for the normalization of endothelial function by sodium nitrite in old mice. That apocynin, an inhibitor of NADPH oxidase, selectively restored EDD in old control mice further suggests that the effects of sodium nitrite in old animals was mediated by reduced NADPH oxide production of superoxide. Lastly, sepiapterin, an exogenous donor of BH4, restored EDD in old control, but not in old nitrite treated mice. This suggests that sodium nitrite may enhance NO bioavailability also by preserving BH4, a critical co-factor for eNOS-dependent NO production, as shown recently in the liver of hypercholesterolemic mice (Stokes et al. 2009).
Together, our data are consistent with the possibility that short-term sodium nitrite treatment ameliorates vascular endothelial dysfunction in old mice by restoring NO bioavailability as a result of reduced NADPH oxidase superoxide production and enhanced bioactivity of BH4.
Large elastic artery stiffness
Our finding that aortic pulse wave velocity was greater in old compared with young cage control mice is consistent with previous reports in both animals and humans that large elastic arteries stiffen with advancing age in the absence of disease (Reddy et al. 2003; Eskurza et al. 2004; Sutton-Tyrrell et al. 2005; Soucy et al. 2006). The present results extend these earlier findings by showing that 3 weeks of sodium nitrite therapy initiated in old age reduces aortic pulse wave velocity to levels that are no longer significantly different from those of young cage control and nitrite treated mice. Nitrite treatment had no effect on aortic pulse wave velocity in young mice, indicating that treatment selectively reduced large elastic artery stiffness in old animals. Our findings in old mice are clinically important because recently it was shown that aortic pulse wave velocity is a major independent risk factor for incident CV events and all-cause mortality in older adults (Mitchell et al. 2010; Vlachopoulos et al. 2010). This provides experimental support for the idea that sodium nitrite treatment holds promise for reducing CVD and all-cause deaths in middle-aged and older humans.
Oxidant stress and inflammation
The present findings are consistent with previous work from our lab (Lesniewski et al. 2009; Rippe et al. 2010) and others (van der Loo et al. 2000; Csiszar et al. 2002; Yang et al. 2009) showing increased arterial oxidative stress with aging as indicated by a marked increase in aortic nitrotyrosine staining in old compared with young control mice. Nitrotyrosine is produced by nitration of tyrosine residues on proteins primarily by peroxynitrite, which is formed when superoxide reacts with NO (Radi 2004). The present findings also confirm recent results from our laboratory (Rippe et al. 2010) showing that this increase in arterial oxidative stress with aging in mice is associated with both increased superoxide production, as measured directly by spin trapping and electron paramagnetic resonance spectroscopy, and reduced activity of SOD, an important anti-oxidant enzyme.
Our findings here suggest that sodium nitrite treatment completely reversed the increase in arterial oxidative stress with aging, as indicated by a reduction of aortic nitrotyrosine abundance in nitrite treated old animals to levels observed in young control mice. An earlier report found that nitrite inhibits myeloperoxidase-mediated modification of low-density lipoprotein (Carr & Frei 2001), consistent with an antioxidant effect. Nitrite treatment appeared to reduce arterial oxidative stress in old mice, at least in part, by normalizing arterial superoxide production. Indeed, to our knowledge this is the first evidence that short-term sodium nitrite administration reduces superoxide formation in arteries, which is consistent with previous observations following ischemia/reperfusion injury in the heart (Dezfulian et al. 2009). Moreover, our results show that nitrite treatment restored SOD activity in aorta of old animals and this also may have contributed to reductions in arterial superoxide bioavailability and oxidative stress in this group. The effect appeared to be the result of an increase in activity of the enzyme and not due to changes in expression, as protein concentrations of the SOD isoforms were unaffected by sodium nitrite treatment in the old animals.
In the present study, we also show that sodium nitrite treatment reduced expression of several pro-inflammatory cytokines in aorta of old mice to concentrations observed in young controls. This is consistent with the results of a previous study in hypercholesterolemic mice, in which sodium nitrite treatment lowered plasma C-reactive protein and reduced leukocyte adhesion and infiltration through venular endothelium (Stokes et al. 2009). Taken together, our results provide the first evidence of a potent combined antioxidant and anti-inflammatory effect of sodium nitrite treatment on arteries.
Plasma and tissue nitrite concentrations
The present data are the first to show that nitrite concentrations are reduced with aging in large elastic arteries (−44% vs. young controls), the heart (−43%) and plasma (−46%). Reductions in nitrite in plasma and the heart have been documented previously in other mouse models of low NO bioavailability including hypercholesterolemia and eNOS deficiency (Bryan et al. 2008; Stokes et al. 2009). In the present study, nitrite supplementation in the drinking water increased arterial, cardiac and plasma nitrite concentrations in old mice to levels not different from (arterial and heart) or even greater than (plasma) young control mice. These findings are consistent with results of earlier studies of nitrite supplementation in states in which baseline nitrite concentrations are reduced (Bryan et al. 2008; Stokes et al. 2009). We did not observe an increase in tissue nitrite above normal control concentrations reported previously in hypercholesterolemic mice (Stokes et al. 2009), but rather only in plasma levels in our old mice. Overall, our results demonstrate that short-term nitrite therapy in old mice restores (arterial and cardiac) or enhances above normal (plasma) nitrite concentrations compared with levels observed in young controls.
Conclusions
The results of the present study show for the first time that nitrite concentrations are reduced with aging in the circulation and cardiovascular tissues, and that short-term sodium nitrite therapy ameliorates vascular endothelial dysfunction and de-stiffens large elastic arteries, two clinically significant expressions of arterial aging, in old C57BL6 mice. Our findings also provide evidence that the improvements in vascular endothelial function in old mice treated with sodium nitrite are mediated by increased NO bioavailability as a result of reduced NADPH oxidase-dependent superoxide bioavailability and enhanced BH4 bioactivity. Moreover, our data provide the first evidence for anti-oxidant and anti–inflammatory influences of sodium nitrite on arterial tissue. Overall, these findings from a preclinical model establish an experimental basis for translational research aimed at determining the efficacy of nitrite therapy for reversing arterial aging and reducing the risk of age-associated CVD in humans.
Experimental procedures
Animals
Young (4-6 months) and old (26-28 months; ~50% survival) male C57BL6 mice were obtained from the National Institute on Aging rodent colony and were fed normal rodent chow ad libitum. After an acclimation period of 2 weeks, the young and old mice were divided into two subgroups: control animals continued on regular drinking water and the other animals that had nitrite supplemented (50 mg/L) drinking water for three weeks. All mice were housed in an animal care facility at the University of Colorado at Boulder on a 12 h:12 h light-dark cycle. All animal procedures conformed to the Guide to the Care and Use of Laboratory Animals (NIH publication n. 85-23, revised 1996) and were approved by the UCB Animal Care and Use Committee.
Carotid artery vasodilatory responses
EDD and endothelium-independent dilation were determined ex vivo in isolated carotid arteries as previously described (Durrant et al. 2009; Lesniewski et al. 2009; Rippe et al.). Briefly, mice were anesthetized using isoflurane and euthanized by exsanguination via cardiac puncture. The carotid arteries were carefully excised, cannulated onto glass micropipettes and secured with nylon (11-0) suture in myograph chambers (DMT Inc.) containing buffered physiological saline solutions. The arteries were pressurized to 50 mmHg at 37° C and were allowed to equilibrate for 1 h. After submaximal preconstriction with phenylephrine (2 μmol/L), increases in luminal diameter in response to acetylcholine (ACh: 1 × 10−9 - 1 × 10−4 mol/L) with and without co-administration of the NO synthase inhibitor N-G-nitro-L-arginine methyl ester (L-NAME), (0.1 mmol/L, 30 min incubation), or the SOD mimetic, TEMPOL, (1 mmol/L, 60 min incubation), were determined. EDD also was determined in the presence of the NADPH oxidase inhibitor, apocynin, (1 mmol/L, 60 min incubation) and the exogenous BH4 donor, sepiapterin, (1 mmol/L, 60 min incubation). Endothelium-independent dilation was determined by vasodilation in response to sodium nitroprusside (SNP: 1 × 10−10 - 1 × 10−4 mol/L). All dose response data are presented on a percent basis. Preconstriction was calculated as a percentage of maximal diameter according to the following formula:
Because of differences in maximal carotid artery diameter between young and old animals, vasodilator responses were recorded as actual diameters expressed as a percentage of maximal response according to the following formula:
Where Dm is maximal inner diameter at 50 mmHg, Ds is the steady-state inner diameter recorded after the addition of drug, and Db is the steady-state inner diameter following preconstriction before the first addition of drug.
NO-dependent dilation was determined from the maximal EDD in the absence or presence of L-NAME according to the following formula:
In vivo aortic pulse wave velocity
Aortic pulse wave velocity was measured as described previously (Kim et al. 2009). Mice were anesthetized with 2% isoflurane and placed supine on a heating board with legs secured to ECG electrodes. Aortic velocity was measured with Doppler probes at the transverse aortic arch and abdominal aorta. Pre-ejection time, the time between the R-wave of the ECG to foot of the Doppler signal, was determined for each site. Aortic pulse wave velocity was calculated by dividing the distance between the transverse and abdominal probes by the difference in the thoracic and abdominal pre-ejection times.
Arterial superoxide production
Production of superoxide was measured by electron paramagnetic resonance (EPR) spectrometry using the spin probe 1-hydroxy-3-methoxycarbonly-2,2,5,5-tetramethylpyrrolidine (CMH, Alexis Biochemicals) as previously described (Rippe et al.). Two-millimeter aortic rings were incubated for 60 min at 37° C in 200 μl of Krebs-HEPES buffer containing 0.55 mmol/L CMH and analyzed immediately on an MS300 X-band EPR spectrometer (Magnettech, Berlin, Germany).
Arterial protein expression and enzyme activities
Aortas were used as a surrogate large elastic artery to provide sufficient tissue for analysis of protein expression by Western blot and enzyme activity as described previously (Durrant et al. 2009; Lesniewski et al. 2009; Rippe et al.). Aortas were excised, cleared of surrounding tissues and frozen in liquid nitrogen before storage at −80°C. The tissue was pulverized over liquid nitrogen and homogenized in ice-cold RIPA lysis buffer containing protease and phosphatase inhibitors [Protease Inhibitor Cocktail Tablet (Roche, Indianapolis, IN, USA) and 0.01% phosphatase inhibitor cocktail (Sigma, St. Louis, MO, USA)]. Ten micrograms of protein was loaded on 4-12% polyacrylamide gels, seperated by electrophoresis and transferred onto nitrocellulose membranes for Western blot analysis. Antibodies for Western blot analysis included anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH 1:1000, Cell Signaling), anti-nitrotyrosine (1:100, Abcam), anti-p67 phox (1:1000, Cell Signaling), anti-MnSOD, anti-CuZn (1:2000 Stressgen), anti-ecSOD (1:500 Sigma) and anti-endothelial NO synthase (eNOS 1:500, BD Biosciences). Total SOD activity in aortic lysates (1 μg protein) was determined using the SOD Activity Assay kit (Cayman Chemical, Ann Arbor, MI, USA) according to the manufacturer’s instructions. Concentrations of the pro-inflammatory cytokines IL-1β, IL-6, IFNγ and TNF-α were determined in aortic whole cell lysates by multiplex ELISA (Searchlight Mouse Inflammatory Cytokine Kit; Aushon Biosystems; Billerica, MA) as previously described (Rippe et al.).
Analysis of nitrite in large elastic arteries, heart and plasma
Nitrite analysis procedures have been described in detail (Bryan et al. 2007; Bryan et al. 2008; Elrod et al. 2008). Nitrite concentrations were quantified by ion chromatography (ENO20 Analyzer, Eicom). Plasma was obtained by centrifugation at 800 g for 10 min.
Statistics
Results are presented as mean ± SEM. Statistical analysis was performed with SPSS 17.0 software. For the ex vivo vasodilatory dose response, group differences were determined by repeated measures ANOVA. A two-way ANOVA was used to analyze stiffness. For maximal dilation, protein expression, enzyme activities, superoxide production and animal characteristics comparisons between groups were made using ANOVA. Significance was determined using p<0.05.
Acknowledgments
Funding
This work supported by the National Institutes of Health (AG013038, HL098481-01, AG000279, HL007822, HL092141 and HL093579) and the American Diabetes Association (7-09-BS-26).
References
- Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- Blackwell KA, Sorenson JP, Richardson DM, Smith LA, Suda O, Nath K, Katusic ZS. Mechanisms of aging-induced impairment of endothelium-dependent relaxation: role of tetrahydrobiopterin. Am J Physiol Heart Circ Physiol. 2004;287:H2448–2453. doi: 10.1152/ajpheart.00248.2004. [DOI] [PubMed] [Google Scholar]
- Brandes RP, Fleming I, Busse R. Endothelial aging. Cardiovasc Res. 2005;66:286–294. doi: 10.1016/j.cardiores.2004.12.027. [DOI] [PubMed] [Google Scholar]
- Bryan NS, Calvert JW, Elrod JW, Gundewar S, Ji SY, Lefer DJ. Dietary nitrite supplementation protects against myocardial ischemia-reperfusion injury. Proc Natl Acad Sci U S A. 2007;104:19144–19149. doi: 10.1073/pnas.0706579104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan NS, Calvert JW, Gundewar S, Lefer DJ. Dietary nitrite restores NO homeostasis and is cardioprotective in endothelial nitric oxide synthase-deficient mice. Free Radic Biol Med. 2008;45:468–474. doi: 10.1016/j.freeradbiomed.2008.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvert JW, Lefer DJ. Clinical translation of nitrite therapy for cardiovascular diseases. Nitric Oxide. 2010;22:91–97. doi: 10.1016/j.niox.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr AC, Frei B. The nitric oxide congener nitrite inhibits myeloperoxidase/H2O2/Cl- -mediated modification of low density lipoprotein. J Biol Chem. 2001;276:1822–1828. doi: 10.1074/jbc.M009082200. [DOI] [PubMed] [Google Scholar]
- Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. Journal of the American College of Cardiology. 1994;24:471–476. doi: 10.1016/0735-1097(94)90305-0. [DOI] [PubMed] [Google Scholar]
- Cosentino F, Patton S, d’Uscio LV, Werner ER, Werner-Felmayer G, Moreau P, Malinski T, Luscher TF. Tetrahydrobiopterin alters superoxide and nitric oxide release in prehypertensive rats. J Clin Invest. 1998;101:1530–1537. doi: 10.1172/JCI650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csiszar A, Ungvari Z, Edwards JG, Kaminski P, Wolin MS, Koller A, Kaley G. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res. 2002;90:1159–1166. doi: 10.1161/01.res.0000020401.61826.ea. [DOI] [PubMed] [Google Scholar]
- Csiszar A, Wang M, Lakatta EG, Ungvari Z. Inflammation and endothelial dysfunction during aging: role of NF-kappaB. J Appl Physiol. 2008;105:1333–1341. doi: 10.1152/japplphysiol.90470.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delp MD, Behnke BJ, Spier SA, Wu G, Muller-Delp JM. Ageing diminishes endothelium-dependent vasodilatation and tetrahydrobiopterin content in rat skeletal muscle arterioles. J Physiol. 2008;586:1161–1168. doi: 10.1113/jphysiol.2007.147686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dezfulian C, Shiva S, Alekseyenko A, Pendyal A, Beiser DG, Munasinghe JP, Anderson SA, Chesley CF, Vanden Hoek TL, Gladwin MT. Nitrite therapy after cardiac arrest reduces reactive oxygen species generation, improves cardiac and neurological function, and enhances survival via reversible inhibition of mitochondrial complex I. Circulation. 2009;120:897–905. doi: 10.1161/CIRCULATIONAHA.109.853267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato AJ, Black AD, Jablonski KL, Gano LB, Seals DR. Aging is associated with greater nuclear NFkappaB, reduced IkappaBalpha, and increased expression of proinflammatory cytokines in vascular endothelial cells of healthy humans. Aging Cell. 2008;7:805–812. doi: 10.1111/j.1474-9726.2008.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE, Seals DR. Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB. Circ Res. 2007;100:1659–1666. doi: 10.1161/01.RES.0000269183.13937.e8. [DOI] [PubMed] [Google Scholar]
- Durrant JR, Seals DR, Connell ML, Russell MJ, Lawson BR, Folian BJ, Donato AJ, Lesniewski LA. Voluntary wheel running restores endothelial function in conduit arteries of old mice: direct evidence for reduced oxidative stress, increased superoxide dismutase activity and down-regulation of NADPH oxidase. J Physiol. 2009;587:3271–3285. doi: 10.1113/jphysiol.2009.169771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elrod JW, Calvert JW, Gundewar S, Bryan NS, Lefer DJ. Nitric oxide promotes distant organ protection: evidence for an endocrine role of nitric oxide. Proc Natl Acad Sci U S A. 2008;105:11430–11435. doi: 10.1073/pnas.0800700105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskurza I, Monahan KD, Robinson JA, Seals DR. Ascorbic acid does not affect large elastic artery compliance or central blood pressure in young and older men. Am J Physiol Heart Circ Physiol. 2004;286:H1528–1534. doi: 10.1152/ajpheart.00879.2003. [DOI] [PubMed] [Google Scholar]
- Eskurza I, Myerburgh LA, Kahn ZD, Seals DR. Tetrahydrobiopterin augments endothelium-dependent dilatation in sedentary but not in habitually exercising older adults. J Physiol. 2005;568:1057–1065. doi: 10.1113/jphysiol.2005.092734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch RM, Vergona R, Sullivan ME, Wang YX. Nitric oxide synthase inhibition increases aortic stiffness measured by pulse wave velocity in rats. Cardiovasc Res. 2001;51:351–358. doi: 10.1016/s0008-6363(01)00299-1. [DOI] [PubMed] [Google Scholar]
- Gonzalez FM, Shiva S, Vincent PS, Ringwood LA, Hsu LY, Hon YY, Aletras AH, Cannon RO, 3rd, Gladwin MT, Arai AE. Nitrite anion provides potent cytoprotective and antiapoptotic effects as adjunctive therapy to reperfusion for acute myocardial infarction. Circulation. 2008;117:2986–2994. doi: 10.1161/CIRCULATIONAHA.107.748814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton CA, Brosnan MJ, Al-Benna S, Berg G, Dominiczak AF. NAD(P)H oxidase inhibition improves endothelial function in rat and human blood vessels. Hypertension. 2002;40:755–762. doi: 10.1161/01.hyp.0000037063.90643.0b. [DOI] [PubMed] [Google Scholar]
- Kim JH, Bugaj LJ, Oh YJ, Bivalacqua TJ, Ryoo S, Soucy KG, Santhanam L, Webb A, Camara A, Sikka G, Nyhan D, Shoukas AA, Ilies M, Christianson DW, Champion HC, Berkowitz DE. Arginase inhibition restores NOS coupling and reverses endothelial dysfunction and vascular stiffness in old rats. J Appl Physiol. 2009;107:1249–1257. doi: 10.1152/japplphysiol.91393.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakatta EG. Age-associated cardiovascular changes in health: impact on cardiovascular disease in older persons. Heart Fail Rev. 2002;7:29–49. doi: 10.1023/a:1013797722156. [DOI] [PubMed] [Google Scholar]
- Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a “set up” for vascular disease. Circulation. 2003;107:139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest. 2003;111:1201–1209. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesniewski LA, Connell ML, Durrant JR, Folian BJ, Anderson MC, Donato AJ, Seals DR. B6D2F1 Mice are a suitable model of oxidative stress-mediated impaired endothelium-dependent dilation with aging. J Gerontol A Biol Sci Med Sci. 2009;64:9–20. doi: 10.1093/gerona/gln049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- Lonn E, Bosch J, Teo KK, Pais P, Xavier D, Yusuf S. The polypill in the prevention of cardiovascular diseases: key concepts, current status, challenges, and future directions. Circulation. 2010;122:2078–2088. doi: 10.1161/CIRCULATIONAHA.109.873232. [DOI] [PubMed] [Google Scholar]
- Lundberg JO, Weitzberg E. Nitrite reduction to nitric oxide in the vasculature. Am J Physiol Heart Circ Physiol. 2008;295:H477–478. doi: 10.1152/ajpheart.00611.2008. [DOI] [PubMed] [Google Scholar]
- Luscher TF, Barton M. Biology of the endothelium. Clin Cardiol. 1997;20:II-3–10. [PubMed] [Google Scholar]
- Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010;121:505–511. doi: 10.1161/CIRCULATIONAHA.109.886655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller-Delp JM, Spier SA, Ramsey MW, Delp MD. Aging impairs endothelium-dependent vasodilation in rat skeletal muscle arterioles. Am J Physiol Heart Circ Physiol. 2002;283:H1662–1672. doi: 10.1152/ajpheart.00004.2002. [DOI] [PubMed] [Google Scholar]
- Nilsson PM, Boutouyrie P, Laurent S. Vascular aging: A tale of EVA and ADAM in cardiovascular risk assessment and prevention. Hypertension. 2009;54:3–10. doi: 10.1161/HYPERTENSIONAHA.109.129114. [DOI] [PubMed] [Google Scholar]
- Radi R. Nitric oxide, oxidants, and protein tyrosine nitration. Proc Natl Acad Sci U S A. 2004;101:4003–4008. doi: 10.1073/pnas.0307446101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy AK, Li YH, Pham TT, Ochoa LN, Trevino MT, Hartley CJ, Michael LH, Entman ML, Taffet GE. Measurement of aortic input impedance in mice: effects of age on aortic stiffness. Am J Physiol Heart Circ Physiol. 2003;285:H1464–1470. doi: 10.1152/ajpheart.00004.2003. [DOI] [PubMed] [Google Scholar]
- Rippe C, Lesniewski L, Connell M, Larocca T, Donato A, Seals D. Short-term calorie restriction reverses vascular endothelial dysfunction in old mice by increasing nitric oxide and reducing oxidative stress. Aging Cell. 2010 doi: 10.1111/j.1474-9726.2010.00557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals DR, Jablonski KL, Donato AJ. Aging and vascular endothelial function in humans. Clin Sci (Lond) 2010 doi: 10.1042/CS20100476. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi W, Meininger CJ, Haynes TE, Hatakeyama K, Wu G. Regulation of tetrahydrobiopterin synthesis and bioavailability in endothelial cells. Cell Biochem Biophys. 2004;41:415–434. doi: 10.1385/CBB:41:3:415. [DOI] [PubMed] [Google Scholar]
- Sindler AL, Delp MD, Reyes R, Wu G, Muller-Delp JM. Effects of ageing and exercise training on eNOS uncoupling in skeletal muscle resistance arterioles. J Physiol. 2009;587:3885–3897. doi: 10.1113/jphysiol.2009.172221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soucy KG, Ryoo S, Benjo A, Lim HK, Gupta G, Sohi JS, Elser J, Aon MA, Nyhan D, Shoukas AA, Berkowitz DE. Impaired shear stress-induced nitric oxide production through decreased NOS phosphorylation contributes to age-related vascular stiffness. J Appl Physiol. 2006;101:1751–1759. doi: 10.1152/japplphysiol.00138.2006. [DOI] [PubMed] [Google Scholar]
- Spier SA, Delp MD, Meininger CJ, Donato AJ, Ramsey MW, Muller-Delp JM. Effects of ageing and exercise training on endothelium-dependent vasodilatation and structure of rat skeletal muscle arterioles. J Physiol. 2004;556:947–958. doi: 10.1113/jphysiol.2003.060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes KY, Dugas TR, Tang Y, Garg H, Guidry E, Bryan NS. Dietary nitrite prevents hypercholesterolemic microvascular inflammation and reverses endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2009;296:H1281–1288. doi: 10.1152/ajpheart.01291.2008. [DOI] [PubMed] [Google Scholar]
- Sun D, Huang A, Yan EH, Wu Z, Yan C, Kaminski PM, Oury TD, Wolin MS, Kaley G. Reduced release of nitric oxide to shear stress in mesenteric arteries of aged rats. Am J Physiol Heart Circ Physiol. 2004;286:H2249–2256. doi: 10.1152/ajpheart.00854.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton-Tyrrell K, Najjar SS, Boudreau RM, Venkitachalam L, Kupelian V, Simonsick EM, Havlik R, Lakatta EG, Spurgeon H, Kritchevsky S, Pahor M, Bauer D, Newman A. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation. 2005;111:3384–3390. doi: 10.1161/CIRCULATIONAHA.104.483628. [DOI] [PubMed] [Google Scholar]
- Taddei S, Virdis A, Ghiadoni L, Salvetti G, Bernini G, Magagna A, Salvetti A. Age-related reduction of NO availability and oxidative stress in humans. Hypertension. 2001;38:274–279. doi: 10.1161/01.hyp.38.2.274. [DOI] [PubMed] [Google Scholar]
- Taddei S, Virdis A, Mattei P, Ghiadoni L, Gennari A, Fasolo CB, Sudano I, Salvetti A. Aging and endothelial function in normotensive subjects and patients with essential hypertension. Circulation. 1995;91:1981–1987. doi: 10.1161/01.cir.91.7.1981. [DOI] [PubMed] [Google Scholar]
- Ungvari Z, Kaley G, de Cabo R, Sonntag WE, Csiszar A. Mechanisms of Vascular Aging: New Perspectives. J Gerontol A Biol Sci Med Sci. 2010 doi: 10.1093/gerona/glq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Labinskyy N, Gupte S, Chander PN, Edwards JG, Csiszar A. Dysregulation of mitochondrial biogenesis in vascular endothelial and smooth muscle cells of aged rats. Am J Physiol Heart Circ Physiol. 2008;294:H2121–2128. doi: 10.1152/ajpheart.00012.2008. [DOI] [PubMed] [Google Scholar]
- van der Loo B, Labugger R, Skepper JN, Bachschmid M, Kilo J, Powell JM, Palacios-Callender M, Erusalimsky JD, Quaschning T, Malinski T, Gygi D, Ullrich V, Luscher TF. Enhanced peroxynitrite formation is associated with vascular aging. J Exp Med. 2000;192:1731–1744. doi: 10.1084/jem.192.12.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasquez-Vivar J, Kalyanaraman B, Martasek P, Hogg N, Masters BS, Karoui H, Tordo P, Pritchard KA., Jr. Superoxide generation by endothelial nitric oxide synthase: the influence of cofactors. Proc Natl Acad Sci U S A. 1998;95:9220–9225. doi: 10.1073/pnas.95.16.9220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vita JA, Keaney JF., Jr. Endothelial function: a barometer for cardiovascular risk? Circulation. 2002;106:640–642. doi: 10.1161/01.cir.0000028581.07992.56. [DOI] [PubMed] [Google Scholar]
- Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55:1318–1327. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- Wilkinson IB, Franklin SS, Cockcroft JR. Nitric oxide and the regulation of large artery stiffness: from physiology to pharmacology. Hypertension. 2004;44:112–116. doi: 10.1161/01.HYP.0000138068.03893.40. [DOI] [PubMed] [Google Scholar]
- Yang YM, Huang A, Kaley G, Sun D. eNOS uncoupling and endothelial dysfunction in aged vessels. Am J Physiol Heart Circ Physiol. 2009;297:H1829–1836. doi: 10.1152/ajpheart.00230.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalba G, Beaumont FJ, San Jose G, Fortuno A, Fortuno MA, Etayo JC, Diez J. Vascular NADH/NADPH oxidase is involved in enhanced superoxide production in spontaneously hypertensive rats. Hypertension. 2000;35:1055–1061. doi: 10.1161/01.hyp.35.5.1055. [DOI] [PubMed] [Google Scholar]


