Abstract
Background and Aims
This study evaluated the responses to soluble epoxide hydrolase (s-EH) inhibition, an essential enzyme in the metabolism of arachidonic acid, on food intake, body weight and metabolic parameters in mice fed a high fat-high fructose diet (HFD) for 10 weeks.
Methods and Results
After 5 weeks of HFD, mice were divided into two groups: 1) s-EH inhibitor (AR9281, 200 mg/kg/day by gavage twice daily), and 2) vehicle (0.3 ml per gavage). Food intake, body weight, oxygen consumption (VO2), carbon dioxide production (VCO2), respiratory quotient (RQ), and motor activity were measured weekly for more 5 weeks. HFD increased body weight (37±1 vs 26±1 g), and plasma of glucose (316±8 vs 188±27 mg/dl), insulin (62.1±8.1 vs 15.5±5.0 µU/ml), and leptin levels (39.4±3.6 vs 7.5±0.1 ng/ml) while reducing VO2, VCO2 and motor activity. s-EH inhibition for 5 weeks decreased caloric intake by ~32% and increased VO2 by ~17% (42.8±1.4 vs. 50.2±1.5 ml/kg/min) leading to significant weight loss. Inhibition of s-EHi also caused significant reductions in plasma leptin levels and visceral fat content. Uncoupling protein 1 (UCP1) content in brown adipose tissue was also elevated by ~50% during s-EH inhibition compared to vehicle treatment.
Conclusion
These results suggest that s-EH inhibition with AR9281 promotes weight loss by reducing appetite and increasing metabolic rate, and that increased UCP1 content may contribute to the increase in energy expenditure.
Keywords: obesity, energy expenditure, epoxide hydrolase inhibition, body weight, oxygen consumption
INTRODUCTION
Soluble epoxide hydrolase (s-EH), an essential enzyme in the metabolism of arachidonic acid, has been implicated in the development of hypertension, inflammatory disease and metabolic abnormalities (2,9,12,14,16). Epoxyeicosatrienoic acids (EETs) are bioactive lipid mediators synthesized from arachidonic acid that cause coronary dilation (6) by acting as an endothelium-derived hyperpolarizing factor. EETs are catabolized by s-EH to form dihydroxyeicosatrienoic acid (DHETs). In pregnancy-induced hypertension, the excretion of DHETs is elevated, suggesting a role for epoxide hydrolase in the regulation of blood pressure (4). Disruption of the s-EH gene in mice reduces blood pressure and inhibition of s-EH decreases blood pressure in several experimental hypertensive models (10,19). s-EH may also be involved in inflammatory processes and inhibition of s-EH seems to be effective in the treatment of inflammatory diseases (14).
Obesity and its related metabolic abnormalities are associated with a complex systemic inflammatory state as well as endothelial dysfunction. Inflammation, endothelial dysfunction and insulin resistance are interconnected and have reciprocal relationships that link cardiovascular and metabolic diseases. This association could lead to increased risk for cardiovascular events and target organ injury. Previous studies have demonstrated that eicosanoids are altered in metabolic syndrome and may contribute to the progression of renal injury, as suggested by increased albumin excretion (5,8,11,24).
The antihypertensive and anti-inflammatory properties of EETs and soluble epoxide hydrolase inhibitors make them a potential therapeutic target for treatment of many complications associated with metabolic syndrome. Although the beneficial cardiovascular actions of s-EH inhibition have been previously studied, and preliminary studies suggest that adipose tissue is an important source of s-EH, the long-term effects of s-EH inhibition on body weight regulation and the metabolic profile in obesity have not, to our knowledge, been previously reported. Therefore, the main objective of the present study was to investigate whether s-EH inhibition alters the regulation of appetite and whole body energy expenditure thus promoting weight loss in a model of diet-induced obesity in mice.
METHODS
All experimental procedures conform to the National Institute of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the University of Mississippi Medical Center. The mice received food and water ad libitum throughout the study and were placed on a 12hr:12hr light-dark cycle.
Animals
Male C57BL/6J mice were housed in individual cages for determination of water and food consumption. Mice were maintained on a normal rodent chow (Purina 5001) containing 0.4% NaCl during the acclimatization and control periods, and offered water ad lib. Mice were then switched to a high fat-high fructose diet (HFD, Research Diets, New Brunswick, NJ; #D12451, 4.7 kcal/g) for the remaining 10 weeks of the study, and water was replaced by degassed 7UP (Pepper/Seven Up, Inc, Plano, TX; 0.416 kcal/mL).
Experimental Protocol
During the control and experimental periods, mice were placed in special metabolic cages (AccuScan Instruments Inc, Columbus, OH) for 48 hours every 10 days. Although we measured oxygen consumption for 2 consecutive days, the first 24 hours were considered as an acclimatization period and only the data collected on the second day was used in the calculations. Mice were housed individually in an acrylic cage (16 cm × 24 cm × 17 cm) equipped with oxygen sensor to measure oxygen consumption (VO2) and infrared beams to determine motor activity. VO2 was measured for 2-min at 10-minute intervals using a Zirconia oxygen sensor. This system also measured energy expenditure and carbon dioxide production (VCO2) and automatically calculated respiratory quotients (RQ). Heat production was derived from the following formula (4.33 + (0.67 * RQ) * VO2 * weight (grams) * 60. Animal motor activity was determined using infrared light beams mounted in the cages in X, Y and Z axes. Precise measurements of food and liquid consumption were performed using a computerized workstation that continuously monitored the weight of the food and water hoppers.
Experimental groups and treatment
After 5 weeks on the HFD, the mice were randomly assigned to one of two groups and maintained on the HFD for the remaining 5 weeks of the study: 1) Vehicle (n=8), mice were dosed by twice-daily oral gavage with vehicle (0.3 ml of a solution containing 1% carboxymethylcellulose + 0.1% Tween 80) and kept on high fat-high fructose diet, and 2) Treated (n=10), mice were dosed by twice-daily (at 8:00 am and 6:00 pm) oral gavage with the s-EH inhibitor, AR9281, at 100 mg/kg/per gavage and kept on high fat-high fructose diet. This treatment regimen results in plasma concentrations of the s-EHi ~12.5 µg/ml, which has been shown to completely block s-EH activity and significantly increases the epoxy:diol lipid ratio in plasma up to 12 hours after each dosing (22,23). Blood samples (100 µl) for determination of glucose, insulin and leptin concentrations were collected at baseline, at week 5 of HFD and again at the end of the 5-week treatment period via a small tail incision. The samples were collected after 6-hour fasting between 2:00 and 3:00 pm.
Western Blotting for Uncoupling Proteins
Interscapular brown adipose tissue (BAT) was homogenized in lysis buffer (KPO4, pH 7.4), sonicated and cleared by centrifugation (3.500 × g, 5 min at 4°C). After protein concentration of supernatant was determined by Bradford method (Bio-rad, Hercules, CA). Fifty micrograms of protein were separated by 4–15% precast linear gradient polyacrylamide gel (Bio-Rad, CA). After being transferred to nitrocellulose membranes, blots were rinsed in PBS and blocked in Odyssey blocking buffer (LI-COR, Lincoln, NE) for 1 h at room temperature, and incubated with mouse monoclonal anti-UCP1 (uncoupling protein 1) (1:1,000; Sigma, Saint Louis, MO) or rabbit monoclonal anti-UCP3 (1:1,000; Sigma, Saint Louis, MO) antibodies overnight at 4°C. The membranes were probed with mouse anti-β-actin (1:5,000; Abcam) as a loading control. Membranes were incubated with IR700-conjugated donkey anti-mouse IgG and IR800-conjugated donkey anti-rabbit (1:2,000; Rockland Immunologicals). Antibody labeling was visualized using the Odyssey Infrared Scanner (LI-COR) for simultaneous detection of two fluorprobes. Fluorescence intensity analyses were performed using Odyssey software (LI-COR). Measurements of UCP1 and UCP3 were normalized to β-actin.
Visceral Fat Content and Adipocyte Size
To determine the effects of treatment on visceral fat content, mesenteric and retroperitoneal (epididymal plus perirenal) fat pads were excised and weighed immediately after euthanasia. For analysis of adipocyte size, epididymal fat pads of mice treated with s-EH inhibitor or vehicle were fixed in formalin and embedded in paraffin, cut in 5 mm section and stained with hematoxylin and eosin. Adipocyte size was determined at 20X magnification using a color video camera attached to a Leica microscope by Imagepro software (Universal Imaging Corporation, PA, USA).
Analytical Methods
Plasma insulin and leptin were determined by ELISA (Linco Insulin ELISA, MO, USA; R&D Leptin ELISA, MN, USA). Plasma glucose was determined by the glucose oxidation method (Beckman glucose analyzer 2, CA, USA).
Statistical Methods
The data are expressed as mean ± SEM. Data obtained on a daily basis were analyzed using ANOVA with repeated measures. Comparisons between groups were performed using one-way or two-way ANOVA followed by the Bonferroni’s post-hoc test or Student’s t-test where appropriate. Statistical significance was accepted at a level of P<0.05.
RESULTS
Effects of high fat-high fructose diet on body weight, caloric intake, oxygen consumption (VO2), respiratory quotient (RQ), motor activity, and plasma glucose, leptin and insulin levels
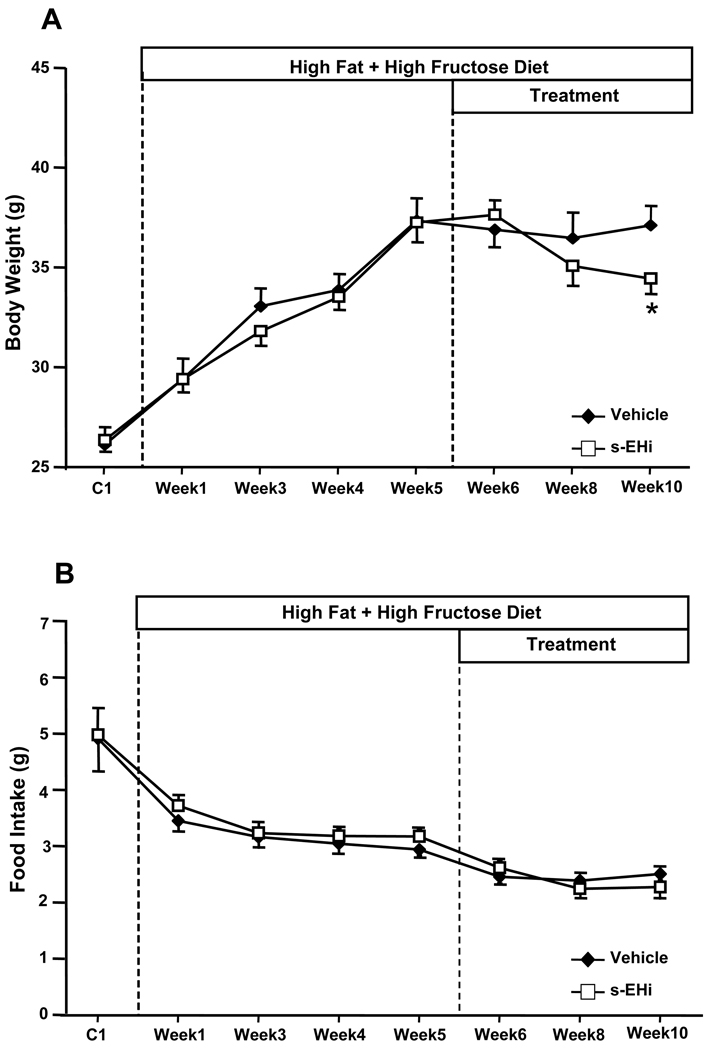
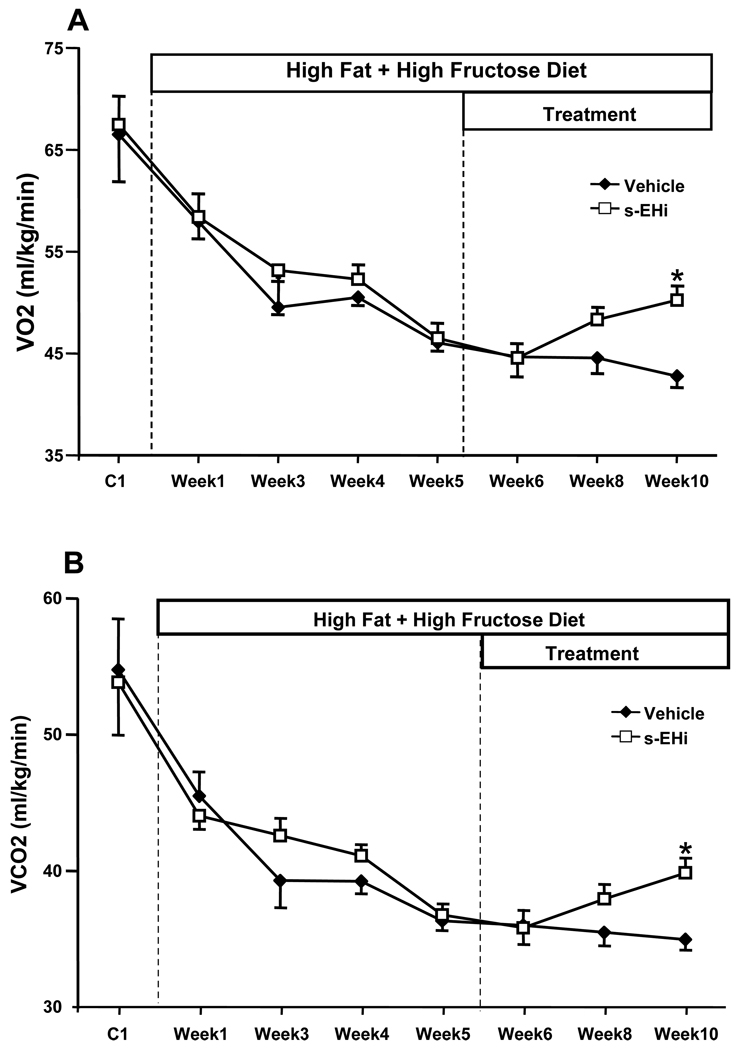
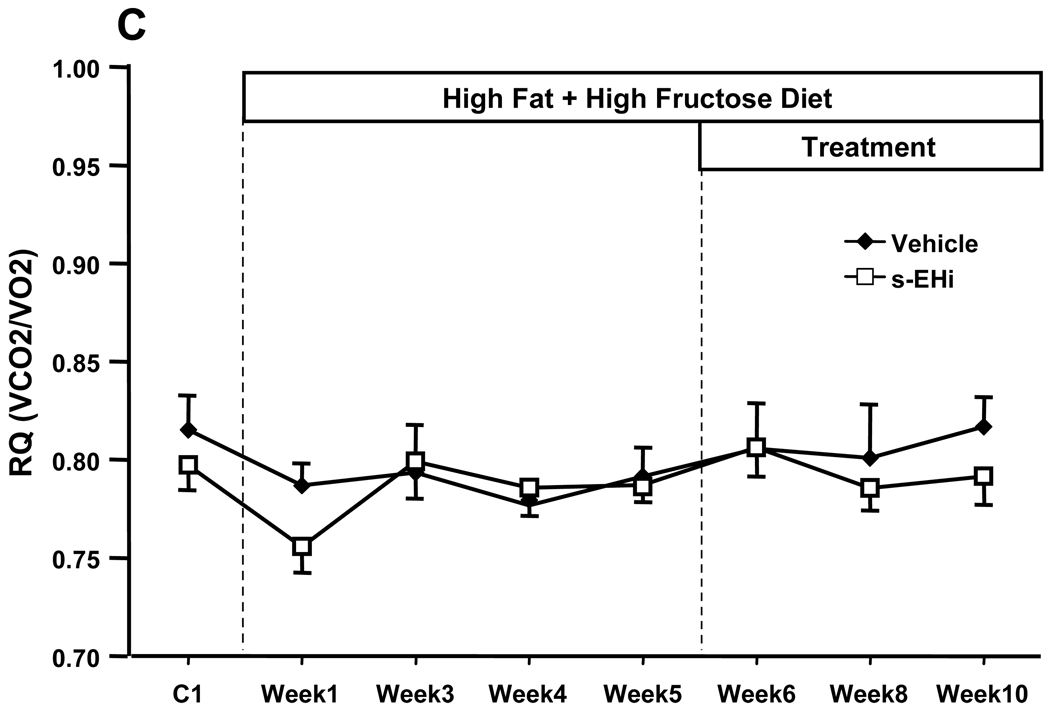
The average pooled data of all animals used in these experiments (n=18), before they were divided into 2 sub-groups, demonstrated that high fat-high fructose feeding for 5 weeks raised plasma glucose (316 ± 8 vs. 188 ± 27 mg/dl), insulin (62.1 ± 8.1 vs. 15.5 ± 5.0 µU/ml), and leptin levels (39.4 ± 3.6 vs. 7.5 ± 0.1 ng/ml) compared to the baseline values measured during the control week on standard diet. High fat-high fructose feeding for 5 weeks also caused approximately a 42% increase in body weight (37 ± 1 vs. 26 ± 1 g, Figure 1A) During the first week of the HFD there was a small increase in total caloric intake (amount of calories consumed per day from the food + fructose intake) which returned to levels comparable to control values by the end of week 5 (Figure 1C). VO2 and motor activity decreased during the HFD (Figures 2A and 3B), which may explain the gain in body weight in the face of unaltered or even slightly reduced caloric intake during HFD. No significant changes in RQ were observed during the 5 weeks of HFD (Figure 2C).
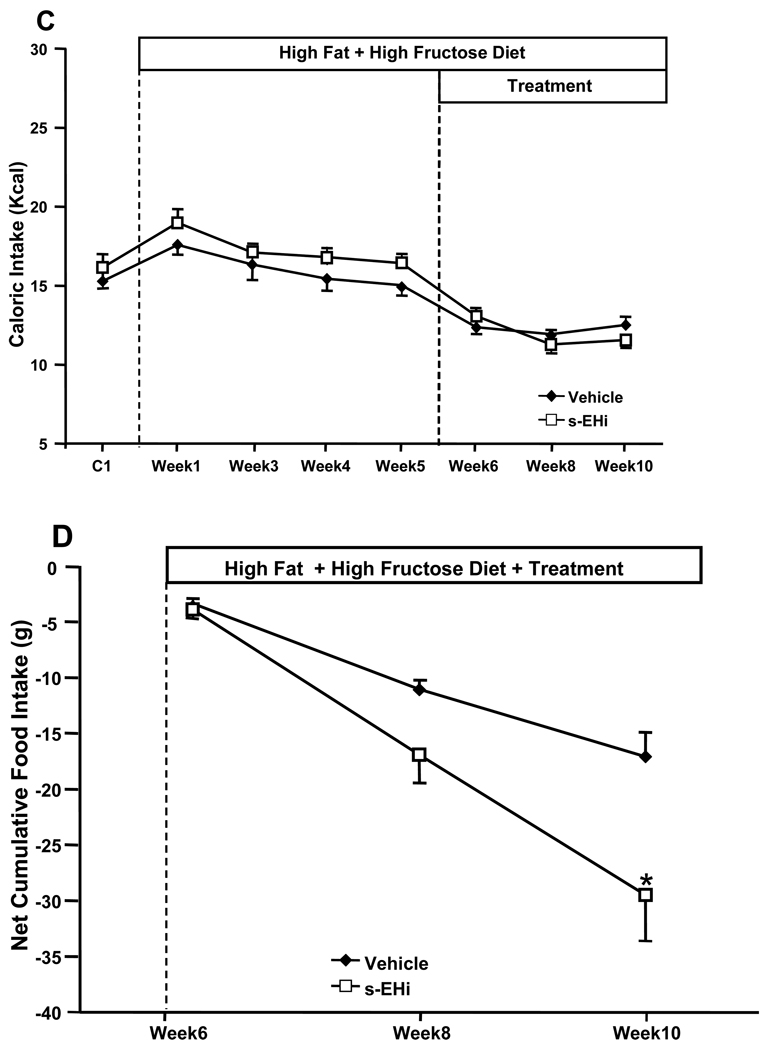
Figure 1.
Effects of the s-EH inhibitor (s-EHi, AR9281) or vehicle in mice fed high fat-high fructose diet on (A) body weight, (B) food intake, (C) caloric intake and (D) net cumulative food intake. Net cumulative food intake was calculated as the sum of the daily difference in food intake after treatment was started minus the average daily food intake during week 5 on the high fat-high fructose diet. * p<0.05 compared to Vehicle.
Figure 2.
Effects of high fat-high fructose diet (HFD) plus daily gavage with vehicle or s-EH inhibitor (s-EHi, AR9281) on (A) oxygen consumption (VO2, ml/kg/min), (B) carbon dioxide production (VCO2, ml/kg/min) and (C) respiratory quotient (RQ) in wild-type mice. * p<0.05 compared to Vehicle on Week 10.
Figure 3.
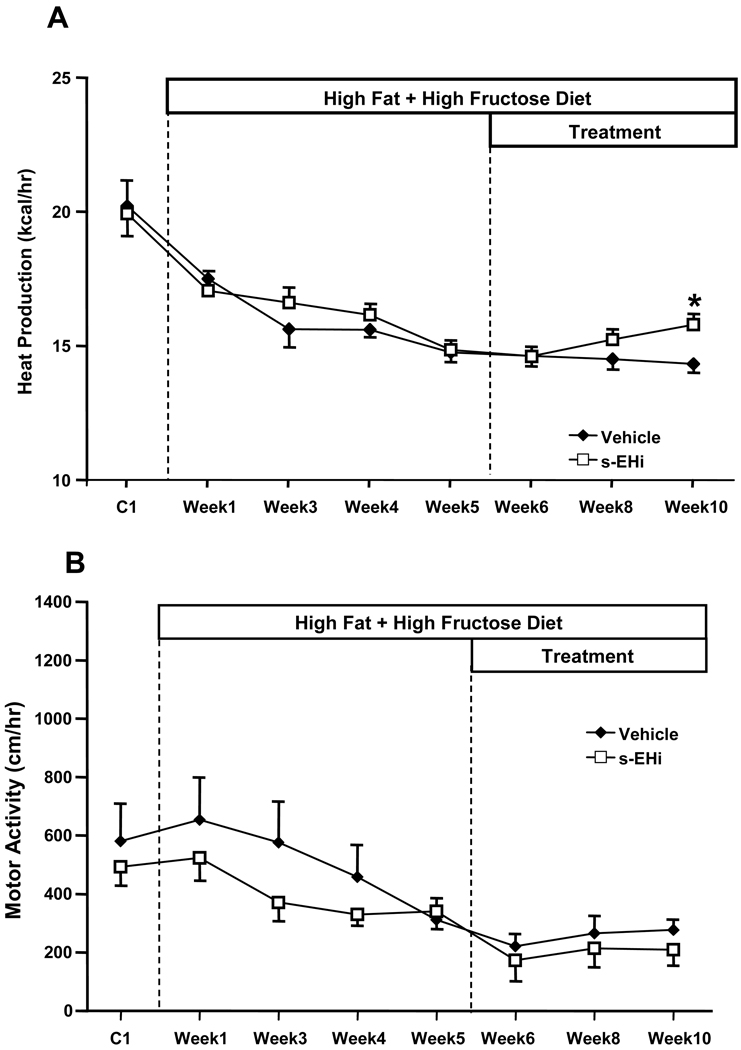
Effects of high fat-high fructose diet plus daily gavage with vehicle or s-EH inhibitor (s-EHi, AR9281) on (A) heat production and (B) motor activity in wild-type mice.
Effects of chronic s-EH inhibition on caloric intake and body weight
Chronic inhibition of s-EH with the s-EH inhibitor AR9281 for 5 weeks reduced caloric intake by ~32%, which may have contributed to the significant weight loss compared to the last week of HFD before treatment was started (Figures 1A and 1C). Despite a slight reduction in caloric intake no significant weight loss was observed in vehicle-treated animals. The greater effect of s-EH inhibition to reduce caloric intake compared to vehicle treatment is more evident when analyzing the net cumulative food intake during the 5 weeks of treatment (Figure 1D), which expresses the sum of the daily difference in food intake after treatment was started minus the average food intake on week 5 of the HFD. These results suggest that s-EH inhibition causes a small but important reduction in caloric intake and promotes weight loss.
Effects of s-EH inhibition on VO2, CO2, RQ and motor activity
s-EH inhibition for 5 weeks caused a 17% increase in VO2 (50.2 ± 1.5 vs 42.8 ± 1.4 ml/kg/min - Figure 2A). In addition to increasing VO2, s-EH inhibition also caused a proportional increase in VCO2 compared to vehicle treatment (Figure 2B). Accordingly, there was no significant difference in respiratory quotient (RQ, expressed as VCO2/VO2) between the groups (Figure 2C). s-EH inhibition also increased heat production by approximately 20% when compared to the vehicle-treated group (Figure 3A), but did not alter motor activity (Figure 3B).
Effects of s-EH inhibition on circulating hormones and plasma glucose levels
s-EH inhibition and vehicle treatment significantly decreased plasma glucose levels (Table 1). s-EH inhibition significantly reduced plasma leptin levels compared to the week before treatment was started, while no significant changes were observed in the vehicle-treated group (Table 1). Although, insulin levels were slightly reduced at the end of the treatment period in both groups, these reductions did not reach statistical significance (Table 1).
Table 1.
Plasma leptin, insulin and glucose concentrations during HFD and HFD plus daily oral gavage with vehicle or s-EH inhibitor (s-EHi, AR9281) in wild-type mice.
| Plasma Leptin (ng/mL) |
Plasma Insulin (µU/mL) |
Plasma Glucose (mg/dL) |
|
|---|---|---|---|
| Vehicle (n=8) | |||
| HFD – Week 5 | 36.3 ± 5.5 | 69.1 ± 9.4 | 310 ± 12 |
| HFD+TT – Week 10 | 44.0 ± 9.3 | 47.8 ± 9.1 | 242 ± 14# |
| s-EHi (n=10) | |||
| HFD – Week 5 | 41.9 ± 4.8 | 56.5 ± 12.6 | 320 ± 11 |
| HFD +TT – Week 10 | 33.4 ± 3.9# | 42.2 ± 7.7 | 227 ± 11# |
HFD = high fat diet; TT = treatment with vehicle or s-EHi. Data expressed as means ± SEM.
p<0.05 compared to HFD – Week 5.
Effect of s-EH inhibition on visceral fat content and adipocyte size
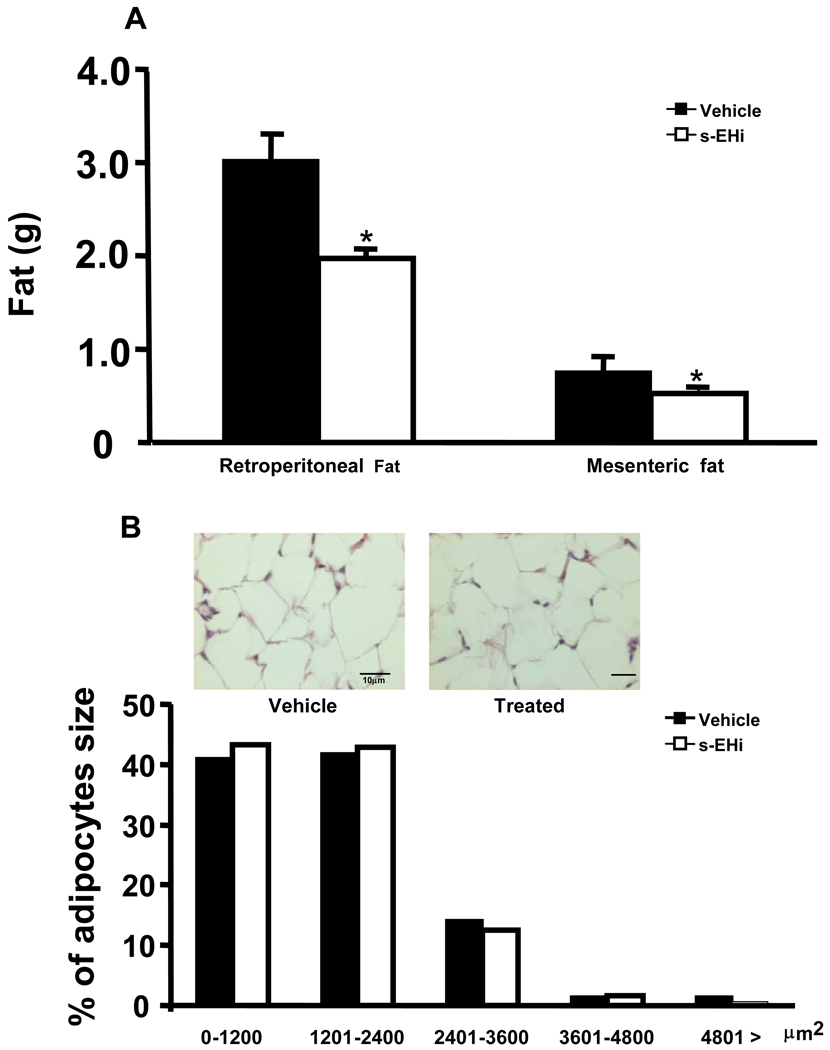
Visceral fat content was significantly reduced by s-EH inhibition compared to vehicle treatment (retroperitoneal, 1.97 ± 0.14 vs. 3.02 ± 0.30 g; and mesenteric fat, 0.52 ± 0.04 vs. 0.76 ± 0.08 g) (Figure 4A). No significant differences in adipocyte size were observed between groups (Figure 4B).
Figure 4.
Effects of high fat-high fructose diet plus daily gavage with vehicle or s-EH inhibitor (s-EHi, AR9281) on (A) visceral adiposity and (B) motor activity (cm/hr) in wild-type mice.
Effect of s-EH inhibition on UCP1 and UCP3 levels in BAT
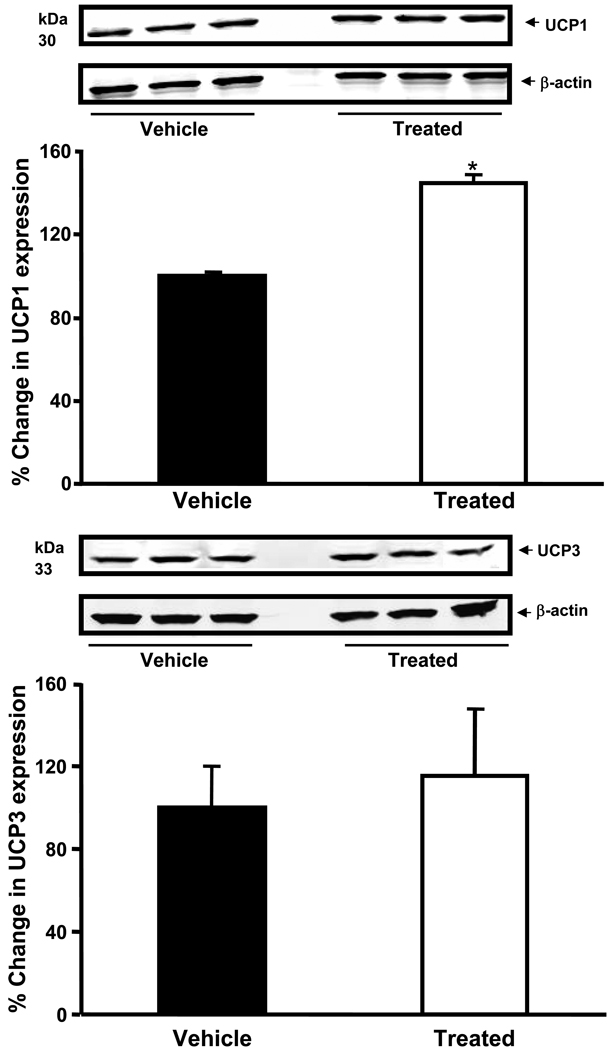
Interscapular BAT UCP1 protein level was elevated by ~50% after s-EH inhibition compared to vehicle treatment (Figure 5A). However, no significant difference was found in the UCP3 content between s-EHi and vehicle-treated groups (Figure 5B).
Figure 5.
Effects of high fat-high fructose diet plus daily gavage with vehicle or s-EH inhibitor (s-EHi, AR9281) on (A) uncoupling protein 1 (UCP-1) and (B) uncoupling protein-3 (UCP-3) in wild-type mice. Values are expressed as percentage change in UCP 1 and 3 expressions compared to vehicle-treated group. * p<0.05 compared to Vehicle on Week 10.
DISCUSSION
The key findings of this study are that s-EH inhibition decreased caloric intake, body weight, abdominal fat, and plasma leptin levels in mice fed a HFD compared to the vehicle group. In addition, s-EH inhibition increased whole body VO2, VCO2 and energy expenditure. Our findings suggest that s-EH inhibition promotes weight loss by increasing energy expenditure and reducing appetite, and we speculate that this class of drugs may provide a novel pharmacological therapy for the treatment of metabolic syndrome.
Mice fed high fat-high fructose diet developed obesity and many characteristics of the metabolic syndrome including increased body weight, increased adiposity, hyperglycemia, hyperinsulinemia and hyperleptinemia. s-EH inhibition stopped the weight gain associated with the HFD and caused a gradual reduction in body weight. This effect of s-EH inhibition to cause weight loss was accompanied by a reduction in caloric intake. The mechanisms by which s-EH inhibition alters appetite regulation are still unknown, but it is clear from our results that the ability of s-EH inhibition to reduce caloric intake plays an important role in mediating the weight loss. Whether the appetite suppressant actions of s-EH inhibition are mediated via direct effects of increased EETs levels in hypothalamic and/or brainstem nuclei involved in the control of food intake, or indirectly via activation of gastrointestinal (GI) nerve afferents that converge to the brainstem or by altering the secretion of GI peptides involved in meal termination such as cholecystokinin or peptide YY is unclear and will require additional studies.
Another potential mechanism that may contribute to some of the effects of chronic s-EH inhibition is activation of AMP-activated protein kinase (AMPK). Recent studies indicate that arachidonic acid epoxygenase metabolites activate AMPK which acts as a sensor of cellular energy status and may contribute to the effects of EETs-mediated improvement of diabetes and insulin resistance (24). Thus, it is possible that the improvement of insulin resistance and weight loss after s-EH inhibition may be due, at least in part, to increased activation of AMPK signaling. Furthermore, s-EH inhibition increases UCP1 content which may contribute to the augmented energy expenditure and also activate the AMPK signaling. Previous studies have shown that mice with overexpression of the uncoupling proteins appear to have persistent activation of AMPK signaling (20). However, the role of AMPK in mediating the metabolic actions s-EH inhibition still remains to be tested.
The reduction in body weight and amelioration of the metabolic changes observed with s-EH inhibition in mice fed a HFD may also be due, in part, to increased energy expenditure. s-EH inhibition caused a small but significant increase in whole body oxygen consumption, without altering motor activity, compared to vehicle treatment. Thus, it appears that a combined effect of the s-EH inhibitor to reduce caloric intake and increase energy expenditure may lead to weight loss in this dietary model of obesity and metabolic syndrome.
As mentioned, previously, one factor that could contribute to increased energy expenditure in the absence of increased motor activity is elevated levels of UCPs. Previous studies have shown that UCP1, a mitochondrial transmembrane protein that dissipates the proton gradient generated by oxidative phosphorylation, enhances energy expenditure and plays an important role in non-shivering thermogenesis. UCP1 is increased in rodents fed highly palatable diets or high-fat diets and increased UCP1 level is associated with increased energy expenditure and BAT hyperplasia (1,3,7,13,17). Therefore, we also investigated whether s-EH inhibition alters UCP1 and UCP3 levels in thermogenic BAT. Although we found no effect of s-EH inhibition on UCP3 levels, UCP1 in interscapular BAT of mice fed a HFD was significantly increased by the s-EH inhibitor compared to vehicle treatment. Thus, our results suggest that s-EH inhibition increases UCP1 content even when UCP1 levels are already elevated due to a HFD, and that enhanced UCP1 activity may contribute to the augmented energy expenditure caused by s-EH inhibition. Since UCP1 activity is regulated mainly by sympathetic activity, it is also possible that s-EH inhibition may increase sympathetic activity to BAT. However, whether s-EH inhibition increases UCP1 levels by stimulating sympathetic outflow to BAT remain to be tested.
Recent observations suggest that inhibition of s-EH may also modulate glucose homeostasis, blood pressure and improve inflammatory disorders (2,14,15,16). However, there is little information on the effects of s-EH inhibition on metabolism and circulating hormones. In the present study, treatment with s-EH inhibitor or vehicle improved insulin sensitivity, evaluated by changes in fasting glucose and insulin levels, in parallel with reductions in body weight. Whether these effects on insulin and glucose were due to the weight loss or to other effects of s-EH inhibition is unclear, although mild weight loss has been shown to enhance insulin sensitivity in obese humans (18,21). Despite similar improvements in glucose and insulin levels, we observed a reduction in leptin levels only in mice treated with s-EH inhibitor. This may be explained by the fact that s-EH inhibition also caused a greater reduction in visceral fat content compared to vehicle treatment. Another possible explanation for reduced retroperitoneal fat pad mass and no change in adipocyte size is that s-EH inhibition could have a proapoptotic function in adipocytes. However, additional experiments are needed to address this question.
Regardless of the precise mechanisms involved, our observations indicate that s-EH inhibition may have beneficial actions, including reductions in appetite, increased energy expenditure and reduced visceral fat, that reverse some components of the metabolic syndrome. Since our main goal in the present study was to determine the actions of s-EH inhibitor on food intake, energy expenditure and other metabolic parameters on mice fed a high fat high-fructose diet we chose mice fed a HFD + vehicle as the best appropriate control for the treated group. In addition, each animal also served as its own control since we measured these parameters before starting the HFD and also after 5 weeks on the diet before treatment was initiated.
In summary, s-EH inhibition for 5 consecutive weeks in mice fed a HFD caused a greater decrease in caloric intake, body weight, visceral fat, and plasma leptin levels compared to vehicle-treated mice. In addition, the s-EH inhibitor increased VO2, VCO2, energy expenditure and UCP1 content when compared to vehicle group, while no differences were found in RQ and motor activity. These observations indicate that the s-EH inhibition has several beneficial metabolic effects in a model of obesity and metabolic syndrome produced by feeding a high fat-high fructose diet.
ACKNOWLEGMENT
We thank Haiyan Zhang for measuring plasma insulin and leptin concentrations.
GRANTS
The authors’ research was supported by a National Heart, Lung, and Blood Institute grant PO1HL-51971. Alexandre A. da Silva is a recipient of a Scientist Development Grant from the American Heart Association.
This study was also supported by grant from Arete Therapeutics Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bukowiecki L, Collet AJ, Follea N, Guay G, Jahjah L. Brown adipose tissue hyperplasia: a fundamental mechanism of adaptation to cold and hyperphagia. Am J Physiol Endocrinol Metab. 1982;242:E353–E359. doi: 10.1152/ajpendo.1982.242.6.E353. [DOI] [PubMed] [Google Scholar]
- 2.Campbell WB. New role for epoxyeicosatrienoic acids as anti-inflammatory mediators. Trends Pharmacol Sci. 2000;21:125–127. doi: 10.1016/s0165-6147(00)01472-3. [DOI] [PubMed] [Google Scholar]
- 3.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 4.Catella F, Lawson JA, Fitzgerald DJ, Fitzgerald GA. Endogenous biosynthesis of arachidonic acid epoxides in humans: increased formation in pregnancy-induced hypertension. Proc Natl Acad Sci USA. 1990;87:5893–5897. doi: 10.1073/pnas.87.15.5893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dorrance AM, Rupp N, Pollock DM, Newman JW, Hammock BD, Imig JD. J Cardiovasc Pharmacol. 2005;46:842–848. doi: 10.1097/01.fjc.0000189600.74157.6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fisslthaler B, Rudiger P, Kiss L, Potente M, Harder DR, Fleming I, Busse R. Cytocrome P450 is an EDHF synthase in coronary arteries. Nature. 1999;401:493–497. doi: 10.1038/46816. [DOI] [PubMed] [Google Scholar]
- 7.Himms-Hagen J. Brown adipose tissue thermogenesis: interdisciplinary studies. FASEB J. 1990;4:2890–2898. [PubMed] [Google Scholar]
- 8.Huang H, Morisseau C, Wang J, Yang T, Falck JR, Hammock BD, Wang MH. Increasing or stabilizing renal epoxyeicosatrienoic acid production attenuates abnormal renal function in hypertension in obese rats. Am J Physiol Renal Physiol. 2007;293:F342–F349. doi: 10.1152/ajprenal.00004.2007. [DOI] [PubMed] [Google Scholar]
- 9.Imig JD, Zhao X, Capdevila JH, Morisseau C, Hammock BD. Soluble epoxide hydrolase inhibition lowers blood pressure in angiotensin II hypertension. Hypertension. 2002;39:690–694. doi: 10.1161/hy0202.103788. [DOI] [PubMed] [Google Scholar]
- 10.Imig JD, Zhao X, Zaharis CZ, Olearczyk JJ, Pollock DM, Newman JW, Kim I, Watanabe T, Hammock BD. An orally active epoxide hydrolase inhibitor loweres blood pressure and provides renal protection in salt-sensitive hypertension. Hypertension. 2005;46:975–981. doi: 10.1161/01.HYP.0000176237.74820.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Imig JD. Eicosanoids and renal damage in cardiometabolic syndrome. Expert Opion Drug Metab Toxicol. 2008;4:165–174. doi: 10.1517/17425255.4.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Imig JD. Eicosanoid regulation of the renal structure. Am J Physiol Renal Physiol. 2000;279:F965–F981. doi: 10.1152/ajprenal.2000.279.6.F965. [DOI] [PubMed] [Google Scholar]
- 13.Inokuma KI, Okamatsu-Ogura Y, Omachi A, Matsushita Y, Kimura K, Yamashita H, Saito M. Indispensable role of mitochondrial UCP1 for antiobesity effect of β3-adrenergic stimulation. Am J Physiol Endocrinol Metab. 2006;290:E1014–E1021. doi: 10.1152/ajpendo.00105.2005. [DOI] [PubMed] [Google Scholar]
- 14.Node K, Huo Y, Ruan X, Yang B, Spieker M, Ley K, Zeldin DC, Liao JK. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science. 1999;285:1276–1279. doi: 10.1126/science.285.5431.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olearczyk JJ, Quigley JE, Yamamoto T, Kim IH, Newman JW, Luria A, Hammock BD, Imig JD. Administration of a substituted adamantly urea inhibitor of soluble epoxide hydrolase protects the kidney from damage in hypertensive Goto-Kakizaki rats. Clin Sci. 2009;116:61–70. doi: 10.1042/CS20080039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev. 2002;82:131–185. doi: 10.1152/physrev.00021.2001. [DOI] [PubMed] [Google Scholar]
- 17.Rothwell NJ, Stock MJ. A role for brown adipose tissue in diet-induced thermogenesis. Nature. 1979;281:31–35. doi: 10.1038/281031a0. [DOI] [PubMed] [Google Scholar]
- 18.Sarafidis PA, Whaley-connell A, Sowers JR, Bakris GL. Cardiometabolic syndrome and chronic kidney disease: What is the link? J Cariometab Synd. 2006;1:58–65. doi: 10.1111/j.0197-3118.2006.05470.x. [DOI] [PubMed] [Google Scholar]
- 19.Sinal CJ, Miyata M, Tohkin M, Nagata K, Bend JR, Gonzales FJ. Target disruption of soluble hydrolase reveals a role in blood pressure regulation. J Biol Chem. 2000;275:40504–40510. doi: 10.1074/jbc.M008106200. [DOI] [PubMed] [Google Scholar]
- 20.Towler M, Hardie DG. AMP-activated protein kinase in metabolilc control and insulin signaling. Cir Res. 2007;100:328–341. doi: 10.1161/01.RES.0000256090.42690.05. [DOI] [PubMed] [Google Scholar]
- 21.Walsh SJ, Dauser-Forrest D. Correlates of weight loss in persons with metabolic syndrome. Prev Med. 2009;15:115–126. doi: 10.1016/j.ypmed.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 22.Whitcomb R, Chen D, Wang Y-X, Anandam S-K, Gless R, Webb HK. AR9281, a Soluble Epoxide Hydrolase Inhibitor - Efficacy in a DIO Mouse Model plus Pharmacokinetics and Pharmacodynamics in Mice and Men. Diabetes. 2009;58 Suppl 1:A165. 612-P. [Google Scholar]
- 23.Wong K, Zhang L-N, Vincelette J, Chen D, Tran V, Mehra U, Gless R, Anadan S-K, Webb HK, MacIntyre E, Wang Y-X. A Novel Inhibitor of Soluble Epoxide Hydrolase, AR9281, Improves Glucose Homeostasis in Diet-Induced Obese Mice. Diabetes. 2009;58 Suppl 1:A436. 1698-P. [Google Scholar]
- 24.Xu X, Zhao CX, Wang L, Tu L, Fang X, Zeng C, Edni ML, Zeldin DC, Wang DW. Increased CYP2J3 expression reduces insulin resistance in fructose-treated rats and db/db mice. Diabetes. 2010;59:997–1005. doi: 10.2337/db09-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao X, Yamamoto T, Newman JW, Kim I, Watanabe T, Hammock BD, Stewart J, Pollock JS, Pollock DM, Imig JD. Soluble epoxide hydrolase protects the kidney from hypertension-induced damage. J Am Soc Nephrol. 2004;15:1244–1253. [PubMed] [Google Scholar]