Abstract
We report a prospective phase II clinical trial in 35 adult patients (median age 40.5 years) with hematologic malignancies who received T-cell depleted, hematopoietic stem cell transplants from HLA-compatible, unrelated donors. The cytoreductive regimen consisted of hyperfractionated total body irradiation, thiotepa, and fludarabine. The preferred graft source was G-CSF-mobilized peripheral blood stem cells (PBSC). PBSC were CD34+ selected, followed by sheep erythrocyte rosetting to deplete residual T cells. Antithymocyte globulin provided graft rejection prophylaxis. No additional graft versus host disease (GvHD) prophylaxis was planned. Estimated disease free survival at 4 years is 56.8% for the entire group and 75% in patients with standard risk disease. The cumulative incidence of relapse is 6%. Acute GvHD grade II-III developed in 9% and chronic GvHD in 29% of patients. Fatal infections occurred in 5 of 35 (14%) patients. There was one late graft failure. This study demonstrates durable engraftment with a low overall incidence of GvHD. Its curative potential is reflected in the remarkably low relapse rate at 4 years.
INTRODUCTION
Several studies have demonstrated the efficacy of TCD allogeneic bone marrow (BM)1-3 and TCD peripheral blood stem cell transplants (PBSCT)4,5 from matched related donors (MRD) in patients with hematologic malignancies. In these reports, reduction in the incidence and severity of acute and chronic GVHD, compared with T-cell replete transplantation, has not compromised the anti-tumor effect of the allograft. Furthermore, a 5 year followup of patients receiving TCD sibling transplants reported excellent performance status and quality of life.6 In contrast, TCD transplants from unrelated donors have been less well studied.3,7,8
Although the addition of antithymocyte globulin (ATG) addressed an early unacceptable rate of immune-mediated graft rejection and provided additional GVHD prophylaxis beyond that of TCD alone 9,10, it has resulted in delayed immune recovery.11 We reported recently that conditioning with hyperfractionated total body irradiation (HFTBI), thiotepa, and fludarabine in TCD PBSCT using MRD eliminated the need for ATG without increasing graft rejections. Furthermore, immune reconstitution improved with a reduction in opportunistic infections (OI).5
In order to extend curative transplantation options to patients without a MRD and to an older population at increased risk of GVHD, we utilized this regimen for matched or mismatched unrelated donor transplants. Only 2 doses of ATG were administered and TCD was performed by automated CD34+ stem cell selection followed by rosetting with sheep red blood cells (sRBC). We report the results of a trial in 35 patients which evaluated the impact of this approach on transplant related morbidity and mortality in patients with hematologic malignancies .Secondary endpoints were to estimate disease-free and overall survival. We sought also to determine if patients could achieve consistent engraftment with durable relapse-free survival and whether T-cell reconstitution could be improved with a low incidence of OIs.
MATERIALS and METHODS
Patient characteristics
Thirty-five adult patients with a variety of malignant hematologic diseases and treatment backgrounds were enrolled on this MSKCC Institutional Review and Privacy Board-approved phase II trial from July 1, 2001, to December 31, 2005, after obtaining informed consent. Analysis was performed as of December 31, 2008, after which there were no censored events. Eligibility included low level of disease or remission, availability of ≥7/10 HLA matched, unrelated donor, Karnofsky performance status (KPS) ≥70; no active infection or extramedullary disease, and satisfactory organ function as previously described. 5
Only those patients with intermediate or high risk AML based on cytogenetics 12, and with ALL high risk cytogenetics [generally t(9;22) with p190 bcr-abl or t(4;11)] underwent transplantation in CR1. Disease status at transplant determined standard or poor risk classification: AML-CR1, -CR2, ALL-CR1, or CML-first chronic phase were standard risk, all others were poor risk.5 HLA matching for -A, -B, -C, -DRB1, and -DQB1 loci was established using DNA sequence-specific oligonucleotide probes. Donors were identified and recruited via the National Marrow Donor Program (NMDP) registry.
Preparative regimen and graft
The cytoreduction consisted of HFTBI followed by thiotepa, ATG, and fludarabine5. HFTBI was administered in 11 fractions of 125 cGy over 4 days, to a total dose of 1,375 cGy. All patients had protective lung shielding after an initial 800cGy, and overlying ribs received an additional 600 cGy boost. Male patients with acute leukemia or lymphoma received an additional 400 cGy testicular boost in a single fraction After completion of HFTBI, thiotepa 5 mg/kg/day was administered over 4 hours on each of 2 consecutive days, with no adjustment for weight. Fludarabine 25 mg/m2/day was administered over 30 minutes for 5 days, beginning on the first day of thiotepa. Patients received 2 doses of equine (60 mg/kg total) or rabbit (5 mg/kg total) ATG divided over the same 2 days as the thiotepa. The source of rabbit ATG was Sangstat until 2003 and there after Genzyme.
Twenty-nine donors underwent G-CSF mobilization of PBSC according to NMDP guidelines. Targeted cell dose was 109 MNC/kg (3 × 106 CD34+/kg) of recipient weight. CD34+ cells were positively selected using the ISOLEX 300i Magnetic Cell Selection System, followed by sRBC-rosette depletion of T cells 5. This achieved an approximate 5 log10 depletion of CD3+ cells. 13 Six donors elected BM harvesting with TCD accomplished by sequential soybean lectin agglutination and sRBC -rosette depletion (SBA-E-) 14. Fresh grafts were infused through a central venous catheter 24-48 hours after completing fludarabine.
GVHD evaluation and management
GVHD was diagnosed clinically and confirmed by biopsy whenever possible. , Patients, who engrafted and survived >30 days, were evaluable for acute GVHD, unless it had already been diagnosed before a terminal event. Scoring was based on CIBMTR criteria15. Patients surviving >100 days were evaluable for chronic GVHD using the Sullivan scoring criteria.16
Supportive care
Patients were managed clinically according to MSKCC standard guidelines 5 including infection prophylaxis for Pneumocystis carinii, Herpes viruses, and fungus. Patients who were seropositive for Toxoplasma gondii or whose donors were seropositive, also received atovaquone prophylaxis after transplantation. Patients who were cytomegalovirus (CMV) negative received seronegative blood products regardless of the donor’s serologic status. If the patient or donor was seropositive, CMV-specific prophylaxis was administered beginning when the absolute neutrophil count (ANC) was self-sustaining > 2000 cells/ul and continuing through d+100. This consisted of maintenance dosing of valganciclovir as peripheral blood counts tolerated and maintenance foscarnet dosing if they did not. Monitoring of CMV reactivation by CMV pp65 antigenemia assay of peripheral blood was performed regularly when either the patient or donor was CMV seropositive, generally once per week during the first 100 days. Epstein-Barr virus (EBV) was monitored similarly by qualitative PCR until 2003 and thereafter by real-time PCR of the BNRF1-p143 locus (Roche Inc) 17.
Prophylactic antibacterial agents were not used until 2005. At that time, the practice of administering vancomycin prophylaxis against Streptococcus viridans at the development of neutropenia or no later than d -2 was initiated. This practice affected 4 patients on this study. Finally patients received G-CSF beginning ≥ d+7, if clinically indicated.
Engraftment, donor chimerism, and immune reconstitution
Standard engraftment criteria were used ANC ≥500/uL without G-CSF support and platelets ≥20,000/uL without transfusion for ≥3 consecutive days. BM aspirates were monitored for disease status and donor chimerism.
Immunophenotyping and T cell proliferative responses to phytohemagglutinin (PHA) mitogen were evaluated every 3-6 months until normal.18 Life-threatening infections were defined as organ-localized infections due to viral, fungal, and/or parasitic pathogens as previously described.5
Biostatistics
Disease free survival (DFS), defined as the interval from transplantation to death, hematological or clinical relapse, or last follow-up, was estimated using the method of Kaplan-Meier. Estimates of the probability of relapse and non-relapse mortality (NRM) were calculated with the cumulative incidence function.19 Wilcoxon rank sum test was used to compare immune reconstitution results between groups of patients. Primary cause of death was determined by previously published criteria20.
RESULTS
Patient and donor characteristics
Table 1 details patient and donor characteristics. Median patient age was 40.5 years, with 10 patients ≥50 years. Eighteen patients were classified as standard risk and 17 were poor risk. Two BM grafts arrived in poor condition and could only be processed with soybean agglutination. This necessitated short term calcineurin inhibitor therapy with these grafts which contained 2.3 and 2.6 × 106CD3+/kg (1-2 logs higher than the CD3+ dose in other grafts). Eighteen donors were HLA disparate at 1-3 of 10 loci (Table 1). Four patients received 2 doses of rabbit ATG by physician choice. Two patients received 1 dose of rabbit and 1 dose of horse ATG due to a reaction during the first dose.
TABLE 1.
PRETRANSPLANT DEMOGRAPHICS AND DISEASE STATUS
| Total # Pts | 35 |
| KPS – range (median) | 70-100 (90) |
| M:F | 21-14 |
| Age – range (median) | 18.2-62.8 (40.5)yr |
| Diseases | |
| AML CR1 | 6 |
| Intermediate Risk* | 3 |
| High Risk | 3 |
| AML CR2 | 10 (1 after MDS) |
| ALL CR1 | 3 |
| ALL CR2 | 7 |
| ALL CR≥3 | 3 |
| AL (other) | 2 (1 CR, 1 extramedullary relapse) |
| CML CP1 | 1 |
| MDS | 2 (1 RA- Intermed IPSS, I RAEB) |
| T-PLL | 1 |
| Donors | |
| Matched: Mismatched | 17:18 |
| M → F | 9 |
| M → M | 12 |
| F → M | 7 |
| F→ F | 7 |
| Age – range (median) | 19-54 (36) yr |
| Grafts | |
| PBSC | 29 |
| BM | 6 |
| HLA Disparity for MM Transplants | 18 |
| 9/10 1 antigen A;C | 2;2 |
| 1 allele A;C;DQ | 2;1;1 |
| 8/10 2 antigen A,C;A,DQ;B,C | 1;1;2 |
| 1 antigen+1 allele A,DQ;A,C; | 1;2 |
| C,DR;DR,DQ | 1;1 |
| 7/10 1 antigen+2 alleles | |
| A,C,DQ | 1 |
AML indicates acute myelogenous leukemia; ALL, acute lymphocytic leukemia; AL, acute leukemia multilineage; CML, chronic myelogenous leukemia; MDS, myelodysplastic syndrome; RA, refractory anemia; RAEB-IT, refractory anemia with excess blasts in transformation; T-PLL, T cell prolymphocytic leukemia; CP, chronic phase; CR, complete remission;
by cytogenetics; KPS, Karnofsky performance status.
Engraftment and donor chimerism
All 34 evaluable patients engrafted neutrophils. One infectious death on day +5 was inevaluable. CD34+ and CD3+ cell dose engraftment data are summarized in Table 2. The median CD34+ dose was within the range targeted by the protocol (3 × 106 CD34+cells/kg). Ten grafts contained less than the targeted dose, half were the TCD BMs. The patient who received the lowest CD34+ dose (TCDBM, 0.8 × 106/kg) exhibited delayed engraftment and required a BM boost. Three of 34 evaluable patients died before achieving platelets >20,000/ul.
TABLE 2.
OUTCOME DATA
| Median Follow-up (range) | 52 (37-83.1)mns |
| Engraftment | 34 (evaluable) |
| Median transplant dose CD34+×106/kg (range) | 3.9 (0.80-11.57) |
| Median transplant dose CD3+×103/kg (range) | 1.52 (0-259) |
| Patients engrafting neutrophils | 34 |
| Median days to ANC ≥ 500/uL (range) | 12 (9-19) days |
| Patients engrafting platelets | 32 |
| Median days to platelets ≥ 20,000/uL (range) | 19 (14-108) days |
| Post Transplant EBV-LPD | 3 |
| GVHD | |
| Acute (overall grade) | 2/34 (1M:1MM) |
| II | 1 (1M) |
| IV | 1 ( 1MM) |
| Chronic | 8/28 (5M:3MM) |
| Limited | 5 (2M: 3MM) |
| Extensive | 3 (3M) |
| Relapses | 2 |
| ABL > CR2 (w/extramedullary disease) | 1 |
| ALL CR2 | 1 |
| Deaths | 14 |
| Matched: Mismatched | 6 : 8 |
| Causes of Death | |
| Infection | 6 |
| Bacterial | 2 |
| Viral (adenovirus) | 2 |
| Toxo/fungal | 1/1 |
| EBV-LPD and secondary complications | 1 |
| Post 2nd transplant complications | 1 |
| GVHD and secondary complications | 3 |
| Late GF | 1 |
| IP | 1 |
| VOD | 1 |
| 100 day NRM | 20% (2M : 5MM) |
| Bacterial | l |
| Viral (adenovirus) | 2 |
| Toxo: bacterial | 1 : 2 |
| VOD | 1 |
| Late GF | 1 |
Mns indicates months; ul, microliter; EBV-LPD, Epstein Barr virus-lymphoproliferative disease; M, matched; MM, mismatched; ABL, acute biphenotypic leukemia; ALL, acute lymphocytic leukemia; CR, complete remission; GVHD, graft versus host disease; toxo, toxoplasmosis; GF, graft failure; IP, interstitial pneumonitis; VOD, venooclusive disease.
One patient with ALL CR2 rejected a 9/10 HLA matched PBSCT. Day +30 evaluation revealed only 55% donor chimerism with multiple new chromosomal abnormalities. This pt died from complications following a second transplant. Thirty of the 34 evaluable patients achieved full donor chimerism in BM by d+100. Four were not evaluated.
At 1 year, 21 of 26 evaluable patients were studied: 19 had full donor chimerism, 2 were ≥ 80% donor. One improved with an infusion of donor lymphocytes (DLI), the other spontaneously. Within 24 months posttransplantation (the last evaluation required by protocol), 21 of 25 evaluable patients studied demonstrated ≥95% donor chimerism. The 1 patient with an HLA matched donor who received low dose DLI for mixed chimerism did not develop GVHD.
GVHD
The median CD3+dose for the 35 patients was 1.52 × 103 CD3+ cells/kg, far below the threshold of 1 × 105 CD3+cells/kg for developing acute GVHD determined by limiting dilution outgrowth using TCD BM from HLA-identical siblings 21. Thirty-four of the 35 patients were evaluable for acute GVHD (Table 2). The incidence of grade II-IV was 5.8%. One of these patients, with an HLA-matched donor, received the highest T cell dose (22.8 × 103CD3+/kg) among the TCD PBSC grafts. The second patient received a mismatched BM allograft containing 15.7 × 103CD3+/kg. Their grade IV lower GI GVHD responded quickly to steroid therapy.
Twenty-eight patients were evaluable for chronic GVHD. Five developed limited and 3 extensive. Three previously had acute GVHD. Incidence was similar in those with matched (18%) vs. mismatched (11%) donors (7-8/10 matches). One patient required calcineurin inhibitor prophylaxis because of the higher residual T cell content in the graft. Three patients died from complications of chronic GVHD. The overall incidence of chronic GVHD was 29%, with only 11% extensive.
DFS and relapse
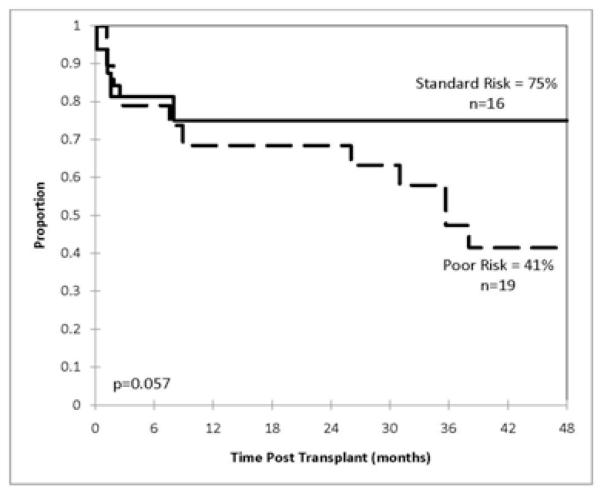
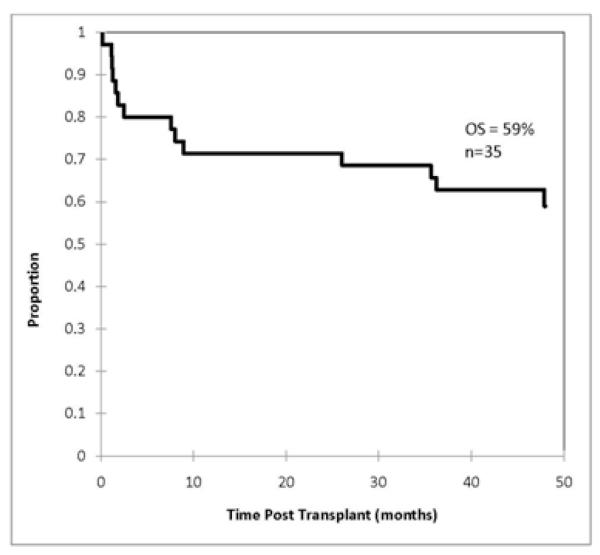
Patients had a variety of diagnoses and treatment histories, and were almost equally divided between ‘standard’ (n=18) and ‘poor’ (n=17) risk disease (Table 1). Estimated probabilities of DFS and overall survival (OS) at 4 years for the entire group were 57% and 59%, respectively (median followup 52 months). Figure 1 illustrates the probability of DFS at 4 years for patients following standard risk - 75%, compared to poor risk transplants – 41% (p=0.057). Figure 2 illustrates OS. DFS and OS for patients ≥ 40 years were not significantly different from that for younger patients. Ten patients were ≥50 years, 4 in the poor and 6 in the standard risk transplant groups. Three of the 10 died from early infections (adenovirus, toxoplasma, and VRE sepsis), 1 each from chronic GVHD, interstitial pneumonitis, and an unrelated gastrointestinal malignancy (at 72 months). No significant difference was observed for matched vs mismatched transplants in DFS and OS showed borderline significance (p=0.54), however, the number of patients is limited.
Figure 1.
Kaplan-Meier estimates of the probability of disease free survival at 4 years based on the status of disease.
Figure 2.
Kaplan-Meier estimates of the probability of overall survival at 4 years for all patients.
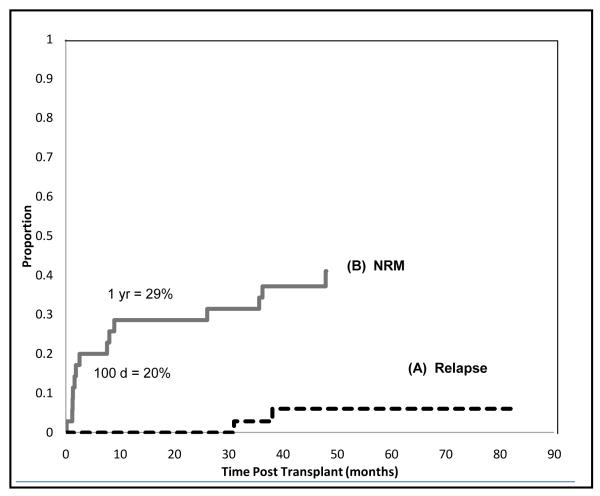
Cumulative incidence of relapse at 4 years is only 6% (Figure 3 (A)). Both patients who relapsed had poor risk disease: 1 with acute biphenotypic leukemia, who developed leukemia cutis during conditioning, relapsed at 38 months; the second with ALL CR2 relapsed at 31 months Both received second (T-replete) transplants and 1 survives in remission at 30 months.
Figure 3.
Cause specific analysis of relapse and mortality. (A) cause specific probability of relapse, adjusting for completing risks of treatment failure by nonrelapse causes (B) cause specific probability of death, adjusting for the competing risk of relapse.
Of the 18 patients who were HLA-mismatched, 4 donor-recipient pairs exhibited KIR ligand incompatibility. Of these, 3 pairs had KIR ligand incompatibility in a host-versus-graft vector, and only one pair had KIR ligand incompatibility in a graft-versus-host vector. For this one pair, however, donor KIR genotyping revealed lack of the relevant inhibitory KIR responsible for sensing the class I ligand lacking in the recipient. . All of the remaining HLA-mismatched patients were KIR ligand compatible.
Posttransplantation EBV-LPD
Three patients (8.5%) developed biopsy-confirmed EBV-LPD, at 3, 6, and 7 months. All recovered with therapy (1 rituximab, 1 rituximab + DLI, 1 EBV-specific cytotoxic T cells). The patient who received DLI from their C allele mismatched donor at 3 months eventually died from complications of GVHD. Death was attributed to complications of EBV-LPD.
Causes of death
Fourteen (40%) of the 35 patients died by the 4 year followup. Five had matched and 9 had mismatched donors. Causes of death are summarized in Table 2. Cause-specific probability of NRM, adjusted for the competing risk of relapse, is 20% at 100 days, and 29% at 1 year (Figure 3(B)).
Immune reconstitution and infections
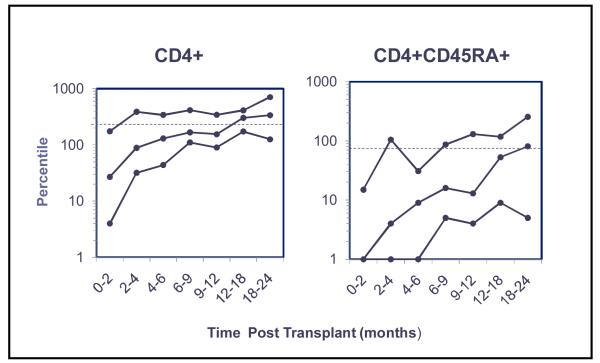
The median time to recovery of normal numbers of circulating CD8+ T cells and to >200 CD4+ T cells/uL was 4-6 and 6-9 months, respectively (Figure 4). Only 4 patients who received steroids within the first 12 months had persistently low CD4+ cells (<100 cells/uL) throughout the first 2 years. By 12-18 months, CD4+CD45RA+ cells recovered in 50% of patients, and the majority of patients’ PHA T cell proliferative responses reached the lower limit of normal (LLN). All patients developed normal numbers of CD56+CD16+ NK cells by 2 months after transplant (data not shown).
Figure 4.
T cell recovery. Shown are the 10-50-90th percentiles of CD4+ and CD45RA+ cells per microliter obtained over 24 months for 26 patients following peripheral blood stem cell transplantation on this study.
The 30 day absolute lymphocyte count (ALC), reported to predict outcomes in TCD HLA-matched sibling transplant patients with acute leukemia or CML22, was analyzed for the entire group. Median ALC at 30 days was 474 cells/uL. There was no difference in the incidence of death or relapse for patients with an ALC at 30 days above or below this median (data not shown). Thirty patients, who achieved a circulating lymphocyte count of 500 cells/uL, did so at a median time (range) of 19.5 (14 to 223) days.
Eleven of 35 patients developed a life-threatening OI due to adenovirus (n=5), EBV (n=3), fungus (n=1), toxoplasmosis (n=1), or Nocardia (n=1). Three of 16 patients at risk for CMV reactivation required treatment: 1 CMV colitis and 2 viremias. Three additional patients experienced nonfatal RSV infections and 1 atypical mycobacterium infection Five died of their OIs: 2 adenovirus, 1 each toxoplasmosis, EBV, and fungus. Four of these patients had received mismatched grafts.
DISCUSSION
This trial addresses the use of myeloablative TCD transplantation from HLA matched and mismatched unrelated donors with current day technology and practices. The goals were to provide effective therapy with a low rate of relapse, to facilitate T cell recovery, and to decrease regimen related toxicity. Despite the high risk nature of many of the transplants and modifications to the regimens, the study demonstrated promising results: only a 6% cumulative incidence of relapse, excellent engraftment, and low rates of GVHD. Confounding contributors to GVHD were incomplete TCD and use of DLI. Death due to infection occurred in 20% of patients, most in the peritransplant setting or in association with GVHD therapy.
Limited reports of TCD unrelated BM or PBSC grafts following myeloablation have been published7,23-26 Most studies utilized partial TCD together with immune suppressive medications25 post transplant. They reported slower immune recovery, a higher incidence of infectious complications, 18,27,28 and relapse in certain diseases compared with T-cell replete transplantation 29-31. Use of ATG has also been associated with similar complications. 1,32 The majority of studies, including the largest trial 33, described patients treated in the 1980’s-2000 using BM allografts. The current study addresses an older patient population, but incorporates improved supportive care, high resolution HLA typing, improved TCD technology, and PBSC graft source.
Studies suggest that higher doses of CD34+ cells facilitate engraftment and immune reconstitution.34-36 In this study, TCD PBSCs grafts had 5.75 ×106/kg median CD34+ cell doses, similar to T-cell replete PBSC37, and much higher than 1.2 × 106/kg for TCD BM grafts (historical, unpublished results) . Log 10 depletion of CD3+ in PBSC was 5.5 compared with 2.5-3 for BM. The level of TCD in this study is approximately 1/2 log greater than that achieved with the currently available CliniMACS device 38.
Median age of the patients was >10 years older than our earlier reports and similar to our recent study of TCD matched, related PBSC transplants.1,2,5 Distribution of diseases was representative of older patients and included many treated at advanced stages. Infectious complications reflect current day pathogens but notably, did not include Streptococcal bacteremias which were observed with this regimen previously5. Early initiation of antibiotics, for fevers during conditioning, may have avoided this complication.
This trial evaluated changes in the regimen and graft. Despite reducing the intensity of the conditioning, the OS and DFS for the 17 ‘standard’ risk patients was excellent, >70% and comparable to our previous results5 and those reported by others. The Kaplan-Meier survival curve for this group did not change after the 8th month. The OS and DFS for the ‘poor’ risk transplant group was 47% and 41%, respectively, again similar to those with T-cell replete grafts, but with less GVHD. Median follow up for this trial was >4 years, encompassing the only 2 relapses. The very low incidence of relapse argues against a loss of graft versus tumor effect with TCD. Deaths on this trial generally occurred within the first 6-8 months and were predominantly infection-related.
The low relapse rate in the setting of a TCD allograft invokes the possibility that NK cells are the primary mediator of the graft-versus-tumor effect. Classic NK alloreactivity due to killer Ig-like receptor (KIR)-mediated recognition of “missing self” occurs when MHC class I KIR ligands present in donor are lacking in the transplant recipient. Only 4 of the 18 HLA-mismatched donor-recipient pairs exhibited KIR ligand incompatibility and only 1 of these pairs had KIR ligand incompatibility in a graft-versus-host vector. For this 1 pair, however, donor KIR genotyping revealed lack of the relevant inhibitory KIR responsible for sensing the class I ligand lacking in the recipient; and thus, no NK alloreactivity due to recognition of missing self would occur. Donor NK alloreactivity due to missing self-MHC, therefore, could not account for the low relapse rate among these patients.
NK alloreactivity due to “missing ligand,” which we have previously described39, can occur when the patient is lacking any class I ligand for donor inhibitory KIR, irrespective of whether the ligand is present in the donor. This receptor-ligand mismatch occurred in 22 of the 35 pairs. Because the event of relapse is low in this small cohort, however, we cannot conclude that NK alloreactivity due to missing ligand is responsible for protection from relapse. A larger cohort would be necessary to confirm this.
A goal of the current study was to reduce the dosing of ATG and potentially improve immune recovery without increasing the incidence of graft rejection or GVHD. Based on previously identified predictors of graft rejection using TCD BM grafts9,40, more than half the patients were at increased risk. However, only 1 HLA mismatched graft recipient, in the poor risk group, experienced late graft failure. Enhanced immunosuppression provided by fludarabine coupled with the higher CD34+ dose achieved with PBSCs likely contributed to the high rate of durable engraftment. These results support further reduction in ATG dosing in future trials.
The TCD PBSC contained >1 log10 lower concentration of T cells compared with TCD BM grafts (median 1.2 vs 42.5 × 103/kg)14 and a 4 log10 lower concentration than T-cell replete PBSC grafts with similar CD34+ numbers . 37 However, the incidence and severity of GVHD in this study, is higher than that previously reported for our TCD BM and PBSC with matched related donors2,5, and for HLA haplotype disparate related donors reported by Aversa et al. 41 Factors contributing to the GVHD might include: 1) additional allogeneic disparities in unrelated and mismatched donors; 2) qualitatively different T cell and NK populations in TCD PBSC vs. TCD BM; and 3) the relatively low dose of ATG employed.
The goal of utilizing lower dose ATG was to improve T cell recovery and potentially reduce life-threatening OIs. Recovery of CD4 counts to ≥200 and normalization of CD8 T cells were similar for the TCD PBSC (6-9 months), compared with unrelated TCD BM (12-18 months) 18. CD4+CD45RA+ cell recovery was also faster than for unrelated TCD BM. Thus, despite including ATG, time to recovery of CD4+ and CD4+CD45RA+cell counts in this trial was similar to that for recipients of related TCD PBSC using identical cytoreduction but without ATG.5 Similarly, patients’ PHA response normalized faster on this trial than in recipients of TCD unrelated BM, who had a PHA response <25% LLN for the first year vs the median PHA response in this study of 60% LLN at 12 months, and normal by 18 months. Keever-Taylor et al. recently published the results of lymphoid recovery in patients transplanted on the National Heart, Lung and Blood Institute randomized TCD versus T-cell replete unrelated BM trial which included posttransplant immunosuppression.42 Despite the older age of the current study patients, recovery of CD4+ cells was similar and CD4+ CD45RA+ cells more rapid compared to that reported by Keever-Taylor et al. (median age 31.2 years).
Infection as the primary cause of death occurred in 17%. This is lower than the 29 and 30% incidence of fatal infection reported by Wagner et al. in recipients of T-cell replete or TCD unrelated stem cell transplants who received immunosuppressives for GVHD prophylaxis. 43 Furthermore, the higher incidence of deaths from CMV and fungus reported by van Burik et al. in TCD unrelated transplant recipients who also received posttransplant GVHD prophylaxis44 was not observed in this study. No patient died of CMV disease and only 1died of a fungal infection.
This study supports the use of TCD PBSC transplantation from both matched and mismatched unrelated donors in appropriately selected adult patients, especially those at increased risk of GVHD. Acknowledging the limited numbers of patients, the median survivals compare favorably with those reported by the NMDP for each disease group45. The risk of relapse was remarkably low, with a median followup of >4 years. The character of complications and death were similar to those observed with T-replete transplantation, but with a lower incidence and severity of GVHD. Adequately TCD PBSC from unrelated donors precludes the need for posttransplant GVHD prophylaxis, allowing more rapid recovery of functional immunity that reduces the risk of lethal opportunistic infections. It provides an alternative form of transplantation for patients without sibling donors including those who are older than 40 years.
Acknowledgments
We are grateful to Drs. Suzanne Wolden and Joachim Yahalom who oversaw the radiation therapy, to the Clinical Immunology and Cytotherapy Laboratory staff, and to Michelle Chiu, Molly Maloy and the research study assistants for coordination and collection of data.
Supported in part by P01 CA23766 from the National Cancer Institute, National Institutes of Health, The Aubrey Fund, The Tow Foundation, The Laura Rosenberg Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Young J, Papadopoulos E, Cunningham I, et al. T-cell-depleted allogeneic bone marrow transplantation in adults with acute nonlymphocytic leukemia in first remission. Blood. 1992;79:3380–3387. [PubMed] [Google Scholar]
- 2.Papadopoulos E, Carabasi M, Castro-Malaspina H, et al. T-cell-depleted allogeneic bone marrow transplantation as postremission therapy for acute myelogenous leukemia: Freedom from relapse in the absence of graft-versus-host disease. Blood. 1998;91:1083–1090. [PubMed] [Google Scholar]
- 3.Alyea EP, Weller E, Fisher DC, et al. Comparable outcome with t-cell depleted unrelated donor versus related donor allogeneic bone marrow transplantation. Biol Blood and Marrow Transplant. 2002;8:601–607. doi: 10.1053/bbmt.2002.v8.abbmt080601. [DOI] [PubMed] [Google Scholar]
- 4.Novitzky N, Thomas V, Hale G, Waldmann H. Myeloablative conditioning is well tolerated by older patients receiving t-cell-depleted grafts. Bone Marrow Transplant. 2005;36:675–682. doi: 10.1038/sj.bmt.1705119. [DOI] [PubMed] [Google Scholar]
- 5.Jakubowski AA, Small TN, Young JW, et al. T cell depleted stem-cell transplantation for adults with hematologic malignancies: Sustained engraftment of hla-matched related donor grafts without the use of antithymocyte globulin. Blood. 2007;110:4552–4559. doi: 10.1182/blood-2007-06-093880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Quan Le R, Bevans M, Savani BN, et al. Favorable outcomes in patients surviving 5 or more years after allogeneic hematopoietic stem cell transplantation for hematologic malignancies. Biology of Blood and Marrow Transplantation. doi: 10.1016/j.bbmt.2010.03.005. In Press, Accepted Manuscript. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wagner JE, Thompson JS, Carter SL, Kernan NA. Trial ftmotUDMT. Effect of graft-versus-host disease prophylaxis on 3-year disease-free survival in recipients of unrelated donor bone marrow (t-cell depletion trial): A multi-centre, randomised phase ii-iii trial. Lancet. 2005;366:733–741. doi: 10.1016/S0140-6736(05)66996-6. [DOI] [PubMed] [Google Scholar]
- 8.von dem Borne PA, Beaumont F, Starrenburg CW, et al. Outcomes after myeloablative unrelated donor stem cell transplantation using both in vitro and in vivo t-cell depletion with alemtuzumab. Haematologica. 2006;91:1559–1562. [PubMed] [Google Scholar]
- 9.Kernan N, Bordignon C, Heller G, et al. Graft failure after t-cell-depleted human leukocyte antigen identical marrow transplants for leukemia: I. Analysis of risk factors and results of secondary transplants. Blood. 1989;74:2227–2236. [PubMed] [Google Scholar]
- 10.Aversa F, Terenzi A, Carotti A, et al. Improved outcome with t-cell–depleted bone marrow transplantation for acute leukemia. J Clin Oncol. 1999;17:1545. doi: 10.1200/JCO.1999.17.5.1545. [DOI] [PubMed] [Google Scholar]
- 11.Small TN, Avigan D, Dupont B, et al. Immune reconstitution following t-cell depleted bone marrow transplantation: Effect of age and posttransplant graft rejection prophylaxis. Biol Blood and Marrow Transplant. 1997;3:65–75. [PubMed] [Google Scholar]
- 12.Byrd JC, Mrozek K, Dodge RK, et al. Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: Results from cancer and leukemia group b (calgb 8461) Blood. 2002;100:4325–4336. doi: 10.1182/blood-2002-03-0772. [DOI] [PubMed] [Google Scholar]
- 13.Collins NH, Fernandez JM, Bleau S. Comparison of bone marrow and g-csf mobilized peripheral blood progenitor cells from single normal donors before and after t cell depletion. Cytotherapy. 1999;1 al. e. [Google Scholar]
- 14.Collins NH, Bleau SA, Kernan NA, O’Reilly RJ. T cell depletion of bone marrow by treatment with soybean agglutinin and sheep red blood cell rosetting. In: Areman HJ, Deeg HJ, Sacher RA, editors. Bone marrow and stem cell processing: A manual of current techniques. F.A. Davis; Philadelphia, PA: 1991. pp. 171–180. [Google Scholar]
- 15.Rowlings PA, Przepiorka D, Klein JP, et al. Ibmtr severity index for grading acute graft-versus-host disease: Retrospective comparison with glucksberg grade. Br J of Haematol. 1997;97:855–864. doi: 10.1046/j.1365-2141.1997.1112925.x. [DOI] [PubMed] [Google Scholar]
- 16.Sullivan K, Shulman H, Storb R, et al. Chronic graft-versus-host disease in 52 patients: Adverse natural course and successful treatment with combination immunosuppression. Blood. 1981;57:267–276. [PubMed] [Google Scholar]
- 17.Niesters HGM, van Esser J, Fries E, Wolthers KC, Cornelissen J, Osterhaus ADME. Development of a real-time quantitative assay for detection of epstein-barr virus. J Clin Microbiol. 2000;38:712–715. doi: 10.1128/jcm.38.2.712-715.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Small TN, Papadopoulos EB, Boulad F, et al. Comparison of immune reconstitution after unrelated and related t-cell-depleted bone marrow transplantation: Effect of patient age and donor leukocyte infusions. Blood. 1999;93:467–480. [PubMed] [Google Scholar]
- 19.Kalbfleisch JD, Prentice RL. The statisical analysis of failure time data. Wiley; New York, NY: 1980. [Google Scholar]
- 20.Copelan E, Casper JT, Carter SL, et al. A scheme for defining cause of death and its application in the t cell depletion trial. Biol Blood and Marrow Transplant. 2007;13:1469–1476. doi: 10.1016/j.bbmt.2007.08.047. [DOI] [PubMed] [Google Scholar]
- 21.Kernan N, Collins N, Juliano L, Cartagena T, Dupont B, O’Reilly R. Clonable t lymphocytes in t cell-depleted bone marrow transplants correlate with development of graft-v-host disease. Blood. 1986;68:770–773. [PubMed] [Google Scholar]
- 22.Savani BN, Mielke SS, Rezvani KK, et al. Absolute lymphocyte count on day 30 is a surrogate for robust hematopoietic recovery and strongly predicts outcome after t cell-depleted allogeneic stem cell transplantation. Biol Blood and Marrow Transplant. 2007;13:1216–1223. doi: 10.1016/j.bbmt.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soiffer RRJ, Weller EE, Alyea EEP, et al. Cd6+ donor marrow t-cell depletion as the sole form of graft-versus-host disease prophylaxis in patients undergoing allogeneic bone marrow transplant from unrelated donors. J Clin Oncol. 2001;19:1152–1159. doi: 10.1200/JCO.2001.19.4.1152. [DOI] [PubMed] [Google Scholar]
- 24.Soiffer RJ, Alyea EP, Hochberg E, et al. Randomized trial of cd8+ t-cell depletion in the prevention of graft-versus-host disease associated with donor lymphocyte infusion. Biol Blood and Marrow Transplant. 2002;8:625–632. doi: 10.1053/bbmt.2002.v8.abbmt080625. [DOI] [PubMed] [Google Scholar]
- 25.Kalaycio M, Rybicki L, Pohlman B, et al. Cd8+ t-cell-depleted, matched unrelated donor, allogeneic bone marrow transplantation for advanced aml using busulfan-based preparative regimens. Bone Marrow Transplant. 2004;35:247–252. doi: 10.1038/sj.bmt.1704736. [DOI] [PubMed] [Google Scholar]
- 26.Patel B, Kirkland KE, Szydlo R, et al. Favorable outcomes with alemtuzumab conditioned unrelated donor stem cell transplantation in adults with high risk philadelphia chromosome negative acute lymphoblastic leukemia in first complete remission. Haematologica. 2009;94:1399–1406. doi: 10.3324/haematol.2009.008649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Casper J, Camitta B, Truitt R, et al. Unrelated bone marrow donor transplants for children with leukemia or myelodysplasia. Blood. 1995;85:2354–2363. [PubMed] [Google Scholar]
- 28.Chown SR, Marks DI, Cornish JM, et al. Unrelated donor bone marrow transplantation in children and young adults with acute myeloid leukaemia in remission. Br J Haematol. 1997;99:36–40. doi: 10.1046/j.1365-2141.1997.3593173.x. [DOI] [PubMed] [Google Scholar]
- 29.Hessner MJ, Endean DJ, Casper JT, et al. Use of unrelated marrow grafts compensates for reduced graft-versus-leukemia reactivity after t-cell-depleted allogeneic marrow transplantation for chronic myelogenous leukemia. Blood. 1995;86:3987–3996. [PubMed] [Google Scholar]
- 30.Drobyski WR, Hessner MJ, Klein JP, Kabler-Babbitt C, Vesole DH, Keever-Taylor CA. T-cell depletion plus salvage immunotherapy with donor leukocyte infusions as a strategy to treat chronic-phase chronic myelogenous leukemia patients undergoing hla-identical sibling marrow transplantation. Blood. 1999;94:434–441. [PubMed] [Google Scholar]
- 31.Sehn LH, Alyea EP, Weller E, et al. Comparative outcomes of t-cell–depleted and non–t-cell–depleted allogeneic bone marrow transplantation for chronic myelogenous leukemia: Impact of donor lymphocyte infusion. J Clin Oncol. 1999;17:561–568. doi: 10.1200/JCO.1999.17.2.561. [DOI] [PubMed] [Google Scholar]
- 32.Meijer E, Bloem AC, Dekker AW, Verdonck LF. Effect of antithymocyte globulin on quantitative immune recovery and graft-versus-host disease after partially t-cell-depleted bone marrow transplantation: A comparison between recipients of matched related and matched unrelated donor grafts. Transplantation. 2003;75:1910–1913. doi: 10.1097/01.TP.0000065737.60591.9D. [DOI] [PubMed] [Google Scholar]
- 33.Wagner JE, Thompson JS, Carter SL, Kernan NA. Effect of graft-versus-host disease prophylaxis on 3-year disease-free survival in recipients of unrelated donor bone marrow (t-cell depletion trial): A multi-centre, randomised phase ii-iii trial. The Lancet. 2005;366:733–741. doi: 10.1016/S0140-6736(05)66996-6. [DOI] [PubMed] [Google Scholar]
- 34.Baron F, Maris MB, Storer BE, et al. High doses of transplanted cd34+ cells are associated with rapid t-cell engraftment and lessened risk of graft rejection, but not more graft-versus-host disease after nonmyeloablative conditioning and unrelated hematopoietic cell transplantation. Leukemia. 2005;19:822–828. doi: 10.1038/sj.leu.2403718. [DOI] [PubMed] [Google Scholar]
- 35.Zaucha JM, Gooley T, Bensinger WI, et al. Cd34 cell dose in granulocyte colony-stimulating factor-mobilized peripheral blood mononuclear cell grafts affects engraftment kinetics and development of extensive chronic graft-versus-host disease after human leukocyte antigen-identical sibling transplantation. Blood. 2001;98:3221–3227. doi: 10.1182/blood.v98.12.3221. [DOI] [PubMed] [Google Scholar]
- 36.Reisner Y, Martelli MF. Transplantation tolerance induced by “mega dose” cd34+ cell transplants. Experimental Hematology. 2000;28:119–127. doi: 10.1016/s0301-472x(99)00132-0. [DOI] [PubMed] [Google Scholar]
- 37.Yakoub-Agha I, Mesnil F, Kuentz M, et al. Allogeneic marrow stem cell transplantation from human leukocyte antigen-identical siblings versus human leukocyte antigen-allelic-matched unrelated donors (10/10) in patients with standard-risk hematologic malignancy: A prospective study from the french society of bone marrow transplantation and cell therapy. J Clin Oncol. 2006;24:5695–5702. doi: 10.1200/JCO.2006.08.0952. [DOI] [PubMed] [Google Scholar]
- 38.Devine SM, Soiffer RJ, Pasquini MC, et al. Hla-identical sibling-matched, cd34+ selected, t cell depleted peripheral blood stem cells following myeloablative conditioning for first or second remission acute myeloid leukemia (aml): Results of blood and marrow transplant clinical trials network (bmt ctn) protocol 0303. Blood. 2009;114:655a. [Google Scholar]
- 39.Hsu KC, Keever-Taylor CA, Wilton A, et al. Improved outcome in hla-identical sibling hematopoietic stem-cell transplantation for acute myelogenous leukemia predicted by kir and hla genotypes. Blood. 2005;105:4878–4884. doi: 10.1182/blood-2004-12-4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bordignon C, Keever C, Small T, et al. Graft failure after t-cell-depleted human leukocyte antigen identical marrow transplants for leukemia: Ii. In vitro analyses of host effector mechanisms. Blood. 1989;74:2237–2243. [PubMed] [Google Scholar]
- 41.Aversa F, Tabilio A, Velardi A, et al. Treatment of high-risk acute leukemia with t-cell-depleted stem cells from related donors with one fully mismatched hla haplotype. N Engl J Med. 1998;339:1186–1193. doi: 10.1056/NEJM199810223391702. [DOI] [PubMed] [Google Scholar]
- 42.Keever-Taylor CA, Wagner JE, Kernan NA, et al. Comparison of immune recovery in recipients of unmanipulated vs t-cell-depleted grafts from unrelated donors in a multicenter randomized phase ii-iii trial (t-cell depletion trial) Bone Marrow Transplant. 2010;45:587–589. doi: 10.1038/bmt.2009.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wagner J, C A, Kollman C. Unrelated donor bone marrow transplantation in 5075 patients with malignant and non-malignant disease: Effect of graft-versus-host disease prophylaxis on treatment outcome. Blood. 1998;92:686a. al. e. [Google Scholar]
- 44.van Burik J-AH, Carter SL, Freifeld AG, et al. Higher risk of cytomegalovirus and aspergillus infections in recipients of t cell-depleted unrelated bone marrow: Analysis of infectious complications in patients treated with t cell depletion versus immunosuppressive therapy to prevent graft-versus-host disease. Biology of Blood and Marrow Transplantation. 2007;13:1487–1498. doi: 10.1016/j.bbmt.2007.08.049. [DOI] [PubMed] [Google Scholar]
- 45.Chatchada K, Gene ON, Pintip C, et al. Twenty years of unrelated donor hematopoietic cell transplantation for adult recipients facilitated by the national marrow donor program. Biol Blood and Marrow Transplant. 2008;14:8–15. doi: 10.1016/j.bbmt.2008.06.006. [DOI] [PubMed] [Google Scholar]