Introduction
Pheochromocytomas and paragangliomas are tumors derived from chromaffin cells in the adrenal medulla or extra-adrenal paraganglia.[1] Pheochromocytomas and sympathetic paragangliomas usually synthesize and secrete norepinephrine and/or epinephrine, while 23% of parasympathetic paraganglia-derived tumors secrete only dopamine.[2-3] Adrenal tumors and tumors located in the sympathetic paraganglia will be referred to as pheochromocytomas. Pheochromocytomas are found in 0.2-0.6% of subjects with hypertension.[4-6] The cost-effectiveness of diagnostic workup of pheochromocytoma is markedly restricted by low specificity of clinical symptoms together with symptoms overlapping within a wide variety of other conditions, including idiopathic hypertension, hyperthyroidism, heart failure, migraines, and anxiety.[7] In a study performed by Mayo Clinic, it was found that out of 54 pheochromocytomas found on autopsy over a 50-year period, only 24% had been diagnosed before death.[8] On the other hand, McNeil et al. reported only one undiagnosed pheochromocytoma out of 2031 autopsies, which may very well be related to both advances in biochemical and anatomical diagnosis and some degree of referral bias.[9]
The most common sign of pheochromocytoma is hypertension, found in approximately 95% of patients and related to catecholamine excess.[10-11] Clinical characteristics of hypertension vary and may show either a sustained or paroxysmal pattern.[12] In some patients, hypertensive paroxysms will occur in the background of sustained hypertension. On the other hand, a small, but significant proportion of patients with pheochromocytoma are normotensive. Pathophysiologic mechanisms of phenotypic characteristics of pheochromocytoma-related hypertension are discussed below. Additional symptoms seen in pheochromocytoma patients include headache, palpitation, anxiety, and sweating.[13]
In addition to being a great mimicker, pheochromocytoma represents one of the most dazzling clinical paradoxes, where symptoms and signs vary to hardly understandable degrees in seemingly comparable clinical settings. To understand this paradox, one has to take into account the multiple pathophysiologic variables that lead to clinically significant complications. Some of these variables mentioned and discussed below include: a]. catecholamine synthesis in different chromaffin-cell organs in health and disease, b]. differences in enzymatic machinery involved in catecholamine synthesis, c]. availability of substrate, d]. size/amount of secreting tissue and its metabolic activity, e]. type of secreted catecholamine, f]. amount of secreted catecholamine, g]. pattern of catecholamine secretion, h]. degree of end-organ damage, and many others. But most importantly, a certain depth of basic knowledge of catecholamine synthesis and metabolism is imperative in understanding pheochromocytoma clinically.
Physiology of Catecholamine Biosynthesis
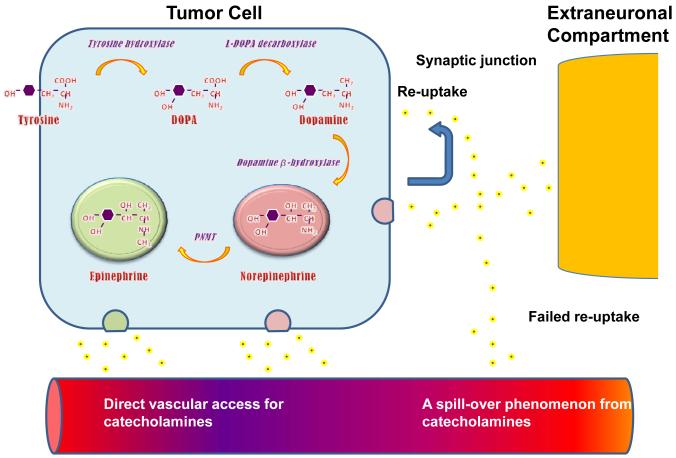
Catecholamines are synthesized in and secreted from chromaffin cells located in the adrenal medulla and sympathetic paraganglia, although catecholamine-positive secretory granules are also found in parasympathetic paraganglia. Enzymatic machinery of catecholamine-producing cells is of great importance for synthesis (Figure 1). The first step is rate-limiting as L-tyrosine is brought into the cell where it is hydroxylated to L-3,4-dihydroxyphenylalanine (dopa) via tyrosine hydroxylase (TH), an enzyme found only in cells that produce catecholamines.[14-15] Normally, molecular oxygen along with tertahydropteridine (TH4) act as co-factors in this step and the oxidation of TH4 by catecholamines represents a negative feedback loop, which inhibits TH from functioning.[16] Subsequently, L-Dopa is decarboxylated to L-dihydroxyphenylethylamine (dopamine) by aromatic L-amino acid decarboxylase (AADC) in the cytoplasm of the cell, with pyridoxal phosphate acting as a co-factor in the reaction. .[2, 13] Dopamine will then enter the neurosecretory vesicles where it is hydroxylated by dopamine-β-hydroxylase (DBH) to L-norepinephrine. Norepinephrine is further converted into epinephrine by the enzyme phenylethanolamine-N-methyl transferase (PNMT).
Figure 1.
Diagram illustrating biosynthesis of the three major catecholamines that are secreted by pheochromocytomas. DOPA, dihydroxyphenylalanine; PNMT, phenylethanolamine-N-methyl transferase. Data from Eisenhofer G, Lenders JWM, Pacak K. Biochemical Diagnosis of Pheochromocytoma. In: Lehnert H, ed. Pheochromocytoma. Pathophysiology and Clinical Management. Front Horm Res. Vol 31. Basel: Karger; 2004:76-106.
The paraganglial content of PMNT is negligible, which means that most of the epinephrine is only produced in the adrenal glands. Under normal conditions, norepinephrine is released into the synaptic space through pre-synaptic exocytosis and re-absorbed through norepinephrine transporters with minimal, if any, systemic spill-off. Norepinephrine and epinephrine are also released from the adrenal medulla through exocytosis in response to cholinergic stimulation by the splanchnic nerves.[13, 17]
Catecholamine Action
Catecholamines – norepinephrine, epinephrine, and dopamine – act through ubiquitously expressed G-protein coupled adrenergic receptors and play important roles in practically every aspect of human physiology. Norepinephrine signals through α1, α2, and β1 receptors, while epinephrine will primarily stimulate only β1 and β2 receptors. At normal levels, dopamine does not have much of an effect on any of the adrenergic receptors; however, as plasma concentrations increase (ex: dopamine-secreting tumor), dopamine can stimulate both α and β receptors.[13]
α1-adrenergic receptors are found primarily on smooth muscle tissue including peripheral (coronary, cerebral, renal, etc.) arteries and veins, causing vasoconstriction upon stimulation. This increases systemic pressure and reduces organ perfusion. It also induces positive inotropic effects in cardiomyocytes. α2-adrenergic receptors are located on the pre-synaptic surface of sympathetic ganglia, acting as a negative feedback loop for norepinephrine release. Stimulation of α2-adrenergic receptors located on smooth muscles will result in arterial vasodilation and coronary vasoconstriction.[13]
β1-adrenergic receptors can be stimulated by both norepinephrine and epinephrine. The positive inotropic effect of β1 activation in cardiomyocytes is significantly more pronounced than one, induced by α1 stimulation. In addition, there is a significant positive chronotropic effect through stimulation of the cardiac pacemaker. Stimulation of β1 receptors will also result in release of renin, which will increase mean arterial blood pressure by converting angiotensinogen to angiotensin I. β2-adrenergic receptors are stimulated mainly by epinephrine and will induce vasodilation of muscular arteries, as well as increase norepinephrine release from the sympathetic ganglia.[13]
Dopamine will target D1 and D2 dopaminergic receptors. Activation of D1 receptors results in vasodilation of the renal arteries, while D2 activation will inhibit norepinephrine secretion from sympathetic nerve terminals and have a mild negative inotropic effect on the heart. The signaling net result would explain the clinical phenomenon of lack of hypertension and palpitations in patients with dopamine-secreting pheochromocytomas. On the other hand, pharmacologically high levels of dopamine will stimulate α and β1 receptors causing vasoconstriction and increased heart rate.[13]
Desensitization of adrenergic receptors has always been a hot topic in both research and clinical care. There have been numerous studies that reported significant desensitization of both α and β adrenergic receptors in either healthy humans or patients with pheochromocytoma, as well as in animal models.[18-22] Clinical experience suggests that a significant number of patients with pheochromocytoma-related hypercatecholaminemia are asymptomatic, despite minimal or no treatment. On the other hand, one needs to remember that the specific mechanisms and actual rate of desensitization in the individual person are still unknown. It can be presumed, however, that paroxysmal hypercatecholaminemia, with a rapid secretion of large amounts of catecholamines, will probably precipitate a significant clinical episode, even in a patient with some degree of desensitization. Desensitization seems to be reversible, followed by resensitization of receptors when catecholamine levels return to normal.
Catecholamine Production and Secretion in Pheochromocytoma
The concentration of dopamine, norepinephrine, and epinephrine varies in every tumor depending on its enzymatic machinery. Pheochromocytomas have been reported, to express very high levels of mRNA for the TH, AADC, and DBH enzymes. The high levels of TH expression specifically correlate with high levels of catecholamines production.[23] PNMT activity, on the other hand, is positively influenced by glucocorticoids and is predominantly restricted to normal adrenal tissue. The glucocorticoids can increase PNMT activity and, therefore, raise epinephrine concentration in the cell.[24-25] PNMT levels have been recorded to be lower in pheochromocytomas than in normal adrenal tissue, although the prevalence of PNMT in adrenal pheochromocytomas is higher than in extra-adrenal tumors, because of proximity to the adrenal cortex.[16, 25]
The secretory profile of pheochromocytoma can be useful as a clinical diagnostic tool. Extra-adrenal tumors tend to predominantly secrete norepinephrine and rarely will be dopamine-secreting.[26-27] Adrenal pheochromocytomas, especially ones associated with multiple endocrine neoplasia type 2 (MEN 2), primarily secrete epinephrine or a mix of norepinephrine with epinephrine. Tumors associated with von Hippel-Lindau disease secrete only norepinephrine.[13] Dopamine-secreting tumors are found on rare occasions and only in extra-adrenal pheochromocytomas.[27] These tumors show decreases in DBH expression, which would result in decreases in norepinephrine production and accumulation of dopamine.[28]
Pheochromocytomas can also produce other hormones and peptides including adrenomedullin, vasoactive intestinal polypeptide, ACTH, neuropeptide Y, endothelin-1, somatostatin, atrial natriuretic factor, and parathyroid hormone-related peptide. The clinical picture related to secretion of either or several additional neuroendocrine products would depend on vasoconstrictive versus vasodilatory properties of the substance, superimposed on baseline hypercatecholaminemia.[13, 29-34]
The pattern of catecholamine secretion from the tumor can be either continuous, episodic or both, and the hypertensive paroxysm can be precipitated by physical activity (exercise, postural change) or tumor manipulation.[13, 35] However, it is still unknown and, therefore, difficult to predict when and how many catecholamines each particular tumor will secrete during each secretory episode.
There is also evidence suggesting a correlation between biochemical phenotype and characteristics of hypertension. Patients with tumors that produce high concentrations of norepinephrine are likely to incur sustained hypertension, while patients with significantly elevated levels of epinephrine are often seen having paroxysmal and orthostatic hypertension.[36] Patients with dopamine secreting tumors are most often normotensive.[27]
Clinical Pattern of Pheochromocytoma-Related Hypertension
Hypertension occurs in about 80-90% of patients with pheochromocytoma. About half of these patients develop sustained hypertension, another 45% present with paroxysmal hypertension, while 5-15% are normotensive.[6, 37] The triad of headaches, palpitations, and sweating represent the classical clinical complex seen in patients with pheochromocytoma. Other symptoms may include tachycardia, anxiety and pallor.[36] Most patients with pheochromocytoma have significantly increased systemic catecholamine levels, with norepinephrine and epinephrine levels reaching 5 to 10 times the upper reference limit; normotension is seen mostly in patients with relatively small amounts of catecholamines in circulation. Clinical phenotype of hypertensive syndrome depends on multiple factors including adrenal content of catecholamines, as well as the pattern and nature of their secretion. While the cellular content can be enormous, intracellular processing can divert significant amounts into metabolites, an apparent clinical paradox of normal or near normal plasma catecholamines and significantly elevated metanephrines.[38]
Sustained hypertension strongly correlates with high levels of plasma norepinephrine continuously released from a tumor. Moreover, it was also seen that patients with tumors that predominantly and continuously secreted norepinephrine had higher 24-hr, daytime and nighttime BP compared to patients with tumors that secreted only epinephrine.[39] Patients with sustained hypertension were found to have high plasma levels of catecholamines during every measurement, suggesting a continuous rather than paroxysmal secretory pattern.[13] It is not uncommon, however, to observe a patient with baseline sustained hypertension presenting and fluctuating blood pressure levels as a result of short-term changes in catecholamine concentrations in circulation.[13] Children are also significantly more likely to present with sustained hypertension than paroxysmal hypertension. Evidence suggests that excess norepinephrine is stored in the axon terminals of the sympathetic ganglia and is released with activation of sympathetic nervous system, causing continuous vasoconstriction and sustained hypertension.[40] The presence of orthostatic hypotension may also occur in patients with sustained hypertension (especially those who also have elevated circulating epinephrine levels), and much less commonly in those who have normotension or paroxysmal hypertension.[13] Decreased blood volume caused by consistent vasoconstriction and diminished sympathetic reflex may be important contributing factors for postural hypotension.[41] Postural tachycardia combined with postural hypotension can cause dizziness, palpitations, and syncope when a patient changes from a supine to an upright position. [42]
Paroxysmal hypertension occurred mostly in patients with high-levels of plasma epinephrine and is highly typical for MEN2-related pheochromocytoma.[13] Episodes of paroxysmal hypertension result from the sudden release of catecholamines by tumors and can be induced by multiple factors including physical activity, smoking, abdominal pressure, postural changes and anxiety.[13, 43] Certain foods or beverages with high concentrations of tyramine (cheeses, beers, and wines), drugs (histamine, phenothiazine, or tricyclic antidepressants), and operative procedures with/without anesthesia can cause paroxysmal hypertension in patients with pheochromocytoma.[12, 34, 44] However, the majority of attacks are unpredictable and their frequency can vary from several times in one day to once in several months with attacks lasting from several minutes to over an hour. The sporadic release of epinephrine from these tumors may contribute to the fluctuation in blood pressure. Patients with paroxysmal hypertension exhibit dramatic increases in both systolic and diastolic blood pressure during the paroxysm.[13]
Rarely, patients will rapidly cycle between hypertension and hypotension. In one case, the patient’s BP fluctuated every 14 minutes with a BP ranging from 52/34 to 242/129 mmHg.[45] As with paroxysmal hypertension, these patients had tumors which secreted primarily epinephrine.[46] While the pathophysiology for this event is unclear, it is suggested that baroreceptors respond to a rapid rise in BP during acute paroxysm with activation of the negative feedback loop via both the sympathetic and parasympathetic systems, causing a rapid decrease in BP.[45-46]
Normotension mostly occurs in patients with familial pheochromocytoma, tumors that may be too small to secrete high levels of catecholamines, or dopamine-secreting tumors. Patients may have no typical symptoms of pheochromocytoma and some tumors are detected incidentally because of a palpable abdominal mass or imaging studies carried out to seek the cause of abdominal pain. A study by the Adrenal Incidentaloma Study Group of the Italian Endocrinology Society (AI-SIE) showed that about half the incidentelomas, later found to be pheochromocytomas, were normotensive. The remaining patients experienced only slight elevations in blood pressure and none had paroxysmal hypertension.[47] Parasympathetic paragangliomas of the head and neck usually do not secrete catecholamines, and about 25% associate with some degree of hypertension.[48] Around 10% of patients with mutations in the B subunit of succinate dehydrogenase complex (SDHB) may have biochemically silent pheochromocytomas due to a lack of TH.[49] This group is especially concerning because patients with SDHB mutation are prone to develop metastatic disease. Pheochromocytomas that synthesize and secrete predominantly dopamine also commonly present with normotension or hypotension. These tumors are found to be extra-adrenal and often malignant. The measurement of plasma free methoxytyramine, the metabolite of dopamine, in addition to dopamine and urinary dopamine, is necessary for detection and diagnosis of dopamine-secreting pheochromocytomas.[50]
Special cases of pheochromocytoma-related hypertension include pediatric and obstetric cases. Children are more susceptible to sustained hypertension than adults: 60-90% of children having the sustained form, a small percentage having paroxysmal hypertension and about 20% incurring normotension.[51] It is important to note that children usually have secondary rather than primary hypertension caused by renal deformities, renal artery disease, and congenital aortic coarctation.[52] Only after excluding these conditions, should children be screened for pheochromocytoma.[53] About 80% of children with pheochromocytomas are found to have orthostatic hypotension alternating with hypertensive episodes.[54] As in adults, signs and symptoms can vary greatly in children with pheochromocytoma, with headache and sweating being most common.[13, 55]
Because of the lack of pheochromocytoma-specific signs and symptoms and the possible adverse effect to both mother and fetus, pregnancy represents an important diagnostic challenge. One of the most common misdiagnoses is pre-eclampsia.[56] In both of these cases, hypertension is usually the telling sign; however, pre-eclampsia also brings about other symptoms not normally seen in pheochromocytoma patients including weight gain, proteinuria, pedal edema, and HELLP syndrome (hemolysis, elevated liver enzymes, and low platelets).[12] In addition, hypertension associated with pre-eclampsia usually develops after the 20th week of gestation, but will be present throughout the entire gestation period in a pheochromocytoma patient.[57] Excess catecholamine release in pregnant patients can arise due to a change in intraabdominal pressure following normal delivery, compression of the tumor during labor, and postural changes.[13, 58] Patients experiencing paroxysmal hypertension, headaches, and resistance to traditional anti-hypertensive therapy, should strongly be suspected of having pheochromocytoma.[56] It is stressed that undiagnosed pheochromocytoma can lead to fatal consequences for both mother and fetus via hypertensive crises; complications that can arise include hemorrhage, congestive heart failure, ischemia, infarction, and intrauterine growth restriction, which can cause hypoxia and death for the fetus.[57] To avoid complications, tumor resection should take place either in the first or second trimester (optimal), or after cesarean section when the fetus comes to term.[59] It is important to have close medical management of the patient during the gestation period.
Organ-Specific Hypertensive Complications of Hypercatecholaminemia
Pheochromocytoma is known for life-threatening acute hypertensive emergencies, as well as clinical consequences of long-lasting hypertension (Table 1).
Table 1.
Organ-specific complications of pheochromocytoma-related hypertension
| Organ | Syndrome | Mechanism | Receptor | Receptor action |
|---|---|---|---|---|
| Heart | Angina Heart attack Cardiomyopathies* Myocarditis Acute failure Arrhythmias |
Coronary spasm Positive inotropy Positive chronotropy Unmatched O2 demand Hypoperfusion |
Coronary α1, β2 Conducting system β1, β2 Conducting system β1, β2 Cardiomyocyte β1, β2 |
Constriction Increased conduction Increased automaticity Increased contractility |
| Brain | Stroke Encephalopathy |
Vasoconstriction Unmatched O2 demand Hypoperfusion |
Cerebral arterioles α1 | Mild constriction Most of effect related to sytemic hypertension |
| Vascular | Shock Postural hypotension Aortic dissection Organ ischemia Limb ischemia |
Vasoconstriction Unmatched O2 demand Hypoperfusion |
Skeletal muscle α1, α2, β2 | Arteriolar constriction Arteriolar dilation Venous dilation |
| Kidneys | ARF Hematuria |
Vasoconstriction Unmatched O2 demand Hypoperfusion |
Vascular α1, α2, β1, β2 | Dilation > Constriction Renin secretion |
| Lungs | Pulmonary edema ARDS Fibrosis (?) Pulmonary HTN (?) |
Cardiac decompensation Increased permeability |
Vascular α1, β2 Smooth muscle β2 |
Dilation > Constriction Bronchodilation |
| GI | Intestinal ischemia (necrosis, peritonitis) |
Vasoconstriction Unmatched O2 demand Hypoperfusion |
Visceral arterioles α1, β2 | Constriction |
| Ocular | Acute blindness Retinopathy |
Vasoconstriction | ? | |
| AMF | All of the above |
ARDS Acute respiratory disstress syndrome
HTN Hypertension
ARF Atute renal failure
AMF Acute multiorgan failure
Cardiomyopathies Including Acute – (dilated, tako-tsubo) and chronic (hypertrophic, ischemic, obstructive)
Data from [1-10]
1. Petrak O, Strauch B, Zelinka T, et al. Factors influencing arterial stiffness in pheochromocytoma and effect of adrenalectomy. Hypertens Res. 2010;33(5):454-459.
2. Zelinka Ta, Strauch Ba, Petrak Oa, et al. Increased blood pressure variability in pheochromocytoma compared to essential hypertension patients. [Article]. Journal of Hypertension November. 2005;23(11):2033-2039.
3. O’Callaghan CJa, Williams Bb. The regulation of human vascular smooth muscle extracellular matrix protein production by [alpha]- and [beta]-adrenoceptor stimulation. [Article]. Journal of Hypertension February. 2002;20(2):287-294.
4. Brouwers FM, Lenders JWM, Eisenhofer G, et al. Pheochromocytoma as an Endocrine Emergency. Reviews in Endocrine & Metabolic Disorders. 2003;4(2):121-128.
5. Brilakis ES, YoungJr WF, Wilson JW, et al. Reversible catecholamine-induced cardiomyopathy in a heart transplant candidate without persistent or paroxysmal hypertension. The Journal of Heart and Lung Transplantation. 1999;18(4):376-380.
6. Tack CJJ, Lenders JWM. Pheochromocytoma as a Cause of Blue Toes. Arch Intern Med. September 13, 1993 1993;153(17):2061-a-.
7. Januszewicz W, Wocial B. Clinical and Biochemical Aspects of Pheochromocytoma. Cardiology. 1985;72(Suppl. 1):131-136.
8. Radtke WE, Kazmier FJ, Rutherford BD, et al. Cardiovascular complications of pheochromocytoma crisis. The American Journal of Cardiology. 1975;35(5):701-705.
9. Kwong YL, Yu YL, Lam KSL, et al. CT appearance in hypertensive encephalopathy. Neuroradiology. 1987;29(2):215-215.
10. Newell KA, Prinz RA, Pickleman J, et al. Pheochromocytoma Multisystem Crisis: A Surgical Emergency. Arch Surg. August 1, 1988 1988;123(8):956-959.
Cardiovascular
A large number of cardiovascular complications arise from hypertension. It is suggested that catecholamines, mainly norepinephrine, are capable of structurally changing the vasculature, leading to increased arterial stiffness.[60] Petrak et al. found that the degree of arterial stiffness correlated with high levels of urinary norepinephrine in pheochromocytoma patients. It was also seen that higher BP variability could increase arterial stiffness.[61] Studies suggest that an increase in α-adrenergic receptor stimulation is capable of increasing extra-cellular matrix protein production including collagen and fibronectin, which can result in hypertrophy and can lead to further cardiovascular complications.[62]
Activation of α- and β-adrenergic receptors can also cause vasoconstriction of the coronary arteries and stimulate positive inotropic effects in the heart resulting in tachyarrhythmia. The increase in cardiac contractility along with myocardial hypoxia can result in acute or chronic ischemia and myocardial infarction.[63] Unlike myocardial infarction due to heart disease, pheochromocytoma patients with no past medical history of heart disease will appear to have normal coronary arteries during the angiography along with normal levels of cardiac enzymes.[64] Long-standing hypertension, chronic myocardial hypoxia and metabolic myocarditis of hypercatecholaminemia are known to cause cardiomyopathy that, in the case of pheochromocytoma, can be of either variety – chronic (hypertrophic, dilated, obstructive), or acute (ischemic, tako-tsubo).
Peripheral vascular disease can be acute, caused by intense vasoconstriction and resulting in limb ischemia, necrosis and gangrene or aortic dissection.[63, 65-66] On the other hand, chronic disease will resemble peripheral arterial insufficiency, presenting with limb pallor, pain and intermittent claudication.[67]
Cerebrovascular
Pheochromocytoma can also associate with neurological complications including hypertensive encephalopathy and stroke.[68] In a healthy person, increased systemic blood pressure will cause activation of the α1-adrenergic receptors located in the cerebral resistance vessels, which will constrict.[69] This autoregulation, called Bayliss response, is set to maintain cerebral blood flow. Evidence suggests that in pheochromocytoma, continuously elevated BP “breaks through” this autoregulation and causes vasodilation, resulting in hypoperfusion and ischemia, which leads to hypertensive encephalopathy.[70] In addition, paroxysmal hypertension can cause hemorrhagic, while postural hypotension can result in acute ischemic stroke.[71] Signs and symptoms of hypertensive encephalopathy include headache, papilledema, altered mental status, and even cerebral infarction.[69, 72-73]
Renal
There is evidence suggesting that pheochromocytoma associates with renal complications including renal failure. A study reports that a patient with a norepinephrine-secreting tumor and hypertension also developed renal failure, which resolved after tumor removal.[74]
Vasoconstriction can cause muscle ischemia in pheochromocytoma patients. As a result of the lack of oxygen flowing to the skeletal muscles, the patient can develop rhabdomyolysis and acute tubular necrosis caused by myoglobinuria.[63] High serum levels of creatine kinase together with high levels of urinary myoglobin may be reported in these patients.[75]
Renal artery stenosis can develop in patients with pheochromocytoma and hypertension. Vasoconstriction of the renal artery caused by high levels of catecholamines may lead to impaired kidney function and perhaps renal failure. The majority of tumors that cause this have been found to be extra-adrenal and derived from sympathetic tissue.[76] These tumors have been found mostly in adults and have been reported as benign pheochromocytomas.[77]
Gastrointestinal
Pheochromocytoma can cause gastrointestinal complications, including intestinal ischemia. Patients with intestinal ischemia usually present with severe, sharp, radiating pain in the abdomen.[78] These tumors are usually large and secrete high amounts of catecholamines, resulting in vasospasm in the visceral arterioles, which causes decreased blood flow and visceral ischemia.[78-79]
Ocular
Pheochromocytoma patients that have arterial hypertension may also present with hypertensive retinopathy, caused by retinal vasoconstriction, increased vascular permeability, and secondary arteriosclerosis.[80-81] A retinal exam may reveal retinal microaneurysms, hemorrhages, cotton wool spots, and venous bleeding.[82] Patients with malignant hypertension more commonly show signs of vision problems than other groups.[81]
Multisystem organ failure
Pheochromocytoma patients will rarely present with cardiogenic shock, which is seen mainly in epinephrine-secreting tumors.[83-85] It has been suggested that β-receptor down regulation, caused by continuously increased catecholamine levels, can lead to a decrease in contractility, which results in cardiogenic shock.[86]
Patients can also display pheochromocytoma multi-system crisis (PMC). Different from cardiogenic shock, this complication consists of multiple organ system failure, a fever over 40°C, encephalopathy, and severe hypertension and/or hypotension.[87] PMC can be masked to present as sepsis, which can delay surgery. surgery and resection of the tumor have proven to be the ideal choice in regards to treatment.[88] While the specific cause of PMC is unknown, all of the previously discussed complications in this section may present in PMC and it is therefore important to mention them.
Diagnostic Approach
Pheochromocytoma has been called many names – from a friendly “great mimicker” to a treacherous “cold-blooded killer” (the associated hypermetabolic state would actually make it rather “warm-blooded”) and whatever fits in between, which mostly translates into our fear of missing it, because of highly feasible fatal complications. Although the most important part of pheochromocytoma diagnosis still remains timely clinical suspicion, the level of its threshold continues to fluctuate. So, it is not just “think about it”, but rather “think about it, when …” with the latter being a subject of debate. It is probably reasonable to work up a hypertensive patient who does not respond to appropriate therapeutic trial (the key word being “appropriate”), or has paroxysmal episodes, rapidly progressive or resistant hypertension, severe hypertension, hypertension associated with systemic (sweating, pallor, etc.), as well as postural reactions (hypotension or tachycardia). While this selection will significantly reduce the number of patients with hypertension that will need workup for pheochromocytoma, the addition of adrenal incidentalomas with any elevation in BP shoot up the number of workups exponentially. The cost-effectiveness of the current approach is still to be established. Diagnostic workup includes plasma or urinary metanephrines with or without catecholamines and chromogranin A, followed if positive, by imaging studies. A detailed diagnostic approach is outside the scope of this article and can be found elsewhere.[12, 89-91]
Pharmacological Treatment of Pheochromocytoma
While the ultimate treatment of pheochromocytoma is surgical, pharmacological therapy still remains of vital significance in pre-operative and operative control of BP, as well as in cases of inoperable metastatic disease (Table 2). It also important to remember that tumor manipulations during surgery may associate with the release of tremendous amounts of catecholamines into circulation, which might may be capable of overpowering the pharmacological blockade. In order to control hypertension levels prior to an operation, even in pre-operative normotensive patients, it is recommended that patients undergo pre-operative pharmacological treatment.[92] Phenoxybenzamine (Dibenzyline) is a non-competitive α1 and α2 blocker that reduces blood pressure fluctuation and eases vasoconstriction, preventing an intraoperative hypertensive crisis.[93-95] Patients are usually prescribed 10-30 mg twice daily until BP and other symptoms have stabilized, which normally takes 10-14 days.[34, 95]
Table 2.
Approach to treatment of pheochromocytoma-related hypertension
| Stage | Goal | Primary Rx | Supplementary/Alternative* Rx |
|---|---|---|---|
| Initial trx (A) | Normalization of BP Minimal organ complications |
In the following order: Phenoxybenzamine PO β blocker PO Metyrosine PO |
Alternative α blocker (see text) Calcium channel blocker Labetalol PO |
| Pre-operative trx | Normal BP Normovolemia Optimized cardiac performance |
As in (A) Fluids to normovolemia |
As in (A) |
| Intra-operative trx |
Prevention of the following: Severe hypercatecholaminemia Severe hypertension Severe hypotension |
Nitroprusside IV β blocker IV Aggressive fluid replacement |
Labetalol IV Nitroprusside IV |
| Post-operative trx | Prevention of hypotension Prevention of hypoglycemia |
Aggressive fluid replacement Glucose Replacement |
|
| Trx of inoperable disease | Maintanence of normal BP Treatment of metastatic disease |
Chemotherapy Radiotherapy Debulking |
Experimental Therapy |
Trx Treatment
BP Blood pressure
Supplementary Rx Added to meet the goal for BP control
Alternative Rx Denotes alternative treatment based on center-specific preferences
Metyrosine α-methyl-L-tyrosine (α-MPT, Demser). For mechanism of action, please see text
Data from [1-8]
1. Pacak K, Eisenhofer G, Ahlman H, et al. Pheochromocytoma: recommendations for clinical practice from the First International Symposium. Nat Clin Pract End Met. 2007;3(2):92-102.
2. Manger W, Eisenhofer G. Pheochromocytoma: Diagnosis and management update. Current Hypertension Reports. 2004;6(6):477-484.
3. Bravo EL, Tagle R. Pheochromocytoma: State-of-the-Art and Future Prospects. Endocr Rev. August 1, 2003 2003;24(4):539-553.
4. Ross E, Prichard B, Kaufman L, et al. Preoperative and operative management of patients with phaeochromocytoma. Br Med J. 1967;1(5534):191-198.
5. Desmonts JM, Marty J. Anaesthetic management of patients woth phaeochromocytoma. Br. J. Anaesth. July 1, 1984 1984;56(7):781-789.
6. Ulchaker JC, Goldfarb DA, Bravo EL, et al. Successful outcomes in pheochromocytoma surgery in the modern era. The Journal of Urology. 1999;161(3):764-767.
7. Bravo EL. Evolving Concepts in the Pathophysiology, Diagnosis, and Treatment of Pheochromocytoma. Endocr Rev. June 1, 1994 1994;15(3):356-368.
8. Tokioka H, Takahashi T, Kosogabe Y, et al. Use of diltiazem to control circulatory fluctuations during resection of a phaeochromocytoma. Br. J. Anaesth. April 1, 1988 1988;60(5):582-587.
An alternative pre-operative approach is to avoid use of phenoxybenzamine, while aggressively utilizing potent, short acting, intravenous, anti-hypertensive agents - sodium nitropruside (Nitropress) and nitroglycerin (Nitrostat) - to control sudden changes in blood pressure during surgery.[93, 96] This approach is based on several reports of the seeming inefficacy of phenoxybenzamine during surgery, as well as well-known postoperative hypotension and tachycardia, related to its use.[7, 97] On the other hand, one should remember that in the event of incomplete α-adrenergic receptor blockade and massive release of catecholamines during surgery, phenoxybenzamine would be only partially efficient. It could also be speculated that treatment with phenoxybenzamine was only in part to blame for a case of postoperative hypovolemia and rapid cessation of hypercatecholaminemia, with associated hypotension and tachycardia.
Specific α1-adrenergic receptor blockers, like prazosin hydrochloride (Minipres), terazosin (Hytrin), or doxazosin (Cardura) can be used instead of phenoxybenzamine in an attempt to prevent occurrence of tachycardia by allowing the natural negative feedback mechanism through the unopposed α2-adrenergic receptors. They also are of a shorter duration of action, which decreases the length of post-operative hypotension.
Calcium channel blockers - like diltiazem (Cardizem) - are effective vasodilators and can be used in patients with pheochromocytoma to control blood pressure.[98] Unlike phenoxybenzamine, calcium channel blockers will not cause dangerous hypotension and are relatively short acting. It is especially useful for normotensive patients or those with very mild hypertension. Combination of calcium channel blockers with prazosin, doxazosin, or another α1-adrenergic receptor blocker would complement each other well during tumor resection.
The use of a β-blockade can be utilized concomitantly with any of the α-adrenergic receptor blockades, especially when used with phenoxybenzamine, to prevent reflex tachycardia.[94] It is important, however, that β-blockade only be used subsequent to an α-blockade, because unopposed β-blockade can result in a significant increase in arterial pressure.
Use of metyrosine (Demser) proved to be useful in decreasing the amount of synthesized catecholamines by preoccupying tyrosine hydroxylase with substrate that is hydroxylated to inactive metabolite. It is effective in pre-operative treatment, as well as in treatment of patients with metastatic and inoperable disease.[97] The last group is also treated with adrenergic blockers for symptomatic disease, as well as with chemo- and radiotherapy.
Conclusions
Pheochromocytoma is a tumor of the chromaffin cells in the adrenal medulla and sympathetic paraganglia, which synthesizes and secretes catecholamines. Norepinephrine, epinephrine, and dopamine all act on their target receptors, which causes a physiological change in the body. High, circulating levels of catecholamines can lead to severe hypertension and can have devastating effects on multiple body systems (cardiovascular, cerebrovascular, etc.), and can lead to death if untreated. Although surgical treatment represents the only modality of ultimate cure, pharmacological pre-operative treatment remains the mainstay of successful outcome.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have nothing to disclose.
References
- 1.DeLellis RA, Lloyd RV, Heitz PU, et al. World Health Organization Classification of Tumours. Pathophysiology and Genetics of Tumours of Endocrine Organs. IARC Press; Lyon: 2004. [Google Scholar]
- 2.Lehnert H. Pheochromocytoma. Pathophysiology and Clinical Management. Vol. 31. Karger; Basel: 2004. [PubMed] [Google Scholar]
- 3.van Duinen N, Steenvoorden D, Kema IP, et al. Increased Urinary Excretion of 3-Methoxytyramine in Patients with Head and Neck Paragangliomas. J Clin Endocrinol Metab. 2010 January 1;95(1):209–214. doi: 10.1210/jc.2009-1632. 2010. [DOI] [PubMed] [Google Scholar]
- 4.Omura M, Saito J, Yamaguchi K, et al. Prospective Study on the Prevalence of Secondary Hypertension among Hypertensive Patients Visiting a General Outpatient Clinic in Japan. Hypertens Res. 2004;27(3):193–202. doi: 10.1291/hypres.27.193. [DOI] [PubMed] [Google Scholar]
- 5.Ariton M, Juan CS, AvRuskin TW. Pheochromocytoma: Clinical observations from a brooklyn tertiary hospital. Endocrine Practice. 2000;6(3):249–252. doi: 10.4158/EP.6.3.249. [DOI] [PubMed] [Google Scholar]
- 6.Manger WM. The Protean Manifestations of Pheochromocytoma. Horm Metab Res. 2009;41(09):658–663. doi: 10.1055/s-0028-1128139. [DOI] [PubMed] [Google Scholar]
- 7.Lenders JWM, Eisenhofer G, Mannelli M, et al. Phaeochromocytoma. The Lancet. 2005;366(9486):665–675. doi: 10.1016/S0140-6736(05)67139-5. [DOI] [PubMed] [Google Scholar]
- 8.Sutton M, Sheps SG, Lie JL. Prevalence of clinically unsuspected pheochromocytoma. Review of a 50-year autopsy series. Mayo Clinic Proceedings. 1981;56(6):354–360. [PubMed] [Google Scholar]
- 9.McNeil AR, Blok BH, Koelmeyer TD, et al. Phaeochromocytomas discovered during coronial autopsies in Sydney, Melbourne and Auckland. Internal Medicine Journal. 2000;30(6):648–652. doi: 10.1111/j.1445-5994.2000.tb04358.x. [DOI] [PubMed] [Google Scholar]
- 10.Calhoun DA, Jones D, Textor S, et al. Resistant Hypertension: Diagnosis, Evaluation, and Treatment: A Scientific Statement From the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension. 2008 June 1;51(6):1403–1419. doi: 10.1161/HYPERTENSIONAHA.108.189141. 2008. [DOI] [PubMed] [Google Scholar]
- 11.Guérin M, Guillemot J, Thouënnon E, et al. Granins and their derived peptides in normal and tumoral chromaffin tissue: Implications for the diagnosis and prognosis of pheochromocytoma. Regulatory Peptides. 2010 doi: 10.1016/j.regpep.2010.06.003. In Press, Corrected Proof. [DOI] [PubMed] [Google Scholar]
- 12.Manger WM. An Overview of Pheochromocytoma. Annals of the New York Academy of Sciences. 2006;1073:1–20. doi: 10.1196/annals.1353.001. Pheochromocytoma First International Symposium. [DOI] [PubMed] [Google Scholar]
- 13.Manger W, Gifford RW. Clinical and Experimental Pheochromocytoma. Second Edition Blackwell Science; Cambridge: 1996. [Google Scholar]
- 14.Nagatsu T, Levitt M, Udenfriend S. Tyrosine Hydroxylase. Journal of Biological Chemistry. 1964 September 1;239(9):2910–2917. 1964. [PubMed] [Google Scholar]
- 15.Kaufman S, Friedman S. Dopamine-β-hydroxylase. Pharmacological Reviews. 1965 June;17(2):71–100. 1965. [PubMed] [Google Scholar]
- 16.Lehnert H. Regulation of catecholamine synthesizing enzyme gene expression in human pheochromocytoma. Eur J Endocrinol. 1998 April 1;138(4):363–367. doi: 10.1530/eje.0.1380363. 1998. [DOI] [PubMed] [Google Scholar]
- 17.Jameson JL, DeGroot LJ. Endocrinology: Adult and Pediatric. 6th ed. Saunders; Philadelphia: 2010. [Google Scholar]
- 18.Greenacre J, Conolly M. Desensitization of the beta-adrenoceptor of lymphocytes from normal subjects and patients with phaeochromocytoma: studies in vivo. Br J Clin Pharmacol. 1978;5(3):191–197. doi: 10.1111/j.1365-2125.1978.tb01623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsujimoto G, Manger W, Hoffman B. Desensitization of beta-adrenergic receptors by pheochromocytoma. Endocrinology. 1984;114(4):1272–1278. doi: 10.1210/endo-114-4-1272. [DOI] [PubMed] [Google Scholar]
- 20.Tsujimoto G, Honda K, Hoffman B, et al. Desensitization of postjunctional alpha 1- and alpha 2-adrenergic receptor-mediated vasopressor responses in rat harboring pheochromocytoma. Circ Res. 1987;61(1):86–98. doi: 10.1161/01.res.61.1.86. [DOI] [PubMed] [Google Scholar]
- 21.Krause M, Reinhardt D, Kruse K. Phaeochromocytoma without symptoms: desensitization of the alpha- and beta-adrenoceptors. Eur J Pediatr. 1988;147(2):121–122. doi: 10.1007/BF00442207. [DOI] [PubMed] [Google Scholar]
- 22.Jones C, Hamilton C, Whyte K, et al. Acute and chronic regulation of alpha 2-adrenoceptor number and function in man. Clin Sci. 1985;68(Suppl 10):129–132. doi: 10.1042/cs068s129. [DOI] [PubMed] [Google Scholar]
- 23.Isobe K, Nakai T, Yukimasa N, et al. Expression of mRNA coding for four catecholamine-synthesizing enzymes in human adrenal pheochromocytomas. Eur J Endocrinol. 1998 April 1;138(4):383–387. doi: 10.1530/eje.0.1380383. 1998. [DOI] [PubMed] [Google Scholar]
- 24.Wurtman RJ, Axelrod J. Control of Enzymatic Synthesis of Adrenaline in the Adrenal Medulla by Adrenal Cortical Steroids. Journal of Biological Chemistry. 1966 May 25;241(10):2301–2305. 1966. [PubMed] [Google Scholar]
- 25.Betito K, Diorio J, Meaney MJ, et al. Adrenal Phenylethanolamine <i>N</i>-Methyltransferase Induction in Relation to Glucocorticoid Receptor Dynamics: Evidence that Acute Exposure to High Cortisol Levels Is Sufficient to Induce the Enzyme. Journal of Neurochemistry. 1992;58(5):1853–1862. doi: 10.1111/j.1471-4159.1992.tb10062.x. [DOI] [PubMed] [Google Scholar]
- 26.Brown WJ, Barajas L, Waisman J, et al. Ultrastructural and biochemical correlates of adrenal and extra-adrenal pheochromocytoma. Cancer. 1972;29(3):744–759. doi: 10.1002/1097-0142(197203)29:3<744::aid-cncr2820290331>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 27.Proye C, Fossati P, Fontaine P, et al. Dopamine-secreting pheochromocytoma: an unrecognized entity? Classification of pheochromocytomas according to their type of secretion. Surgery. 1986;100(6):1154–1162. [PubMed] [Google Scholar]
- 28.Yasunari K, Kohno M, Minami M, et al. A Dopamine-Secreting Pheochromocytoma. Journal of Cardiovascular Pharmacology. 2000;36:S75–S77. doi: 10.1097/00005344-200000006-00016. [DOI] [PubMed] [Google Scholar]
- 29.Letizia C, De Toma G, Caliumi C, et al. Plasma Adrenomedullin Concentrations in Patients with Adrenal Pheochromocytoma. Horm Metab Res. 2001;33(05):290–294. doi: 10.1055/s-2001-15281. [DOI] [PubMed] [Google Scholar]
- 30.Sano T, Saito H, Inaba H, et al. Immunoreactive somatostatin and vasoactive intestinal polypeptide in adrenal pheochromocytoma an immunochemical and ultrastructural study. Cancer. 1983;52(2):282–289. doi: 10.1002/1097-0142(19830715)52:2<282::aid-cncr2820520215>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 31.Nijhoff MF, Dekkers OM, Vleming LJ, et al. ACTH-producing pheochromocytoma: Clinical considerations and concise review of the literature. European Journal of Internal Medicine. 2009;20(7):682–685. doi: 10.1016/j.ejim.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 32.deS Senanayake P, Denker J, Bravo EL, et al. Production, characterization, and expression of neuropeptide Y by human pheochromocytoma. The Journal of Clinical Investigation. 1995;96(5):2503–2509. doi: 10.1172/JCI118310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oishi S, Sasaki M, Sato T. Elevated immunoreactive endothelin levels in patients with pheochromocytoma. 1994;7(8):717–722. doi: 10.1093/ajh/7.8.717. [DOI] [PubMed] [Google Scholar]
- 34.Manger W, Eisenhofer G. Pheochromocytoma: Diagnosis and management update. Current Hypertension Reports. 2004;6(6):477–484. doi: 10.1007/s11906-004-0044-2. [DOI] [PubMed] [Google Scholar]
- 35.Bravo EL. Pheochromocytoma: current perspectives in the pathogenesis, diagnosis, and management. Arquivos Brasileiros de Endocrinologia & Metabologia. 2004;48:746–750. doi: 10.1590/s0004-27302004000500021. [DOI] [PubMed] [Google Scholar]
- 36.Ito Y, Fuimoto Y, Obara T. The role of epinephrine, norepinephrine, and dopamine in blood pressure disturbances in patients with pheochromocytoma. World Journal of Surgery. 1992;16(4):759–763. doi: 10.1007/BF02067379. [DOI] [PubMed] [Google Scholar]
- 37.Zelinka T, Eisenhofer G, Pacak K. Pheochromocytoma as a catecholamine producing tumor: Implications for clinical practice. Stress: The International Journal on the Biology of Stress. 2007;10(2):195–203. doi: 10.1080/10253890701395896. [DOI] [PubMed] [Google Scholar]
- 38.Pacak K. Preoperative Management of the Pheochromocytoma Patient. J Clin Endocrinol Metab. 2007 November 1;92(11):4069–4079. doi: 10.1210/jc.2007-1720. 2007. [DOI] [PubMed] [Google Scholar]
- 39.O’Rourke MF, Staessen JA, Vlachopoulos C, et al. Clinical applications of arterial stiffness; definitions and reference values. Am J Hypertens. 2002;15(5):426–444. doi: 10.1016/s0895-7061(01)02319-6. [DOI] [PubMed] [Google Scholar]
- 40.Bravo E, Tarazi R, Fouad F, et al. Blood pressure regulation in pheochromocytoma. Hypertension. 1982 May 1;4(3):193–199. 1982. [PubMed] [Google Scholar]
- 41.Engleman K, Zelis R, Waldmann T, et al. Meachanism of orthostatic hypotension in pheochromocytoma. Circulation. 1968;38(Suppl. 6):VI–72. [Google Scholar]
- 42.Streeten DHP, Anderson GH. Mechanisms of orthostatic hypotension and tachycardia in patients with pheochromocytoma. American Journal of Hypertension. 1996;9(8):760–769. doi: 10.1016/0895-7061(96)00057-x. [DOI] [PubMed] [Google Scholar]
- 43.Widimský J., Jr Recent Advances in the Diagnosis and Treatment of Pheochromocytoma. Kidney and Blood Pressure Research. 2006;29(5):321–326. doi: 10.1159/000097262. [DOI] [PubMed] [Google Scholar]
- 44.Bittar D. Innovar-induced hypertensive crises in patients with pheochromocytoma. Anesthesiology. 1979;50(4):366–369. doi: 10.1097/00000542-197904000-00019. [DOI] [PubMed] [Google Scholar]
- 45.Ionescu CN, Sakharova OV, Harwood MD, et al. Cyclic Rapid Fluctuation of Hypertension and Hypotension in Pheochromocytoma. The Journal of Clinical Hypertension. 2008;10(12):936–940. doi: 10.1111/j.1751-7176.2008.00046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kobal SL, Paran E, Jamali A, et al. Pheochromocytoma: cyclic attacks of hypertension alternating with hypotension. Nat Clin Pract Cardiovasc Med. 2008;5(1):53–57. doi: 10.1038/ncpcardio1054. [DOI] [PubMed] [Google Scholar]
- 47.Mantero F, Albiger N. A comprehensive approach to adrenal incidentalomas. Arquivos Brasileiros de Endocrinologia & Metabologia. 2004;48:583–591. doi: 10.1590/s0004-27302004000500003. [DOI] [PubMed] [Google Scholar]
- 48.Erickson D, Kudva YC, Ebersold MJ, et al. Benign Paragangliomas: Clinical Presentation and Treatment Outcomes in 236 Patients. J Clin Endocrinol Metab. 2001 November 1;86(11):5210–5216. doi: 10.1210/jcem.86.11.8034. 2001. [DOI] [PubMed] [Google Scholar]
- 49.Timmers HJLM, Kozupa A, Eisenhofer G, et al. Clinical Presentations, Biochemical Phenotypes, and Genotype-Phenotype Correlations in Patients with Succinate Dehydrogenase Subunit B-Associated Pheochromocytomas and Paragangliomas. J Clin Endocrinol Metab. 2007 March 1;92(3):779–786. doi: 10.1210/jc.2006-2315. 2007. [DOI] [PubMed] [Google Scholar]
- 50.Eisenhofer G, Goldstein DS, Sullivan P, et al. Biochemical and Clinical Manifestations of Dopamine-Producing Paragangliomas: Utility of Plasma Methoxytyramine. J Clin Endocrinol Metab. 2005 April 1;90(4):2068–2075. doi: 10.1210/jc.2004-2025. 2005. [DOI] [PubMed] [Google Scholar]
- 51.Barontini M, Levin G, Sanso G. Characteristics of Pheochromocytoma in a 4- to 20-Year-Old Population. Annals of the New York Academy of Sciences. 2006;1073:30–37. doi: 10.1196/annals.1353.003. Pheochromocytoma First International Symposium. [DOI] [PubMed] [Google Scholar]
- 52.Londe S. Causes of hypertension in the young. Pediatr Clin North Am. 1978;25(1):55–65. doi: 10.1016/s0031-3955(16)33532-5. [DOI] [PubMed] [Google Scholar]
- 53.Ross JH. Pheochromocytoma: Special Considerations in Children. Urologic Clinics of North America. 2000;27(3):393–402. doi: 10.1016/s0094-0143(05)70088-4. [DOI] [PubMed] [Google Scholar]
- 54.Beltsevich DG, Kuznetsov NS, Kazaryan AM, et al. Pheochromocytoma Surgery: Epidemiologic Peculiarities in Children. World Journal of Surgery. 2004;28(6):592–596. doi: 10.1007/s00268-004-7134-9. [DOI] [PubMed] [Google Scholar]
- 55.Ludwig AD, Feig DI, Brandt ML, et al. Recent advances in the diagnosis and treatment of pheochromocytoma in children. The American Journal of Surgery. 2007;194(6):792–797. doi: 10.1016/j.amjsurg.2007.08.028. [DOI] [PubMed] [Google Scholar]
- 56.Oliva R, Angelos P, Kaplan E, et al. Pheochromocytoma in Pregnancy: A Case Series and Review. Hypertension. 2010 March 1;55(3):600–606. doi: 10.1161/HYPERTENSIONAHA.109.147579. 2010. [DOI] [PubMed] [Google Scholar]
- 57.Dugas G, Fuller J, Singh S, et al. Pheochromocytoma and pregnancy: a case report and review of anesthetic management. Canadian Journal of Anesthesia / Journal canadien d’anesthésie. 2004;51(2):134–138. doi: 10.1007/BF03018772. [DOI] [PubMed] [Google Scholar]
- 58.Ahlawat SK, Jain S, Kumari S, et al. Pheochromocytoma Associated With Pregnancy: Case Report and Review of the Literature. Obstetrical & Gynecological Survey. 1999;54(11):728. doi: 10.1097/00006254-199911000-00025. [DOI] [PubMed] [Google Scholar]
- 59.George J, Tan JYL. Pheochromocytoma in pregnancy: a case report and review of literature. Obstet Med. 2010 June 1;3(2):83–85. doi: 10.1258/om.2010.090063. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Petrak O, Strauch B, Zelinka T, et al. Factors influencing arterial stiffness in pheochromocytoma and effect of adrenalectomy. Hypertens Res. 2010;33(5):454–459. doi: 10.1038/hr.2010.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zelinka Ta, Strauch Ba, Petrak Oa, et al. Increased blood pressure variability in pheochromocytoma compared to essential hypertension patients. Journal of Hypertension November. 2005;23(11):2033–2039. doi: 10.1097/01.hjh.0000185714.60788.52. Article. [DOI] [PubMed] [Google Scholar]
- 62.O’Callaghan CJa, Williams Bb. The regulation of human vascular smooth muscle extracellular matrix protein production by [alpha]- and [beta]-adrenoceptor stimulation. Journal of Hypertension February. 2002;20(2):287–294. doi: 10.1097/00004872-200202000-00019. Article. [DOI] [PubMed] [Google Scholar]
- 63.Brouwers FM, Lenders JWM, Eisenhofer G, et al. Pheochromocytoma as an Endocrine Emergency. Reviews in Endocrine & Metabolic Disorders. 2003;4(2):121–128. doi: 10.1023/a:1022981801344. [DOI] [PubMed] [Google Scholar]
- 64.Brilakis ES, Young WF, Jr, Wilson JW, et al. Reversible catecholamine-induced cardiomyopathy in a heart transplant candidate without persistent or paroxysmal hypertension. The Journal of Heart and Lung Transplantation. 1999;18(4):376–380. doi: 10.1016/s1053-2498(98)00014-x. [DOI] [PubMed] [Google Scholar]
- 65.Tack CJJ, Lenders JWM. Pheochromocytoma as a Cause of Blue Toes. Arch Intern Med. 1993 September 13;153(17):2061. 1993. a. [PubMed] [Google Scholar]
- 66.Januszewicz W, Wocial B. Clinical and Biochemical Aspects of Pheochromocytoma. Cardiology. 1985;72(Suppl. 1):131–136. doi: 10.1159/000173959. [DOI] [PubMed] [Google Scholar]
- 67.Radtke WE, Kazmier FJ, Rutherford BD, et al. Cardiovascular complications of pheochromocytoma crisis. The American Journal of Cardiology. 1975;35(5):701–705. doi: 10.1016/0002-9149(75)90060-0. [DOI] [PubMed] [Google Scholar]
- 68.Kwong YL, Yu YL, Lam KSL, et al. CT appearance in hypertensive encephalopathy. Neuroradiology. 1987;29(2):215–215. doi: 10.1007/BF00327557. [DOI] [PubMed] [Google Scholar]
- 69.Dinsdale H. Hypertensive encephalopathy. Stroke. 1982 September 1;13(5):717–719. doi: 10.1161/01.str.13.5.717. 1982. [DOI] [PubMed] [Google Scholar]
- 70.Strandgaard S, Olesen J, Skinhoj E, et al. Autoregulation of Brain Circulation in Severe Arterial Hypertension. Br Med J. 1973 March 3;1(5852):507–510. doi: 10.1136/bmj.1.5852.507. 1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lin P, Hsu J, Chung C, et al. Pheochromocytoma underlying hypertension, stroke, and dilated cardiomyopathy. Tex Heart Inst J. 2007;34(2):244–246. [PMC free article] [PubMed] [Google Scholar]
- 72.Campellone JV, Kolson DL, Wells GB, et al. Cerebral infarction during hypertensive encephalopathy: Case report with pathologic and atypical radiographic findings. Journal of Stroke and Cerebrovascular Diseases. 1995;5(2):66–71. doi: 10.1016/S1052-3057(10)80348-9. [DOI] [PubMed] [Google Scholar]
- 73.Majic T, Aiyagari V. Cerebrovascular Manifestations of Pheochromocytoma and the Implications of a Missed Diagnosis. Neurocritical Care. 2008;9(3):378–381. doi: 10.1007/s12028-008-9105-8. [DOI] [PubMed] [Google Scholar]
- 74.Fujiwara M, Imachi H, Murao K, et al. Improvement in renal dysfunction and symptoms after laparoscopic adrenalectomy in a patient with pheochromocytoma complicated by renal dysfunction. Endocrine. 2009;35(1):57–62. doi: 10.1007/s12020-008-9119-1. [DOI] [PubMed] [Google Scholar]
- 75.Shemin D, Cohn PS, Zipin SB. Pheochromocytoma Presenting as Rhabdomyolysis and Acute Myoglobinuric Renal Failure. Arch Intern Med. 1990 November 1;150(11):2384–2385. 1990. [PubMed] [Google Scholar]
- 76.Hill FS, Jander HP, Murad T, et al. The coexistence of renal artery stenosis and pheochromocytoma. Ann Surg. 1983;197(4):484–490. doi: 10.1097/00000658-198304000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Crowe A, Jones N, Carr P. Five ways to be fooled by phaeochromocytoma-renal and urological complications. Nephrol. Dial. Transplant. 1997 February 1;12(2):337–340. doi: 10.1093/ndt/12.2.337. 1997. [DOI] [PubMed] [Google Scholar]
- 78.Salehi A, Legome EL, Eichhorn K, et al. Pheochromocytoma and bowel ischemia. Journal of Emergency Medicine. 1996;15(1):35–38. doi: 10.1016/s0736-4679(96)00241-7. [DOI] [PubMed] [Google Scholar]
- 79.Carr ND, Hulme A, Sheron N, et al. Intestinal ischaemia associated with phaeochromocytoma. Postgraduate Medical Journal. 1989 August 1;65:594–596. doi: 10.1136/pgmj.65.766.594. 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chatterjee S, Chattopadhyay S, Hope-Ross M, et al. Hypertension and the eye: changing perspectives. J Hum Hypertens. 2002;16(10):667–675. doi: 10.1038/sj.jhh.1001472. [DOI] [PubMed] [Google Scholar]
- 81.Petkou D, Petropoulos IK, Kordelou A, et al. Severe Bilateral Hypertensive Retinopathy and Optic Neuropathy in a Patient with Pheochromocytoma. Schwere beidseitige hypertensive Retinopathie und Optikusneuropathie bei einem Patienten mit Phäochromozytom. 2008;225(05):500–503. doi: 10.1055/s-2008-1027355. [DOI] [PubMed] [Google Scholar]
- 82.Schubert HD. Ocular manifestations of systemic hypertension. Current Opinion in Ophthalmology. 1998;9(6):69–72. doi: 10.1097/00055735-199812000-00012. [DOI] [PubMed] [Google Scholar]
- 83.Bergland BE. Pheochromocytoma presenting as shock. The American Journal of Emergency Medicine. 1989;7(1):44–48. doi: 10.1016/0735-6757(89)90084-3. [DOI] [PubMed] [Google Scholar]
- 84.Galetta F, Franzoni F, Bernini G, et al. Cardiovascular complications in patients with pheochromocytoma: A mini-review. Biomedicine & Pharmacotherapy. 2009 doi: 10.1016/j.biopha.2009.09.014. In Press, Corrected Proof. [DOI] [PubMed] [Google Scholar]
- 85.Olson SW, Deal LE, Piesman M. Epinephrine-Secreting Pheochromocytoma Presenting with Cardiogenic Shock and Profound Hypocalcemia. Annals of Internal Medicine. 2004 May 18;140(10):849–851. doi: 10.7326/0003-4819-140-10-200405180-00033. 2004. [DOI] [PubMed] [Google Scholar]
- 86.Cryer P. Physiology and pathophysiology of the human sympathoadrenal neuroendocrine system. N Engl J Med. 1980 August 21;303(8):436–444. doi: 10.1056/NEJM198008213030806. 1980. [DOI] [PubMed] [Google Scholar]
- 87.Newell KA, Prinz RA, Pickleman J, et al. Pheochromocytoma Multisystem Crisis: A Surgical Emergency. Arch Surg. 1988 August 1;123(8):956–959. doi: 10.1001/archsurg.1988.01400320042007. 1988. [DOI] [PubMed] [Google Scholar]
- 88.Moran ME, Rosenberg DJ, Zornow DH. Pheochromocytoma multisystem Crisis. Urology. 2006;67(4):846.e819–846.e820. doi: 10.1016/j.urology.2005.10.046. [DOI] [PubMed] [Google Scholar]
- 89.Barron J. Phaeochromocytoma: diagnostic challenges for biochemical screening and diagnosis. Journal of Clinical Pathology. 2010 doi: 10.1136/jcp.2009.071647. [DOI] [PubMed] [Google Scholar]
- 90.Eisenhofer G, Siegert G, Kotzerke J, et al. Current Progress and Future Challenges in the Biochemical Diagnosis and Treatment of Pheochromocytomas and Paragangliomas. Horm Metab Res. 2008;40(05):329–337. doi: 10.1055/s-2008-1073156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Adler JT, Meyer-Rochow GY, Chen H, et al. Pheochromocytoma: Current Approaches and Future Directions. Oncologist. 2008 July 10; doi: 10.1634/theoncologist.2008-0043. 2008:theoncologist.2008-0043. [DOI] [PubMed] [Google Scholar]
- 92.Pacak K, Eisenhofer G, Ahlman H, et al. Pheochromocytoma: recommendations for clinical practice from the First International Symposium. Nat Clin Pract End Met. 2007;3(2):92–102. doi: 10.1038/ncpendmet0396. [DOI] [PubMed] [Google Scholar]
- 93.Bravo EL, Tagle R. Pheochromocytoma: State-of-the-Art and Future Prospects. Endocr Rev. 2003 August 1;24(4):539–553. doi: 10.1210/er.2002-0013. 2003. [DOI] [PubMed] [Google Scholar]
- 94.Ross E, Prichard B, Kaufman L, et al. Preoperative and operative management of patients with phaeochromocytoma. Br Med J. 1967;1(5534):191–198. doi: 10.1136/bmj.1.5534.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Desmonts JM, Marty J. Anaesthetic management of patients woth phaeochromocytoma. Br. J. Anaesth. 1984 July 1;56(7):781–789. doi: 10.1093/bja/56.7.781. 1984. [DOI] [PubMed] [Google Scholar]
- 96.Ulchaker JC, Goldfarb DA, Bravo EL, et al. Successful outcomes in pheochromocytoma surgery in the modern era. The Journal of Urology. 1999;161(3):764–767. [PubMed] [Google Scholar]
- 97.Bravo EL. Evolving Concepts in the Pathophysiology, Diagnosis, and Treatment of Pheochromocytoma. Endocr Rev. 1994 June 1;15(3):356–368. doi: 10.1210/edrv-15-3-356. 1994. [DOI] [PubMed] [Google Scholar]
- 98.Tokioka H, Takahashi T, Kosogabe Y, et al. Use of diltiazem to control circulatory fluctuations during resection of a phaeochromocytoma. Br. J. Anaesth. 1988 April 1;60(5):582–587. doi: 10.1093/bja/60.5.582. 1988. [DOI] [PubMed] [Google Scholar]