Abstract
Purpose of review
Deletion of the α1,3-galactosyltransferase (GalT) gene in pigs has removed a major xenoantigen but has not eliminated the problem of dysregulated coagulation and vascular injury. Rejecting GalT KO organ xenografts almost invariably show evidence of thrombosis and platelet sequestration, and primate recipients frequently develop consumptive coagulopathy (CC). This review examines recent findings that illuminate potential mechanisms of this current barrier to successful xenotransplantation.
Recent findings
The coagulation response to xenotransplantation differs depending on the type of organ and quite likely the distinct vasculatures. Renal xenografts appear more likely to initiate CC than cardiac xenografts, possibly reflecting differential transcriptional responses. Liver xenografts induce rapid and profound thrombocytopenia resulting in recipient death within days due to bleeding; ex vivo data suggest that liver endothelial cells and hepatocytes are responsible for platelet consumption by a coagulation-independent process.
It has been proposed that expression of recipient tissue factor on platelets and monocytes is an important trigger of CC. Finally, pigs transgenic for human anticoagulants and antithrombotics are slowly but surely coming on line, but have not yet been rigorously tested to date.
Summary
Successful control of coagulation dysregulation in xenotransplantation may require different combinatorial pharmacological and genetic strategies for different organs.
Keywords: Coagulation, inflammation, thrombosis, xenograft
Introduction
While deletion of the GalT gene represented a significant advance in the field of xenotransplantation, it did not provide a complete solution to the problem of thrombotic vascular injury to xenografts and the development of coagulopathy in recipients. In this review, we will present an overview of coagulation and particularly its relationship to inflammation and its role in transplant rejection; outline several factors that may predispose xenografts to thrombosis; describe recent data from the pig-to-primate preclinical model that provide insights into the mechanisms of coagulation dysregulation and discuss progress on potential pharmacological and genetic strategies.
Coagulation: activation, regulation, and links to innate immunity and inflammation
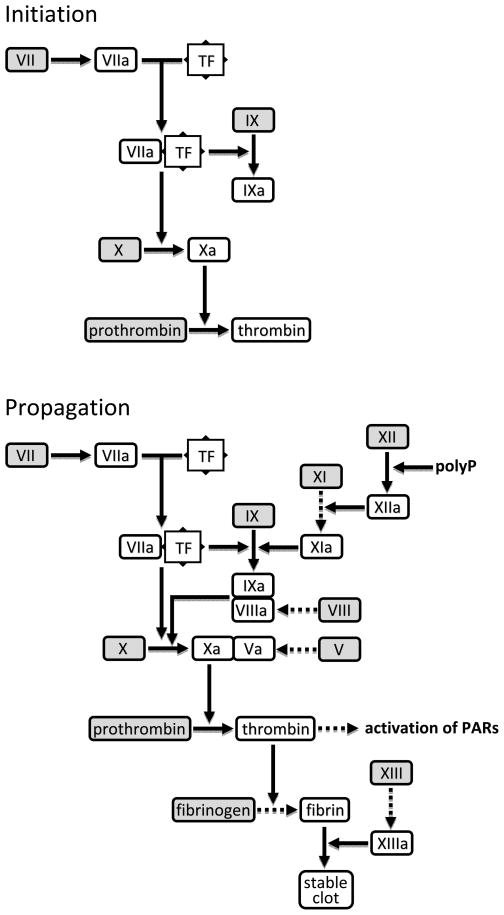
Coagulation is integral to the normal hemostatic response to vascular injury (1, 2). Exposure of tissue factor (TF) to the circulation triggers initiation and propagation of highly coordinated processes that seal the endothelial breach with a fibrin-enmeshed platelet plug (Fig. 1). Three major anticoagulant mechanisms cooperate to tightly regulate and localize clot formation. Tissue factor pathway inhibitor-1 is the primary regulator of the initiation phase (3). Membrane-bound (TFPIβ) and circulating (TFPIα) isoforms contain two Kunitz-type domains that bind and neutralize factor Xa (FXa) and TF/FVIIa. TFPIα has a third Kunitz domain that interacts with protein S to enhance the inactivation of FXa (4). Antithrombin inhibits coagulation at multiple levels by targeting several serine proteases including FXa and thrombin; it is present in the circulation but is far more active when associated with the endothelial cell glycocalyx (5). The protein C pathway comprises several membrane-bound and circulating proteins that inhibit the propagation phase (6). The endothelial protein thrombomodulin (TBM) binds thrombin and alters its substrate specificity, blocking its procoagulant activities and acting as a cofactor for activation of protein C by thrombin. Activated protein C (APC), with its cofactor protein S, inactivates FVa and FVIIIa to arrest further production of thrombin. Endothelial protein C receptor (EPCR) enhances APC generation by the thrombin/TBM complex (7).
Figure 1. Initiation and propagation of coagulation.
Interaction of tissue factor (TF) with traces of circulating FVIIa triggers the initiation phase (top), resulting in the generation of a ‘trickle’ of thrombin. Thrombin activates components of the intrinsic pathway and signals via protease-activated receptors (PARs) on platelets, leukocytes and endothelial cells, resulting in propagation of coagulation and formation of a stable fibrin clot (bottom). Dashed arrows signify thrombin-mediated reactions. PolyP; inorganic polyphosphate released by activated platelets.
Coagulation is intricately connected to innate immunity and inflammation (8–10)•. Thrombin not only activates PARs to cause wide-ranging procoagulant and proinflammatory effects, but also promotes further complement activation by cleaving C3 (11) and C5 (12). Reciprocally, complement activation amplifies coagulation by several mechanisms including C5a-mediated induction of TF on neutrophils (13). While TF is recognized as a key player in inflammation-induced coagulation, recent evidence suggests that TF-independent mechanisms also play a role. Inorganic polyphosphate (polyP) released by activated platelets directly activates the intrinsic coagulation pathway by cleaving FXII (Fig. 1), resulting in thrombin generation and fibrin formation (14)••.
Coagulation is exacerbated during inflammation by the down regulation and degradation of critical endothelial anticoagulant and anti-platelet systems. This is best illustrated by the impact of inflammatory mediators on components of the protein C pathway, with reduced TBM gene expression and mRNA stability, shedding of TBM and EPCR from the endothelial surface, and proteolytic inactivation of EPCR (15–19). Circulating anticoagulants are also affected by inflammation. Activated neutrophils release nucleosomes (DNA/histone complexes), which act as scaffolds for the degradation of TFPIα by neutrophil elastase (20)•. In acute inflammatory states such as sepsis or with liver failure, widespread expression of proinflammatory cytokines and TF can overwhelm regulatory mechanisms, resulting in disseminated intravascular coagulation (DIC). Paradoxically, the systemic consumption of clotting factors and platelets in DIC leaves patients susceptible to life-threatening bleeding.
Coagulation in transplantation
Transplantation of vascularized organs is by its very nature a procedure that generates ischemia, varying degrees of inflammation and, consequently, thrombosis. Graft endothelial cells can be activated by numerous interconnected immune and non-immune factors including ischemia-reperfusion injury (IRI), antibody binding, and complement activation (21). The procoagulant phenotype adopted by activated endothelial cells can in some circumstances precipitate graft loss. Thrombotic microangiopathy (TMA) is a complication of renal allotransplantation that can be triggered by IRI, immunosuppressive drugs, viral infections, or acute antibody-mediated rejection (22). Recipients with mutations affecting particular complement regulatory proteins are at greater risk of TMA (22), reflecting the interplay between the coagulation and complement systems.
Coagulation is a major problem in intraportal islet allo (and xeno) transplantation. Isolated islets contain TF and collagen, and their infusion into the portal vein triggers a powerful innate response known as the instant blood-mediated inflammatory reaction (IBMIR) (23, 24). The features of IBMIR include platelet binding, complement activation, thrombosis and infiltration by neutrophils.
Special coagulation problems presented by xenotransplantation
The tendency for graft intravascular coagulation in xenotransplantation is exacerbated by pre-existing antibodies and by a number of cross-species differences, as discussed below.
Pre-existing antibodies
Pre-existing human anti-pig antibodies pose a significant challenge to solid organ xenotransplantation. Although anti-Gal antibodies predominate and are the primary drivers of hyperacute rejection, they are no longer considered a problem because their target has been eliminated by deletion of the GalT gene. The remaining antibodies are collectively referred to as anti-non-Gal, but their specificities are still mostly unknown and their titers (both IgM and IgG) show considerable variability between individuals (25). A recent study found that some human sera samples had high levels of pre-existing anti-non-Gal IgG antibodies that activated porcine aortic endothelial cells (PAEC) to express TF in a complement-independent manner (26)••. This finding emphasizes that careful screening of recipients will be important in xenotransplantation, just as it is in allotransplantation.
Molecular incompatibilities affecting the control of coagulation and inflammation
As described at the start of this review, coagulation is a rapid and powerful process that requires multiple levels of regulation. Defects in anticoagulant mechanisms can disrupt this fine balance and result in an increased risk of thrombosis (viz. thrombophilia). Most mutations associated with thrombophilia in humans have been found to affect the protein C system (27). The importance of APC in controlling coagulation is demonstrated by the Factor V Leiden mutation, which encodes an APC-resistant FV and predisposes to venous thrombosis. Recent findings have also highlighted APC’s expanding anti-inflammatory role; for example, extracellular histones released by activated macrophages are toxic to endothelial cells, but can be proteolytically inactivated by endogenously generated APC (28)•.
In vitro and ex vivo studies in the 1990s suggested that APC generation in porcine solid organ xenografts may be significantly compromised by cross-species incompatibility in the protein C pathway (29, 30). This was confirmed by a molecular analysis showing that pig TBM binds human thrombin but is a poor cofactor for activation of human protein C (31). The APC-generating capacity of pig TBM in the xenogeneic setting has been estimated at only 1–10% that of human TBM (29–32)•. This defect makes it conceivable that even a relatively small stimulus might give rise to a self-perpetuating cycle of inflammation and coagulation in xenografts.
A second pig-human molecular incompatibility, between von Willebrand Factor (vWF) and platelet glycoprotein Ib (GPIb), has also been documented. Contact with pig vWF spontaneously aggregates human platelets, in the absence of shear stress, due to an aberrant interaction between the O-glycosylated A1 domain and human GPIb (33).
A third potential element relates to porcine endothelial expression of tissue factor pathway inhibitor which does not effectively neutralize human FXa (34, 35), albeit the recombinant forms expressed in primate cells have full anticoagulant activity (36). Several other factors may account for the thromboregulatory failure of pig endothelium and aberrant tissue factor activity in xenograft rejection.
Expression of recipient tissue factor (TF)
Monocyte-associated TF may be induced by both allo- or xenogeneic interactions with endothelium, and is in turn regulated by cell-associated TFPI. Kopp and Robson et al proposed that monocytes may modulate the thrombotic process at sites of vascular injury in association with both allo- and xenograft rejection (35). Dorling and colleagues reported that human platelets and monocytes are likewise activated to express TF by incubation with resting wild-type PAEC, even in the absence of human plasma (37). They proposed this as a procoagulant mechanism generating blood-borne TF that may contribute to the systemic coagulopathy frequently observed in solid organ xenotransplantation.
Direct prothrombinase activity
Pig endothelial cells inducibly express an enzyme that directly activates human prothrombin to thrombin in the absence of other coagulation factors (38). Immunohistochemical analysis of rejected pig-to-baboon renal xenografts revealed upregulation of this enzyme, fgl2, in small vessels and glomerular capillaries, and the intensity of fgl2 staining corresponded to the degree of vascular injury (38). Treatment of PAEC with 20 ng/ml human TNFα has been shown to upregulate fgl2 at the mRNA and protein level (38) and to increase direct prothrombinase activity more than 20-fold (32). Together these data point to an inflammation-mediated mechanism for TF-independent clotting in xenografts.
Coagulation dysregulation in the pig-to-nonhuman primate (NHP) model
The pig-to-NHP model is recognized as the gold standard for preclinical xenotransplantation, but like all models it has limitations, one of which may be exaggeration of the tendency for coagulation specifically in the baboon. A comparison of primate and human whole blood by thromboelastometry, which accurately measures the dynamic process of clot formation and lysis, indicated that primates have a more hypercoagulable profile than humans; in particular, clotting times were shorter, and clot firmness was higher despite lower fibrinogen levels (39)•. However, this species difference at least gives confidence that any solution to coagulation dysregulation identified in the NHP model is likely to be effective in the clinic.
Coagulation dysregulation in GalT KO pig-to-NHP xenotransplantation has been addressed in a number of recent reviews (40–44). What is generally agreed is that failure to completely control the anti-non-Gal response is associated with unregulated coagulation, manifested by TMA within the graft and (frequently) the development of consumptive coagulopathy (CC) in recipients.
It seems likely that the molecular incompatibilities described earlier contribute to this disproportionate reaction, although this has not yet been formally proven given the multiple other pathways that might activate coagulation within the xenograft e.g. humoral immunity. What is less clear is whether refinements in immunosuppression or tolerance induction will provide a solution to the problem. In this section, we will review recent data from pig-to-NHP solid organ xenotransplantation using GalT KO donors.
Kidney
The Pittsburgh group reported that GalT KO pig kidneys transplanted into baboons under suboptimal immunosuppression were rejected at days 2 and 5, with TMA and infiltration by innate immune cells (45). CC was diagnosed in both recipients on the basis of laboratory tests and/or clinical bleeding. The same group subsequently transplanted Gal KO/CD46 pig kidneys into baboons treated with a more effective immunosuppressive regimen and anticoagulation with heparin (46)••. Four recipients died or were euthanased between days 9 and 16 because of the development of CC. The creatinine level was relatively stable in two animals at the time of death; most of the grafts showed minimal evidence of an immune response, and there was no increase in serum anti-pig antibodies, at least by day 10. Interestingly, however, TF expression was detected on platelets within 2 days, on peripheral blood mononuclear cells (PBMCs) from day 4–6 onwards, and in recipient heart and liver at euthanasia. The authors hypothesized that platelets are activated to express TF early after transplantation either in response to inflammatory mediators or simply by contact with pig endothelial cells (37). PBMCs are subsequently activated by thrombin or by adhesion to activated platelets, and the resulting systemic elaboration of TF causes CC. If substantiated, this implies that recipient-derived TF may be a major significant contributor to CC.
The Boston group reported a substantially different outcome following transplantation of seven composite thymus-kidney (thymokidney) grafts from GalT KO miniature swine into immunosuppressed baboons (47)••. The tolerance-directed protocol appeared to block the T cell-mediated xenogeneic response, and graft function remained relatively stable until the death of recipients at 18 to 83 days from causes unrelated to rejection. Although glomerulopathy and TMA was observed in the grafts, none of the recipients developed frank CC. It is difficult to explain why CC was so pronounced in one study (46)•• but not in the other (47)••; potential contributing factors include differences in donor characteristics (domestic outbred GalT KO/CD46 versus miniature inbred GalT KO), treatment protocols inclusive of approaches to achieve tolerance, and surgical techniques.
Heart
Coagulation disturbances have been reported as a feature of cardiac xenotransplantation, although again CC is not always reported. GalT KO miniature swine hearts transplanted into immunosuppressed baboons were rejected after 59 to 179 days by a slowly progressing humoral process involving the development of TMA, but none of the recipients developed CC (48). In a more recent study, hearts from GalT KO outbred pigs were rejected at 5 and 8 weeks, with evidence of TMA and innate immune cell infiltration, and both recipients developed CC (45). However, to further complicate matters, CC was not exhibited in an immunosuppressed baboon that maintained a cardiac xenograft from an outbred GalT KO/CD46 pig for 7 weeks (49), although coagulation parameters were not reported.
Transcriptional analysis has suggested that recipients of cardiac xenografts may be less prone to developing CC and platelet sequestration than renal xenograft recipients due to differential gene responses in the vasculature of the two organs (50)•. The solution to the problem of coagulation dysregulation may therefore need to account for organ-specific differences.
Liver
Coagulopathy and thrombocytopenia are particularly severe problems in liver xenotransplantation. Four immunosuppressed baboons receiving GalT KO/CD46 pig livers developed profound thrombocytopenia within 5 hrs and were euthanased or died at day 5–7 due to CC, with “functional” grafts (51)••4. A study in which wild-type pig livers were perfused ex vivo with purified human platelets has provided insights into the mechanism of thrombocytopenia (52)••. The authors reported that 93% of the platelets disappeared from the perfusate within 15 min, and early biopsies showed phagocytosis of intact platelets by liver sinusoidal endothelial cells (LSECs), a process confirmed in vitro. Later biopsies showed degraded platelets within hepatocytes, suggesting that platelets were transferred from LSECs to hepatocytes via transcytosis. This process appears unrelated to antibody binding and/or complement activation since neither was present in the system. While the exact mechanism is unclear, it was suggested to involve an aberrant interaction between a porcine lectin receptor and a human platelet glycoprotein (52)••.
Treatment options to control coagulation dysregulation
Systemic therapy with traditional anticoagulant and anti-platelet agents including heparin and aspirin appears to be largely ineffective against the development of graft TMA and CC in recipients of GalT KO solid organ xenografts (45, 46, 48, 51)••.
Studies on sepsis-associated DIC, which shares some features with xenotransplantation-associated CC, suggest that manipulation of the protein C pathway may be a more fruitful approach. Indeed, APC infusion reduced mortality in severely septic patients (53) and is now the recommended treatment for this condition (54); benefits of TFPI have been less pronounced (55).
APC produced promising results in an ex vivo perfusion model of renal xenotransplantation, blocking the activation of coagulation in wild type kidneys (56). However, the appeal of APC treatment is reduced by links to increased bleeding risk (53), the likely need for continuous administration, and the fact that it has more potent protective effects when generated endogenously on endothelial cells than when provided exogenously (57). A better alternative may be soluble TBM, which acts ‘on-demand’ as a facilitator of APC production. In rodent models, treatment with recombinant human TBM has been shown to protect against sepsis (58, 59) and ischemia-induced renal dysfunction (60), and liposomal rabbit TBM protected islet grafts from IBMIR (61)•. In the long run, though, genetic overexpression of human TBM on the xenograft seems preferable (see following section).
Statins are a relatively safe class of cholesterol-lowering drugs that have shown promise in small animal and in vitro models of thrombosis, due to their effects on TF expression. Pravastatin was shown to downregulate glomerular TF expression and stabilize renal function in a mouse model of antiphospholipid antibody-mediated TMA (62). Atorvastatin at high concentrations almost completely blocked upregulation of TF transcription in PAEC induced by incubation with sensitized baboon serum, although the corresponding increase in TF activity was only partly prevented (26)••.
For islets, several approaches hold promise. Low molecular weight dextran sulphate protected pig islets from IBMIR in vitro (63) and has been used as an anticoagulant in pig-to-monkey islet xenotransplantation (64). Surface heparinization protected islets from IBMIR in an allogeneic setting (65). Encapsulation offers a means of protecting the xenograft from direct exposure to recipient blood and thus avoiding IBMIR altogether (66, 67), as does the use of alternative transplant sites (68–70).
Further genetic modification of the donor to control coagulation dysregulation
The GalT KO background, preferably bolstered by transgenic expression of one or more complement regulatory proteins, has become the platform for additional genetic modifications to inhibit coagulation and inflammation, several of which are discussed below.
TBM
Overexpression of human TBM in xenografts has obvious appeal: to compensate for the molecular incompatibility and inflammation-mediated downregulation of pig TBM, to neutralize thrombin generated by TF-dependent and -independent mechanisms, and because TBM has potent anti-inflammatory effects distinct from its capacity to promote generation of APC (71, 72). Wild type PAEC transfected to express human TBM significantly suppressed classical and direct prothrombinase activity and delayed clotting of human plasma compared to vector-transfected cells (32), consistent with earlier transduction studies (73).
Expression of human TBM from the mouse H-2Kb promoter in transgenic mice resulted in an anticoagulant phenotype but importantly without any overt bleeding tendency, and modestly prolonged cardiac xenograft survival in a mouse-to-rat model (74)••. Human TBM has also been expressed from the pig TBM promoter in transgenic pigs, resulting in strong endothelial-specific expression in various organs including heart and kidney (75), but functional consequences have not yet been reported.
CD39
The ectonucleotidase CD39 has anti-platelet activity by degrading the platelet agonist ADP (76). Transgenic mice overexpressing human CD39 are healthy and possess an anticoagulant phenotype similar to that of human TBM transgenic mice (77). CD39 overexpression was shown to protect transgenic mouse islets in an ex vivo model of IBMIR (78), hearts in a model of antibody-mediated rejection (77), and kidneys from IRI and transplant-related vascular injury (79)••.
TFPI
Overexpression of TFPI has great potential to control TF-dependent initiation of coagulation in xenotransplantation. Cardiac xenografts from transgenic mice expressing a membrane-anchored form of human TFPIα survived long-term in immunosuppressed rats (80). Transgenic pigs generated using the same construct showed more modest expression of TFPI, but this was sufficient to partially block anti-non-Gal antibody-mediated upregulation of TF transcription and activity in PAEC (26)••. Another group has recently reported the generation of transgenic pigs expressing a modified human TFPIβ attached to the cell surface with a transmembrane linkage (81).
Conclusions
Solving the problem of coagulation dysregulation in xenotransplantation requires careful consideration of the tightly integrated and regulated processes of blood clotting and inflammation. Many natural anticoagulants are also anti-inflammatory, so deficiencies in anticoagulant function resulting from immune activation and/or molecular incompatibility may provoke an excessive self-perpetuating response to even modest proinflammatory signals. Controlling these signals by more effective immunosuppression and/or tolerance induction, and strengthening the xenograft anticoagulant protective defenses, would seem to be the logical and desired approach.
Acknowledgments
The work of the authors described in this review was supported by funding from the National Health and Medical Research Council of Australia, by JDRF and by National Institutes of Health.
References
- 1.Mackman N. Triggers, targets and treatments for thrombosis. Nature. 2008 Feb 21;451(7181):914–8. doi: 10.1038/nature06797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Furie B, Furie BC. Mechanisms of thrombus formation. N Engl J Med. 2008 Aug 28;359(9):938–49. doi: 10.1056/NEJMra0801082. [DOI] [PubMed] [Google Scholar]
- 3.Lwaleed BA, Bass PS. Tissue factor pathway inhibitor: structure, biology and involvement in disease. J Pathol. 2006 Feb;208(3):327–39. doi: 10.1002/path.1871. [DOI] [PubMed] [Google Scholar]
- 4.Ndonwi M, Tuley EA, Broze GJ., Jr The Kunitz-3 domain of TFPI-alpha is required for protein S-dependent enhancement of factor Xa inhibition. Blood. 2010 Aug 26;116(8):1344–51. doi: 10.1182/blood-2009-10-246686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rau JC, Beaulieu LM, Huntington JA, Church FC. Serpins in thrombosis, hemostasis and fibrinolysis. J Thromb Haemost. 2007 Jul;5( Suppl 1):102–15. doi: 10.1111/j.1538-7836.2007.02516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dahlback B, Villoutreix BO. The anticoagulant protein C pathway. FEBS Lett. 2005 Jun 13;579(15):3310–6. doi: 10.1016/j.febslet.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Taylor FB, Jr, Peer GT, Lockhart MS, et al. Endothelial cell protein C receptor plays an important role in protein C activation in vivo. Blood. 2001 Mar 15;97(6):1685–8. doi: 10.1182/blood.v97.6.1685. [DOI] [PubMed] [Google Scholar]
- 8.Danese S, Vetrano S, Zhang L, et al. The protein C pathway in tissue inflammation and injury: pathogenic role and therapeutic implications. Blood. 2010 Feb 11;115(6):1121–30. doi: 10.1182/blood-2009-09-201616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010 Sep;11(9):785–97. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10•.Delvaeye M, Conway EM. Coagulation and innate immune responses: can we view them separately? Blood. 2009 Sep 17;114(12):2367–74. doi: 10.1182/blood-2009-05-199208. This is an excellent overview of the intricate interplay between coagulation, inflammation and innate immunity. [DOI] [PubMed] [Google Scholar]
- 11.Clark A, Weymann A, Hartman E, et al. Evidence for non-traditional activation of complement factor C3 during murine liver regeneration. Mol Immunol. 2008 Jun;45(11):3125–32. doi: 10.1016/j.molimm.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huber-Lang M, Sarma JV, Zetoune FS, et al. Generation of C5a in the absence of C3: a new complement activation pathway. Nat Med. 2006 Jun;12(6):682–7. doi: 10.1038/nm1419. [DOI] [PubMed] [Google Scholar]
- 13.Ritis K, Doumas M, Mastellos D, et al. A novel C5a receptor-tissue factor cross-talk in neutrophils links innate immunity to coagulation pathways. J Immunol. 2006 Oct 1;177(7):4794–802. doi: 10.4049/jimmunol.177.7.4794. [DOI] [PubMed] [Google Scholar]
- 14••.Muller F, Mutch NJ, Schenk WA, et al. Platelet polyphosphates are proinflammatory and procoagulant mediators in vivo. Cell. 2009 Dec 11;139(6):1143–56. doi: 10.1016/j.cell.2009.11.001. This study identifies inorganic polyphosphate released by activated platelets as a key driver of the Factor XII-mediated intrinsic pathway of coagulation, linking primary to secondary hemostasis. It is important in light of the increasing recognition of the role of platelets in dysregulated coagulation in xenotransplantation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lentz SR, Tsiang M, Sadler JE. Regulation of thrombomodulin by tumor necrosis factor-alpha: comparison of transcriptional and posttranscriptional mechanisms. Blood. 1991 Feb 1;77(3):542–50. [PubMed] [Google Scholar]
- 16.Navarro A, Frevel M, Gamero AM, et al. Thrombomodulin RNA is destabilized through its 3′-untranslated element in cells exposed to IFN-gamma. J Interferon Cytokine Res. 2003 Dec;23(12):723–8. doi: 10.1089/107999003772084833. [DOI] [PubMed] [Google Scholar]
- 17.Takano S, Kimura S, Ohdama S, Aoki N. Plasma thrombomodulin in health and diseases. Blood. 1990 Nov 15;76(10):2024–9. [PubMed] [Google Scholar]
- 18.Villegas-Mendez A, Montes R, Ambrose LR, et al. Proteolysis of the endothelial cell protein C receptor by neutrophil proteinase 3. J Thromb Haemost. 2007 May;5(5):980–8. doi: 10.1111/j.1538-7836.2007.02480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Menschikowski M, Hagelgans A, Eisenhofer G, Siegert G. Regulation of endothelial protein C receptor shedding by cytokines is mediated through differential activation of MAP kinase signaling pathways. Exp Cell Res. 2009 Sep 10;315(15):2673–82. doi: 10.1016/j.yexcr.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 20•.Massberg S, Grahl L, von Bruehl ML, et al. Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat Med. 2010 Aug;16(8):887–96. doi: 10.1038/nm.2184. This study identifies a new pathway by which inflammation contributes to thrombosis by downregulating the activity of circulating tissue factor pathway inhibitor. [DOI] [PubMed] [Google Scholar]
- 21.Aird WC. Endothelium and allotransplantation. Transplantation. 2006 Jul 15;82(1 Suppl):S6–8. doi: 10.1097/01.tp.0000231345.23918.bd. [DOI] [PubMed] [Google Scholar]
- 22.Noris M, Remuzzi G. Thrombotic microangiopathy after kidney transplantation. Am J Transplant. 2010 Jul;10(7):1517–23. doi: 10.1111/j.1600-6143.2010.03156.x. [DOI] [PubMed] [Google Scholar]
- 23.Bennet W, Sundberg B, Lundgren T, et al. Damage to porcine islets of Langerhans after exposure to human blood in vitro, or after intraportal transplantation to cynomologus monkeys: protective effects of sCR1 and heparin. Transplantation. 2000 Mar 15;69(5):711–9. doi: 10.1097/00007890-200003150-00007. [DOI] [PubMed] [Google Scholar]
- 24.Johansson H, Lukinius A, Moberg L, et al. Tissue factor produced by the endocrine cells of the islets of Langerhans is associated with a negative outcome of clinical islet transplantation. Diabetes. 2005 Jun;54(6):1755–62. doi: 10.2337/diabetes.54.6.1755. [DOI] [PubMed] [Google Scholar]
- 25.Baumann BC, Stussi G, Huggel K, et al. Reactivity of human natural antibodies to endothelial cells from Galalpha(1,3)Gal-deficient pigs. Transplantation. 2007 Jan 27;83(2):193–201. doi: 10.1097/01.tp.0000250478.00567.e5. [DOI] [PubMed] [Google Scholar]
- 26••.Lin CC, Ezzelarab M, Hara H, et al. Atorvastatin or transgenic expression of TFPI inhibits coagulation initiated by anti-nonGal IgG binding to porcine aortic endothelial cells. J Thromb Haemost. 2010 Sep;8(9):2001–10. doi: 10.1111/j.1538-7836.2010.03950.x. An in vitro study identifying two approaches to tackle the coagulation problem in xenotransplantation: statin treatment to downregulate TF expression, and transgenic expression of TFPI to inhibit TF activity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dahlback B. Advances in understanding pathogenic mechanisms of thrombophilic disorders. Blood. 2008 Jul 1;112(1):19–27. doi: 10.1182/blood-2008-01-077909. [DOI] [PubMed] [Google Scholar]
- 28•.Xu J, Zhang X, Pelayo R, et al. Extracellular histones are major mediators of death in sepsis. Nat Med. 2009 Nov;15(11):1318–21. doi: 10.1038/nm.2053. This article describes the ability of activated protein C to degrade toxic histones released by activated macrophages, adding to the list of APC’s anti-inflammatory activities. It is relevant because molecular incompatibility in the protein C pathway results in a reduced capacity by pig endothelial cells to activate human protein C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Siegel JB, Grey ST, Lesnikoski BA, et al. Xenogeneic endothelial cells activate human prothrombin. Transplantation. 1997 Sep 27;64(6):888–96. doi: 10.1097/00007890-199709270-00017. [DOI] [PubMed] [Google Scholar]
- 30.Lawson JH, Daniels LJ, Platt JL. The evaluation of thrombomodulin activity in porcine to human xenotransplantation. Transplant Proc. 1997 Feb-Mar;29(1–2):884–5. doi: 10.1016/s0041-1345(96)00192-3. [DOI] [PubMed] [Google Scholar]
- 31.Roussel JC, Moran CJ, Salvaris EJ, et al. Pig thrombomodulin binds human thrombin but is a poor cofactor for activation of human protein C and TAFI. Am J Transplant. 2008 Jun;8(6):1101–12. doi: 10.1111/j.1600-6143.2008.02210.x. [DOI] [PubMed] [Google Scholar]
- 32•.Miwa Y, Yamamoto K, Onishi A, et al. Potential value of human thrombomodulin and DAF expression for coagulation control in pig-to-human xenotransplantation. Xenotransplantation. 2010 Jan–Feb;17(1):26–37. doi: 10.1111/j.1399-3089.2009.00555.x. This in vitro study shows that overexpression of human thrombomodulin in pig endothelial cells blocks both classical and direct prothrombinase activity, providing further support for the generation of transgenic pigs expressing this protein. [DOI] [PubMed] [Google Scholar]
- 33.Schulte Am Esch J, 2nd, Robson SC, Knoefel WT, et al. O-linked glycosylation and functional incompatibility of porcine von Willebrand factor for human platelet GPIb receptors. Xenotransplantation. 2005 Jan;12(1):30–7. doi: 10.1111/j.1399-3089.2004.00187.x. [DOI] [PubMed] [Google Scholar]
- 34.Kopp CW, Siegel JB, Hancock WW, et al. Effect of porcine endothelial tissue factor pathway inhibitor on human coagulation factors. Transplantation. 1997 Mar 15;63(5):749–58. doi: 10.1097/00007890-199703150-00023. [DOI] [PubMed] [Google Scholar]
- 35.Kopp CW, Robson SC, Siegel JB, et al. Regulation of monocyte tissue factor activity by allogeneic and xenogeneic endothelial cells. Thromb Haemost. 1998 Mar;79(3):529–38. [PubMed] [Google Scholar]
- 36.Lee KF, Salvaris EJ, Roussel JC, et al. Recombinant pig TFPI efficiently regulates human tissue factor pathways. Xenotransplantation. 2008 May;15(3):191–7. doi: 10.1111/j.1399-3089.2008.00476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin CC, Chen D, McVey JH, et al. Expression of tissue factor and initiation of clotting by human platelets and monocytes after incubation with porcine endothelial cells. Transplantation. 2008 Sep 15;86(5):702–9. doi: 10.1097/TP.0b013e31818410a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ghanekar A, Mendicino M, Liu H, et al. Endothelial induction of fgl2 contributes to thrombosis during acute vascular xenograft rejection. J Immunol. 2004 May 1;172(9):5693–701. doi: 10.4049/jimmunol.172.9.5693. [DOI] [PubMed] [Google Scholar]
- 39•.Spiezia L, Bertini D, Boldrin M, et al. Reference values for thromboelastometry (ROTEM(R)) in cynomolgus monkeys (Macaca fascicularis) Thromb Res. 2010 Oct;126(4):e294–7. doi: 10.1016/j.thromres.2010.07.016. This study reports that one of the commonly used recipient species in pig-to-primate preclinical studies has a more hypercoagulable profile than humans, suggesting that dysregulated coagulation may be particularly difficult to correct in this model. [DOI] [PubMed] [Google Scholar]
- 40.Lin CC, Cooper DK, Dorling A. Coagulation dysregulation as a barrier to xenotransplantation in the primate. Transpl Immunol. 2009 Jun;21(2):75–80. doi: 10.1016/j.trim.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cowan PJ, d’Apice AJ. Complement activation and coagulation in xenotransplantation. Immunol Cell Biol. 2009 Mar–Apr;87(3):203–8. doi: 10.1038/icb.2008.107. [DOI] [PubMed] [Google Scholar]
- 42.Pierson RN, 3rd, Dorling A, Ayares D, et al. Current status of xenotransplantation and prospects for clinical application. Xenotransplantation. 2009 Sep–Oct;16(5):263–80. doi: 10.1111/j.1399-3089.2009.00534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ekser B, Cooper DK. Overcoming the barriers to xenotransplantation: prospects for the future. Expert Rev Clin Immunol. 2010 Mar;6(2):219–30. doi: 10.1586/eci.09.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schmelzle M, Schulte Esch J, 2nd, Robson SC. Coagulation, platelet activation and thrombosis in xenotransplantation. Curr Opin Organ Transplant. 2010 Apr;15(2):212–8. doi: 10.1097/MOT.0b013e3283373ccc. [DOI] [PubMed] [Google Scholar]
- 45.Ezzelarab M, Garcia B, Azimzadeh A, et al. The innate immune response and activation of coagulation in alpha1,3-galactosyltransferase gene-knockout xenograft recipients. Transplantation. 2009 Mar 27;87(6):805–12. doi: 10.1097/TP.0b013e318199c34f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46••.Lin CC, Ezzelarab M, Shapiro R, et al. Recipient tissue factor expression is associated with consumptive coagulopathy in pig-to-primate kidney xenotransplantation. Am J Transplant. 2010 Jul;10(7):1556–68. doi: 10.1111/j.1600-6143.2010.03147.x. This study tracks the elaboration of TF during early development of consumptive coagulopathy (CC) in baboons receiving GalT KO/CD46 renal xenografts. It points to TF expression by recipient platelets and monocytes rather than by xenograft endothelial cells as being the major contributor to CC in this model. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47••.Griesemer AD, Hirakata A, Shimizu A, et al. Results of gal-knockout porcine thymokidney xenografts. Am J Transplant. 2009 Dec;9(12):2669–78. doi: 10.1111/j.1600-6143.2009.02849.x. This study reports the survival of functional GalT KO thymokidneys in baboons for up to 83 days in the absence of overt CC. It suggests that coagulopathic complications are at least dampened by tolerance-inducing protocols. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shimizu A, Hisashi Y, Kuwaki K, et al. Thrombotic microangiopathy associated with humoral rejection of cardiac xenografts from alpha1,3-galactosyltransferase gene-knockout pigs in baboons. Am J Pathol. 2008 Jun;172(6):1471–81. doi: 10.2353/ajpath.2008.070672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bauer A, Postrach J, Thormann M, et al. First experience with heterotopic thoracic pig-to- baboon cardiac xenotransplantation. Xenotransplantation. 2010 May;17(3):243–9. doi: 10.1111/j.1399-3089.2010.00587.x. [DOI] [PubMed] [Google Scholar]
- 50•.Knosalla C, Yazawa K, Behdad A, et al. Renal and cardiac endothelial heterogeneity impact acute vascular rejection in pig-to-baboon xenotransplantation. Am J Transplant. 2009 May;9(5):1006–16. doi: 10.1111/j.1600-6143.2009.02602.x. This study examines changes in the transcription of certain genes involved with coagulation, fibrinolysis and platelet function. It identifies a differential response in the vasculature of renal and cardiac xenografts, which may account for the apparently different course of coagulation dysregulation in recipients of these organs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51••.Ekser B, Long C, Echeverri GJ, et al. Impact of thrombocytopenia on survival of baboons with genetically modified pig liver transplants: clinical relevance. Am J Transplant. 2010 Feb;10(2):273–85. doi: 10.1111/j.1600-6143.2009.02945.x. This study demonstrates that while GalT KO/CD46 liver xenografts will function for up to a week in baboons, they induce very rapid consumption of platelets and severe bleeding complications. It highlights that different pharmacological and/or genetic strategies may be required for different organs. [DOI] [PubMed] [Google Scholar]
- 52••.Burlak C, Paris LL, Chihara RK, et al. The fate of human platelets perfused through the pig liver: implications for xenotransplantation. Xenotransplantation. 2010 Sep–Oct;17(5):350–61. doi: 10.1111/j.1399-3089.2010.00605.x. This paper reports on a potential mechanism for the profound thrombocytopenia in primate recipients of pig liver xenografts. It describes an ex vivo model showing that human platelets appear to be rapidly phagocytosed by liver endothelial cells before transfer to hepatocytes for degradation. [DOI] [PubMed] [Google Scholar]
- 53.Bernard GR, Vincent JL, Laterre PF, et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001 Mar 8;344(10):699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- 54.Dellinger RP, Levy MM, Carlet JM, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Intensive Care Med. 2008 Jan;34(1):17–60. doi: 10.1007/s00134-007-0934-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.LaRosa SP, Opal SM. Tissue factor pathway inhibitor and antithrombin trial results. Crit Care Clin. 2005 Jul;21(3):433–48. doi: 10.1016/j.ccc.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 56.Ramackers W, Friedrich L, Tiede A, et al. Effects of pharmacological intervention on coagulopathy and organ function in xenoperfused kidneys. Xenotransplantation. 2008 Feb;15(1):46–55. doi: 10.1111/j.1399-3089.2008.00443.x. [DOI] [PubMed] [Google Scholar]
- 57.Feistritzer C, Schuepbach RA, Mosnier LO, et al. Protective signaling by activated protein C is mechanistically linked to protein C activation on endothelial cells. J Biol Chem. 2006 Jul 21;281(29):20077–84. doi: 10.1074/jbc.M600506200. [DOI] [PubMed] [Google Scholar]
- 58.Iba T, Nakarai E, Takayama T, et al. Combination effect of antithrombin and recombinant human soluble thrombomodulin in a lipopolysaccharide induced rat sepsis model. Crit Care. 2009;13(6):R203. doi: 10.1186/cc8210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nagato M, Okamoto K, Abe Y, et al. Recombinant human soluble thrombomodulin decreases the plasma high-mobility group box-1 protein levels, whereas improving the acute liver injury and survival rates in experimental endotoxemia. Crit Care Med. 2009 Jul;37(7):2181–6. doi: 10.1097/CCM.0b013e3181a55184. [DOI] [PubMed] [Google Scholar]
- 60.Sharfuddin AA, Sandoval RM, Berg DT, et al. Soluble thrombomodulin protects ischemic kidneys. J Am Soc Nephrol. 2009 Mar;20(3):524–34. doi: 10.1681/ASN.2008060593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61•.Cui W, Wilson JT, Wen J, et al. Thrombomodulin improves early outcomes after intraportal islet transplantation. Am J Transplant. 2009 Jun;9(6):1308–16. doi: 10.1111/j.1600-6143.2009.02652.x. The results in this paper suggest that increasing the capacity to activate protein C may be protective for islet grafts, not just solid organ grafts. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Seshan SV, Franzke CW, Redecha P, et al. Role of tissue factor in a mouse model of thrombotic microangiopathy induced by antiphospholipid antibodies. Blood. 2009 Aug 20;114(8):1675–83. doi: 10.1182/blood-2009-01-199117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Goto M, Johansson H, Maeda A, et al. Low molecular weight dextran sulfate prevents the instant blood-mediated inflammatory reaction induced by adult porcine islets. Transplantation. 2004 Mar 15;77(5):741–7. doi: 10.1097/01.tp.0000114872.26990.4f. [DOI] [PubMed] [Google Scholar]
- 64.van der Windt DJ, Bottino R, Casu A, et al. Long-term controlled normoglycemia in diabetic non-human primates after transplantation with hCD46 transgenic porcine islets. Am J Transplant. 2009 Dec;9(12):2716–26. doi: 10.1111/j.1600-6143.2009.02850.x. [DOI] [PubMed] [Google Scholar]
- 65.Cabric S, Sanchez J, Lundgren T, et al. Islet surface heparinization prevents the instant blood- mediated inflammatory reaction in islet transplantation. Diabetes. 2007 Aug;56(8):2008–15. doi: 10.2337/db07-0358. [DOI] [PubMed] [Google Scholar]
- 66.Cui H, Tucker-Burden C, Cauffiel SM, et al. Long-term metabolic control of autoimmune diabetes in spontaneously diabetic nonobese diabetic mice by nonvascularized microencapsulated adult porcine islets. Transplantation. 2009 Jul 27;88(2):160–9. doi: 10.1097/TP.0b013e3181abbfc1. [DOI] [PubMed] [Google Scholar]
- 67.Dufrane D, Gianello P. Pig islets for clinical islet xenotransplantation. Curr Opin Nephrol Hypertens. 2009 Nov;18(6):495–500. doi: 10.1097/MNH.0b013e328331a8e3. [DOI] [PubMed] [Google Scholar]
- 68.Berman DM, O’Neil JJ, Coffey LC, et al. Long-term survival of nonhuman primate islets implanted in an omental pouch on a biodegradable scaffold. Am J Transplant. 2009 Jan;9(1):91–104. doi: 10.1111/j.1600-6143.2008.02489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van der Windt DJ, Echeverri GJ, Ijzermans JN, Cooper DK. The choice of anatomical site for islet transplantation. Cell Transplant. 2008;17(9):1005–14. doi: 10.3727/096368908786991515. [DOI] [PubMed] [Google Scholar]
- 70.Echeverri GJ, McGrath K, Bottino R, et al. Endoscopic gastric submucosal transplantation of islets (ENDO-STI): technique and initial results in diabetic pigs. Am J Transplant. 2009 Nov;9(11):2485–96. doi: 10.1111/j.1600-6143.2009.02815.x. [DOI] [PubMed] [Google Scholar]
- 71.Conway EM, Van de Wouwer M, Pollefeyt S, et al. The lectin-like domain of thrombomodulin confers protection from neutrophil-mediated tissue damage by suppressing adhesion molecule expression via nuclear factor kappaB and mitogen-activated protein kinase pathways. J Exp Med. 2002 Sep 2;196(5):565–77. doi: 10.1084/jem.20020077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Van de Wouwer M, Plaisance S, De Vriese A, et al. The lectin-like domain of thrombomodulin interferes with complement activation and protects against arthritis. J Thromb Haemost. 2006 Aug;4(8):1813–24. doi: 10.1111/j.1538-7836.2006.02033.x. [DOI] [PubMed] [Google Scholar]
- 73.Kopp CW, Grey ST, Siegel JB, et al. Expression of human thrombomodulin cofactor activity in porcine endothelial cells. Transplantation. 1998 Jul 27;66(2):244–51. doi: 10.1097/00007890-199807270-00019. [DOI] [PubMed] [Google Scholar]
- 74••.Crikis S, Zhang XM, Dezfouli S, et al. Anti-inflammatory and anticoagulant effects of transgenic expression of human thrombomodulin in mice. Am J Transplant. 2010 Feb;10(2):242–50. doi: 10.1111/j.1600-6143.2009.02939.x. An important study showing that overexpression of human thrombomodulin in mice results in graft- protective effects without impacting on animal health. An even greater effect might be expected in pigs because of the documented incompatibility of pig thrombomodulin with the human protein C pathway. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Aigner B, Klymiuk N, Wolf E. Transgenic pigs for xenotransplantation: selection of promoter sequences for reliable transgene expression. Curr Opin Organ Transplant. 2010 Apr;15(2):201–6. doi: 10.1097/MOT.0b013e328336ba4a. [DOI] [PubMed] [Google Scholar]
- 76.Kaczmarek E, Koziak K, Sevigny J, et al. Identification and characterization of CD39/vascular ATP diphosphohydrolase. J Biol Chem. 1996 Dec 20;271(51):33116–22. doi: 10.1074/jbc.271.51.33116. [DOI] [PubMed] [Google Scholar]
- 77.Dwyer KM, Robson SC, Nandurkar HH, et al. Thromboregulatory manifestations in human CD39 transgenic mice and the implications for thrombotic disease and transplantation. J Clin Invest. 2004 May;113(10):1440–6. doi: 10.1172/JCI19560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dwyer KM, Mysore TB, Crikis S, et al. The transgenic expression of human CD39 on murine islets inhibits clotting of human blood. Transplantation. 2006 Aug 15;82(3):428–32. doi: 10.1097/01.tp.0000229023.38873.c0. [DOI] [PubMed] [Google Scholar]
- 79••.Crikis S, Lu B, Murray-Segal LM, et al. Transgenic Overexpression of CD39 Protects Against Renal Ischemia-Reperfusion and Transplant Vascular Injury. Am J Transplant. 2010 Sep 14; doi: 10.1111/j.1600-6143.2010.03257.x. CD39 degrades the platelet agonist ADP and promotes generation of adenosine. This paper extends earlier findings to show that overexpression of CD39 protects mouse renal grafts from ischemia-reperfusion injury via anti-inflammatory adenosine receptor signalling, reinforcing CD39’s appeal as a transgene to be expressed in pigs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen D, Weber M, McVey JH, et al. Complete inhibition of acute humoral rejection using regulated expression of membrane-tethered anticoagulants on xenograft endothelium. Am J Transplant. 2004 Dec;4(12):1958–63. doi: 10.1111/j.1600-6143.2004.00625.x. [DOI] [PubMed] [Google Scholar]
- 81.Lee H, Lee B, Kim Y, et al. Characterization of Transgenic Pigs That Express Human Decay Accelerating Factor and Cell Membrane-tethered Human Tissue Factor Pathway Inhibitor. Reprod Domest Anim. 2010 Jul 4; doi: 10.1111/j.1439-0531.2010.01670.x. [DOI] [PubMed] [Google Scholar]