Abstract
The present investigation was undertaken to evaluate the bronchodilating effect and bronchial hyperreactivity of alcoholic extract of Taxus baccata Linn. (AET) leaves in experimental animals. Bronchodilator activity of AET was studied on the histamine and acetylcholine aerosol induced bronchospasm in guinea pigs and bronchial hyperreactivity was studied on bronchoalveolar lavage fluid (BALF) in the egg albumin sensitized guinea pigs and by histopathological studies. In vitro mast cell stabilizing activity was studied using compound 48/80 as a degranulating agent. Treatment with AET (200 and 400 mg/kg, p.o., for 7 days) showed significant protection against histamine and acetylcholine aerosol induced bronchospasm in guinea pigs. Significant decrease in the total leukocyte and differential leukocyte count in the BALF of the egg albumin sensitized guinea pigs was observed by administration of AET (200 and 400 mg/kg, p.o., for 15 days). AET dose dependently protected the mast cell disruption induced by compound 48/80. These results suggest that AET not only has bronchodilating activity but also decreases bronchial hyperreactivity by decreasing the infiltration of inflammatory cells in the airway and inhibiting the release of histamine like mediators from the mast cell by stabilizing it.
Keywords: Anti-asthmatic activity, alcoholic extract, leaves, Taxus baccata
INTRODUCTION
Asthma is one of the most common disorders encountered in clinical medicine in both children and adults, characterized by inflammation of the airway that is central to airway dysfunction. It is known that asthma can be triggered by various factors: allergens, drugs, respiratory infection, dust, cold air, exercise, emotions, occupational stimuli, chemicals, histamine, etc.[1] Histological examination of bronchial biopsies and cytology of bronchoalveolar lavage fluid (BALF) have demonstrated infiltrating inflammatory cells in the tracheobronchial mucosa and airway lumen of patients with asthma, even those with mild disease.[2,3] The influx of inflammatory cells is accompanied by marked and characteristic pathophysiological changes to the airways, including thickening of the airway wall, which have been implicated in the restriction of airflow and the development of airway hyperresponsiveness.[3] The disease statistics clearly necessitates the increasing need for drugs targeting the mechanisms involved in eosinophil and neutrophil activation and accumulation, for the management of asthma. Glucocorticosteroids are the only drugs currently available that effectively reduce airway inflammation in asthma.[4]
As a result, there is high prevalence of usage of complementary and alternative medicines for treatment of this disease.[5] Ayurveda, an ancient system of Indian medicine, has recommended a number of drugs from indigenous plant sources for the treatment of bronchial asthma and allergic disorders.[6] Taxus baccata Linn. (Taxaceae) is an evergreen tree, usually 6 m in height and 1.5–1.8 m in width, found in the temperate Himalayas at an altitude between 1800 and 3300 m and in the hills of Meghalaya and Manipur at an altitude of 1500 m.[7] T. baccata has been used in the Ayurvedic system for the treatment of cancer, diarrhea, asthma, hemoptysis and also used as carminative, expectorant, stomachic, etc.[8] T. baccata leaves are reported to be used in traditional medicine as abortifacient, antimalarial, antirheumatic and for bronchitis,[9–11] and dried leaves and barks are used against asthma.[12] Anticancer,[13] anti-inflammatory and antinociceptive,[14] antifungal,[15] antimycobacterial[16] activity of T. baccata has been reported. Many Ayurvedic practitioners prescribe decoction of leaves of T. baccata for the treatment of asthma. However, no scientific studies have been carried out to investigate anti-asthmatic effect in the form of bronchorelaxation and inhibition of bronchial hyperreactivity of leaves of T. baccata. In present study, the anti-asthmatic activity of AET was evaluated in experimental animals by using various in vivo and in vitro models.
MATERIALS AND METHODS
Chemicals
Compound 48/80 was purchased from Sigma-Aldrich Chemical Co. (Bangalore, India). Acetylcholine, egg albumin and other chemicals were purchased from S. D. Fine Chem. Ltd. (Mumbai, India) and histamine was purchased from Himedia Laboratories Pvt. Ltd. (Mumbai, India). Ketotifen was obtained as gift sample from Elysium Pharmaceutical Ltd. (Baroda, India). All other chemicals used were of analytical grade.
Plant material
Dried leaves of T. baccata were purchased from a commercial supplier of Mumbai, India. The plant was authenticated by Prof. Minoo Parabia, Head of Department of Bioscience, Veer Narmad South Gujarat University, Gujarat, India, where a plant specimen has been deposited with the no. HMG/0404/2007.
Preparation of extract
The leaves were reduced to coarse powder and macerated with alcohol (ethanol) for 48 hours, filtered and filtrate was evaporated under reduced pressure to obtained brown crystalline powder. The extract was stored in cool and dry place and used for pharmacological evaluation (alcohol extractive value 4.5% w/w). After obtaining the dry extract, qualitative preliminary phytochemical screening was performed to find out the presence of various phytochemicals.[17] For pharmacological evaluation, the extract was dissolved in distilled water prior to its use.
Experimental animals
Wistar rats (175–200 g) and guinea pigs (400–600 g) of either sex, housed in standard conditions of temperature (22 ± 2°C), relative humidity (55 ± 5%) and light (12 hours light/dark cycles), were used. They were fed with standard pellet diet and water ad libitum. In addition to pellet diet, the guinea pigs were supplemented with Lucerne. The experimental protocol was approved by Institutional Animal Ethical Committee as per the guidance of CPCSEA, Ministry of Social Justice and Empowerment, Government of India (Protocol No. Project 5005). A minimum of six animals were used in each group. Throughout the experiments, the animals were processed according to the suggested ethical guideline for the care of laboratory animals.
Acute toxicity study
Acute toxicity study was performed on female albino rats according to Organization for Economic Cooperation and Development-425 (OECD-425) guideline. Animals were observed for the next 14 days. There was no sign of changes in behavioral and autonomic profiles and any sign of toxicity or mortality up to a dose of 2000 mg/kg.
Histamine and acetylcholine aerosol induced bronchospasm in guinea pigs
Experimental bronchial asthma was induced in guinea pigs by exposing them to histamine and acetylcholine aerosol.[18] Guinea pigs were selected and divided into four groups, each containing six animals, out of which groups I and group II were exposed to 0.1% w/v of histamine dihydrochloride aerosol and group III and group IV were exposed to 0.5% w/v of acetylcholine bromide aerosol in histamine chamber (Inco Ltd., Ambala, India). The animals exposed to histamine and acetylcholine aerosol showed progressive dyspnea. The end point preconvulsion dyspnea (PCD) was determined from the time of aerosol exposure to the onset of dyspnea leading to the appearance of convulsion. As soon as the PCD commenced, the animals were removed from chamber and placed in fresh air. This time of PCD was taken as day 0 value. The guinea pigs of group I and group III were treated with the AET 200 mg/kg, p.o. and group II and group IV animals were treated with 400 mg/kg, p.o., once a day for 7 days, after aerosol exposure on day 0. On the 7th day, 2 hours after the last dose, the time for the onset of PCD was recorded as on day 0. The percentage increase in the time of PCD was calculated using following formula:[19]
percentage increase in the time of PCD =
where T1= time for PCD onset on day 0, T2= time for PCD onset on day 7.
Studies on BALF in egg albumin sensitized guinea pigs[20]
Guinea pigs were selected and divided into five groups, i.e., group I (control: distilled water 10 ml/kg); group II (sensitized); group III (Sensitized + prednisolone 5mg/kg, i.p.), group IV (sensitized + T. baccata 200 mg/kg, p.o.); and group V (sensitized + T. baccata 400 mg/kg, p.o.), each containing six animals. The guinea pigs of group II, group III, group IV and group V were sensitized with egg albumin (1 ml, 10% w/v, i.p.) on the 1st day. The animals of group III were dosed once daily for 15 days with prednisolone 5 mg/kg, while group IV and group V animals were dosed once daily for 15 days with AET. Two hours after the last dose of drug administration (on 15th day), all the animals of group II, group III, group IV and group V were again challenged with egg albumin (0.5 ml, 2% w/v, i.v.) through saphenous vein. After 3 hours of administration of egg albumin or just prior to death of animals, whichever was earlier, the trachea was immediately cannulated after anesthetization and the airways lavaged with saline at 25°C (two aliquots of 1 ml/100 g body weight). Bronchoalveolar cells were collected in two successive lavages using saline and recovered through a tracheal cannula. The BALF was stored on ice and total WBC cell counts were performed using a light microscope. Dilutions of lavage fluid (1 in 10) were made in saline, and differential WBCs were counted by light microscopy stained with Leishman’s stain. At least 200 cells were counted on each slide. Cells were differentiated using standard morphological criteria. All differential cell counts were performed blind and in randomized order at the end of the study. The results obtained were compared between control and sensitized groups and sensitized and treated groups.
Lung histology
The same animals of the above model, i.e., used for studies on the BALF, were used for the histological study of the lungs. Left bronchi were tied before collection of BALF to avoid possible traumatic damage due to BALF. The lungs were removed and then fixed by slowly inflating with buffer formalin and subsequently embedded in paraffin. A transverse section (2–4 μm thick) was cut from each of the collected lungs and stained with hematoxylin and eosin. Histopathology assessment under light microscope was performed on sections.
In vitro mast cell degranulation by compound 48/80
The effect of T. baccata on in vitro mast cell degranulation by compound 48/80 was studied following the method of Gupta and Srimal.[21] Normal saline (5 ml/kg) containing 5 units/ml of heparin was injected in the peritoneal cavity of male rats (n = 6) lightly anesthetized with ether. After a gentle abdominal massage, the peritoneal fluid containing mast cells was collected in centrifuge tubes placed over ice. Peritoneal fluid of rats was collected and centrifuged at 2000 rpm for 5 min. Supernatant solution was discarded and the cells was washed twice with saline and resuspended in 1 ml of saline. All the solutions were prepared in normal saline.
The peritoneal cell suspension was divided into six parts, viz., –ve control, +ve control, reference standard (ketotifen 10 μg/ml), AET of three concentrations, i.e., 500, 750, 1000 μg/ml, each containing 0.1 ml of cell suspension and incubated at a constant temperature in a water bath at 37°C for 15 min. Then, 0.1 ml of compound 48/80 (10 μg/ml) was added to all the samples except in –ve control and the suspensions were further incubated for 10 min at 37°C. The cells were then stained with 10% of Toluidine blue solution and observed under the high power of light microscope. The percentage granulated and percentage degranulated mast cells were counted. In +ve control group, compound 48/80 was added without the addition of test agents, i.e., ketotifen and T. baccata, and in –ve control group neither compound 48/80 nor the test agents were added to correct for spontaneous degranulation of mast cells without any degranulating agent.
Statistical analysis
The results of various studies were expressed as mean ± SEM and analyzed statistically using Student’s t-test to find out the level of significance. Data were considered statistically significant at minimum level of P < 0.05.
RESULTS
Acute toxicity study
AET did not produce mortality and any sign of toxicity up to dose of 2000 mg/kg.
Phytochemical screening
Preliminary qualitative phytochemical screening of AET showed the presence of lignans, flavonoids, glycosides, sugars, amino acids and triterpenoids.
Effect on histamine and acetylcholine aerosol induced bronchospasm in guinea pigs
AET significantly and dose dependently increased the time of PCD following histamine (P < 0.001) and acetylcholine (P < 0.01) aerosol induced bronchospasm in guinea pigs [Table 1]. Increase in the time of PCD was more against histamine aerosol as compared to acetylcholine aerosol, following administration of T. baccata leaves extract.
Table 1.
Effect of Taxus baccata (p.o., for 7 days) on histamine and acetylcholine aerosol induced bronchospasm in guinea pigs
| Groups | Preconvulsion dyspnea time (sec) |
||
|---|---|---|---|
| Before treatment (control) | After treatment | % Increase in the time of PCD | |
| Histamine aerosol (0.1% w/v) | |||
| I- T. baccata (200 mg/kg) | 125.8 ± 18.56 | 47.2 ± 25.34* | 72.82 ± 3.14 |
| II- T. baccata (400 mg/kg) | 127.4 ± 20.32 | 632.7 ± 53.47* | 80.67 ± 6.23 |
| Acetylcholine aerosol (0.5% w/v) | |||
| III- T. baccata (200 mg/kg) | 149.27 ± 11.23 | 354.5 ± 32.09# | 58.73 ± 3.09 |
| IV- T. baccata (400 mg/kg) | 137.4 ± 23.60 | 438.57 ± 43.71* | 67.98 ± 4.61 |
Values are expressed as mean ± SEM for six guinea pigs in each group
P < 0.001
P < 0.001 when compared with control group
Effect on BALF in egg albumin sensitized guinea pigs
After 15 days, the guinea pigs were again challenged with egg albumin. In the BALF, significant increases in the total leukocyte count and differential leukocytes count were observed in the sensitized, i.e., group II (P < 0.001) animals as compared to the control, i.e., group I animals. AET (200 and 400 mg/kg, p.o., for 15 days) significantly and dose dependently decreased the total leukocyte count (P < 0.05) and differential leukocyte count (P < 0.001) in group IV and group V as compared to group II [Table 2] animals. Prednisolone also significantly decreased total leukocytes count (P < 0.001) and differential leukocytes count (P < 0.001) compared to sensitized group.
Table 2.
Effect of Taxus baccata (p.o., for 15 days) on BALF in egg albumin sensitized guinea pigs
| Control | Sensitized | Prednisolone (5 mg/kg) | Sensitized + T. baccata (200 mg/kg) | Sensitized + T. baccata (400 mg/kg) | |
|---|---|---|---|---|---|
| TLC/mm3 | 8842 ± 429 | 14,740 ± 670.9* | 9247 ± 467.7$ | 11,680 ± 313.5@ | 9680 ± 248.32$ |
| Neutrophil count/mm3 | 2774 ± 304.2 | 4100 ± 169.9* | 2894 ± 189.6$ | 3921 ± 287.25# | 3081 ± 267.34$ |
| Lymphocyte count/mm3 | 4472 ± 384.8 | 9130 ± 235.2* | 4872 ± 328.4$ | 6772 ± 343.2$ | 5892 ± 327.82$ |
| Eosinophil count/mm3 | 184.8 ± 14.82 | 506.2 ± 36.71* | 278 ± 19.67$ | 420.3 ± 19.45$ | 337.78 ± 13.26$ |
| Monocyte count/mm3 | 109.3 ± 6.28 | 263.1 ± 21.52* | 113.6 ± 8.67$ | 200.5 ± 17.08$ | 153.6 ± 8.31$ |
Values are expressed as mean ± SEM for six guinea pigs in each group
P < 0.001 when compared with control group
P < 0.05
P < 0.001
P < 0.001 when compared with sensitized group, BALF: Bronchoalveolar lavage fluid, TLC: Total leukocyte count
Lung histology
Histological analysis of the lungs from non-sensitized, i.e., group I animals, showed normal lung histology [Figure 1a]. In contrast, similar to the BALF study, histological sections of lung tissue from group II guinea pigs exhibited airway inflammation, infiltration of eosinophils, lymphocytes and submucosal edema of the lungs, and bronchoconstriction shown as lumen plugging by mucus and cells [Figure 1b]. Treatment with prednisolone and T. baccata, i.e., group IV and group V animals, prevented the tissue edema, epithelial cell hypertrophy, infiltration of inflammatory cell, and airway lumen plugging, thereby decreasing inflammation and broncoconstriction, which led to normal lumen size [Figure 1c–e].
Figure 1.
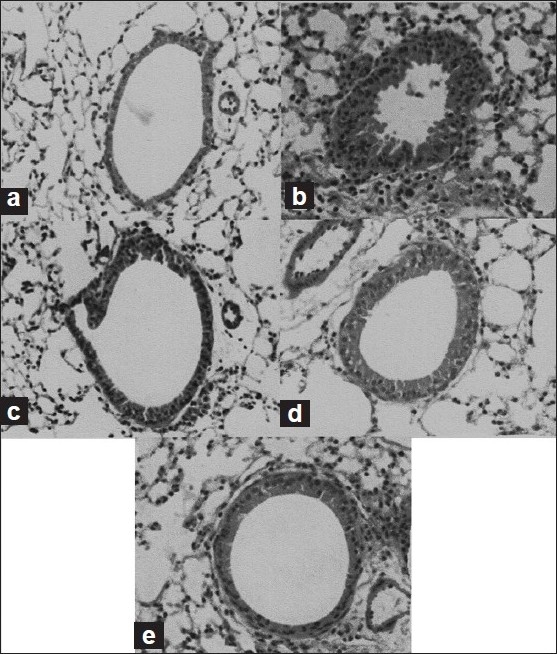
Effect of Taxus baccata on the histology of lung tissue. (a) Control group I, (b) egg albumin sensitized group II, (c) egg albumin sensitized + prednisolone (5 mg/kg) group III, (d) egg albumin sensitized + T. baccata treated (200 mg/kg, p.o.) group IV and (e) egg albumin sensitized + T. baccata treated (400 mg/kg, p.o.) group V. Prednisolone and T. baccata treatment was given for 15 days
Effect on compound 48/80 induced mast cell degranulation
AET and ketotifen were found to significantly (P < 0.001) inhibit rat peritoneal mast cell degranulation induced by compound 48/80 in vitro as compared to baseline value, i.e., +ve control group [Table 3].
Table 3.
Effect of Taxus baccata on compound 48/80 induced mast cell degranulation
| Treatment | Concentration (μg/ml) | Mast cells |
|
|---|---|---|---|
| % Granulated | % Degranulated | ||
| –ve control | – | 91.83 ± 0.654 | 8.167 ± 0.654 |
| +ve control | – | 26.5 ± 1.176 | 73.5 ± 1.176 |
| Ketotifen | 10 | 80.5 ± 0.957* | 19.5 ± 0.957 |
| Taxus baccata | 500 | 41.17 ± 0.945* | 58.83 ± 0.945 |
| Taxus baccata | 750 | 50.67 ± 0.666* | 49.33 ± 0.666 |
| Taxus baccata | 1000 | 63.17 ± 0.945* | 36.83 ± 0.945 |
Values are expressed as mean ± SEM, n = 6 in each group
P < 0.001 when compared with baseline value, i.e., +ve control
DISCUSSION
Bronchial asthma is commonly characterized by increased airway reactivity to spasmogens. An initial event in asthma appears to be the release of inflammatory mediators like histamine, triggered by exposure to allergens that directly cause acute bronchoconstriction.[22,23] In the present study, histamine and acetylcholine were used as spasmogens in the form of aerosol to cause immediate bronchoconstriction in the form of PCD in guinea pigs. Bronchodilating effect of AET was evaluated by observing its effects at the time of PCD. In our study, we found that the time of occurrence of PCD was significantly increased, suggestive of bronchodilating activity following treatment with T. baccata against spasmogens.
Increasing evidence suggests that the frequently observed association between activated T lymphocytes and eosinophils plays a major role in the development of airway inflammation and in the accompanying bronchial hyperreactivity.[24,25] Neutrophils and monocytes play a pivotal role in the disease process as they are a source of variety of inflammatory mediators which are responsible for bronchial hyperresponsiveness and airway inflammation.[26] In association with asthma, elevated numbers of these inflammatory cells like eosinophils, neutrophils, lymphocytes, monocytes have been identified in various tissue compartments like blood, biopsies of lung tissue, in BALF and in sputum. In the present study, sensitization using egg albumin (1 ml, 10% w/v, i.p.) and then second exposure to the same antigen, i.e., egg albumin (0.5 ml, 2% w/v), through saphenous vein caused acute anaphylactic shock resembling the acute asthmatic attack resulting in the release of various mediators and cellular infiltration. Antigen challenge resulted in significant increase in the number of eosinophils in the BALF. This was accompanied by intense eosinophil infiltration, accumulation and degranulation in the guinea pig lungs as evident in histopathology study, which is consistent with human asthmatic lungs. In our study, we found that treatment with T. baccata in antigen challenged animals significantly inhibited antigen induced hyperreactivity by preventing increased infiltration of total leukocyte count and eosinophils count. After antigen challenge, airway hyperresponsiveness is supported by inflammatory pathology, suggesting the involvement of other mediators in the pathogenesis of asthma. Neutrophil numbers have also been reported to increase in bronchial lavage fluid in asthmatics, but neutrophilia is generally of shorter duration than eosinophilia.[27,28] This was observed in our result where treatment with T. baccata resulted in significant inhibition of antigen induced bronchial hyperreactivity by decreasing the neutrophil count. The participation of T lymphocytes in the pathogenesis of bronchial asthma and the accompanying bronchial hyperreactivity has been widely demonstrated.[25] Indeed, activated CD4+ T lymphocytes are found in the blood and bronchial lumen from asthmatics.[29] Recently, interest has been focused on the characterization of CD4+ T lymphocytes based on their repertoire of secreted cytokines and their possible role in the pathogenesis of allergic disorders. Thus, CD4+ T cells from asthmatics preferentially elaborate Th2-derived cytokines, such as IL-4 and IL-5, which have been shown to enhance IgE synthesis,[30] and to act specifically on eosinophil survival, activation, and secretion of proinflammatory mediators.[31] Large numbers of T lymphocytes, mainly of the CD4+ subset, have been identified in the bronchial mucosa of antigen challenged guinea pigs.[32] In accordance with the above, the present finding shows that treatment with T. baccata in the sensitized animals produced a significant decrease in the lymphocyte count as compared to the sensitized animals without treatment. The predominant cells in BALF recovered from unchallenged guinea pigs were those of the monocytes. The numbers of these cells were increased after antigen challenge.[33] In line with the above context, treatment with T. baccata significantly decreased monocytes as compared to that in sensitized guinea pigs. The results of our study suggest that in guinea pig airways, antigen challenge induced eosinophil, neutrophil, monocyte and lymphocyte infiltration and activation is similar to that reported in human asthmatics. These show that T. baccata exerts its protective effect by preventing the infiltration of inflammatory cell, thereby decreasing the release of preformed inflammatory mediators, which can prevent the direct damage to airway, which in turn prevents airway hyperresponsiveness.
Various processes involved in bronchial asthma such as inflammatory response can explain various histopathological alterations observed in the biopsy of asthmatic patients. In asthma, chronic inflammation is responsible for the bronchoconstriction which leads to airway narrowing and decrease in the lumen size of the bronchiole.[34] This can be clearly seen by observing the cross-section of bronchi in the histopathological studies of the lung tissue. In the present study, the sections of the lung tissues of animals sensitized with egg albumin depicted marked bronchitis and severe bronchoconstriction. Treatment with T. baccata prevented the inflammation and bronchoconstriction, which led to normal lumen size and normal cellular structure, compared to antigen-sensitized guinea pigs.
Mast cell degranulation is important in the initiation of immediate responses following exposure to allergens.[35] Once binding of allergen to cell-bound IgE occurs, mediators such as histamine; eosinophil and neutrophil chemotactic factors; leukotrienes C4, D4, and E4; prostaglandins; platelet-activating factor; and others are released from mast cells, which are responsible for the development of airway inflammation and bronchoconstriction. An attempt was made to find out whether AET has any effect on the rate of disruption of mast cells following exposure to compound 48/80, an agent which causes histamine release.[36] It has been assumed that the process leading to histamine secretion may be mediated by calcium release from an intracellular store of mast cells.[37] In this study, T. baccata offered significant protection against compound 48/80 induced mast cell degranulation by stabilizing it, which is responsible for the decreasing airway inflammation by preventing the release of various inflammatory mediators.
Phytochemical screening of T. baccata showed the presence of lignans, flavonoids, sugar derivatives, etc.[38,39] Lignans are known to possess various biological activities including antibacterial, antioxidant, anticancer, spasmolytic and anti-inflammatory effects.[40] Flavonoids are known to possess various biological activities including antibacterial, antifungal, spasmolytic, antiviral, anticancer, and anti-inflammatory effects.[41–43] Anti-asthmatic activity of T. baccata may be due to the presence of the above constituents. In conclusion, our data suggest that the alcoholic extract of the leaves of T. baccata possesses significant anti-asthmatic activity and has beneficial effect in asthma by causing bronchorelaxation and decreasing bronchial hyperreactivity.
Footnotes
Source of Support:Nil
Conflict of Interest:None declared
REFERENCES
- 1.Kelly HW, Sorknes CA. Asthma. In: Dipiro JT, Talbert RL, Yee GC, Matzke TR, Wells BG, Posey LM, editors. Pharmacotherapy – A Pathophysiological Aproch. 6th ed. New York: The McGraw-Hill; 2005. p. 504. [Google Scholar]
- 2.Djukanović R, Roche WR, Wilson JW, Beasley CR, Twentyman OP, Howarth RH, et al. Mucosal inflammation in asthma. Am Rev Respir Dis. 1990;142:434–57. doi: 10.1164/ajrccm/142.2.434. [DOI] [PubMed] [Google Scholar]
- 3.Beasley R, Burgess C, Crane J, Pearce N, Roche W. Pathology of asthma and its clinical implications. J Allergy Clin Immunol. 1993;92:148–54. doi: 10.1016/0091-6749(93)90097-y. [DOI] [PubMed] [Google Scholar]
- 4.Barnes PJ, Pederson S. Efficacy and safety of inhaled corticosteroids in asthma. Report of a workshop held in Eze, France, October 1992. Am Rev Respir Dis. 1993;148:S1–26. doi: 10.1164/ajrccm/148.4_Pt_2.S1. [DOI] [PubMed] [Google Scholar]
- 5.Salib RJ, Drake Lee A, Howarth PH. Allergic rhinitis: past, present and the future. Clin Otolaryngol Allied Sci. 2003;28:291–303. doi: 10.1046/j.1365-2273.2003.00706.x. [DOI] [PubMed] [Google Scholar]
- 6.Shri Gulabkunverba Ayurvedic Society. vol. 4. Jamangar: Ayurvedic Mundranalaya; 1949. Anonymous. Charaka Samhita; pp. 1952–2032. [Google Scholar]
- 7.Anonymous. Wealth of India, Raw Materials. vol. 10. New Delhi, India: CSIR; 2005. pp. 132–4. [Google Scholar]
- 8.Yelne MB, Dennis TJ, Billore KV, Chaudhari BG. Database of Medicinal Plants Used in Ayurveda. Central Council for Research in Ayurveda and Siddha. 2005;vol. 7:452–75. [Google Scholar]
- 9.Bryan-Brown T. The pharmacological actions of taxine. Quart J Pharm Pharmacol. 1932;5:205–19. [Google Scholar]
- 10.Appendino G. Taxol (paclitaxel): Historical and ecological aspects. Fitoterapia. 1993;64:5–25. [Google Scholar]
- 11.Ballero M, Fresu I. Le piante di uso officinale nella Barbagia di seni (Sardegna Centrale) Fitoterapia. 1993;64:141–50. [Google Scholar]
- 12.Sing V. Traditional remedies to treat asthma in North West and Trans-Himalayan region in J and K state. Fitoterapia. 1995;66:507–9. [Google Scholar]
- 13.Jennewein S, Croteau R. Taxol: Biosynthesis, molecular genetics, and biotechnological applications. App Micro Biotech. 2001;57:13–9. doi: 10.1007/s002530100757. [DOI] [PubMed] [Google Scholar]
- 14.Kupeli E, Erdemoglu N, Yesilada E, Sener B. Antiinflammatory and antinociceptive activity of taxoids and lignans from the heartwood of Taxus baccata L. J Ethnopharmacol. 2003;89:265–70. doi: 10.1016/j.jep.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 15.Mirosława KB, Marian W. Antifungal Activity of Bioflavones from Taxus baccata and Ginkgo biloba. Zeitschrift für Naturforschung. 2003;58:5–9. doi: 10.1515/znc-2003-1-212. [DOI] [PubMed] [Google Scholar]
- 16.Erdemoglu N, Sener B. Antimicrobial activity of the heartwood of Taxus baccata. Fitoterapia. 2001;72:59–61. doi: 10.1016/s0367-326x(00)00233-1. [DOI] [PubMed] [Google Scholar]
- 17.Herbone JB. 3rd ed. London, UK: Champman and Hall; 1998. Phytochemical methods. [Google Scholar]
- 18.Sheth UK, Dadkar NK, Kamat NG. Bombay: Kothari Book Depot; 1972. Selected topics in experimental pharmacology; p. 63. [Google Scholar]
- 19.Mitra SK, Gopumadhavan S, Venkataranganna MV, Anturlikar SD. Anti-asthmatic and anti-anaphylactic effect of E-721B, a polyherbal formulation. Ind J Pharmacol. 1999;31:133–7. [Google Scholar]
- 20.Thomas G, Aroajo CC, Agra F. Preliminary studies on the hydroalcoholic extract of the root of Cissampelos sympodialis Eichl in guinea pig tracheal strip and bronchoalveolar leucocytes. Phytotherapy Res. 1995;9:473–7. [Google Scholar]
- 21.Gupta PP, Srimal RC. Anti-allergic activity of alkyl substituted Pyrazolo (3,4-d) Pyrimidine (Compound 88-765) Ind J Exp Bio. 1995;33:38–40. [PubMed] [Google Scholar]
- 22.Church MK, Bradding P, Walls AF. Oxford; 1997. Allergy and allergic diseases, Human mast cells and basophils; pp. 149–70. [Google Scholar]
- 23.Barnes PJ, Chung KF, Page CP. Inflammatory mediators of asthma: An update. Pharmacol Rev. 1998;50:515–96. [PubMed] [Google Scholar]
- 24.Azzwi M, Bradley B, Jeffery PK, Frew AJ, Wardlaw AJ, Knowles G, et al. Identification of activated T lymphocytes and eosinophils in bronchial biopsies in stable atopic asthmatics. Am Rev Respir Dis. 1990;142:1407–13. doi: 10.1164/ajrccm/142.6_Pt_1.1407. [DOI] [PubMed] [Google Scholar]
- 25.Corriagan CJ, Kay AB. T cells and eosinophils in pathogenesis of asthma. Immunol Today. 1992;13:501–7. doi: 10.1016/0167-5699(92)90026-4. [DOI] [PubMed] [Google Scholar]
- 26.Sur S, Crotly TB, Kephart GM. Sudden-onset fatal asthma: A distinct entity with few wosinophils and relatively more neutrophils in airway submucosa. Am Rev Respir Dis. 1993;148:713–9. doi: 10.1164/ajrccm/148.3.713. [DOI] [PubMed] [Google Scholar]
- 27.Diaz P, Gonzalez MC, Gallenguillos FR. Leukocytes and mediators in bronchoalveolar lavage during allergen induced late-phase asthmatic reactions. Am Rev Respir Dis. 1989;139:1383–8. doi: 10.1164/ajrccm/139.6.1383. [DOI] [PubMed] [Google Scholar]
- 28.Metzger WJ, Zavala D, Richerson HB. Local allergen challenge and bronchoalveolar lavage of allergic asthmatic lungs: description of the model and local airway inflammation. Am Rev Respir Dis. 1987;135:433–40. doi: 10.1164/arrd.1987.135.2.433. [DOI] [PubMed] [Google Scholar]
- 29.Walker C, Virchow JC, Jr, Bruijnzeel PLB, Blaser K. T-cell subsets and their soluble products regulate eosinophilia in allergic and non-allergic asthma. J Immunol. 1991;146:1829–35. [PubMed] [Google Scholar]
- 30.Pene J, Rousset F, Briere F, Chr6tien I, Bonnefoy JY, Spits H, et al. IgE production by human B cells is induced by ID4 and suppressed by interferons 3’ and ot and prostaglandin E2. Proc Natl Acad Sci USA. 1988;85:8166. doi: 10.1073/pnas.85.18.6880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanderson CJ. Interleukin-5, eosinophils, and disease. Blood. 1992;79:3101–9. [PubMed] [Google Scholar]
- 32.Lapa e Silva JR, Bachelet CM, Pretolani M, Baker D, Scheper RJ, Vargaftig BB. Immunopathologic alterations in the bronchi of immunized guinea-pigs. Am J Respir Cell Mol Biol. 1993;9:44–53. doi: 10.1165/ajrcmb/9.1.44. [DOI] [PubMed] [Google Scholar]
- 33.Tarayre JP, Aliaga M, Barbara M, Tisseyre N, Vieu S, Tisne-Versailles J. Model of bronchial hyperreactivity after active anaphylactic shock in conscious guineapigs. J Pharmacol Methods. 1990;23:13–9. doi: 10.1016/0160-5402(90)90004-5. [DOI] [PubMed] [Google Scholar]
- 34.Kelly HW, Sorknes CA. Asthma. In: Dipiro JT, Talbert RL, Yee GC, Matzke TR, Wells BG, Posey LM, editors. Pharmacotherapy- A Pathophysiological Aproch. 6th ed. New York: The McGraw-Hill; 2005. p. 508. [Google Scholar]
- 35.Bethesda, MD, US Department of Health and Human Services. Washington: NIH Publication; 1997. NAPP-National Asthma Education and Prevention Program. Expert Panel Report 2. Guidelines for the Diagnosis and Management of Asthma; p. 97.p. 4051. [Google Scholar]
- 36.Jippo-Kanemoto T, Kasugai T, Yamatodani A, Ushio H, Mochizuki T, Tohya K, et al. A supernormal histamine release and normal cytotoxic activity of beige rats mast cells with giant granules. Inter Arch Aller Immunol. 1993;100:99–106. doi: 10.1159/000236395. [DOI] [PubMed] [Google Scholar]
- 37.Lee YM, Kim DK, Kim SH, Shin TY, Kim HM. Anti-anaphylactic activity if Poncirus trifoliate formit extract. J Ethnopharmacol. 1996;54:77–84. doi: 10.1016/s0378-8741(96)01451-1. [DOI] [PubMed] [Google Scholar]
- 38.Baloglu E, Kingstone DG. The taxane diterpenoids. J Nat Pro. 1999;62:1448–72. doi: 10.1021/np990176i. [DOI] [PubMed] [Google Scholar]
- 39.Parmar VS, Jha A, Bisht KS, Taneja P, Singh SK, Kumar A, et al. Constituents of yew trees. Phytochem. 1999;50:1267–304. doi: 10.1016/s0031-9422(98)00702-x. [DOI] [PubMed] [Google Scholar]
- 40.Cho JY, Park J, Kim PS, Yoo ES, Baik KU, Park MH. Savinin, a lignan from Pterocarpus santalinus inhibits tumor necrosis factor-alpha production and T cell proliferation. Bio Pharm Bull. 2001;24:167–71. doi: 10.1248/bpb.24.167. [DOI] [PubMed] [Google Scholar]
- 41.Kim HK, Cheon BS, Kim YH, Kim SY, Kim HP. Effects of naturally occurring flavonoids on nitric oxide production in the macrophage cell line RAW 264.7 and their structure-activity relationships. Biochem Pharmacol. 1999;58:759–65. doi: 10.1016/s0006-2952(99)00160-4. [DOI] [PubMed] [Google Scholar]
- 42.Srinivas KV, Koteswara Rao Y, Mahender I, Das B, Krishna KV, Kishore KH. Flavonoids from Caesalpinia pulcherrima. Phytochem. 2003;63:789–93. doi: 10.1016/s0031-9422(03)00325-x. [DOI] [PubMed] [Google Scholar]
- 43.Matsuda H, Morikawa T, Ando S, Toguchida I, Yoshikawa M. Structural requirements of flavonoids for nitric oxide production inhibitory activity and mechanism of action. Bioorg Med Chem. 2003;11:1995–2000. doi: 10.1016/s0968-0896(03)00067-1. [DOI] [PubMed] [Google Scholar]


