Abstract
Rationale
Relapse to smoking is often precipitated by stress, yet little is known about the effects of nicotine withdrawal on responses to acute stress, or whether nicotine replacement reverses withdrawal-induced changes in stress response.
Objectives
The aim of the present study is to use an effective social stressor, the Trier Social Stress Test (TSST), to study subjective, cardiovascular and hormonal responses to stress during withdrawal, and examine whether nicotine replacement moderates responses to stress during withdrawal.
Methods
Forty-nine current regular smokers were randomly assigned to smoke as normal (SM), 12-h abstention with placebo patch (PL), or 12-h abstention with nicotine patch (NIC). They participated in a single session using the TSST, during which subjective affect, heart rate (HR), mean arterial blood pressure (MAP) and salivary cortisol were measured.
Results
The TSST produced expected increases in subjective negative affect, HR, MAP, and cortisol. Groups did not differ in subjective or cardiovascular responses, but the PL group exhibited larger stress-induced increase in cortisol than the other groups.
Conclusions
The increased cortisol response might indicate a greater hormonal stress response during nicotine withdrawal. Alternatively, considering that cortisol also provides negative feedback to the stress system, and blunted cortisol responses are predictive of smoking relapse, the lower cortisol responses in the NIC and SM groups might indicate chronic dysregulation of the stress system. In this case, restoration of cortisol response by nicotine treatment to the lower levels seen during regular smoking may actually represent an undesired side effect of nicotine replacement.
Keywords: Nicotine withdrawal, Stress, Nicotine replacement, Cortisol
Introduction
Stress plays an important role in smoking and relapse to smoking. External stressors are important triggers of relapse (Shiffman et al. 1996), and nicotine withdrawal itself produces a “stress-like state” of negative affect (Hughes 2007). There is evidence that smoking withdrawal may influence responses to acute stress, which may add to the difficulty of remaining abstinent. Moreover, prolonged use of nicotine produces neuroadaptations in stress systems (Sinha 2001) that may leave smokers vulnerable to stress during abstinence. However, little is known about how nicotine withdrawal influences responses to stress during early abstinence, when risk for relapse is high. The available literature is inconclusive, suggesting that withdrawal may either increase or decrease responses to stress (as part of a more general blunting of emotional response). Even less is known about whether available treatments, such as nicotine replacement, moderate responses to acute stress during withdrawal. Thus, the present study investigates how smoking withdrawal influences reactions to a potent social stressor, and whether this effect is modulated by nicotine replacement.
Acute stress produces a multitude of subjective, physiological, and endocrine changes. Subjective responses include increased negative affect (NA) and decreased positive affect (PA) (Stone 1995). Physiological effects include activation of the sympathetic/adrenomedullary (SAM) system and a cascade of responses in the hypothalamic– pituitary–adrenocortical (HPA) axis (Gunnar and Quevedo 2007). In the SAM system, acute stress causes the adrenal medulla to secrete epinephrine and norepinephrine, which increases heart rate, blood pressure, and levels of free glucose (Gunnar and Quevedo 2007). In the HPA axis, acute stress causes secretion of corticotrophin-releasing hormone (CRH) from the hypothalamus, which stimulates adrenocorticotropic hormone (ACTH) release from the pituitary, which, in turn, leads to release of glucocorticoids (primarily cortisol in humans) by the adrenal cortex. Cortisol, the primary stress-related hormone, affects gene transcription, initiating changes that unfold minutes to hours after the stressor (Gunnar and Quevedo 2007). Thus, acute stress acts on many physiological systems, some of which overlap with systems involved in nicotine dependence and withdrawal (al’Absi 2006).
It is reasonable to suppose that nicotine withdrawal, which is itself stressful, will increase responses to stress. However, there is also a reason to believe that responses to stress may be decreased during nicotine abstinence, consistent with dampened responses to other emotional events. Thus, on the one hand, abstinent smokers may experience external stressors as more potent, because their coping resources are depleted by the “stress-like” state of withdrawal (Baker et al. 2004), or because of neural changes resulting from prolonged exposure to nicotine (Sinha 2001). A heightened response to stress during withdrawal would be particularly problematic for smokers attempting to quit, since coping with negative emotions is frequently cited as a reason for smoking (Copeland et al. 1995). On the other hand, there is also evidence that nicotine abstinence may actually reduce responses to stress. In laboratory animals and humans, exposure to nicotine enhances physiological and hormonal responses to stress and other emotional stimuli (Chaudhri et al. 2006; Chen et al. 2008; Perkins et al. 1986; Pomerleau and Pomerleau 1990), and conversely, there is some evidence that responses to both positive and negative emotional stimuli are dampened during withdrawal (Dawkins et al. 2007; Dawkins et al. 2009; Semba et al. 2004). Indeed, there is convincing preclinical evidence that nicotine acts as a “salience enhancer”, that is, that it increases reactivity to environmental stimuli (Caggiula et al. 2002; Chaudhri et al. 2006). By the same process, it might enhance reactivity to a stressor. Consistent with this, laboratory animals exhibit dampened HPA axis reactions to an “uncontrollable” restraint stressor during withdrawal (Semba et al. 2004). Interestingly, dampened responses to stress during withdrawal are blocked by bupropion, a drug that effectively reduces smoking (Kotlyar et al. 2006). Thus, perhaps it is not enhanced stress responses, but rather blunted responses to stress (and other emotional stimuli) that are important targets for smoking-cessation aids. Thus, there is evidence that stress response may either be enhanced, or reduced, during acute withdrawal.
Several laboratory studies with humans have examined acute stress responses during withdrawal, with mixed results (al’Absi et al. 2002; al’Absi et al. 2003; Attwood et al. 2008; Girdler et al. 1997; Robinson and Cinciripini 2006; Tsuda et al. 1996; VanderKaay and Patterson 2006; Vujanovic and Zvolensky 2009). These mixed findings may stem from differences in the types of stressors used, which have included cognitive, physical, and social interventions. Studies using “cognitive” stressors (e.g., difficult math or progressive matrices) have generally found cardiovascular and subjective stress responses to be enhanced during withdrawal (al’Absi et al. 2002; Tsuda et al. 1996; VanderKaay and Patterson 2006 but c.f. Girdler et al. 1997; Robinson and Cinciripini 2006), and that nicotine replacement ameliorates this enhanced stress response (VanderKaay and Patterson 2006). However, cognitive stressors typically have only mild emotional effects, and may depend on performance ability, which may be impaired during withdrawal (Dickerson and Kemeny 2004). Studies using “physical” stressors, including the cold pressor (in which participants hold their hands in ice water), and carbon dioxide (CO2) inhalation (a model of anxiety; van Duinen et al. 2005) have produced both decreases (VanderKaay and Patterson 2006) and increases (Attwood et al. 2008; Vujanovic and Zvolensky 2009) in stress responses. However, the cold pressor does not reliably increase cortisol levels (McRae et al. 2006), and in at least one study, CO2 inhalation reduced withdrawal symptoms, which could also have reduced stress responses (Attwood et al. 2008). Thus, findings using “cognitive” and “physical” stressors during nicotine withdrawal have been inconsistent, perhaps because of variations in the effectiveness of the stress induction procedures.
The procedures that most effectively activate both the SAM and HPA systems (Dickerson and Kemeny 2004) are “social” stressors, especially those with multiple methods of social evaluation (e.g., videotaping and a live audience) and an element of uncontrollability (Dickerson and Kemeny 2004). One such social stressor is Trier Social Stress Test (TSST; Kirschbaum et al. 1993a), during which participants deliver a 5-min speech and perform difficult mental arithmetic in front of a video camera and an audience trained to be unaffirming. Although the TSST itself has not been tested during withdrawal, studies have investigated stress responses during withdrawal using somewhat similar speech tasks (al’Absi et al. 2003; Girdler et al. 1997). For example, al’Absi et al. (2003) found that withdrawal did not affect responses to a speech delivered in front of a video camera. However, this modified task may not have been as effective as the standard TSST, since it did not increase cortisol levels in smokers compared to a control task. The TSST reliably increases cortisol levels compared to baseline, even in cigarette smokers who typically have a blunted cortisol response to stress (Buchmann et al. 2010; Kirschbaum et al. 1993b). This suggests the TSST may be superior to previously used speech tasks, especially when studying smokers whose cortisol responses are blunted. Until now, the TSST has not been tested during acute withdrawal from smoking.
Here, we examined the effects of withdrawal on stress response using the TSST. The TSST has advantages over previously used tasks, in that it does not depend on the participants’ cognitive performance and assures an emotional component by providing negative feedback regardless of the subject’s performance. Thus, the task effectively engages both the SAM and HPA axis. We hypothesize that when this highly effective stressor is used, acute abstinence from smoking will reduce responses to stress. We further predict that this dampened response to stress will be reversed by administration of transdermal nicotine.
Methods
Participants
Participants were current regular smokers (N=49; 39 males; minimum 10 cigarettes per day) ages 18 to 42. They were recruited using flyers and online advertisements and screened by physical examination, electrocardiogram, modified Structured Clinical Interview for DSM-IV, and health and drug history. Other inclusion criteria were: body mass index between 18 and 35, no serious medical conditions or contraindications to administration of nicotine, no past year DSM-IV Axis I disorder (aside from nicotine dependence), and at least high school education, to ensure comprehension of questionnaires. Participants were recruited without regard to race or ethnicity. All participants gave informed consent, and the University of Chicago Institutional Review Board approved all procedures. Qualifying participants attended an orientation, at which they practiced questionnaires and tasks and completed the Beck Depression Inventory (BDI) and Fagerstrom Test for Nicotine Dependence (FTND).
Design
Participants were randomly assigned to three groups: smoke as normal (SM; n=16), 12-h abstention with placebo patch (PL; n=17), or 12-h abstention with nicotine patch (NIC; n= 16). They participated in a single session using the TSST.
Abstinence and nicotine manipulations
Abstinence from nicotine in the PL and NIC groups was verified with exhaled carbon monoxide (CO) readings on a piCO+ Smokerlyzer (Bedfont, Rochester, UK) of <10 ppm at the start of the experimental session. Individuals in the NIC group received transdermal patches designed to deliver individually determined doses of nicotine (10.5, 14, 17.5, or 21 mg; Nicoderm CQ, GlaxoSmithKline, Pittsburgh, PA, USA). Doses were based on the subjects’ habitual smoking level (i.e., cigarettes per day and orientation CO). The average dose was 14.5 mg/24 h (SD=2.82). The PL group received patches consisting of a gauze pad with a small amount of capsaicin. Patches were prepared and applied by an experimenter who did not conduct any other procedures, and were covered with athletic wrap to preserve double-blindness.
An effort was made to match plasma levels of nicotine at the time of the TSST in the NIC and SM groups. Thus, subjects in the SM group smoked a single cigarette at 9:30 a.m., at the same time when patches were applied to the NIC group, 130 min prior to the TSST. Plasma levels of nicotine rise after Nicoderm patch placement to a steady peak concentration 2–4 h after application (Gorsline 1993; Gupta et al. 1995), whereas cigarettes produce an immediate spike in plasma nicotine that asymptotically declines. Based on available information, we projected that nicotine plasma concentrations in the smoking and nicotine patch conditions would be roughly equivalent at the start of the stress procedure (Henningfield 1995).
Measures
We measured three main aspects of the stress response: self-report measures, SAM indices (HR and blood pressure), and HPA reactivity (salivary cortisol).
Subjective
Subjects reported on their mood states using the Positive and Negative Affect Scale (PANAS; Watson et al. 1988). NA and PA were assessed before patch administration or smoking (time 0), time 1 (1 h after smoking/patch), time 2 (before stress), time 5 (after stress), and times 6–10 (every 10 min during the 50-min “recovery” period). Participants also reported withdrawal symptoms at time 2 (before stress) using the Minnesota Nicotine Withdrawal Scale (MNWS; Hughes 1992), which asked about DSM-IV symptoms of nicotine withdrawal over the last 18 h.
Cardiovascular
HR was measured continuously by a Mini-Logger monitor with chest band (Mini Mitter, Bend, OR, USA), which produced an estimate of beats per minute (BPM) for each minute. Ten-minute averages of HR were taken at baseline, time 1 (1 h after smoking/patch), time 2 (before stress), times 3–4 (during stress), time 5 (after stress), and times 6–10 (during the 50-min recovery period). BP was measured using a LifeSource portable blood pressure cuff (A&D Company, Tokyo, Japan) at baseline, time 1 (1 h after smoking/patch), time 2 (before stress), time 5 (after stress), and time 6 (first recovery time point).
Hormonal
Salivary cortisol was obtained using Salivettes (Sarstedt AG, Numbrecht, Germany) at baseline, time 1 (1 h after smoking/patch), time 2 (before stress), time 5 (after stress), and times 6–10 (every 10 min during recovery). Samples were stored in a −80 freezer and then assayed using the Salimetrics HS-Cortisol kit (sensitivity, 0.003 µg/dL).
Procedure
Participants arrived for individually run sessions at 9:00 a.m. They were instructed to refrain from alcohol for 24 h and other illicit drugs for 48 h prior to sessions, which was verified using breath alcohol (Alcosensor III, Intoximeters Inc., St. Louis, MO, USA) and urine drug testing (ToxCup, Branan Medical Corportation, Irvine, CA, USA). Women not on hormonal birth control were scheduled during the follicular phase, while women taking hormonal birth control were scheduled any time during active pill use. Women were tested for pregnancy before the session (AimStrip, Germaine Laboratories, San Antonio, TX, USA). After drug and pregnancy testing, participants completed baseline measures (See above or below). At 9:30 a.m., the patch was placed (NIC and PL groups), or subjects in the SM group smoked a single cigarette. Participants relaxed for 2 h, completing measurements at 10:30 and 11:30 (times 1 and 2). At 11:40 (time 3), participants were given TSST instructions per Kirschbaum et al. (1993a) and allowed a ten-minute preparation period. At 11:50 (time 4), they were escorted into the adjacent stress room. Two confederates, whom they had not previously encountered, acted as interviewers, and participants were able to see themselves on a video monitor for the duration of the task. Participants completed a 5-min speech, and 5 min of mental arithmetic (serial subtraction). Confederates maintained a cold demeanor throughout the task, with specific interruptions/prompts as in Kirschbaum et al. (1993b). Immediately after the task (at 12:00), participants were brought back to the study room and completed time 5 measurements. After this, participants completed measurements at 10-min intervals (times 6–10) until 12:50, at which time they were debriefed.
Data analysis strategy
The analysis addressed several questions. A regression approach to ANOVA with planned comparisons was used (Judd et al. 2009), using two different sets of orthogonal contrasts, depending on the phase of the analysis. First, we examined the groups at baseline on the day of the stress session to answer two questions: (1) Did the SM group differ from the two abstaining groups (NIC and PL) at baseline on the day of the session? (2) Did the two abstaining groups differ before administration of the patch? We contrasted the SM group with NIC and PL groups (“Abstaining” contrast), to examine effects of overnight withdrawal. We also contrasted the two abstaining groups to look for any differences that might indicate failure of randomization (“Randomization” contrast). These, together formed the “Baseline” set of contrasts. Then we examined responses to administration of nicotine (the “Treatment” set of contrasts) to answer two questions: (1) Did the responses of participants with recent nicotine exposure (SM and NIC) differ from those in overnight withdrawal (PL)? (2) Did the method of nicotine delivery (smoking vs. patch) affect responses to stress? Thus, for time points after administration of nicotine or placebo, we contrasted the combined SM and NIC groups with the PL group to capture our primary hypothesis (“Nicotine” contrast). We also contrasted the SM vs. NIC group to evaluate effects of method of nicotine delivery (the “Delivery” contrast). To reduce multiple comparisons over a large number of time points, change scores were constructed over key events: administration, stress, and recovery. The “Administration” period was the difference between baseline and time 1 (1 h after patch or smoking). The “Stress” period was the difference between time 2 and 5 (or between time 2 and area under the curve for times 3–5 for HR). Finally, the “Recovery” period consisted of time 5 relative to the area under the curve for times 6–10 (or the difference between times 5 and 6 for BP). Differences at baseline, immediately before stress (time 2), and at the outset of recovery (time 5) were included as covariates in the change score analyses if necessary (Jin 1992).
Results
Demographic, substance use, and smoking variables by group (Table 1)
Table 1.
Demographics, substance use and smoking variables by group
| Smoke as normal Percentage of sample or mean (SD) |
Placebo patch Percentage of sample or mean (SD) |
Nicotine patch Percentage of sample or mean (SD) |
|
|---|---|---|---|
| Demographics | |||
| Sex | 69% Male | 88% Male | 81% Male |
| Ethnicity | 6% Hispanic | 6% Hispanic | 7% Hispanic |
| Race | 44% White | 59% White | 81% White |
| 25% Black | 29% Black | 6% Black | |
| 31% Other/mixed race | 12% Other/mixed race | 13% Other/mixed race | |
| Age | 25.19 (5.61) | 27.18 (6.94) | 25.94 (5.00) |
| BDI scores | 4.93 (6.39) | 2.18 (3.05) | 2.80 (3.73) |
| Drug use last 30 days | |||
| Caffeinated beverages per week | 13.32 (7.47) | 20.21 (14.41) | 14.13 (11.83) |
| Alcoholic drinks per week | 9.47(9.55) | 9.55 (7.50) | 15.17 (10.48) |
| Cannabis occasions last 30 days | 6.25 (10.58) | 7.19 (8.00) | 8.00 (11.97) |
| Drug use lifetime (ever used) | |||
| Lifetime use of cannabis | 100% | 100% | 100% |
| Lifetime use of tranquilizers | 25% | 18% | 44% |
| Lifetime use of stimulants | 53% | 59% | 62% |
| Lifetime use of opiates | 31% | 35% | 50% |
| Lifetime use of hallucinogens | 50% | 59% | 56% |
| Lifetime use of inhalants | 0% | 29% | 25% |
| Smoking-related variables | |||
| Cigarettes per day | 16.00 (7.59) | 14.94 (4.84) | 12.78 (2.43) |
| FTND scores | 4.69 (1.14) | 4.76 (2.14) | 4.44 (1.75) |
The three groups did not differ on any of the variables tested (one-way ANOVA and chi-square tests; Table 1).
Compliance
Six participants in the PL group and one in the NIC group exceeded the predetermined breath CO level (CO at all greater than 10 ppm) but were retained in the study because their CO was lower than their orientation session. All analyseswere run with and without these individuals, and exclusion of these individuals from the analysis did not affect results, with one exception reported later in the text. CO differences between abstaining and non-abstaining groups at baseline were highly significant, B=−10.81, t (1, 48)=−5.10, p<0.001, but there were not significant differences between the NIC and PL groups, even with the possibly non-compliant individuals included, B=−3.38, t (1, 48)=−1.39, p=0.17.
Self-report
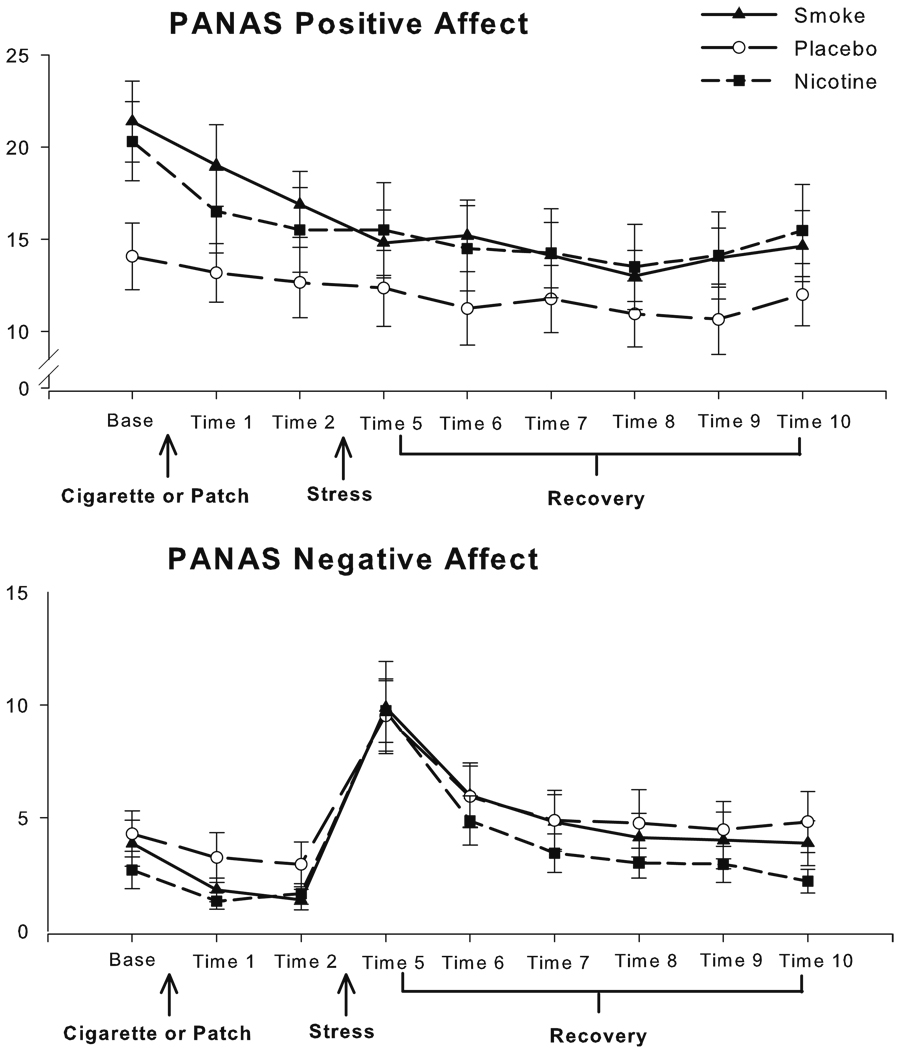
Negative affect
The groups did not differ at baseline in self-reported NA (see Fig. 1), and the groups also did not differ after administration of nicotine before the stress. Overall NA declined during the administration period, B=−1.50, t (1, 48)=−3.51, p=0.001. The groups did not differ over the TSST, although NA increased markedly during the TSST, B=7.73, t (1, 48)=9.91, p<0.001. One participant in the nicotine group was omitted from recovery analyses due to missing data. The groups did not differ at the outset of the recovery period (time 5), and over recovery, although there were significant overall decreases in NA from peak stress during recovery (seen as a negative area under the curve), B=−239.58, t (1, 47)=−8.36, p<0.001.
Fig. 1.
Subjective PANAS ratings at each time point across the study, with standard error of the mean (SEM) error bars
Positive affect
Although the abstaining groups and the SM group did not differ in PA at baseline, there was an unexpected significant difference between the NIC and PL groups, with the PL group reporting lower PA at baseline, B=6.25, t (1, 48)= 2.17, p=0.04 (see Fig. 1). Baseline PA was thus entered as a covariate in the analysis of change over administration. PA did not significantly change over administration. By time 2, immediately before stress, there were no significant differences between the groups on PA, and PA also did not change over stress or recovery in any of the groups.
Withdrawal
When the possibly non-compliant individuals were included, the groups did not differ on the MNWS, which was administered at time 2, immediately before stress. With the possibly non-compliant individuals excluded, the PL group reported more withdrawal symptoms than the nicotine exposed groups, B=4.72, t (1, 41)=2.37, p=0.02.
Cardiovascular
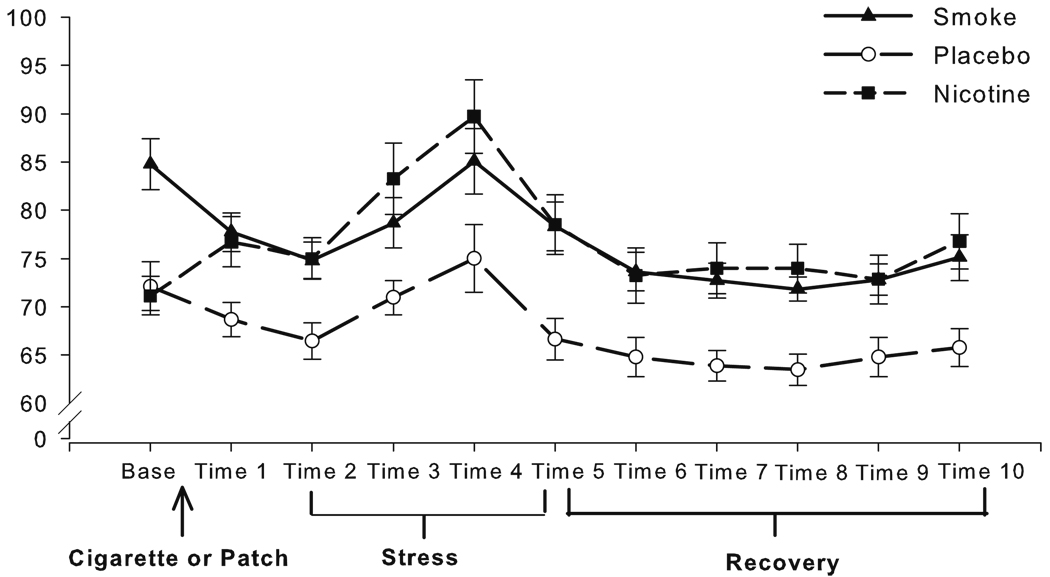
Heart rate
Data for three individuals (two in PL and one in the NIC group) were lost due to MiniLogger malfunctions. At baseline, the abstaining groups had lower HR than the SM group, an effect typical of withdrawal (al’Absi et al. 2003; VanderKaay and Patterson 2006), B=−13.15, t (1, 45)= −4.52, p<0.001 (see Fig. 1). The abstaining NIC and PL groups did not differ at baseline. Baseline HR was entered as a covariate in analyses of the administration period. Over administration, HR increased in the NIC group, bringing their HR up to the higher level of the SM group, while the PL group remained low, relative to the other groups (nicotine and delivery contrasts, B=−4.78, t (1, 45)=3.23, p=0.02 and B=7.85, t (1, 45)=3.23, p=0.002, respectively). Examining Fig. 2, this pattern persisted at time 2 (before stress), such that the nicotine-exposed groups had higher HR than the PL group, B=−8.47, t (1, 45)=−3.48, p=0.003, whereas the SM and NIC group did not differ. Given this, time 2 HR was entered as a covariate in the stress analysis. Examining change over the TSST, HR increased over time regardless of group (seen as a positive area under the curve), B=179.77, t (1, 45)=6.74, p<0.001. Significant absolute differences between the nicotine-exposed and PL group persisted at the outset of recovery, B=−11.81, t (1, 45)=−3.67, p<0.001, so time 5 HR was used as a covariate in recovery analyses. HR declined over the period of recovery (B=1040.75, t (1, 44)=4.44, p<0.001), but the groups did not differ in this phase.
Fig. 2.
Mean heart rate (bpm) for each 10-min time point across the study, with SEM error bars
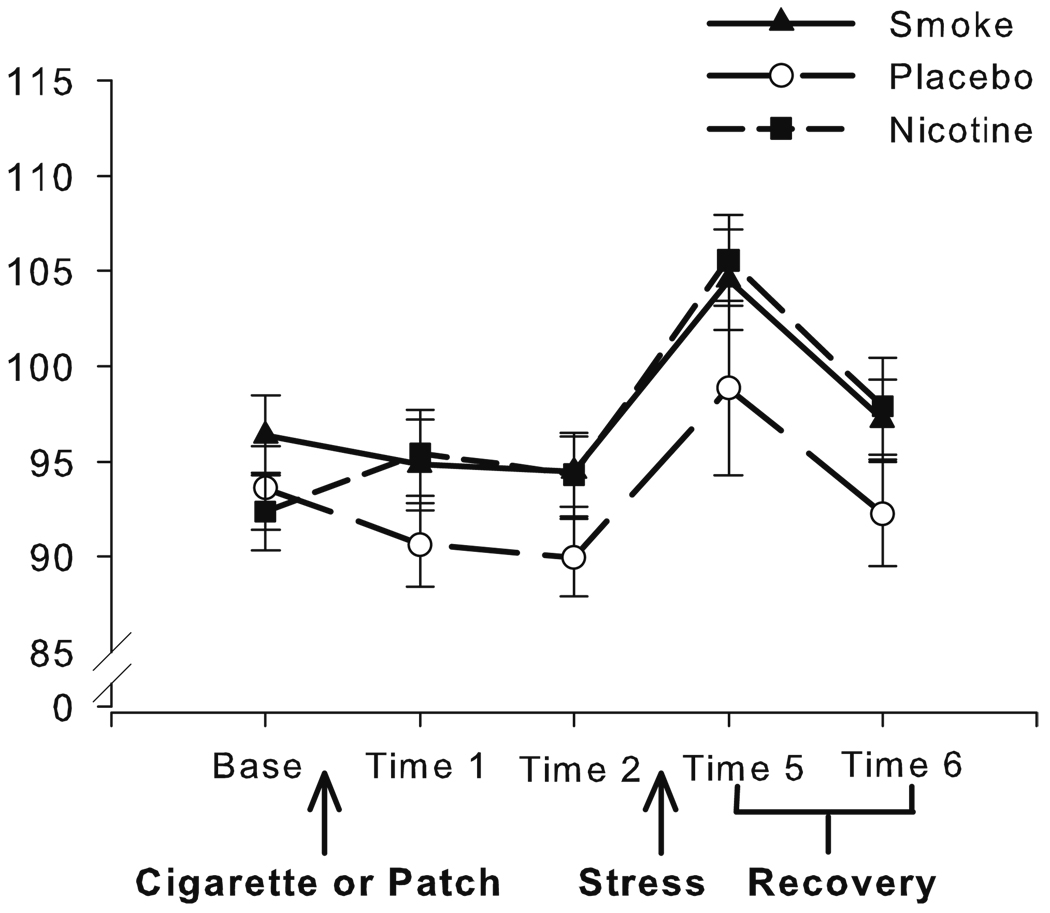
Blood pressure
We did not have an a-priori reason to expect differential effects on systolic vs. diastolic blood pressure, so we used mean arterial pressure (MAP; [systolic BP+2*diastolic BP]/3) for all analyses. The groups did not differ at baseline. During the administration phase, the MAP of the NIC group increased marginally, up to the level of the SM group, while the MAP of the PL group remained low (Fig. 3; nicotine and delivery contrasts, B=−3.74, t (1, 48)=−1.85, p=0.07, and B=4.60, t (1, 48)=1.93, p=0.06, respectively). This pattern persisted at time 2, with a marginal difference between the combined nicotine-exposed and PL group, B=−4.46, t (1, 48)=−1.84, p=0.07, but no difference between the SM and NIC group. Time 2 scores were thus used as covariates in the stress analysis. The TSST increased MAP to a similar extent in all three groups (MAP, B=10.07, t (1, 48)=6.34, p<0.001). MAP declined in a similar manner during recovery in all groups (B=−7.20, t (1, 48)=−6.79, p<0.001).
Fig. 3.
Mean arterial pressure (mmHg) at each time point across the study, with SEM error bars
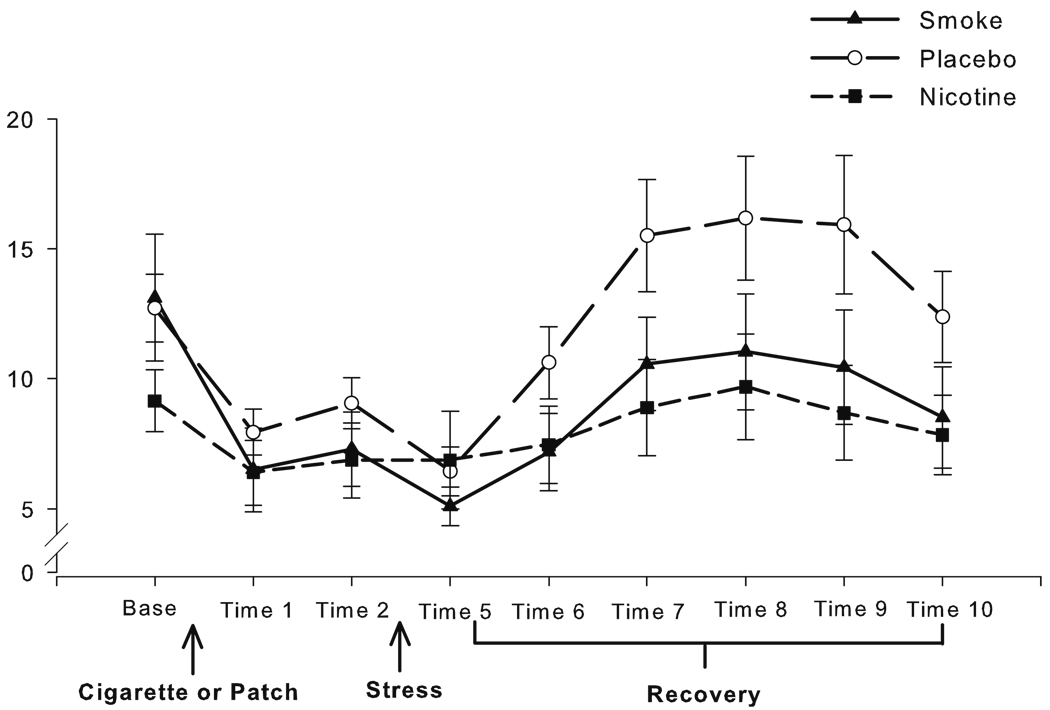
Hormonal
A positive skew in the cortisol data was adequately corrected by square root transformation. Data from one individual in the PL group were lost due to sample contamination. The groups did not differ at baseline. Over administration, the decline in cortisol levels was slightly steeper in the SM group compared to the other two groups (see Fig. 4; delivery contrast, B=0.14, t (1, 47)=2.16, p=0.04). Despite this, the groups did not differ immediately before stress. Cortisol levels declined across the stress period (B=−0.062, t (1, 47)=−2.42, p= 0.02) consistent with the delay in circulating cortisol levels after acute stress. The groups did not differ at the outset of the recovery period (time 5), but during the recovery period, the PL group had a significantly larger increase in cortisol compared to the nicotine-exposed groups (nicotine contrast, B=5.41, t (1, 47)=2.44, p=0.02). The NIC and SM groups did not differ during recovery.
Fig. 4.
Raw values of salivary cortisol levels (nmol/L) at each time point across the study, with SEM error bars (Note: analyses were conducted on square root transformations of raw values to correct for positive skew)
Discussion and conclusions
The effects of withdrawal on acute stress varied across the outcome measures of subjective, cardiovascular, and hormonal effects. First, on the measure of subjective responses, stress increased NA to a similar extent in all three groups. Although the PL group scored unexpectedly lower on PA at baseline, this difference resolved prior to the stress procedure. On the cardiovascular measures, the NIC and PL groups exhibited the expected lower HR and marginally lower MAP after overnight abstinence, and the nicotine patch ameliorated these effects. All three groups exhibited the expected increases in HR and MAP after the TSST and this effect did not differ across the groups. On the HPA measure, the increase in cortisol was greater in the PL group than either of the nicotine-exposed groups. Thus, using a standardized social stress procedure that effectively and reliably elicits SAM and HPA responses, we found that nicotine withdrawal increased the HPA response, but not SAM or subjective response to stress.
Congruent with our first prediction, the use of a very effective social stressor that strongly engages the HPA axis revealed differences in stress response between nicotine-exposed and overnight-withdrawn groups. Further, nicotine replacement ameliorated these differences. The differences observed were specific to the functioning of the HPA axis, so our results may differ from the null results of previous studies utilizing social stress (al’Absi et al. 2003), because our stressor is more effectively engaged in the HPA axis, making underlying differences in functioning visible.
Regarding our second prediction that withdrawal would decrease stress response, the observed increase in cortisol response in the PL group would not appear to support this idea. However, increased cortisol response to stress during withdrawal in the absence of changes in other measures, is open to several interpretations. First, greater cortisol production could indicate greater reactivity stress. However, cortisol also provides negative feedback to the HPA axis, and thus, lower cortisol response in the NIC and SM groups might also indicate a dysregulated stress response with negative implications for coping. Indeed, smokers typically demonstrate a blunted cortisol response when compared to non-smokers (al’Absi et al. 2003), and individual differences in cortisol response to stress during smoking withdrawal predict relapse, with blunted responses, predicting faster returns to smoking (al’Absi 2006). Thus, the increased cortisol response in the PL group may be an indicator of rapid recovery in stress systems chronically dysregulated by nicotine. In this case, restoration of cortisol responses by the nicotine patch to the (typically blunted) levels seen during regular smoking may be an unfortunate side effect of nicotine replacement and a reason to pursue combination pharmacotherapy with bupropion, which, in other studies, restored stress responses during abstinence (Kotlyar et al. 2006).
The study had several limitations. First, there were limitations to the manipulations of withdrawal. A small number of individuals were questionably compliant with the abstinence requirement, which may have attenuated group differences in withdrawal symptoms. Additionally, the measure of withdrawal queried participants about symptoms in the previous 18 h, and thus, included periods of time outside the 12-h abstinence period, which might further reduce our ability to discern differences in withdrawal. Future studies might consider using a more precise measure for assessing withdrawal symptoms at various points in the procedure to document the effectiveness of the withdrawal manipulations. The absence of withdrawal-induced negative affect may be an indicator that the abstinence duration was insufficient in our study to produce a full withdrawal syndrome. However, there was also evidence that our chosen time frames for both abstinence and smoking did effectively distinguish the nicotine exposed from the abstaining groups, in the form of clear cardiovascular withdrawal effects in the abstaining groups that persisted throughout the stress procedure for the PL group. Additionally, analyses run with and without potentially non-compliant individuals produced substantively similar results. A second limitation was the absence of nicotine plasma levels in the SM and NIC patch groups to verify that comparable nicotine levels were attained. However, the fact that the nicotine patch effectively ameliorated the cardiovascular effects of abstinence (NIC group) would suggest that the patch yielded the expected increase in nicotine concentrations. Another limitation of the study was the lack of a nonstress control session or nonsmoker control group. The inclusion of a within-subject nonstress session might increase the sensitivity of the procedure; however, the obvious immediate reactions to the TSST in subjective, cardiovascular measures, and cortisol levels argue that the effects observed are a result of the combined effects of stress and withdrawal, and not due to withdrawal alone. The sessions were conducted from 9:00 a.m. to 1:00 p.m., at a time when diurnal cortisol levels are expected to fall, not rise (Smyth et al. 1997). Further, administration of nicotine would generally be expected to increase cortisol, not decrease it (Wilkins et al. 1982). Inclusion of a non-smoking control group would help to determine whether withdrawal “normalizes” or exaggerates the effects of stress. Finally, our sample size was modest, and replication of our results in a larger sample size would provide greater confidence in the robustness of our findings, as well as improved power to detect smaller differences in other response variables (i.e., cardiovascular or self-report) that may not have been evident in the current study.
In future studies, it will be important to examine responses to stress and coping with stress over longer periods of abstinence and with other stress procedures. In our study, we used a single stressor scheduled very soon after initiation of abstinence. Because the cortisol response is of longer latency and duration than other stress responses, its effects on subjective emotional response, craving, or other relapse-related measures may not be manifested until the system is repeatedly stressed, or stressed over a prolonged period. In addition, future studies should explicitly examine gender differences and differences across the menstrual cycle in women, as both cortisol response and nicotine withdrawal symptoms differ systematically across the menstrual cycle (Allen et al. 2000; Kudielka and Kirschbaum 2005). Thus, further controlled studies, with multiple stressors over longer time frames, should be combined with more naturalistic determinations of responses to acute stress during real quit attempts to elucidate the significance of this finding for relapse.
Contributor Information
Margaret C. Wardle, Department of Psychiatry and Behavioral Neuroscience, University of Chicago, 5841 S. Maryland Ave. MC 3077, Chicago, IL 60637, USA
Marcus R. Munafò, Department of Experimental Psychology, University of Bristol, 12a Priory Rd., Bristol BS8 1TU, UK
Harriet de Wit, Email: hdew@uchicago.edu, Department of Psychiatry and Behavioral Neuroscience, University of Chicago, 5841 S. Maryland Ave. MC 3077, Chicago, IL 60637, USA.
References
- al’Absi M. Hypothalamic-pituitary-adrenocortical responses to psychological stress and risk for smoking relapse. Int J Psychophysiol. 2006;59:218–227. doi: 10.1016/j.ijpsycho.2005.10.010. [DOI] [PubMed] [Google Scholar]
- al’Absi M, Amunrud T, Wittmers LE. Psychophysiological effects of nicotine abstinence and behavioral challenges in habitual smokers. Pharmacol Biochem Behav. 2002;72:707–716. doi: 10.1016/s0091-3057(02)00739-6. [DOI] [PubMed] [Google Scholar]
- al’Absi M, Wittmers LE, Erickson J, Hatsukami D, Crouse B. Attenuated adrenocortical and blood pressure responses to psychological stress in ad libitum and abstinent smokers. Pharmacol Biochem Behav. 2003;74:401–410. doi: 10.1016/s0091-3057(02)01011-0. [DOI] [PubMed] [Google Scholar]
- Allen SS, Hatsukami D, Christianson D, Brown S. Effects of transdermal nicotine on craving, withdrawal and premenstrual symptomatology in short-term smoking abstinence during different phases of the menstrual cycle. Nicotine Tob Res. 2000;2:231–241. doi: 10.1080/14622200050147493. [DOI] [PubMed] [Google Scholar]
- Attwood A, Saeed F, Bailey J, Nutt D, Munafò MR. Effects of carbon dioxide challenge on craving and anxiety in abstinent and non-abstinent cigarette smokers. Poster presented at the Society for Res on Nicotine and Tob; Rome. 2008. [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychol Rev. 2004;111:33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Buchmann AF, Laucht M, Schmid B, Wiedemann K, Mann K, Zimmermann US. Cigarette craving increases after a psychosocial stress test and is related to cortisol stress response but not to dependence scores in daily smokers. J Psychopharmacol. 2010;24:247–255. doi: 10.1177/0269881108095716. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, Chaudhri N, Perkins KA, Evans-Martin FF, Sved AF. Importance of nonpharmacological factors in nicotine self-adminstration. Physiol Behav. 2002;77:683–687. doi: 10.1016/s0031-9384(02)00918-6. [DOI] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Palmatier MI, Liu X, Sved AF. Complex interactions between nicotine and nonpharmacological stimuli reveal multiple roles for nicotine in reinforcement. Psychopharmacology. 2006;184:353–366. doi: 10.1007/s00213-005-0178-1. [DOI] [PubMed] [Google Scholar]
- Chen H, Fu Y, Sharp BM. Chronic nicotine self-administration augments hypothalamic-pituitary-adrenal responses to mild acute stress. Neuropsychopharmacology. 2008;33:721–730. doi: 10.1038/sj.npp.1301466. [DOI] [PubMed] [Google Scholar]
- Copeland AL, Brandon TH, Quinn EP. The Smoking Consequences Questionnaire-Adult: measurement of smoking outcome expectancies of experienced smokers. Psychol Assess. 1995;7:484–494. [Google Scholar]
- Dawkins L, Acaster S, Powell JH. The effects of smoking and abstinence on experience of happiness and sadness in response to positively valenced, negatively valenced, and neutral film clips. Addict Behav. 2007;32:425–431. doi: 10.1016/j.addbeh.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Dawkins L, Powell JH, Pickering A, Powell J, West R. Patterns of change in withdrawal symptoms, desire to smoke, reward motivation and response inhibition across 3 months of smoking abstinence. Addiction. 2009;104:850–858. doi: 10.1111/j.1360-0443.2009.02522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol Bull. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Girdler SS, Jamner LD, Jarvik M, Soles JR, Shapiro D. Smoking status and nicotine administration differentially modify hemodynamic stress reactivity in men and women. Psychosom Med. 1997;59:294–306. doi: 10.1097/00006842-199705000-00012. [DOI] [PubMed] [Google Scholar]
- Gorsline J. Nicotine pharmacokinetics of four nicotine transdermal systems. Health Values. 1993;17:20–24. [Google Scholar]
- Gunnar M, Quevedo K. The neurobiology of stress and development. Annu Rev Psychol. 2007;58:145–173. doi: 10.1146/annurev.psych.58.110405.085605. [DOI] [PubMed] [Google Scholar]
- Gupta SK, Okerholm RA, Eller M, Wei G, Rolf CN, Gorsline J. Comparison of the pharmacokinetics of two nicotine transdermal systems: nicoderm and habitrol. J Clin Pharmacol. 1995;35:493–498. doi: 10.1002/j.1552-4604.1995.tb04093.x. [DOI] [PubMed] [Google Scholar]
- Henningfield JE. Nicotine medications for smoking cessation. N Engl J Med. 1995;333:1196–1203. doi: 10.1056/NEJM199511023331807. [DOI] [PubMed] [Google Scholar]
- Hughes JR. Tobbacco withdrawal in self-quitters. J Consult Clin Psychol. 1992;60:689–697. doi: 10.1037//0022-006x.60.5.689. [DOI] [PubMed] [Google Scholar]
- Hughes JR. Effects of abstinence from tobacco: etiology, animal models, epidemiology, and significance: a subjective review. Nicotine Tob Res. 2007;9:329–339. doi: 10.1080/14622200701188927. [DOI] [PubMed] [Google Scholar]
- Jin P. Toward a reconceptualization of the law of initial value. Psychol Bull. 1992;111:176–184. doi: 10.1037/0033-2909.111.1.176. [DOI] [PubMed] [Google Scholar]
- Judd CM, McClelland GH, Ryan CS. Data analysis: A model comparison approach. 2nd edn. New York: Routledge/Taylor & Francis Group; 2009. [Google Scholar]
- Kirschbaum C, Pirke K-M, Hellhammer DH. The ‘Trier Social Stress Test’—a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993a;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Strasburger CJ, Langkrär J. Attenuated cortisol response to psychological stress but not to CRH or ergometry in young habitual smokers. Pharmacol Biochem Behav. 1993b;44:527–531. doi: 10.1016/0091-3057(93)90162-m. [DOI] [PubMed] [Google Scholar]
- Kotlyar M, Brauer LH, al’Absi M, Adson DE, Robiner W, Thuras P, Harris J, Finocchi ME, Bronars CA, Candell S, Hatsukami DK. Effect of bupropion on physiological measures of stress in smokers during nicotine withdrawal. Pharmacol Biochem Behav. 2006;83:370–379. doi: 10.1016/j.pbb.2006.02.017. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Kirschbaum C. Sex differences in HPA axis responses to stress: a review. Biol Psychol. 2005;69:113–132. doi: 10.1016/j.biopsycho.2004.11.009. [DOI] [PubMed] [Google Scholar]
- McRae AL, Saladin ME, Brady KT, Upadhyaya H, Back SE, Timmerman MA. Stress reactivity: biological and subjective responses to the cold pressor and Trier Social stressors. Hum Psychopharmacol. 2006;21:377–385. doi: 10.1002/hup.778. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Epstein LH, Jennings JR, Stiller R. The cardiovascular effects of nicotine during stress. Psychopharmacology. 1986;90:373–378. doi: 10.1007/BF00179194. [DOI] [PubMed] [Google Scholar]
- Pomerleau OF, Pomerleau CS. Cortisol response to a psychological stressor and/or nicotine. Pharmacol Biochem Behav. 1990;36:211–213. doi: 10.1016/0091-3057(90)90153-9. [DOI] [PubMed] [Google Scholar]
- Robinson JD, Cinciripini PM. The effects of stress and smoking on catecholaminergic and cardiovascular response. Behav Med. 2006;32:13–18. doi: 10.3200/BMED.32.1.13-18. [DOI] [PubMed] [Google Scholar]
- Semba J, Wakuta M, Maeda J, Suhara T. Nicotine withdrawal induces subsensitivity of hypothalamic-pituitary-adrenal axis to stress in rats: implications for precipitation of depression during smoking cessation. Psychoneuroendocrinology. 2004;29:215–226. doi: 10.1016/s0306-4530(03)00024-6. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Paty JA, Gnys M, Kassel JA, Hickcox M. First lapses to smoking: within-subjects analysis of real-time reports. J Consult Clin Psychol. 1996;64:366–379. doi: 10.1037//0022-006x.64.2.366. [DOI] [PubMed] [Google Scholar]
- Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology. 2001;158:343–359. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- Smyth JM, Ockenfels MC, Gorin AA, Catley D, Porter LS, Kirschbaum C, Hellhammer DH, Stone AA. Individual differences in the diurnal cycle of cortisol. Psychoneuroendocrinology. 1997;22:89–105. doi: 10.1016/s0306-4530(96)00039-x. [DOI] [PubMed] [Google Scholar]
- Stone AA. Measurement of affective response measuring stress: a guide for health and social scientists. New York: Oxford University Press; 1995. pp. 148–171. [Google Scholar]
- Tsuda A, Steptoe A, West R, Fieldman G, Kirschbaum C. Cigarette smoking and psychophysiological stress responsiveness: effects of recent smoking and temporary abstinence. Psychopharmacology. 1996;126:226–233. doi: 10.1007/BF02246452. [DOI] [PubMed] [Google Scholar]
- van Duinen MA, Schruers KRJ, Maes M, Griez EJL. CO2 challenge results in hypothalamic-pituitary-adrenal activation in healthy volunteers. J Psychopharmacol. 2005;19:243–247. doi: 10.1177/0269881105051527. [DOI] [PubMed] [Google Scholar]
- VanderKaay MM, Patterson SM. Nicotine and acute stress: effects of nicotine versus nicotine withdrawal on stress-induced hemoconcentration and cardiovascular reactivity. Biol Psychol. 2006;71:191–201. doi: 10.1016/j.biopsycho.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Vujanovic AA, Zvolensky MJ. Anxiety sensitivity, acute nicotine withdrawal symptoms, and anxious and fearful responding to bodily sensations: a laboratory test. Exp Clin Psychopharmacol. 2009;17:181–190. doi: 10.1037/a0016266. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Wilkins J, Carlson H, Vunakis H, Hill M, Gritz E, Jarvik M. Nicotine from cigarette smoking increases circulating levels of cortisol, growth hormone, and prolactin in male chronic smokers. Psychopharmacol. 1982;78:305–308. doi: 10.1007/BF00433730. [DOI] [PubMed] [Google Scholar]