Abstract
Background & Aims
Hepatitis C virus (HCV) screening can provide opportunities to reduce disease progression through counseling against alcohol use, but empirical data on this issue are sparse. We determined the efficacy of a behavioral intervention in reducing alcohol use among young, HCV-infected injection drug users (IDUs) (n=355) and assessed whether changes in liver enzymes were associated with changes in alcohol consumption.
Methods
Both the intervention and attention-control groups were counseled to avoid alcohol use, but the intervention group received enhanced counseling. Logistic regression, ANOVA, and continuous time Markov models were used to identify factors associated with alcohol use, changes in mean ALT and AST levels and change in alcohol use post-intervention.
Results
Six months post-intervention, alcohol abstinence increased 22.7% in both groups, with no difference by intervention arm. Transition from alcohol use to abstinence was associated with a decrease in liver enzymes, with a marginally greater decrease in the intervention group (p=0.05 for ALT; p=0.06 for AST). In multivariate Markov models, those who used marijuana transitioned from alcohol abstinence to consumption more rapidly than non-users (RR=3.11); those who were homeless transitioned more slowly to alcohol abstinence (RR=0.47); and those who had ever received a clinical diagnosis of liver disease transitioned more rapidly to abstinence (RR=1.88).
Conclusions
Although, behavioral counseling to reduce alcohol consumption among HCV-infected IDUs had a modest effect, reductions in alcohol consumption were associated with marked improvements in liver function. Interventions to reduce alcohol use among HCV-infected IDUs may benefit from being integrated into clinical care and monitoring of HCV infection.
Introduction
The most compelling reason for screening of hepatitis C virus (HCV) infection among high-risk populations is to appropriately direct interventions in order to 1) reduce the spread of infection to others; 2) increase uptake of HCV treatment; and 3) reduce additional liver damage to infected individuals through alcohol cessation. Although all three aspects are critical in preventing significant HCV-related morbidity and mortality among high-risk populations, few studies have focused on alcohol cessation as a measure of liver preservation in injection drug users (IDUs).
Among HCV-infected persons, excessive alcohol use has been proposed to result in more severe histological injury, more rapid disease progression, and a higher frequency of cirrhosis and hepatocellular carcinoma [1]. Moderate (<80 grams alcohol/day) and heavy alcohol use (80 grams +/day) have been associated with increased risk of cirrhosis and hepatocellular carcinoma [2]. Lower alcohol consumption (<140 grams/week [3] and > 50 grams/day [4]) has been associated with increased fibrosis. Since IDUs may experience more barriers to HCV treatment than other populations [5, 6], abstinence from alcohol use could be extremely important in sustaining liver health.
Although alcohol use has been shown to increase alanine aminotransferase (ALT) levels in the general population [7] and among anti-HCV-positive individuals [8], few studies have examined liver enzymes in IDUs in relation to alcohol consumption and active HCV-infection (i.e., detectable HCV RNA). Clinical evidence suggests that HCV replication and alcohol metabolism may interact synergistically to exacerbate liver damage [9, 10], and therefore could result in increased hepatic injury over a shorter period. To our knowledge, no study has compared change in serum ALT or asparatate aminotransferase (AST) levels with change in alcohol behavior among young HCV-infected IDUs. Developing a thorough understanding of the relation between alcohol consumption, HCV infection, and liver enzymes is very important for HCV-infection management, as HCV-infected individuals may withhold disclosure of their alcohol use to a clinician for fear of disapproval, and heavy alcohol use may be common among young IDUs [11].
Different patterns of alcohol use in response to standard counseling at diagnosis have been reported among HCV-infected IDUs [12] and other patients [13], including cessation of alcohol use; modification of use without cessation; and continued high levels of alcohol consumption. High levels of alcohol use have been reported prior to HCV infection, with marked reductions in use immediately following HCV diagnosis, and rebounds in use six and 12 months post-diagnosis have been reported among young IDUs [14]. Brief alcohol interventions among IDUs [15] and HCV-infected IDUs in treatment [16] have also demonstrated short-term efficacy. These studies suggest that alcohol cessation interventions may be highly effective among IDUs.
We previously reported that a behavioral intervention was successful in reducing distributive needle sharing among young, HCV-infected IDUs in three U.S. cities [17]. A secondary outcome of this intervention was to reduce alcohol consumption. Herein, we report on the efficacy of this intervention in reducing alcohol consumption among 355 young, HCV-infected IDUs. Furthermore we examine changes in ALT and AST with change in alcohol use and patterns and predictors of changes in alcohol use.
Patients and Methods
Participants & Study Design
The data for this study were collected as part of the Study to Reduce Intravenous Exposures (STRIVE), a randomized, attention-control trial, which aimed to determine the efficacy of an intervention on reducing distributive needle sharing and increasing healthcare utilization among HCV-infected IDUs, which has been described [17, 18].
Between April 2002 and May 2004, young, HCV-antibody positive IDUs were recruited from Baltimore, Maryland; New York City, New York; and Seattle, Washington. IDUs were eligible if they were 18–35 years-old; injected drugs within the previous six months; planned to stay in their recruitment location for 12 months; had documented HCV antibody-positive and HIV antibody-negative tests; and consented to provide a blood sample for HCV-related tests. Participants were not excluded if they were hepatitis B antibody-positive or if they had known liver disease. HCV antibody testing was conducted in other studies focusing on prevention of HCV acquisition, and HCV-infected subjects who were ineligible for those studies were referred to STRIVE. All participants enrolled in STRIVE received standard-of-care HCV counseling, including information on alcohol cessation, immediately before they were randomized.
In total, 952 IDUs were referred from other studies, 630 enrolled and completed baseline assessments, and 418 were randomized to intervention or control groups [17, 18]. Among randomized subjects, 13.6% (N=57) did not complete any follow-up interviews, 26.5% (N=115) completed one follow-up and 59.8% (N=250) completed both follow-up visits. Of the 365 individuals completing at least one follow up visit, 10 lacked complete data on alcohol use, leaving 355 who were eligible for analyses. All study activities were approved by institutional review boards at participating sites and participants provided written, informed consent.
Data Collection, Intervention, and Follow-up
Prior to randomization, STRIVE participants completed a baseline assessment interview using audio computer-assisted self-interview (A-CASI) to reduce socially desirable responding [19, 20]. The assessment included injection behaviors, alcohol use, the alcohol use disorders identification test (AUDIT) [21], healthcare utilization, patient-doctor interactions, HCV treatment readiness, depression, and utilization of alcohol and drug treatment. Participants were scheduled to attend a randomization visit within 15 weeks of their baseline visit.
Participants underwent their first intervention or control session at the randomization appointment to reduce attrition [17, 18]. Those in the attention-control arm watched a television docudrama about the lives of IDUs, followed by a facilitator-led discussion about family, education, self-respect, relationships, violence, parenting, and employment.
The intervention arm participated in facilitated exercises focusing on the natural history of HCV infection, HCV treatment, and how to maintain liver health, including avoiding alcohol use. Although the intervention was not specific to alcohol cessation, all intervention group sessions included HCV prevention messages targeted toward participants, encouraging dissemination to their HCV-infected peers, and stressed the dangers of alcohol use and benefits of cessation for HCV-infected individuals.
Both trial arms consisted of six two-hour sessions held biweekly by public health professionals for three weeks. Detailed scripts were followed by facilitators in order to reduce variation in delivery of the intervention. Participants were asked to return for follow-up three and six months after completing the intervention.
Measures Assessed
At baseline and six-month follow-up, standard liver enzyme panels, including ALT and AST, were completed on fresh serum samples at commercial laboratories. All participants were counseled on results by physicians who were study investigators and referred for treatment if indicated. No serum samples were drawn at the three-month follow-up visit. Presence of HCV RNA was determined at six-month follow-up using polymerase change reaction (PCR). Serum samples from all sites were shipped to John’s Hopkins University for assessment using a standardized protocol.
Actual homelessness was defined as an affirmative response to having slept outside, in a car, or abandon building for seven or more days consecutively. Perceived homelessness was defined as the participant indicating that s/he thought of him/herself as homeless [22]. Participants were asked if they received any type of alcohol treatment in the preceding three months and if they were currently receiving drug treatment, but type of treatment was not specified.
AUDIT scores were based on a ten item scale [21] with three questions each on alcohol consumption and dependence, and four on external problems related to alcohol consumption (problem drinking). AUDIT scores can range from 0–40, with a score of 8 or more indicating problem drinking [21]. Baseline AUDIT measures pertained to alcohol behaviors in the past year, however, three- and six-month follow-up measures pertained to the past three months only. For general alcohol use measures, we used a single AUDIT question pertaining to how often participants drank alcohol prior to that particular interview.
Variables that remained constant over time were measured at baseline. Time-varying factors were measured at each visit and those assessed at follow-up pertain only to the three-month time period prior to the visit.
Statistical Analysis
Analyses were restricted to the 355 individuals who completed at least one follow-up interview and had complete data on alcohol use. For analyses examining liver enzymes, 318 participants with complete data were included. Contingency tables and univariate logistic regression were used to examine differences in demographics, injection behaviors, life situations and baseline liver enzymes between those who did and did not report alcohol use at baseline. Effects of the intervention on alcohol use, AUDIT score, and liver enzymes were examined using separate univariate and multivariate logistic regression models.
Predictors of change in alcohol use status at consecutive visits were analyzed using continuous time Markov models, which provide hazard estimates while allowing for repeated changes in outcome that are bidirectional. These models were used to estimate the transition rate between alcohol use and abstinence in terms of sociodemographic predictors, interventions and personal health status information, life situations, and substance use.
ANOVA was used to examine overall change in liver enzyme scores from baseline to six-month follow-up according to category of change in alcohol use (i.e., 1) no change in alcohol use (whether using or abstaining), 2) change from use at baseline to abstinence at follow-up, or 3) change from abstinence at baseline to use at follow-up). Those who did not change their alcohol use were grouped into a single category as we were examining change in liver enzymes with change in alcohol use; however, differences in liver enzymes for users and non-users in this group were compared. Analyses were stratified by presence of HCV RNA to determine if associations were limited to individuals who were likely to have active HCV infection, which would suggest a synergistic effect of alcohol metabolism and HCV replication.
Results
Characteristics of the Study Sample
The mean age of participants was 26.7 years, 55.8% were White, and approximately three quarters were male (Table 1). More than half regarded themselves as homeless and slightly more were actually homeless. Non-injection drug use in the three months prior to baseline was common. Sixty-two percent of participants were diagnosed with HCV for the first time within less than one year of baseline (median: 3 months). At HCV diagnosis, most recalled being counseled on cirrhosis (84.4%) and being advised to stop consuming alcohol (75.1%). Nearly 15% reported receiving alcohol treatment in the previous three months, and 16.9% had ever been diagnosed with liver disease of any etiology.
Table 1.
Alcohol use at baseline by demographics, other substance use, life situation, and liver function tests among HCV-infected IDUs
| TOTAL (n=355) % (n) mean (median) |
No Alcohol Use (n=97) % (n) mean (median) |
Alcohol Use (n=258) % (n) mean (median) |
OR | p | |
|---|---|---|---|---|---|
| Age | 26.7 (27) | 27.2 (27) | 26.5 (26) | 0.96 | 0.15 |
| White ethnicity | 55.8 (198) | 57.3 (56) | 55.0 (142) | 0.90 | 0.65 |
| Female sex vs. Male | 24.8 (88) | 26.8 (26) | 24.0 (62) | 0.86 | 0.59 |
| Intervention vs. control | 52.7 (187) | 61.9 (60) | 41.2 (127) | 0.60 | 0.03 |
| Perceived homelessness | 56.3 (200) | 54.6 (53) | 57.0 (147) | 1.10 | 0.69 |
| Actual homelessness | 42.8 (152) | 38.1 (37) | 44.6 (115) | 1.30 | 0.28 |
| Incerated | 80.0 (284) | 76.3 (74) | 81.4 (210) | 1.36 | 0.29 |
| Thought about taking own life | 17.2 (61) | 12.4 (12) | 19.0 (49) | 1.66 | 0.16 |
| Smoked marijuana | 85.4 (303) | 71.1 (69) | 87.6 (226) | 2.87 | <0.01 |
| Snorted or smoked cocaine | 83.1 (295) | 76.3 (74) | 88.8 (229) | 2.45 | <0.01 |
| Snorted, swallowed, or smoked heroin | 92.7 (329) | 88.7 (86) | 94.2 (243) | 2.07 | 0.08 |
| Days since first HCV diagnosis | 579.4 (91) | 628.5 (251) | 574.4 (61) | 0.99 | 0.62 |
| Told HCV caused cirrhosis at diagnosis | 83.7 (297) | 80.4 (78) | 84.9 (219) | 1.37 | 0.31 |
| Diagnosed with HCV in research study | 50.7 (180) | 52.6 (51) | 50.0 (129) | 0.90 | 0.67 |
| Told to stop drinking at diagnosis | 75.5 (268) | 71.1 (69) | 77.1 (199) | 1.37 | 0.24 |
| Had regular physician at baseline | 32.1 (114) | 29.9 (29) | 33.0 (85) | 1.15 | 0.58 |
| Recent alcohol treatment | 14.7 (52) | 8.3 (8) | 17.1 (44) | 2.29 | 0.04 |
| In drug treatment at interview | 18.6 (66) | 19.6 (19) | 18.2 (47) | 0.91 | 0.77 |
| Physician diagnosed liver disease | 16.9 (60) | 16.5 (16) | 17.1 (44) | 1.04 | 0.90 |
| Would like HCV treatment | 77.5 (275) | 79.4 (77) | 76.7 (198) | 0.86 | 0.60 |
| ALT | 96.1 (51) | 69.6 (44) | 105.6 (55) | 0.04 | |
| AST | 61.8 (35) | 48.3 (29) | 66.8 (37) | 0.11 |
ALT – alanine aminotransferase; AST – asparatate aminotransferase
Of those with complete liver function tests (N=318), median ALT levels at baseline were 51 IU/L (mean 96.1; IQR 27–109) and AST was 35 IU/L (mean 61.8; IQR 23–65). The baseline median AUDIT score was 5 (mean 7.7: IQR: 0–34). Overall, 72.3% of participants reported using any alcohol at baseline; 47.8% of those who consumed alcohol had an AUDIT score of 8 or more.
Although individuals were randomly assigned to trial arms, those in the intervention arm were less likely to use alcohol at baseline (Table 1). Baseline alcohol users were also significantly more likely to report smoking marijuana or using cocaine in the previous three months and having had recent alcohol treatment than those who reported no alcohol use. Baseline alcohol use was also associated with higher baseline mean ALT levels, but not AST levels.
Effect of STRIVE Intervention
The mean number of intervention sessions attended by all participants was 5 (median 6); with 61% attending all 6 sessions and 72% attending 5 sessions. The median time for the first follow-up visit was 12 weeks and 27 weeks for the second follow up visit. The intervention had a marginal effect on ALT and AST measures at six months follow-up, but had no apparent effect on alcohol use, overall AUDIT score, or specific AUDIT score (i.e., consumption, problem, dependence) in univariate models (Table 2). After controlling for presence of HCV RNA at six months, age, sex, and drug and alcohol use in the three months prior to the final follow-up visit there was a marginally significant association between receiving the intervention and reduced ALT (OR=0.94, p=0.05) or reduced AST (OR=0.91, p=0.06), but not for self-reported alcohol use or AUDIT score.
Table 2.
Unadjusted and adjusted logistic regression models assessing associations between alcohol use at 3 and 6 months follow-up and receiving HCV Peer Mentoring Intervention.
| Unadjusted | Adjusted* | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Alcohol use outcome | 3 Month Follow-up (n=271) |
6 Month Follow-up (n=334) |
6 Month Follow-up (n=334) |
||||||
| OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p | |
| Any alcohol use in preceding 3 months | 1.26 | 0.78, 2.04 | 0.35 | 0.83 | 0.54, 1.27 | 0.38 | 1.23 | 0.57, 2.69 | 0.60 |
| AUDIT score | 1.01 | 0.98, 1.04 | 0.74 | 0.82 | 0.95, 1.01 | 0.16 | 0.99 | 0.96, 1.01 | 0.50 |
| AUDIT score ≥8 | 1.20 | 0.79, 1.84 | 0.39 | 0.98 | 0.49, 1.36 | 0.44 | 1.01 | 0.55, 1.85 | 0.99 |
| AUDIT alcohol consumption score | 1.04 | 0.96, 1.12 | 0.39 | 0.98 | 0.92, 1.05 | 0.56 | 1.03 | 0.94, 1.13 | 0.58 |
| AUDIT alcohol dependence score | 0.98 | 0.88, 1.08 | 0.65 | 0.92 | 0.84, 1.01 | 0.07 | 0.94 | 0.85, 1.03 | 0.19 |
| AUDIT alcohol problem score | 1.01 | 0.94, 1.09 | 0.75 | 0.95 | 0.89, 1.02 | 0.15 | 0.96 | 0.89, 1.04 | 0.34 |
| ALT (per 25 IU/L) | N/A | 0.96 | 0.91, 1.01 | 0.10 | 0.94 | 0.89, 0.99 | 0.05 | ||
| AST (per 25 IU/L) | N/A | 0.93 | 0.85, 1.02 | 0.12 | 0.91 | 0.83, 1.01 | 0.06 | ||
Any type of alcohol use is defined as consuming any alcohol in the previous 3 months. AUDIT is a standardized scale, made up of 3 components, alcohol consumption, alcohol dependence, and alcohol problems, used to determine problem alcohol use; a score of 8 or more is regarded as problem alcohol use.
Adjusted for HCV RNA at six months, age, sex, drug use three months prior to the final follow-up visit, and self-reported alcohol use
Patterns of Alcohol Use
Patterns of self-reported alcohol use were dynamic and complex (Figure 1). At baseline, 97 participants reported no alcohol use in the past year, of whom, 50 (52%) continued to abstain while 22 (23%) commenced use at three-month follow-up. At the six month follow up, 43 of the 50 (86%) abstainers at baseline and three-month follow-up continued to report abstinence. Of the 22 who commenced alcohol use at the three-month follow-up, 13 (59%) reported abstaining at six months, while 9 (41%) reported continued alcohol use. In comparison, among the 258 participants who reported alcohol use at baseline, 133 (52%) continued to report alcohol use at the three-month follow-up, and 66 (26%) reported abstaining. By the six-month follow-up, 97 participants (73%) had reported alcohol use at all three visits and 27 (20%) who reported use at baseline and three months reported abstinence at six months. Of the 66 participants who transitioned to abstinence at three months, 18 (27%) resumed alcohol use at six months. Within the entire sample, there was a 15.3% reduction in proportion of participants who reported alcohol use at three months and a 22.7% reduction at six months.
Figure 1.
Changes in alcohol use patterns of STRIVE participants at baseline, three-month, and six month follow-up periods, including lost to follow-up
Changes in Liver Enzymes by Alcohol Use
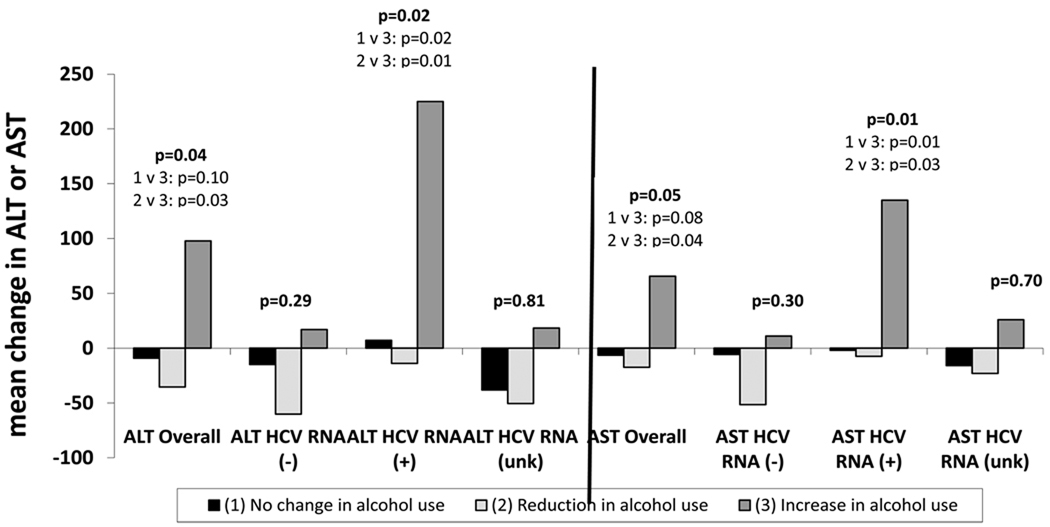
For participants who reported transitioning from alcohol use at baseline to abstinence at 6-month follow-up, mean ALT and AST levels were lower at 6-month follow-up than at baseline (Figure 2). Among those who reported using alcohol during follow-up but were abstinent at baseline, large increases in mean ALT and AST values were observed. Overall differences in the mean change in ALT and AST were significant for the group reporting abstaining from alcohol compared to those starting alcohol use, with a mean change in ALT of −35.5 IU/L for those reporting alcohol cessation and +97.8 IU/L for those who reported starting use after abstinence (p=0.03) and a mean change in AST of −17.9 IU/L and +65.6 IU/L, respectively (p=0.05). Marijuana and other substance use were not associated with AST or ALT or changes in these enzymes.
Figure 2.
Mean change in ALT or AST from baseline to 6-month follow-up by alcohol use and HCV RNA status
After stratifying by presence of HCV RNA in sera, significant changes were only observed in those who were HCV RNA positive by PCR. Among HCV RNA positive individuals, mean changes in ALT from baseline to 6-month follow-up were significantly different between those reporting uptake of alcohol use (+225.4 IU/L) and those reporting cessation of alcohol use (−13.9 IU/L; p=0.01). Similar results were observed for AST, with significant differences between those reporting uptake of alcohol use (+135 IU/L) and cessation of alcohol use (−7.4 IU/L; p=0.03). For those who reported no change in alcohol use, there was very little variation in ALT and AST levels from baseline to follow-up, except when stratified by those of unknown RNA status. ALT (+1.5 IU/L v. −14.3 IU/L, p=0.53) and AST (−1.4 IU/L v. −0.99 IU/L, p=0.93) levels were similar between abstainers and users respectively.
Predictors of Changes in Alcohol Use
A change from abstinence to alcohol use was associated with incarceration in the three months prior to interview and smoking marijuana in univariate models (Table 3). Switching from alcohol use to abstinence was less likely among those who were homeless, those who were advised to stop drinking alcohol at the time of HCV diagnosis, and those recently attending alcohol treatment. Those who had ever been diagnosed as having liver disease by a physician were more likely to switch from alcohol use to abstinence. In multivariate continuous time Markov models, a change from abstinence to alcohol use was three times more rapid among those reporting marijuana use, while a change from use to abstinence was half as rapid among homeless participants and almost twice as rapid if the participant was diagnosed with liver disease by a physician after controlling for being incarcerated, recently attending an alcohol treatment program, and being instructed to stop using alcohol at HCV diagnosis. Age, perceived homelessness, contemplating suicide, cocaine use, non-injection heroin use, current drug treatment, and any non-injection drug use, were not associated with change in alcohol use.
Table 3.
Univariate and multivariate Markov models of factors associated with transition between alcohol use and non-use states
| Unadjusted Models | Fully Adjusted Model | |||||||
|---|---|---|---|---|---|---|---|---|
| Direction of Change of Alcohol Consumption: | Abstinence to Use | Use to Abstinence | Abstinence to Use | Use to Abstinence | ||||
| RR | 95% CI | RR | 95% CI | RR | 95% CI | RR | 95% CI | |
| Age | 1.00 | 0.92, 1.08 | 0.99 | 0.94, 1.04 | - | - | ||
| White ethnicity | 1.76 | 0.89, 3.49 | 1.44 | 0.94, 2.19 | - | - | ||
| Male vs. Female | 0.63 | 0.30, 1.33 | 0.92 | 0.58, 1.45 | - | - | ||
| Intervention vs. control | 1.14 | 0.60, 2.16 | 1.06 | 0.71, 1.58 | - | - | ||
| Perceived homelessness | 1.36 | 0.72, 2.56 | 0.73 | 0.48, 1.10 | - | - | ||
| Actual homelessness | 0.74 | 0.38, 1.44 | 0.51 | 0.33, 0.79 | 0.68 | 0.31, 1.49 | 0.47 | 0.28, 0.78 |
| Incarcerated | 2.50 | 1.26, 4.94 | 0.92 | 0.61, 1.39 | 1.65 | 0.75, 3.60 | 0.90 | 0.55, 1.47 |
| Injection drug use last 30 days | 0.74 | 0.36, 1.51 | 1.10 | 0.64, 1.91 | - | - | ||
| Injection drug use last 3 months | 1.11 | 0.41, 3.01 | 1.05 | 0.46, 2.40 | - | - | ||
| Thought about taking own life | 1.72 | 0.84, 3.55 | 0.75 | 0.46, 1.21 | - | - | ||
| Smoked marijuana | 3.03 | 1.49, 6.17 | 0.94 | 0.60, 1.49 | 3.11 | 1.33, 7.23 | 1.05 | 0.62, 1.80 |
| Snorted/smoked cocaine | 1.22 | 0.62, 2.40 | 0.69 | 0.43, 1.09 | - | - | ||
| Snorted/swallowed/smoked heroin | 1.01 | 0.50, 2.05 | 0.73 | 0.44, 1.23 | - | - | ||
| Used any non-injection drugs | 0.87 | 0.36, 2.06 | 0.75 | 0.33, 1.68 | - | - | ||
| Told HCV caused cirrhosis at diagnosis | 1.16 | 0.47, 2.84 | 0.67 | 0.37, 1.19 | - | - | ||
| Diagnosed with HCV in research study | 1.03 | 0.54, 2.00 | 1.26 | 0.83, 1.91 | - | - | ||
| Told to stop drinking at diagnosis | 0.89 | 0.43, 1.88 | 0.53 | 0.32, 0.88 | 0.79 | 0.31, 2.00 | 0.60 | 0.32, 1.12 |
| Had a regular physician at diagnosis | 0.83 | 0.43, 1.58 | 0.79 | 0.52, 1.20 | - | - | ||
| Physician diagnosed liver disease | 1.98 | 0.87, 4.51 | 1.77 | 1.03, 3.03 | 1.60 | 0.58, 4.41 | 1.88 | 1.00, 3.54 |
| Recent alcohol treatment | 0.56 | 0.19, 1.63 | 0.57 | 0.32, 1.00 | 1.11 | 0.38, 3.28 | 0.69 | 0.33, 1.42 |
| In drug treatment at the time of interview | 0.91 | 0.43, 1.90 | 1.11 | 0.71, 1.75 | - | - | ||
Markov models estimate the likelihood of transition between alcohol abstinence and use from baseline to the first follow-up or the first to the second followup, as compared to remaining in a current state, allowing transition to occur in either direction; relative risks less than one indicate a lower likelihood of transition from one state to the next, conversely relative risks greater than one indicate a greater likelihood of change. Two relative risks are reported for every variable fitted because we have not assumed that the rate of changing from abstinence to alcohol use is the same as changing from alcohol use to abstinence.
Discussion
In this study young, HCV-infected IDUs had much higher ALT levels than normal. This is the first known study to demonstrate that cessation in alcohol use is associated with decreased ALT and AST among young, HCV-infected IDUs, whereas uptake of alcohol use is associated with an increase in ALT and AST. These changes were particularly pronounced in those with presence of HCV RNA in sera, providing evidence of syngeristic effects of HCV replication and alcohol metabolism on liver damage. Although self-reported alcohol use did not differ by intervention arm, there was modest evidence of intervention efficacy based on ALT and AST. However, other factors seemed to have a greater influence on change in alcohol use behaviors including, ever receiving a diagnosis of liver disease, marijuana use, and homelessness. While there was an encouraging increase in alcohol abstinence at three and six months follow-up among all of the HCV-infected IDUs in this study, many reported fluctuating between use and abstinence, which has been observed previously among a mixed sample of non-IDU and IDU in drug treatment [23].
These findings support current guidelines recommending testing of high-risk individuals for HCV and providing counseling for alcohol cessation. One of our novel findings was a dose-response effect between ALT or AST and alcohol use (i.e., reduction in ALT and AST levels with abstinence and increases in levels with uptake of alcohol consumption) among young, HCV-infected individuals. After stratifying by presence of HCV RNA in sera, this effect was observed only in those with detectable of HCV RNA. Given that these were all young IDUs who had injected drugs for a mean and median of 6 years, they were not likely have been infected with HCV for prolonged durations, suggesting that alcohol use and active HCV-infection may synergistically damage the liver over a short period, which is supported by evidence from clinical studies [9, 10]. These findings highlight the importance of active case identification, HCV RNA testing in HCV antibody-positive individuals, and alcohol cessation counseling for HCV-infected IDUs of all ages who are RNA positive.
Normal ALT levels are generally accepted as less than 40 IU/L [7]. In this study, median ALT scores at baseline were 28% higher than this overall, and 38% higher among those reporting alcohol use; which is surprising given that ALT and AST in younger individuals tends to be lower [24]. Elevated ALT is suggestive of liver fibrosis [25, 26] and higher HCV viral load [27]. Very few participants (4% at baseline and follow-up) had AST/ALT ratios >2, but 22% had AST/ALT ratios > 1 which may suggest higher viral loads and active HCV damage. Participants’ young age, and relatively short period of injecting, suggests a short duration of HCV infection, implying that changes observed in AST and ALT with alcohol use may be representative of the synergistic damage in HCV RNA positive individuals.
Given the importance of alcohol cessation demonstrated in our study, it is disappointing that our intervention only showed modest associations with reduction in ALT and AST and no associations with self-reported alcohol use. However, the intervention was not primarily designed for this purpose. Given the intervention’s efficacy in reducing distributive needle sharing [17], additional tailoring to incorporate liver function test results into intervention messages may yield greater reductions in alcohol consumption. Brief alcohol interventions may be highly effective in achieving reductions in alcohol use [28, 29]. In particular, short interventions incorporating motivational interviewing (MI) have been shown to reduce alcohol consumption among IDU [15] and HCV-infected IDU in treatment [16]. A combination of approaches may be necessary for one intervention to address both injection equipment sharing and alcohol use, as evidenced in a recent control trial comparing MI and education, which demonstrated efficacy in reducing alcohol consumption in both arms, but not receptive needle sharing [30]. Incorporating MI into the STRIVE intervention might be effective in combining alcohol cessation with reductions in distributive needle sharing.
The differences we observed in biological and behavioral measures of alcohol consumption may be due to greater accuracy of biological measures, as compared to self-reported behaviors; participants may be reluctant to report behaviors or may not remember when they consumed alcohol. It is more likely that ALT and AST levels were more sensitive to reductions in alcohol use, which was not captured by our survey measures. These findings also suggest the need for both behavioral and biological markers in measuring alcohol consumption in interventions targeting HCV-infected individuals.
Most participants recalled having received counseling to reduce alcohol use at HCV diagnosis and those who had a previous diagnosis of liver disease transitioned to alcohol abstinence more rapidly, reinforcing the notion that medical counseling may have some impact on behavior change for young IDUs. The increase in the number of participants reporting abstaining from alcohol use in either study arm from baseline through six-month follow up is encouraging and suggests that educational messages aimed at liver wellness may influence young IDUs’ alcohol use behavior.
Not surprisingly, we found that those who were homeless transitioned to abstinence more slowly. Homelessness is related to decreased resources and increased risk behaviors among drug users [22, 31] and was previously associated with higher AUDIT scores in a previous analysis of the STRIVE baseline sample [32]. It is critical to provide additional resources to homeless IDUs in order to support and encourage opportunities for drug and alcohol treatment.
Similarly, associations observed between transitioning to alcohol use and smoking marijuana were not unexpected. Simultaneous poly-substance use, or using more than one substance at once, is commonly reported with marijuana and alcohol [33, 34]. While this finding suggests that alcohol cessation interventions may be more effective with inclusion of marijuana cessation, other studies suggest that cessation of marijuana may result in increased alcohol use [35, 36]. Future studies examining the effects of marijuana use on alcohol cessation in current and former IDUs could assist in developing more effective alcohol cessation interventions among HCV-infected IDUs.
Although this study involved a randomized controlled trial, there were some limitations. Since alcohol cessation was not the primary outcome of our intervention, the study was neither powered nor designed to measure alcohol cessation as an endpoint. Dynamic changes in alcohol use observed over this six-month period suggest that this duration was too short for estimating long-term trends in alcohol use. Studies of alcohol use patterns over a longer period of time could provide more information on predictors of changes in alcohol use and durability of a behavior change intervention. Additionally, more precise measures of amount of alcohol used daily, would have been helpful in assessing reductions in use, not just cessation, and determining thresholds of dangerous amounts of alcohol use in HCV-infected IDUs. Changes in alcohol use among a mixed sample of IDU and non-IDU in drug treatment over a 4–5 year period, demonstrated similar fluctuations in alcohol use, but noted that abstainers were unlikely to become heavy users when transitioning to alcohol use [23]. By chance, participants randomized to the intervention group reported less alcohol use at baseline than did those in the control arm. This lack of balance in randomization was also noted when examining efficacy of this intervention in reducing distributive needle sharing [17]. However, this was unlikely to affect our analyses as there were no observed effects of the intervention on reported alcohol use, self-reported alcohol use was adjusted for in models examining the effect of the intervention on ALT and AST, and other measures of alcohol use examined changes in behavior, which would have been detected regardless of baseline use.
Since HCV RNA was only measured at six-month follow-up, data was missing for some participants, and we were unable to measure changes in HCV RNA positivity from baseline to follow-up. However, it is unlikely that HCV RNA status changed over the relatively short study period. Additionally, AST and ALT were measured at only two time points, and natural fluctuation may occur; however, it is unlikely that fluctuation in AST and ALT would follow the patterns observed by changes in alcohol use and would be less extreme among those not changing alcohol use patterns. In addition, some factors known to affect AST and ALT (e.g., obesity, coffee, etc ) [24] were not measured in this study. In light of the young age of the sample, it is reasonable to assume that AST and ALT should be relatively low [24], and given the relationship between alcohol use and increased ALT and AST specific to HCV RNA-positive IDUs, it is unlikely that the associations observed could be explained by these unmeasured factors. Follow-up data were only available on 85% of the originally randomized sample; however, no significant differences were observed between those who were followed up and those who were not. All self-reported outcome measures may suffer from socially desirable response bias; however, this was likely to be minimized by the use of A-CASI [19, 20].
This study provides further critical evidence that alcohol cessation is an important goal for chronically HCV-infected individuals of any age, providing an important justification for screening high-risk populations. Our data suggest that alcohol cessation can have profound effects on reducing ALT and AST among young IDUs with active HCV infection. Some young, HCV-infected IDUs are motivated to reduce their alcohol use, but may require more intensive alcohol cessation interventions to realize meaningful, long-term reductions. Interventions aimed at reducing alcohol consumption in this population may be particularly successful if they are integrated into clinical care and monitoring of HCV infection.
Acknowledgements
We are grateful to all of the STRIVE participants for making this study possible. This work was supported by funding from NIDA (R01 DA14499). The following individuals were part of the STRIVE study team who developed and conducted the intervention and collected the follow-up data (affiliations shown at the time of the intervention) : Johns Hopkins University - Steffanie A. Strathdee (currently Chief of the Division of Global Public Health, University of California San Diego School of Medicine), Elizabeth T. Golub, David Thomas, Marie Bailey-Kloch, Yvette Bowser, Peter O’Driscoll, Janet Reeves, Marcella Sapun, Dale Netski, McCay Moiforay, Fleesie Hubbard, Coralee Meslin, Karen Yen-Hobelmann, Marie Bailey-Kloch, Eddie Poole, David Hudson, Gina Gant, and Eric Hendren; New York Academy of Medicine - Mary Latka, Farzana Kapadia, David Vlahov, Danielle Ompad, Micaela Coady, Sebastian Bonner, Joanna Cruz, Sandra DelVecchio, Dirk Jackson, Gregory Malave, Joan Monserrate, Clarisse Miller O’Shea, and Manny Yonko; Seattle–King County Department of Public Health - Holly Hagan, Jennifer V. Campbell, Eileen Hough, Hanne Thiede, Rong Lee, Susan Nelson, Jeff St. De Lore, Kimberly Houk, Sarah Brooks, Carrie Shriver, Jeanette Frazier, Jean Pass, and Paul Swenson; and Centers for Disease Control and Prevention-Richard S. Garfein (currently Associate Professor in the Division of Global Public Health, University of California San Diego School of Medicine
List of Abbreviations
- HCV
hepatitis C virus
- IDUs
injection drug users
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- US
United States
- RNA
ribonucleic acid
- STRIVE
Study to Reduce Intravenous Exposures
- A-CASI
audio- computer assisted self interview
- AUDIT
Alcohol Use Disorders Identification Test
- PCR
polmerase chain reaction
- ANOVA
analysis of variance
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Clinical trials registration number: NCT00391482.
Financial Disclosures: none of the authors have a commercial relationship or financial conflict interest in relation to this study.
References
- 1.Bhattacharya R, Shuhart MC. Hepatitis C and alcohol - Interactions, outcomes, and implications. Journal of Clinical Gastroenterology. 2003;36:242–252. doi: 10.1097/00004836-200303000-00012. [DOI] [PubMed] [Google Scholar]
- 2.Khan KN, Yatsuhashi H. Effect of alcohol consumption on the progression of hepatitis C virus infection and risk of hepatocellular carcinoma in Japanese patients. Alcohol and Alcoholism. 2000;35:286–295. doi: 10.1093/alcalc/35.3.286. [DOI] [PubMed] [Google Scholar]
- 3.Pessione F, Degos F, Marcellin P, Duchatelle V, Njapoum C, et al. Effect of alcohol consumption on serum hepatitis C virus RNA and histological lesions in chronic hepatitis C. Hepatology. 1998;27:1717–1722. doi: 10.1002/hep.510270635. [DOI] [PubMed] [Google Scholar]
- 4.Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. Lancet. 1997;349:825–832. doi: 10.1016/s0140-6736(96)07642-8. [DOI] [PubMed] [Google Scholar]
- 5.Mehta SH, Genberg BL, Astemborski J, Kavasery R, Kirk GD, et al. Limited uptake of hepatitis C treatment among injection drug users. Journal of Community Health. 2008;33:126–133. doi: 10.1007/s10900-007-9083-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stoove MA, Gifford SM, Dore GJ. The impact of injecting drug use status on hepatitis C-related referral and treatment. Drug and Alcohol Dependence. 2005;77:81–86. doi: 10.1016/j.drugalcdep.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Clark JM, Brancati FL, Diehl AM. The prevalence and etiology of elevated aminotransferase levels in the United States. The American journal of gastroenterology. 2003;98:960–967. doi: 10.1111/j.1572-0241.2003.07486.x. [DOI] [PubMed] [Google Scholar]
- 8.Inglesby TV, Rai R, Astemborski J, Gruskin L, Nelson KE, et al. A prospective, community-based evaluation of liver enzymes in individuals with hepatitis C after drug use. Hepatology. 1999;29:590–596. doi: 10.1002/hep.510290219. [DOI] [PubMed] [Google Scholar]
- 9.McCartney EM, Beard MR. Impact of alcohol on hepatitis C virus replication and interferon signaling. World Journal of Gastroenterology. 2010;16:1337–1343. doi: 10.3748/wjg.v16.i11.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seronello S, Ito C, Wakita T, Choi J. Ethanol enhances hepatitis C virus replication through lipid metabolism and elevated NADH/NAD+ Journal of Biological Chemistry. 2010;285:845–854. doi: 10.1074/jbc.M109.045740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hahn JA, Page-Shafer K, Ford J, Paciorek A, Lum PJ. Traveling young injection drug users at high risk for acquisition and transmission of viral infections. Drug and Alcohol Dependence. 2008;93:43–50. doi: 10.1016/j.drugalcdep.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ompad DC, Fuller CM, Vlahov D, Thomas D, Strathdee SA. Lack of Behavior change after disclosure of hepatitis C virus infection among young injection drug users in Baltimore, Maryland. Clinical Infectious Diseases. 2002;35:783–788. doi: 10.1086/342063. [DOI] [PubMed] [Google Scholar]
- 13.Stoller EP, Hund AJ, Webster NJ, Blixen CE, Perzynski AT, et al. Alcohol consumption within the context of hepatitis C: A qualitative study of non-problematic drinkers. Alcohol and Alcoholism. 2006;41:546–552. doi: 10.1093/alcalc/agl055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsui JI, Vittinghoff E, Hahn JA, Evans JL, Davidson PJ, Page K. Risk behaviors after hepatitis C virus seroconversion in young injection drug users in San Francisco. Drug and Alcohol Dependence. 2009;105:160–163. doi: 10.1016/j.drugalcdep.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stein MD, Charuvastra A, Maksad J, Anderson BJ. A randomized trial of a brief alcohol intervention for needle exchangers (BRAINE) Addiction. 2002;97:691–700. doi: 10.1046/j.1360-0443.2002.00102.x. [DOI] [PubMed] [Google Scholar]
- 16.Watson B, Conigrave KM, Wallace C, Whitfield JB, Wurst F, Haber DPS. Hazardous alcohol consumption and other barriers to antiviral treatment among hepatitis C positive people receiving opioid maintenance treatment. Drug and Alcohol Review. 2007;26:231–239. doi: 10.1080/09595230701247681. [DOI] [PubMed] [Google Scholar]
- 17.Latka MH, Hagan H, Kapadia F, Golub ET, Bonner S, et al. A randomized intervention trial to reduce the lending of used injection equipment among injection drug users infected with hepatitis C. American Journal of Public Health. 2008;98:853–861. doi: 10.2105/AJPH.2007.113415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kapadia F, Latka MH, Hagan H, Golub ET, Campbell JV, et al. Design and feasibility of a randomized behavioral intervention to reduce distributive injection risk and improve health-care access among hepatitis C virus positive injection drug users: The Study to Reduce Intravenous Exposures (STRIVE) Journal of Urban Health-Bulletin of the New York Academy of Medicine. 2007;84:99–115. doi: 10.1007/s11524-006-9133-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Des Jarlais DC, Paone D, Milliken J, Turner CF, Miller H, et al. Audio-computer interviewing to measure risk behaviour for HIV among injecting drug users: a quasi-randomised trial. The Lancet. 1999;353:1657–1661. doi: 10.1016/s0140-6736(98)07026-3. [DOI] [PubMed] [Google Scholar]
- 20.Murphy DA, Durako S, Muenz LR, Wilson CM. Marijuana use among HIV-positive and high-risk adolescents: a comparison of self-report through audio computer-assisted self-administered interviewing and urinalysis. American Journal of Epidemiology. 2000;152:805–813. doi: 10.1093/aje/152.9.805. [DOI] [PubMed] [Google Scholar]
- 21.Babor TF, Higgins-Biddle JC, Saunders JB, Monteiro MG. AUDIT: The Alcohol Use Disorders Identification Test: Guidelines for Use in Primary Care. second ed. Geneva: World Health Organization; 2001. [Google Scholar]
- 22.Coady MH, Latka MH, Thiede H, Golub ET, Ouellet L, et al. Housing status and associated differences in HIV risk behaviors among young injection drug users (IDUs) AIDS and Behavior. 2007;11:854–863. doi: 10.1007/s10461-007-9248-1. [DOI] [PubMed] [Google Scholar]
- 23.Gossop M, Browne N, Stewart D, Marsden J. Alcohol use outcomes and heavy drinking at 4–5 years among a treatment sample of drug misusers. Journal of substance abuse treatment. 2003;25:135–143. doi: 10.1016/s0740-5472(03)00129-6. [DOI] [PubMed] [Google Scholar]
- 24.Conigrave KM, Davies P, Haber P, Whitfield JB. Traditional markers of excessive alcohol use. Addiction. 2003;98:31–43. doi: 10.1046/j.1359-6357.2003.00581.x. [DOI] [PubMed] [Google Scholar]
- 25.Rai R, Wilson LE, Astemborski J, Anania F, Torbenson M, et al. Severity and correlates of liver disease in hepatitis C virus-infected injection drug users. Hepatology. 2002;35:1247–1255. doi: 10.1053/jhep.2002.33151. [DOI] [PubMed] [Google Scholar]
- 26.Monto A, Patel K, Bostrom A, Pianko S, Pockros P, et al. Risks of a range of alcohol intake on hepatitis C-related fibrosis. Hepatology. 2004;39:826–834. doi: 10.1002/hep.20127. [DOI] [PubMed] [Google Scholar]
- 27.Jamal MM, Soni A, Quinn PG, Wheeler DE, Arora S, Johnston DE. Clinical features of hepatitis C-infected patients with persistently normal alanine transaminase levels in the Southwestern United States. Hepatology. 1999;30:1307–1311. doi: 10.1002/hep.510300526. [DOI] [PubMed] [Google Scholar]
- 28.Bien TH, Miller WR, Tonigan JS. Brief interventions for alcohol problems: a review. Addiction. 1993;88:315–336. doi: 10.1111/j.1360-0443.1993.tb00820.x. [DOI] [PubMed] [Google Scholar]
- 29.Wachtel T, Staniford M. The effectiveness of brief interventions in the clinical setting in reducing alcohol misuse and binge drinking in adolescents: a critical review of the literature. Journal of Clinical Nursing. 19:605–620. doi: 10.1111/j.1365-2702.2009.03060.x. [DOI] [PubMed] [Google Scholar]
- 30.Zule WA, Costenbader EC, Coomes CM, Wechsberg WM. Effects of a Hepatitis C Virus Educational Intervention or a Motivational Intervention on Alcohol Use, Injection Drug Use, and Sexual Risk Behaviors Among Injection Drug Users. Am J Public Health. 2009;99:S180–S186. doi: 10.2105/AJPH.2007.126854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Galea S, Vlahov D. Social Determinants and the Health of Drug Users: Socioeconomic Status, Homelessness, and Incarceration. Public Health reports. 2002;117:S135–S145. [PMC free article] [PubMed] [Google Scholar]
- 32.Campbell JV, Hagan H, Latka MH, Garfein RS, Golub ET, et al. High prevalence of alcohol use among hepatitis C virus antibody positive injection drug users in three US cities. Drug and Alcohol Dependence. 2006;81:259–265. doi: 10.1016/j.drugalcdep.2005.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shillington AD, Clapp JD. Beer and Bongs: differential problems experienced by older adolescents using alcohol only compared to combined alcohol and marijuana use. American Journal of Drug and Alcohol Abuse. 2002;28:379–397. doi: 10.1081/ada-120002980. [DOI] [PubMed] [Google Scholar]
- 34.Shillington AM, Clapp JD. Heavy alcohol use compared to alcohol and marijuana use: Do college students experience a Difference in Substance use problems? Journal of Drug Education. 2006;36 doi: 10.2190/8PRJ-P8AJ-MXU3-H1MW. [DOI] [PubMed] [Google Scholar]
- 35.Jones C, Weatherburn D. Reducing cannabis consumption. Crime and Justice Bulletin. 2001;60:1–8. [Google Scholar]
- 36.Peters E. University of Vermont PhD Dissertation. 2009. Stopping marijuana increases alcohol use: an experimental verification of drug substitution. URI: http://hdl.handle.net/123456789/229. [Google Scholar]