Abstract
In tropical forests, regional differences in annual rainfall correlate with differences in plant species composition. Although water availability is clearly one factor determining species distribution, other environmental variables that covary with rainfall may contribute to distributions. One such variable is light availability in the understory, which decreases towards wetter forests due to differences in canopy density and phenology. We established common garden experiments in three sites along a rainfall gradient across the Isthmus of Panama in order to measure the differences in understory light availability, and to evaluate their influence on the performance of 24 shade-tolerant species with contrasting distributions. Within sites, the effect of understory light availability on species performance depended strongly on water availability. When water was not limiting, either naturally in the wetter site or through water supplementation in drier sites, seedling performance improved at higher light. In contrast, when water was limiting at the drier sites, seedling performance was reduced at higher light, presumably due to an increase in water stress that affected mostly wet-distribution species. Although wetter forest understories were on average darker, wet-distribution species were not more shade-tolerant than dry-distribution species. Instead, wet-distribution species had higher absolute growth rates and, when water was not limiting, were better able to take advantage of small increases in light than dry-distribution species. Our results suggest that in wet forests the ability to grow fast during temporary increases in light may be a key trait for successful recruitment. The slower growth rates of the dry-distribution species, possibly due to trade-offs associated with greater drought tolerance, may exclude these species from wetter forests.
Electronic supplementary material
The online version of this article (doi:10.1007/s00442-010-1832-9) contains supplementary material, which is available to authorized users.
Keywords: Panama, Shade tolerance, Drought tolerance, Tropical dry forest, Tropical wet forest
Introduction
Changes in species composition along environmental gradients increase species diversity at regional scales (Chave 2008). For that reason, a central question in plant ecology is how biotic and abiotic interactions determine why species grow where they do, and to what extent species’ adaptations to environmental niches can limit their spatial distributions (Suding et al. 2003; Gaston 2009; Sexton et al. 2009). At the regional scale, an important correlate of species turnover is annual rainfall, which in tropical ecosystems can vary tenfold between wet and dry forests. Change in forest composition along rainfall gradients has been well documented in the literature (Clinebell et al. 1995; Swaine 1996; Bongers et al. 1999; Pyke et al. 2001; Davidar et al. 2007). However, there are many environmental variables that covary with annual rainfall and may contribute to determining species geographic distributions. Because the interactions of these variables are complex and also differ among locations, understanding the mechanisms that limit species distributions along this gradient is challenging.
Here, we address the mechanisms that promote species turnover along a rainfall gradient as two questions. What prevents wet-distribution species from colonizing dry forests, and what prevents dry-distribution species from colonizing wet forests? Recent evidence suggests that intolerance to seasonal drought is the main factor limiting the distributions of wet-forest species. Drought tolerance correlates with species distributions along rainfall gradients (Engelbrecht et al. 2007). Also, wet-distribution species have fewer adaptations to cope with water stress (Baltzer et al. 2008; Kursar et al. 2009) and, in addition, suffer higher dry-season mortality if experimentally transplanted to a dry forest (Brenes-Arguedas et al. 2009). In contrast, the mechanisms that prevent dry-distribution species from establishing in wet forests are less clear. Among other possibilities, wetter sites tend to have poorer soils (ter Steege et al. 1993; Santiago et al. 2005), higher pest-pressure (Coley and Barone 1996; Givnish 1999; Brenes-Arguedas et al. 2009), and lower light availability (see “Discussion”). Our studies suggest that soil and herbivore effects are subtle relative to the effects of water limitation and, if present, may only be demonstrated in long-term experiments (Brenes-Arguedas et al. 2008, 2009). The present study focuses on whether light availability influences the distribution of species along a rainfall gradient on the Isthmus of Panama.
Dry forests may have higher light availability in the understory for a number of reasons. Dry forests can have fewer trees and less basal area per hectare than wetter forests (Murphy and Lugo 1986; Losos and the CTFS Working Group 2004). Adaptations for water balance and temperature control can favor small leaves (Givnish 1984), and this may result in lower leaf area index. Deciduousness during the dry season should result in more canopy openness during part of the year (Condit et al. 2000). Finally, lower rainfall may correlate with lower cloudiness and higher canopy-level sunlight (Wright and van Schaik 1994). While each of these factors, alone or in combination with others, is likely to result in decreasing understory light availability with increasing rainfall, to our knowledge, the magnitude of this light gradient has not been measured.
The importance of light limitation and adaptations to contrasting light environments within a site are well documented (Bloor and Grubb 2003; Balderrama and Chazdon 2005; Baltzer and Thomas 2007). Here, we ask if a similar mechanism of niche partitioning based upon light availability contributes to the turnover of shade-tolerant species along a rainfall/light gradient. To address this, we measured understory light along a rainfall gradient, and studied the responses of shade-tolerant plants that occur in understory light environments, as these are the most common species and micro-habitats in tropical forests.
Dry-distribution species may have adaptations that allow them to take advantage of higher understory light in drier forests and, due to trade-offs, these may be less shade-tolerant than wet-distribution species (Smith and Huston 1989; Givnish 1999). Hence, dry-distribution species may be excluded from wetter forests by their inability to tolerate lower understory light. However, more light-demanding species tend to have faster growth rates and superior competitive ability relative to shade-tolerators (Kitajima 1994), and previous analyses in our study system suggest that growth rates are faster for wet-distribution species (Brenes-Arguedas et al. 2009). Thus, an alternative hypothesis is that competition for a limiting resource, light or nutrients, may be a major determinant of individual success in wetter forests (Goldberg 1990). In tropical rainforests, competition has received much attention from the perspective of shade-tolerant versus gap-requiring species at a single site, with limited consideration of how competition among shade-tolerant species may change along a light gradient.
Understory light availability may also interact with other environmental variables that covary with annual rainfall to influence species performance. For example, lower water availability in drier sites may result in higher probability of desiccation in high-light microsites, especially for drought-intolerant species. Wetter, tropical forests may have higher pathogen and herbivore pressure than dry forests (Coley and Barone 1996; Givnish 1999; Brenes-Arguedas et al. 2009). Pathogen attack may interact with low light availability in wetter forests, but it is not clear how herbivory may interact with understory light availability.
We used light, growth and mortality data from common garden experiments in three different sites along a rainfall gradient in central Panama to evaluate seedling responses to understory light variation. In two of these sites, we also evaluated the interactions between light availability, insect herbivore attack and water limitations using experimental herbivore exclusion and water supplementation treatments. Specifically, we address the following questions: (1) do understory light levels decrease with increasing rainfall; (2) does variation in understory light influence the growth and mortality of seedlings; (3) do differences in water availability and pest pressure along the rainfall gradient influence seedling responses to light; and (4) do light responses, competitive ability or shade tolerances differ between wet- and dry-distribution species? These results are used to address the issue of whether or not variation in understory light plays a role in determining species distributions along the rainfall gradient.
Materials and methods
Study sites
The study sites were in the Isthmus of Panama where continuous forest stretches between the Atlantic and Pacific Oceans. Although the Isthmus of Panama is only 60 km wide, there is a gradient from drier forests with less than 2,000 mm of rainfall per year near the Pacific side, to wetter forests with more than 3,000 mm rainfall per year on the Atlantic side. This rainfall gradient results in a clear turnover of species, such that there is almost no overlap in the 50 most common species in opposite sides of the Isthmus (Pyke et al. 2001). We established common gardens in three sites along this rainfall gradient. These are dry (Pacific side), moist (middle), and wet (Atlantic side) sites (Fig. S1), all with elevations <150 m above sea level and average daily temperatures of 27–28°C. The drier site, with annual rainfall of 1,740 mm, was in Gunn Hill in Ciudad del Saber, Clayton (9°0′50″N, 79°35′W). The moist site, with annual rainfall of 2,600 mm, was in Buena Vista peninsula of the Barro Colorado Nature Monument (9°11′N, 79°49′W). The vegetation at both sites is typical of lowland, semi-deciduous, tropical moist forest. The wetter site, with annual rainfall of 3,020 mm, was in the Fort Sherman Canopy Crane site within San Lorenzo National Park (9°17′N, 79°58′W). The vegetation at this site is typical of lowland, evergreen tropical moist forest. Soil properties are described in Brenes-Arguedas et al. (2008) for the wet and dry sites and in Baillie et al. (2007) for a site near Buena Vista.
Study species
The experiment used 24 species with contrasting distributions along the rainfall gradient (Table S1). We collected seedlings in 2005 in Parque Nacional San Lorenzo (wet forest), Parque Nacional Soberanía (moist forest), Ciudad del Saber in Clayton and Parque Natural Metropolitano (dry forests). Using the sources described elsewhere (Brenes-Arguedas et al. 2008, 2009), species were classified as wet- or dry-distribution when their range was limited to the wet or the dry forests or when they were widespread but clearly more abundant in one of the two regions. Seedlings were potted temporarily and maintained at low light in a shade house before being transplanted to the study sites. For most plots, this was only a few weeks after collection. However, the moist site plots were established 1 year later, and these seedlings were 1 year older at the time of planting. Further details on seedling collection and age are in Brenes-Arguedas et al. (2009).
Common garden experiments
The results reported here represent the analysis of two separate common garden experiments. The first experiment, at the wet and dry sites, was planted between August and December 2005. Seedling performance was followed for 12–17 months, until December 2006. There were ten plots per site, although one plot from the wet site had to be discarded 6 months into the experiment due to a tree fall. The plot locations were chosen to obtain a variety of understory light environments (avoiding gaps or gap-edges), and were selected based on subjective estimates of light availability. In both sites, each plot was subdivided into four 1 m × 1 m subplots with fully crossed watering and herbivore exclusion treatments. Two sub-plots were watered manually during the dry season to supplement rainfall to 50 mm week−1 (W), sufficient to prevent most desiccation-induced mortality (Brenes-Arguedas et al. 2009), and two were unwatered controls (C). One watered and one control subplot were protected with mesh cages to exclude herbivores. The year 2006 was a wet year, although within the normal range of long-term variation. At the dry site, dry-season rainfall, from January to March, was 80% higher than average (based on data in Kaufmann and Paton 2008; Fig. S2). The details of the experimental methods, and the data on seedling growth and mortality in these experimental treatments have been reported elsewhere (Brenes-Arguedas et al. 2009).
The second experiment, at the moist site, was planted 1 year later, in August 2006. Seedling performance was followed for 17 months, until December 2007. The moist site had 20 plots distributed in 3 locations, all within 1 ha of forest. Plot location was also chosen to obtain a variety of understory light environments, but for this site light was measured before plot placement. All of the moist-site plots were watered during the dry season. Thus, moist-site plots are comparable to the watered and uncaged subplots in the dry and wet sites. In contrast to the first experiment, 2007 was drier than average. At the moist site, dry season rainfall, from January to March 2007, was 35% lower than the long-term average for the same months, and 50% lower than the rainfall observed during the 2006 dry season (based on data in Kaufmann and Paton 2008; Fig. S2).
In each plot or sub-plot in each site, we planted one individual from each of the 24 species about 20 cm apart from each other to avoid shading. To maintain sample size, we replaced seedlings that died during the first 6 months of the experiments.
Light measurements
To compare understory light within and among forest types, we measured instantaneous photosynthetic photon flux density in the experimental plots in units of μmol m−2 s−1 from 0600 to 1800 hours. We used two types of quantum sensors: LI-190 sensors (LI-COR, Lincoln, NE, USA), and QSO sensors (Apogee Instruments, Logan, UT, USA) and CR200 and CR1000 data-loggers (Campbell Scientific, Logan, UT, USA). QSO sensors were modified by Apogee to provide sensitivities of 0.1 or 0.3 μmol of photons m−2 s−1 per mV. In order to avoid overriding the voltage range of the data-loggers (2.5 V), the 0.1-μmol sensors were used in sites with very low light and the 0.3-μmol sensors were used in sites with intermediate light.
Sensors were placed 0.5 m above the forest floor. In the moist site, sensors were placed at the beginning of the experiment, in August 2006, and kept permanently in each plot until June 2007. Each month, sensors were rotated to different corners of the plot. Thus, for the first 11 months of the moist-site experiment, we measured light for each plot daily. For the wet and dry sites, light measurements started 6 months into the experiment, in January 2006, and continued until the end of the experiment, in December 2006. For these two sites, light in each plot was not measured daily as in the moist site; instead, sensors were rotated among plots and sub-plots. Thus, for each sub-plot, we collected light data for a number of random days throughout the experiment spanning the dry and the wet seasons.
Instantaneous light data were integrated to daily total photosynthetic photon flux in the understory (PPFU, with units of mol m−2 day−1). We calculated percent transmittance (%T) in each plot and sub-plot as PPFU/PPFO. Where PPFO is the daily, integrated photosynthetic photon flux that is incident in the open (measured above the canopy). PPFO data were obtained from the weather stations of Barro Colorado Island (BCI) canopy tower, Parque Natural Metropolitano Canopy Crane, and Fort Sherman Canopy Crane, 2, 4 and 1 km from the moist, dry and wet sites, respectively (Kaufmann and Paton 2008). PPFO from Parque Natural Metropolitano had gaps, which we complemented using data from a different light sensor and from the BCI station.
Seedling measurements
Mortality
We censused each seedling for survival once a month for the duration of the experiments. Because death due to transplant stress was not easy to separate from other causes of death, we included in the survival analysis only those seedlings that had survived at least one census after planting. The start date for each seedling was the first day they were censused alive after they were planted. If a seedling was found dead, we noted the cause of death whenever possible (i.e., drought, pathogens, herbivores).
Growth
For each individual seedling, we calculated three measures of growth: stem height growth (StmHtGr), net leaf growth (NetLfGr), and new leaf production (NewLfPr). Once a month, we measured height (in cm) and counted the total number of leaves and the number of new leaves produced since the last census. These growth rates were best quantified using a linear regression because the experiments were relatively short, all seedlings had similar sizes, and seedling growth in the understory was very slow. Thus, mean StmHtGr was calculated as the slope of the linear regression of height as a function of time in months (units of mm/month). NetLfGr was the slope of the total number of leaves at each census as a function of time in months, and NewLfPr was calculated by summing all new leaves produced since planting and dividing by the total number of months the plant was in the experiment. Leaf numbers were converted to leaf area by multiplying by the average leaf size per species, measured at the end of the experiments. Thus, both leaf growth measures are in units of cm2 month−1. Because the mean leaf area for each species was larger at the dry relative to the wet site due to differences in growth rates, we used different species means per site.
Data analysis
Site differences in understory light
All data were analyzed using R software (R Development Core Team 2009). We did not run statistical tests to compare understory light among sites because the moist site experiment was run during a different time period and because plot placement was not random with respect to light. Thus, means and variances could have been inflated. Instead we discuss the differences among sites by comparing the seasonal variation in %T, PPFU, and PPFO and by visual inspection of the mean PPFU of each plot in each site.
To evaluate seasonal variation, we estimated the dry season to be from 1 January to 1 May and analyzed each site separately. Seasonal variation in %T and PPFU was evaluated using linear mixed-effect models (‘lme’ function of the ‘nlme’ package; Pinheiro and Bates 2004). The fixed effect was season (2 levels: dry vs wet) and the random blocking factor was plot or sub-plot (20 levels in the moist site, 40 in the dry site and 36 in the wet site). Seasonal variation in PPFO was evaluated using a simple linear model (R ‘lm’ function) using weekly averages to limit the temporal autocorrelation in the data. To calculate the mean PPFU in each plot for the duration of the experiment, we filled in all missing days in all plots by multiplying the site PPFO for that day by the average %T of the plot, using different %T for the dry and wet seasons.
Seedling responses to light
We asked if understory light variation within each site influenced seedling growth and mortality. We evaluated if the light-response curves with respect to growth or mortality were different for species with different distributions, or if they changed in response to water supplementation and herbivore exclusion treatments in the dry and wet sites. Thus, for all sites, there was one continuous covariate: plot PPFU, one fixed effect: species distribution (wet- or dry-distribution), and one random effect: species (24 levels). For the wet and dry sites, there were two additional fixed effects: water treatment (control or watered), and herbivore treatment (control or exclusion). The plot structure was added as a random factor only for the wet and dry sites (four sub-plots per plot), whereas for the moist site, including location in the blocking structure did not significantly improve the models (using the Akaike Information Criterion as a means of model selection).
Mortality
Mortality data were evaluated in different ways. Main effects and interactions of the different variables were tested using Cox proportional hazards models with the random effects due to species differences introduced as cluster factors (‘coxph’ function of the ‘survival’ package, S original by Terry Therneau, maintained by Thomas Lumley). Using Cox models the seedling response to light variation is measured as the hazard ratio (HR), or the percent change in mortality observed per unit increase in PPFU (1.0 mol m−2 day−1). HR = 1 indicates no change in mortality, HR > 1 indicates an increase, and HR < 1 a reduction in mortality with increasing light. To visualize light–mortality responses, we calculated probability curves using logistic mixed-effect models (glmmPQL function of the ‘MASS’ package; Venables and Ripley 2002). Finally, to calculate individual species mortality rates in high and low understory light, we used an exponential model: % survival at t = (ea)t, where t is the time, and 1−ea is the mortality rate per species. Here, we report mortality in units of percent per year.
Growth
Growth data were analyzed using linear mixed-effect models (Pinheiro and Bates 2004). Growth responses to light are known to be non-linear, and are often fit with an asymptotic model. However, because our light variation was limited to understory sites with a small range in light levels, our data were best described with linear models. To compare and visualize the effect of light on growth, we calculated the slopes of the linear models, which indicate an absolute change in growth for 1 mol m−2 day−1 increase in PPFU, such that a slope >0 indicates a positive response.
Results
Do understory light levels decrease with increasing rainfall?
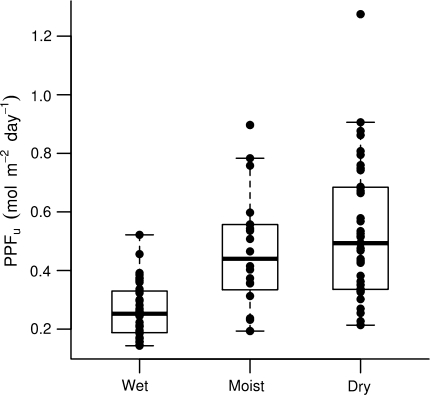
The average annual understory light declined with increasing rainfall from 0.53 mol m−2 day−1 in the dry site to 0.26 mol m−2 day−1 in the two wetter sites (Table 1). This was due to differences in light availability during the dry season, which decreased with increasing rainfall (Table 1). However, it is important to remember that as plot selection was non-random with respect to light, the averages in Table 1 do not necessarily represent the true mean in the sites. Instead, as plot location was established to maximize the variation of understory light environments within each site, it is better to compare the range of understory light microsites available in the three sites (Fig. 1). Clearly, there was considerable overlap in the light availability of the plots in the three study sites, but the ranges increased with decreasing rainfall. The lower boundaries of light availability also increased towards drier sites, but the differences were small compared to the increase in the upper boundaries. Thus, there was increasing spatial variation in light availability with decreasing rainfall. Indeed, despite our bias towards finding high-light microsites in the wet site, most plots there had low light levels (less than 0.5 mol m−2 day−1) and there was relatively little variation among plots relative to the other two sites.
Table 1.
Seasonal and yearly light availability in the three study sites measured as percent transmittance (%T) and integrated daily photosynthetic photon flux in the understory (PPFU) and in the open (PPFO) in mol m−2 day−1
| Site | Season | %T | PPFO | PPFU |
|---|---|---|---|---|
| Drya | Wet | 1.5 ± 0.5%** | 26.0** | 0.46 ± 0.20** |
| Dry | 3.4 ± 2.3% | 37.2 | 0.68 ± 0.29 | |
| Yearly | 2.1 ± 0.9% | 29.6 | 0.53 ± 0.23 | |
| Moistb | Wet | 1.3 ± 0.8%** | 23.3** | 0.22 ± 0.14** |
| Dry | 2.0 ± 0.8% | 34.2 | 0.40 ± 0.17 | |
| Yearly | 1.5 ± 0.7% | 26.7 | 0.26 ± 0.13 | |
| Weta | Wet | 1.0 ± 0.3% ns | 25.3** | 0.24 ± 0.09* |
| Dry | 0.9 ± 0.4% | 33.6 | 0.31 ± 0.11 | |
| Yearly | 1.0 ± 0.3% | 27.7 | 0.26 ± 0.09 |
Values for %T and PPFU are means ± SD for n = 40, 20 and 36 plots and sub-plots in the dry, moist and wet sites, respectively
Asterisks represent the probability that mean daily light availability is the same between the two seasons: *P < 0.05, **P < 0.01, ns not significant (P > 0.05). No tests were done to compare sites
aAverages for the wet and dry sites are from the 2006 light data
bAverages for the moist site are from September 2006 to September 2007 light data
Fig. 1.
Daily photosynthetic photon flux density (PPFU) in the three study sites over the experiment. Each point represents the average of one sub-plot (wet and dry sites) or plot (moist site). The boxes mark the median and quartiles for each site
Part of this difference among sites was due to differences in the seasonality of light, which decreased with increasing rainfall. For all sites, integrated daily photosynthetic photon flux density in the understory (PPFU) was higher in the dry than in the rainy season, and the size of this seasonal difference decreased with increasing rainfall (Table 1). This decrease in the seasonality of light was mostly due to differences in the deciduousness of the canopy, as the seasonal variation in the incident light in the open (PPFO) was similar among the three sites (Table 1). In the evergreen, wet site, there was no seasonal difference in the percent of light transmitted through the canopy (%T), whereas in the two drier sites, there was a seasonal difference that increased with decreasing rainfall, indicating a higher frequency of deciduous trees (Table 1). At the dry site, there was also large among-plot variation in %T during the dry season, likely due to incomplete deciduousness (Table 1).
Does variation in understory light influence the growth and mortality of seedlings?
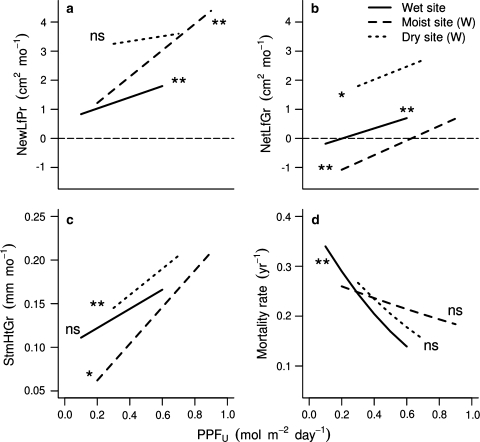
Despite the fact that all of our experiments included only a small fraction of the full range of light availability, about 0.2–3.0% of full sun (Table 1; Fig. 1), seedlings showed significant differences in growth and mortality when grown at different light levels (Fig. 2; Table 2). In the absence of water limitation, either naturally in the wet site or through water supplementation in the other two sites, increasing PPFU correlated positively with all growth variables (slope >0; Table 2a–c; Fig. 2a–c). For all species combined, light responses were strongest at the moist site (Table 2b), maybe because seedlings were larger (1 year older) at the beginning of the experiment. At the wet site, both new leaf production (NewLfPr) and net leaf growth (NetLfGr) correlated significantly with light availability; and at the dryer site, NetLfGr and stem height growth (StmHrGr) correlated significantly with light availability (Table 2a, c).
Fig. 2.
Seedling responses to light in the three study sites: with respect to a new leaf production (NewLfPr), b net leaf growth (NetLfGr), c stem height growth (StmHtGr) and d mortality. The lines represent the mean response for all species combined in the absence of water limitations (water-supplemented seedlings for the dry and moist sites, and both water treatments combined for the wet site) and with herbivores present using mixed-effects models (linear for growth and logistic for mortality). The length of the line represents the range of light levels for each set of plots. In (a) and (b), the line at zero leaf growth is for reference. Asterisks represent the probability that the responses are not different from zero: *P < 0.05, **P < 0.01, ns not significant (P > 0.05). See Table 2 for relevant statistics
Table 2.
Responses to understory light variation with 95% CI (in parenthesis) and sample size (n) for all species combined, and for dry- and wet-distribution species separately, in the (a) wet, (b) moist, (c) water-supplemented, dry site, and (d) unwatered, dry site
| Distribution | a. Wet1 | b. Moist (W)2 | c. Dry (W) | d. Dry (C) | ||||
|---|---|---|---|---|---|---|---|---|
| CI | n | CI | n | CI | n | CI | n | |
| NewLfPr | ||||||||
| All | 1.93 (0.97–2.89) | 422 | 3.74 (2.80–4.67) | 468 | 0.87 (−0.09 to 1.83) | 235 | −0.15 (−0.62 to 0.32) | 224 |
| Dry | 1.06 (0.01–2.11) | 2.88 (1.76–4.00) | 0.41 (−0.62 to 1.45) | 0.07 (−0.43 to 0.58) | ||||
| Wet | 2.79 (1.41–4.16) | 4.55 (3.17–5.94) | 3.49 (1.10–5.87) | −1.50 (−2.72 to −0.27) | ||||
| NetLfGr | ||||||||
| All | 1.76 (0.58–2.93) | 417 | 2.50 (1.50–3.51) | 468 | 2.23 (0.50–3.95) | 234 | −0.36 (−1.33 to 0.60) | 224 |
| Dry | 1.13 (−0.25 to 2.53) | 2.62 (1.28–3.96) | 1.32 (−0.88 to 3.52) | 0.49 (−0.66 to 1.66) | ||||
| Wet | 3.16 (1.02–5.30) | 2.26 (0.64–3.87) | 3.61 (0.86–6.35) | −2.05 (−3.69 to −0.41) | ||||
| StmHtGr | ||||||||
| All | 0.11 (−0.04 to 0.26) | 406 | 0.20 (0.02–0.38) | 467 | 0.15 (0.04–0.26) | 201 | 0.00 (−0.05 to 0.05) | 190 |
| Dry | 0.02 (−0.18 to 0.24) | 0.06 (−0.10 to 0.23) | 0.10 (−0.02 to 0.22) | 0.03 (−0.02 to 0.09) | ||||
| Wet | 0.19 (−0.01 to 0.41) | 0.34 (0.17–0.51) | 0.21 (0.06–0.36) | −0.03 (−0.11 to 0.03) | ||||
| Mortality | ||||||||
| All | 0.16 (0.03–0.86) | 463 | 0.51 (0.24–1.11) | 499 | 0.22 (0.04–1.30) | 250 | 2.97 (1.47–6.03) | 231 |
| Dry | 0.19 (0.02–1.71) | 0.38 (0.12–1.20) | 0.36 (0.03–4.48) | 1.41 (0.45–4.47) | ||||
| Wet | 0.12 (0.01–1.73) | 0.65 (0.23–1.90) | 0.15 (0.01–1.69) | 4.94 (2.00–12.24) | ||||
Only seedlings outside exclusion cages were analyzed at all sites. The growth responses are the slopes of New Leaf Production, Net Leaf Growth (NewLfPr, NetLfGr, both in cm2/month), and Stem Height Growth (StmHtGr, in mm/month) as a function of light from the linear mixed-effects models. The mortality responses are the Hazard Ratios from Cox models (see ‘‘Materials and methods’’). Responses significantly different from zero (or from 1.0 for mortality) at P < 0.05 are in bold
1In the wet site, the water treatments were pooled
2In the moist site, all plots were watered during the dry season and there were no herbivore exclusion cages
The probability of non-desiccation-caused death for all species combined also decreased as light increased (Fig. 2d). This trend was strongest in the wet site where one unit increase in PPFU (1.0 mol m−2 day−1, roughly 3% of full sunlight) reduced the probability of death by 84% (Table 2a; Cox model: n = 463, P = 0.03). In the water-supplemented plots at the dry site, the effect was also strong. One unit increase in PPFU reduced mortality by 78% (Table 2c; HR; Cox model: n = 250, P = 0.09). In the moist site, where all plots were watered during the dry season, one unit increase in PPFU reduced the probability of death by only 49% (Table 2b; HR; Cox model: n = 499, P = 0.09). The reason for the weaker response is explained below.
Do differences in water availability along the rainfall gradient influence seedling responses to light?
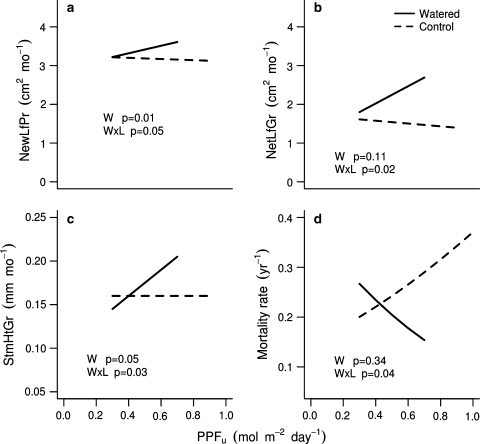
When water was a limiting resource, such as in the control plots at the dry site, higher light had a negative effect on seedling growth and mortality (Table 2d; Fig. 3). This was most striking with respect to mortality, because in the absence of water supplementation both dry- and wet-distribution species had higher mortality at higher light, although this was significant only for the latter (Table 2d). A 1 mol m−2 day−1 increase in PPFU increased seedling mortality by 40% for dry-distribution species, nearly fivefold for wet-distribution species, and nearly three-fold for all species combined (Table 2d). For all species combined there was a significant light × water treatment interaction with respect to mortality (Fig. 3). With respect to the leaf growth variables, performance also decreased with increasing light, but these responses were significantly negative only when wet-distribution species were analyzed separately (Table 2d). While, on average, the dry-distribution species did not have a negative response to increasing light, their light responses were less steep when water was limiting (Table 2c, d), and the light × water treatment interactions of all species combined were significant for all growth variables (Fig. 3).
Fig. 3.
The effect of water addition on the light response of seedlings planted in the dry site. Lines represent the mean response for all species combined: a new leaf production (NewLfPr), b net leaf growth (NetLfGr), c stem height growth (StmHtGr) and d mortality. The mean response was determined using mixed-effects models (linear for growth and logistic for mortality) for uncaged seedlings only. The length of the line represents the range of light levels for each set of plots. In a and b, the line at zero is for reference. The P values in the panels represent the significance of the main effect of watering (W) on seedling performance and the water × light interaction (W × L). See Table 2 for relevant statistics
In the moist site, even though all plots were given supplemental water during the dry season, drought stress also influenced seedling mortality. The moist site was studied one year after the other two sites, and 2007 had a drier dry season than 2006 (Fig. S2). Because the dry season was stronger, the watering treatment in the moist site was not completely effective, and a number of the seedlings were recorded as dead due to desiccation. Such desiccation-caused mortality increased, though not significantly, with increasing light (Cox model: HR = 1.93, n = 499, P = 0.22). This explains why the moist site experienced a relatively smaller reduction in mortality as a function of increasing light (HR = 0.51 vs 0.16 and 0.22 in the other two sites; Table 2a–c). Indeed, when the desiccation-caused mortality was eliminated from the analysis (recoded as a censored observation), each 1.0 mol m−2 day−1 increase in PPFU reduced the probability of seedling death by 83% for all species combined (Cox model: HR = 0.17, n = 499, P = 0.001). This effect was indistinguishable from the effects observed in the wet and the watered dry site (Table 2a, c).
Do differences in pest pressure along the rainfall gradient influence seedling responses to light?
Herbivore exclusion had a weak influence on seedling performance. In a previous report of the same experiment, we had demonstrated that herbivore exclusion influenced the leaf damage observed on the seedlings at the end of the experiment, but we found no effects on seedling growth or mortality (Brenes-Arguedas et al. 2009). Here we find that, when plot light is factored into the model, caging significantly improved seedling growth and survival in the wet site, but in a light-independent manner (see the detailed results in Fig. S3). Caging also influenced growth and mortality in the dry site, but only in the water supplemented plots. As this and other observed effects of caging had limited relevance to understanding light effects in other sites, we will not discuss them here any further.
Do light responses and competitive abilities differ between wet- and dry-distribution species?
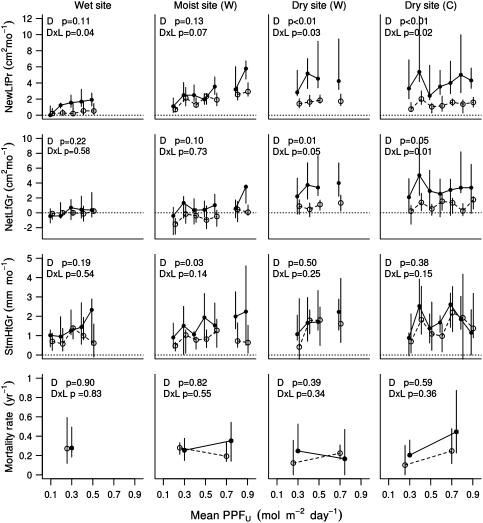
Tests for the differences between wet- and dry-distribution species are less sensitive due to the large variation among species in growth rates, mortality rates, and light responses (Brenes-Arguedas et al. 2009; Figs. S4–S11; Table S3, S5). Nevertheless, we found significant differences in the light responses between dry- and wet-distribution species, with the direction of this difference depending on water availability (Fig. 4; Table 2). When water was available, wet-distribution species had a stronger positive response to light than the dry-distribution species (Table 2a–c). For NewLfPr, the light × distribution interaction was significant at the wet site and at the water-supplemented dry site, and marginally significant at the moist site (Fig. 4). With respect to NetLfGr and StmHtGr, the light responses of wet-distribution species were equal to or stronger than the responses of dry-distribution species at all three sites (Table 2a–c), but the interactions were not significant for either of these variables (Fig. 4). With respect to mortality, the light × distribution interactions were not significant at any site where water was available (Fig. 4).
Fig. 4.
Comparison of the performance of wet- (filled circle) versus dry- (open circle) distribution species grown at different understory light levels in wet, moist and dry sites, with (W) and without water supplementation (C). The symbols represent the medians, and vertical bars represent the inter-quartile range for each group of species at each light category. To improve legibility, plots with similar light levels were combined by averaging seedling performance within species. For mortality rates, we used only two light categories: less than and more than 0.5 mol m−2 day−1. Only data for uncaged seedlings are reported. The P values in the panels represent the significance of the main effect of distribution on seedling performance (D) and the distribution × light interaction (D × L), using linear mixed-effect models for the growth variables and Cox models for mortality
When water was not supplemented at the dry site, the patterns were quite different. With respect to NewLfPr and NetLfGr, light-growth responses were significantly negative for the wet-distribution species, while the dry-distribution species still maintained slightly positive responses (Table 2d). The light × distribution interaction was significant for both variables (Fig. 4). StmHtGr and mortality did not show the same interaction (Table 2d; Fig. 4). However, in the absence of water supplementation, the mortality of the seedlings increased at higher light for both dry- and wet-distribution species (HR > 1; Table 2d), and this increase was 3.5 times stronger and significantly positive only for wet-distribution species (Table 2d).
Growth rate was consistently higher for wet- relative to dry-distribution species. This was most evident at the dry site, where wet-distribution species had faster new leaf production and net leaf growth than dry-distribution species at most light levels (Fig. 4). At the wet and moist sites, NewLfPr was faster for wet-distribution species mostly at high light levels (wet site: PPFU > 0.2: F = 5.2, df = 22, P = 0.03; moist site: PPFU > 0.6: F = 6.25, df = 22, P = 0.02; Fig. 4), while at low light levels both dry- and wet-distribution species performed equally poorly (Fig. 4). This distribution effect was not observed for NetLfGr in either of the two wetter sites, suggesting high levels of leaf loss in the wet-distribution species.
Does shade tolerance differ between wet- and dry-distribution species?
If wet-distribution species were more shade-tolerant, they would have lower mortality rates at very low light. Instead, we found that, at light levels below 0.5 mol m−2 day−1 at the wet and moist sites, mortality rates of wet- and dry-distribution species were indistinguishable (Fig. 4). At similarly low light levels at the dry site, and at light levels above 0.5 mol m−2 day−1 at the moist and dry sites, mortality rates were similar or slightly higher for wet- versus dry-distribution species (Fig. 4). This difference was most likely due to water limitation and not shade tolerance, since the largest effect, 50% more mortality for the wet- than dry-distribution species, was seen for the higher-light, unwatered plots at the dry site (P = 0.03). Overall, mortality rates of the species at low light did not correlate with growth rates at high light (wet-site mortality vs dry-site growth: r 2 = 0.04, P = 0.34; Fig. S12).
Discussion
Based on annual rainfall, all of our sites can be classified as moist forest (Holdridge 1947). However, differences in seasonality along the Isthmus are large enough to result in differences in species composition (Pyke et al. 2001) and performance (Brenes-Arguedas et al. 2009). Here, we also show that differences in canopy structure and phenology result in differences in understory light availability. The performances of dry- and wet-distribution species along this gradient were not consistent with niche partitioning based on these differences in understory light or in levels of shade tolerance. However, there were important differences in the species light responses that probably contribute to explain species distribution along the rainfall gradient.
Differences in light availability across the rainfall gradient
Forest structure, phenology and cloudiness are all variables that determine the amount of light that is transmitted through the canopy (%T) and that reaches the understory (Chazdon and Fetcher 1984; Torquebiau 1988). In tropical regions, cloudiness is more important than latitude in determining incident light at the top of the canopy (Wright and van Schaik 1994). Consistently, we found that incident light decreased with increasing rainfall (Table 1). Forest structure and phenology also vary with rainfall (Wright and van Schaik 1994; Condit et al. 2000) and both influenced understory light availability in our study sites. The lower rainy-season %T at the wetter sites indicates higher leaf area index, and the larger increase in %T during the dry season towards drier sites indicates the presence of deciduous canopy trees (Table 1). Measurements of %T reported in the literature are consistent with this trend, showing even higher %T for sites drier than ours (Table S2).
Not surprisingly, the combination of these three factors resulted in increasing average understory light with decreasing rainfall (Table 1). However, when we consider the variation among plots (Fig. 1), the differences among sites were not very large. Indeed, we found that there was considerable overlap in light microenvironments along the rainfall gradient (Fig. 1), and most of the among-site differences were due to increased light heterogeneity at drier sites (Fig. 1). Wetter forest understories were characterized by more similar, low-light microsites (Fig. 1), with light levels maintained year around (Table 1), whereas the understories of drier forests were more variable (Fig. 1). The variability at the drier sites was partly due to deciduousness, but there was also slightly greater among-plot variation during the wet season (Table 1), likely due to greater variation in canopy structure.
How does variation in understory light influence the growth and mortality of seedlings?
Despite the relatively small ranges in light availability within sites, we found significant growth and survival responses to light availability in all three sites. Positive responses to light are not surprising, as in low light the photosynthetic efficiency per incident photon may be quite high, and CO2 assimilation increases linearly with light availability (Chazdon 1986; Montgomery and Chazdon 2002). However, in this study, we also evaluated how light responses interacted with other environmental factors that varied along the rainfall gradient. We found that the most important environmental factor that influenced the light responses of the seedlings was water limitation. When water was not limiting, either naturally in the wet forest or through water supplementation in the moist and dry forests, small increases in light significantly improved seedling performance (Fig. 2). Instead, when water was limiting in the drier site, seedling performance decreased with increasing light.
Consistent with a number of other studies, we found that in drier sites high light exacerbated seasonal water stress (Gerhardt 1996; Holmgren 2000; McLaren and McDonald 2003; Sack 2004; Sanchez-Gomez et al. 2006). In a previous study, we had shown that water supplementation decreased mortality of the more sensitive, wet-distribution species but we found no effect on growth (Brenes-Arguedas et al. 2009). In this study, we show that when differences in light availability are factored into the model, water supplementation also had a significant effect on growth. This effect was visible only in plots with higher light, such that high light plus drought decreased growth and increased mortality (Fig. 3). Such species responses to drought are clearly very strong if we consider that the 2006 dry season was weak and short relative to the long-term average (Fig. S2). Also, dry-distribution species responded weakly to this drought × light interaction, mostly with respect to mortality (Table 2c–d), suggesting variability in the drought adaptations among these species. Herbivore exclusion cages also influenced growth and mortality in both habitats, but such effects were contingent on water availability and largely independent of light (Fig. S3).
It is possible that the seasonality of light also influenced the performance of the seedlings. For instance, with respect to new leaf production, seedlings showed a weaker response to variation in light availability in the dry site, relative to the other two sites, even when water was supplemented (Fig. 2). It is unlikely that this difference is due to lack of light limitations, because seedlings perform much better even in those plots where average light is as low as in the wet forest (Fig. 2). Instead, the difference is most likely due to the generally better growing conditions (better soils and fewer pests; Brenes-Arguedas et al. 2008, 2009) and to the difference in the seasonality of light (Table 1). In drier forests, the plots with lower light availability, where drought effects were not so strong (Fig. 3), probably benefited more from the seasonal increase in light. Instead, the plots with higher light availability, where drought effects were stronger (Fig. 3), suffered more from the seasonal increase in light. These observations suggest that the best method for quantifying understory light and its effect on seedling performance may depend on forest type. In wetter forests, annual average light would be most relevant, and higher light would generally improve growth and survival. In drier forests, wet and dry season light levels have opposite effects on seedling performance, with the dominant result being the negative effect of high, dry-season light on sensitive species.
Are wet-distribution species more shade-tolerant or better competitors than dry-distribution species?
The wet- and dry-distribution species did not differ in shade tolerance. Given that the understories of wetter forests were darker, we hypothesized that wet-distribution species might be more shade tolerant. Although many traits have been used to describe shade tolerators (Kobe and Coates 1997; Baltzer and Thomas 2007; Pompa and Bongers 1988; Valladares and Niinemets 2008), the most common mechanism is higher survival at low light (Baltzer and Thomas 2007). Although the mortality rates of our seedlings varied among species between 0 and 50% per year (Table S4), wet-distribution species were not better able to survive in the lowest light plots than dry-distribution species (Fig. 4). Instead, many wet-distribution species had higher mortality rates than dry-distribution species (Fig. 4). Also, there was little or no difference in growth rates at very low light especially in the two wetter sites (Fig. 4).
Instead, our results suggest that there are differences in the competitive ability of dry- and wet-distribution species. Competitive ability can be characterized by the species’ performance in good growing conditions. Despite the low average light in wet forest understories, wet-distribution species showed steeper responses to light increases (Table 2a–c), and had higher growth rates at higher light (Fig. 4). This is inconsistent with other definitions of shade tolerance, such as a trade-off in growth at high light versus mortality at low light (Gilbert et al. 2006; Kitajima and Poorter 2008; Dent and Burslem 2009), or a rank reversal among the species in the growth at high versus low light (Sack and Grubb 2003). Instead, our results are consistent with temperate studies where a key difference among shade-tolerant species is the rate of growth in higher light (Pacala et al. 1996). Note, however, that in our study, higher light in the understory can still be as low as a 3–4% of total sunlight.
While our measurements are limited to seedlings, this result may be more general. Other studies suggest that trees in wetter forests may also grow more quickly (Condit et al. 2004) and that the species rank order in growth rate does not differ between seedlings, saplings and trees (Cornelissen et al. 1998; Gilbert et al. 2006; but see Kitajima and Poorter 2008). There have been other reports of lower growth rates, smaller responses to soil nutrients, and less leaf-level acclimation to light for species from drier forests (Baltzer et al. 2007; Markesteijn et al. 2007). Thus, some of the adaptations of dry-distribution species to seasonal water stress may constrain fast growth. For example, intrinsic limitations in shoot water transport may reduce CO2 uptake and photosynthesis (Grace 1990; Liancourt et al. 2005; Hacke et al. 2006; Markesteijn 2010). Also, while we did not measure root growth, if dry-distribution species had higher allocation to roots to increase drought survival, this would also result in lower competitive ability in light-limited environments.
Does light availability contribute to species turnover along the rainfall gradient?
Although the critical processes of seed germination and early seedling establishment remain to be determined, our results for transplanted seedlings suggest that variations in light availability along the rainfall gradient do contribute to shape species distributions. However, the two main effects that we observed were not mediated by shade tolerance or light limitation, but instead by performance at higher understory light availability. Higher understory light in the drier sites tended to reinforce water stress and placed strong constraints on the growth and survival of wet-distribution species, while having a lesser effect on the drought-tolerant, dry-distribution species (Table 2d). Hence, the distribution of wet-forest species is mostly constrained by water stress and higher understory light exacerbates this effect. On the other hand, shade tolerance did not differ for wet- versus dry-distributions species, probably because low light microsites are common to understories along the rainfall gradient. Instead, it is possible that the ability to take advantage of small increases in light is more important for seedling establishment in darker, wetter forests. In our study, slight increases in understory light in the darker, wet site improved the growth and survival of the wet-distribution species, while having a lesser effect on the dry-distribution species (Table 2a). Indeed, the majority of shade-tolerant species do require higher light for regeneration (Rüger et al. 2009).
Hence, the distribution of dry-forest species may be limited by their lower competitive ability in wet-forest microsites with good growing conditions, such as brighter spots in the understory and possibly also light gaps. Although it has been suggested that high-light sites in wet forests could reduce the importance of competition, making many species ecologically equivalent (Hubbell and Foster 1986), plants in sites with low abiotic stress may experience greater competition for light and nutrients (Gerhardt 1996; Greiner La Peyre et al. 2001; Barberis and Tanner 2005; Liancourt et al. 2005). Thus, we prefer the hypothesis that trade-offs between stress tolerance and competitive ability may better explain community assembly and distribution along a rainfall gradient.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
Funding was provided by the National Science Foundation grant DEB-0444590 to T.A.K. and P.D.C. We thank Cecilia Blundo, Marcos Ríos, Gonzalo Rivas, Natalia Anaya, and Lissette Jimenez who conducted the fieldwork; Salomon Aguilar and Rolando Perez helped with species identification. We thank the Smithsonian Tropical Research Institute’s Environmental Monitoring Program and the Autoridad del Canal de Panama for making climate data publicly available; similarly the Center for Tropical Forest Science, the Missouri Botanical Gardens and InBio made species information, herbarium and distribution maps available. We thank T. Paine and an anonymous reviewer for valuable comments on the manuscript. The Peregrine Fund kindly allowed us access to Gunn Hill. The Smithsonian Tropical Research Institute provided logistical support and research facilities. All work was done in compliance with the laws of Panama and the Autoridad Nacional del Ambiente. Open access to this article was provided by the Berkeley Research Impact Initiative of the University of California-Berkeley.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Baillie I, Elsenbeer H, Barthold F, Grimm R, Stallard R (2007) Semi-detailed soil survey of Barro Colorado Island, Panama. http://biogeodb.stri.si.edu/bioinformatics/bci_soil_map/
- Balderrama SIV, Chazdon RL. Light-dependent seedling survival and growth of four tree species in Costa Rican second-growth rain forests. J Trop Ecol. 2005;21:383–395. doi: 10.1017/S026646740500235X. [DOI] [Google Scholar]
- Baltzer JL, Thomas SC. Determinants of whole-plant light requirements in Bornean rain forest tree saplings. J Ecol. 2007;95:1208–1221. doi: 10.1111/j.1365-2745.2007.01286.x. [DOI] [Google Scholar]
- Baltzer JL, Davies SJ, Noor NSM, Kassim AR, LaFrankie JV. Geographical distributions in tropical trees: can geographical range predict performance and habitat association in co-occurring tree species? J Biogeogr. 2007;34:1916–1926. doi: 10.1111/j.1365-2699.2007.01739.x. [DOI] [Google Scholar]
- Baltzer JL, Davies SJ, Bunyavejchewin S, Noor NSM. The role of desiccation tolerance in determining tree species distributions along the Malay–Thai Peninsula. Funct Ecol. 2008;22:221–231. doi: 10.1111/j.1365-2435.2007.01374.x. [DOI] [Google Scholar]
- Barberis IM, Tanner EVJ. Gaps and root trenching increase tree seedling growth in Panamanian semi-evergreen forest. Ecology. 2005;86:667–674. doi: 10.1890/04-0677. [DOI] [Google Scholar]
- Bloor JMG, Grubb PJ. Growth and mortality in high and low light: trends among 15 shade-tolerant tropical rain forest tree species. J Ecol. 2003;91:77–85. doi: 10.1046/j.1365-2745.2003.00743.x. [DOI] [Google Scholar]
- Bongers F, Poorter L, Van Rompaey RSAR, Parren MPE. Distribution of twelve moist forest canopy tree species in Liberia and Côte d’Ivoire: response curves to a climatic gradient. J Veg Sci. 1999;10:371–382. doi: 10.2307/3237066. [DOI] [Google Scholar]
- Brenes-Arguedas T, Ríos M, Rivas-Torres G, Blundo C, Coley PD, Kursar TA. The effect of soil on the growth performance of tropical species with contrasting distributions. Oikos. 2008;117:1453–1460. doi: 10.1111/j.0030-1299.2008.16903.x. [DOI] [Google Scholar]
- Brenes-Arguedas T, Coley PD, Kursar TA. Pests versus drought as determinants of plant distribution along a tropical rainfall gradient. Ecology. 2009;90:1751–1761. doi: 10.1890/08-1271.1. [DOI] [PubMed] [Google Scholar]
- Chave J. Spatial variation in tree species composition across tropical forests: pattern and process. In: Carson WP, Schnitzer SA, editors. Tropical forest community ecology. West Sussex: Wiley/Blackwell; 2008. pp. 11–30. [Google Scholar]
- Chazdon RL. Light variation and carbon gain in rain forest understorey palms. J Ecol. 1986;74:995–1012. doi: 10.2307/2260229. [DOI] [Google Scholar]
- Chazdon RL, Fetcher N. Photosynthetic light environments in a lowland tropical rain forest in Costa Rica. J Ecol. 1984;72:553–564. doi: 10.2307/2260066. [DOI] [Google Scholar]
- Clinebell RR, II, Phillips OL, Gentry AH, Stark N, Zuuring H. Prediction of neotropical tree and liana species richness from soil and climatic data. Biodivers Conserv. 1995;4:56–90. doi: 10.1007/BF00115314. [DOI] [Google Scholar]
- Coley PD, Barone JA. Herbivory and plant defenses in tropical forests. Annu Rev Ecol Syst. 1996;27:305–335. doi: 10.1146/annurev.ecolsys.27.1.305. [DOI] [Google Scholar]
- Condit R, Watts K, Bohlman SA, Perez R, Foster RB, Hubbell SP. Quantifying the deciduousness of tropical forest canopies under varying climates. J Veg Sci. 2000;11:649–658. doi: 10.2307/3236572. [DOI] [Google Scholar]
- Condit R, Aguilar S, Hernandez A, Perez R, Lao S, Angehr G, Hubbell SP, Foster RB. Tropical forest dynamics across a rainfall gradient and the impact of an El Niño dry season. J Trop Ecol. 2004;20:51–72. doi: 10.1017/S0266467403001081. [DOI] [Google Scholar]
- Cornelissen JHC, Castro-Diez P, Carnelli AL. Variation in relative growth rate among woody species. In: Lambers H, Poorter H, van Vuuren MMI, editors. Inherent variation in plant growth: physiological mechanisms and ecological consequences. Leiden: Backhuys; 1998. pp. 363–392. [Google Scholar]
- Davidar P, Rajagopal B, Mohandass D, Puyravaud JP, Condit R, Wright SJ, Leigh EG. The effect of climatic gradients, topographic variation and species traits on the beta diversity of rain forest trees. Glob Ecol Biogeogr. 2007;16:510–518. doi: 10.1111/j.1466-8238.2007.00307.x. [DOI] [Google Scholar]
- Dent DH, Burslem DFRP. Performance trade-offs driven by morphological plasticity contribute to habitat specialization of Bornean tree species. Biotropica. 2009;41:424–434. doi: 10.1111/j.1744-7429.2009.00505.x. [DOI] [Google Scholar]
- Engelbrecht BMJ, Comita L, Condit R, Kursar TA, Tyree MT, Turner BL, Hubbell SP. Drought sensitivity shapes species distribution patterns in tropical forests. Nature. 2007;447:80–83. doi: 10.1038/nature05747. [DOI] [PubMed] [Google Scholar]
- Gaston KJ. Geographic range limits: achieving synthesis. Proc R Soc Lond B. 2009;276:1395–1406. doi: 10.1098/rspb.2008.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt K. Effects of root competition and canopy openness on survival and growth of tree seedlings in a tropical seasonal dry forest. For Ecol Manag. 1996;82:33–48. doi: 10.1016/0378-1127(95)03700-4. [DOI] [Google Scholar]
- Gilbert B, Wright SJ, Muller-Landau HC, Kitajima K, Hernandez A. Life history trade-offs in tropical trees and lianas. Ecology. 2006;87:1281–1288. doi: 10.1890/0012-9658(2006)87[1281:LHTITT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Givnish TJ. Leaf and canopy adaptations in tropical forests. In: Medina E, Mooney HA, Vásquez-Yánes C, editors. Physiological ecology of plants of the wet tropics. The Hague: Junk; 1984. pp. 51–84. [Google Scholar]
- Givnish TJ. On the causes of gradients in tropical tree diversity. J Ecol. 1999;87:193–210. doi: 10.1046/j.1365-2745.1999.00333.x. [DOI] [Google Scholar]
- Goldberg DE. Components of resource competition in plant communities. In: Grace JB, Tilman D, editors. Perspectives on plant competition. New York: Academic; 1990. pp. 27–49. [Google Scholar]
- Grace JB. On the relationship between plant traits and competitive ability. In: Grace JB, Tilman D, editors. Perspectives on plant competition. New York: Academic; 1990. pp. 51–65. [Google Scholar]
- Greiner La Peyre MK, Grace JB, Hahn E, Mendelssohn IA. The importance of competition in regulating plant species abundance along a salinity gradient. Ecology. 2001;82:62–69. doi: 10.1890/0012-9658(2001)082[0062:TIOCIR]2.0.CO;2. [DOI] [Google Scholar]
- Hacke UG, Sperry JS, Wheeler JK, Castro L. Scaling of angiosperm xylem structure with safety and efficiency. Tree Physiol. 2006;26:689–701. doi: 10.1093/treephys/26.6.689. [DOI] [PubMed] [Google Scholar]
- Holdridge LR (1947) Determination of world plant formations from simple climatic data. Science 105:367–368 [DOI] [PubMed]
- Holmgren M. Combined effects of shade and drought on tulip polar seedlings: trade-off in tolerance or facilitation? Oikos. 2000;90:67–78. doi: 10.1034/j.1600-0706.2000.900107.x. [DOI] [Google Scholar]
- Hubbell SP, Foster RB. Biology, chance, and history and the structure of tropical rain forest tree communities. In: Diamond J, Case TJ, editors. Community ecology. New York: Harper and Row; 1986. pp. 314–329. [Google Scholar]
- Kaufmann K, Paton S (2008) Physical monitoring database. http://striweb.si.edu/esp/physical_monitoring/index_phy_mon.htm
- Kitajima K. Relative importance of photosynthetic traits and allocation patterns as correlates of seedling shade tolerance of 13 tropical trees. Oecologia. 1994;98:419–428. doi: 10.1007/BF00324232. [DOI] [PubMed] [Google Scholar]
- Kitajima K, Poorter L. Functional basis for resource niche differentiation by tropical trees. In: Carson WP, Schnitzer SA, editors. Tropical forest community ecology. Oxford: Blackwell; 2008. pp. 160–181. [Google Scholar]
- Kobe RK, Coates KD. Models of sapling mortality as a function of growth to characterize interspecific variation in shade tolerance of eight tree species of northwestern British Colombia. Can J For Res. 1997;27:227–236. doi: 10.1139/x96-182. [DOI] [Google Scholar]
- Kursar TA, Engelbrecht BMJ, Burke A, Tyree MT, El Omari B, Giraldo JP. Tolerance to low leaf water status of tropical tree seedlings is related to drought performance and distribution. Funct Ecol. 2009;23:93–102. doi: 10.1111/j.1365-2435.2008.01483.x. [DOI] [Google Scholar]
- Liancourt P, Callaway RM, Michalet R. Stress tolerance and competitive-response ability determine the outcome of biotic interactions. Ecology. 2005;86:1611–1618. doi: 10.1890/04-1398. [DOI] [Google Scholar]
- Losos EC, The CTFS Working Group . The structure of tropical forests. In: Losos EC, Leigh EG Jr, editors. Tropical forest diversity and dynamism. Findings from a large-scale plot network. Chicago: University of Chicago Press; 2004. pp. 69–78. [Google Scholar]
- Markesteijn L (2010) Drought tolerance of tropical tree species: functional traits, trade-offs and species distribution, PhD thesis, Wageningen University, Wageningen, The Netherlands
- Markesteijn L, Poorter L, Bongers F. Light-dependent leaf trait variation in 43 tropical dry forest tree species. Am J Bot. 2007;94:515–525. doi: 10.3732/ajb.94.4.515. [DOI] [PubMed] [Google Scholar]
- McLaren KP, McDonald MA. The effects of moisture and shade on seed germination and seedling survival in a tropical dry forest in Jamaica. For Ecol Manag. 2003;183:61–75. doi: 10.1016/S0378-1127(03)00100-2. [DOI] [Google Scholar]
- Montgomery RA, Chazdon RL. Light gradient partitioning by tropical tree seedlings in the absence of canopy gaps. Oecologia. 2002;131:165–174. doi: 10.1007/s00442-002-0872-1. [DOI] [PubMed] [Google Scholar]
- Murphy PG, Lugo AE. Ecology of tropical dry forest. Annu Rev Ecol Syst. 1986;17:67–88. doi: 10.1146/annurev.es.17.110186.000435. [DOI] [Google Scholar]
- Pacala SW, Canham CD, Saponara J, Silander JA, Jr, Kobe RK, Ribbens E. Forest models defined by field measurements: estimation, error analysis and dynamics. Ecol Monogr. 1996;66:1–43. doi: 10.2307/2963479. [DOI] [Google Scholar]
- Pinheiro JC, Bates DM. Mixed-effects models in S and S-PLUS. New York: Springer; 2004. [Google Scholar]
- Pompa J, Bongers F. The effect of canopy gaps on growth and morphology of seedlings of rain forest species. Oecologia. 1988;75:625–632. doi: 10.1007/BF00776429. [DOI] [PubMed] [Google Scholar]
- Pyke CR, Condit R, Lao S. Floristic composition across a climatic gradient in a neotropical lowland forest. J Veg Sci. 2001;12:553–566. doi: 10.2307/3237007. [DOI] [Google Scholar]
- R Development Core Team . R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2009. [Google Scholar]
- Rüger N, Huth A, Hubbell SP, Condit R. Response of recruitment to light availability across a tropical lowland rain forest community. J Ecol. 2009;97:1360–1368. doi: 10.1111/j.1365-2745.2009.01552.x. [DOI] [Google Scholar]
- Sack L. Responses of temperate woody seedlings to shade and drought: do trade-offs limit potential niche differentiation? Oikos. 2004;107:110–127. doi: 10.1111/j.0030-1299.2004.13184.x. [DOI] [Google Scholar]
- Sack L, Grubb PJ. Crossovers in seedling relative growth rates between low and high irradiance: analyses and ecological potential (reply to Kitajima & Bolker 2003) Funct Ecol. 2003;17:281–287. doi: 10.1046/j.1365-2435.2003.07402.x. [DOI] [Google Scholar]
- Sanchez-Gomez D, Valladares F, Zavala MA. Performance of seedlings of Mediterranean woody species under experimental gradients of irradiance and water availability: trade-offs and evidence for niche differentiation. New Phytol. 2006;170:795–805. doi: 10.1111/j.1469-8137.2006.01711.x. [DOI] [PubMed] [Google Scholar]
- Santiago LS, Schuur EAG, Silvera K. Nutrient cycling and plant-soil feedbacks along a precipitation gradient in lowland Panama. J Trop Ecol. 2005;21:461–470. doi: 10.1017/S0266467405002464. [DOI] [Google Scholar]
- Sexton JP, McIntyre PJ, Angert AL, Rice KJ. Evolution and ecology of species range limits. Annu Rev Ecol Evol Syst. 2009;40:415–436. doi: 10.1146/annurev.ecolsys.110308.120317. [DOI] [Google Scholar]
- Smith T, Huston M. A theory of spatial and temporal dynamics of plant communities. Vegetation. 1989;83:49–69. doi: 10.1007/BF00031680. [DOI] [Google Scholar]
- Suding KN, Goldberg DE, Hartman KM. Relationships among species traits: separating levels of response and identifying linkages to abundance. Ecology. 2003;84:1–16. doi: 10.1890/0012-9658(2003)084[0001:RASTSL]2.0.CO;2. [DOI] [Google Scholar]
- Swaine MD. Rainfall and soil fertility as factors limiting forest species distributions in Ghana. J Ecol. 1996;84:419–428. doi: 10.2307/2261203. [DOI] [Google Scholar]
- ter Steege H, Jetten VG, Polak AM, Werger MJA. Tropical rain forest types and soil factors in a watershed area in Guyana. J Veg Sci. 1993;4:705–716. doi: 10.2307/3236137. [DOI] [Google Scholar]
- Torquebiau EF. Photosynthetically active radiation environment, patch dynamics and architecture in a tropical rainforest in Sumatra. Aust J Plant Physiol. 1988;15:327–342. doi: 10.1071/PP9880327. [DOI] [Google Scholar]
- Valladares F, Niinemets Ü. Shade tolerance, a key plant feature of complex nature and consequences. Annu Rev Ecol Evol Syst. 2008;39:237–257. doi: 10.1146/annurev.ecolsys.39.110707.173506. [DOI] [Google Scholar]
- Venables WN, Ripley BD. Modern applied statistics with S. 4. NY: Springer; 2002. [Google Scholar]
- Wright SJ, van Schaik CP. Light and the phenology of tropical trees. Am Nat. 1994;143:192–199. doi: 10.1086/285600. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.