Abstract
Background
Metal-on-metal bearings in surface arthroplasty are associated with prolonged periods of elevated ion circulation. However, there exists some controversy regarding the effect of different surgical variables on the concentration of metal ions in whole blood of patients after hip resurfacing.
Questions/purposes
We sought to confirm which clinical and radiographic parameters are associated with elevated levels of cobalt, chromium, and molybdenum after unilateral metal-on-metal surface arthroplasty.
Methods
We retrospectively reviewed 91 patients with a minimum followup of 24 months (mean, 37 months; range, 24–55 months). The clinical variables consisted of age, gender, preoperative severity of osteoarthritis, component size, and functional outcome measures using the Harris hip score and UCLA activity score. The radiographic parameters included acetabular inclination and version as well as femoral component alignment from both the anteroposterior and lateral radiographs.
Results
A smaller femoral head diameter was associated with larger levels of cobalt and chromium. We observed a negative correlation between ion levels and the Harris hip score or UCLA score. A larger acetabular inclination showed a direct relationship with the concentration of metal ions. Severity of preoperative osteoarthritis, acetabular version, femoral stem-shaft and valgus angle, and anterior orientation of the femoral component had no effect on the circulating metal ion levels.
Conclusions
The data suggest a smaller implant diameter, larger cup inclination, and lower postoperative functional scores are associated with increased cobalt and chromium levels after metal-on-metal hip resurfacing.
Introduction
Metal-on-metal bearings in surface arthroplasty are associated with prolonged periods of elevated metal ions in the blood [3, 7, 19, 30]. The biologic response to metal alloys is poorly understood but can result in DNA damage, cellular toxicity, and chromosomal changes in vitro [9, 18, 20, 22, 27]. The long-term effects of raised metal ion levels are also unknown, although elevated levels are reportedly related to altered lymphocyte concentrations, metal hypersensitivity, and pseudotumors [11, 13, 16, 17, 26, 31]. Hip resurfacing has become a promising option for young patients with higher activity levels and longer average lifespans, because of the relatively higher failure rate with THA in this patient population. Therefore, it is essential to understand the causes resulting in elevated levels of cobalt (Co), chromium (Cr), and molybdenum (Mo).
A number of factors reportedly increase the concentration of metal ions after surface arthroplasty. Most of the current research has focused on the size and orientation of the acetabular component. A greater component size appears to result in lower volumetric wear rates and a decrease in metal ion release [10, 23, 24]. An increase in the acetabular inclination angle has been associated with elevated ion levels, although some studies are conflicting with many authors recommending the cup be inserted below a wide range from 40° to 55° [10, 15, 21]. Furthermore, two retrieval studies suggest higher acetabular inclination angles are associated with increased wear [4, 32]. The effect of the acetabular version has also been studied showing a rise in metal ions at both extreme anteversion and retroversion [24]. However, the effects of the femoral component alignment as well as other clinical parameters on the circulating metal ion levels remain unclear. Identifying such risk factors may allow for better patient selection and avoiding the technical pitfalls that are associated with an increase in metal ion concentration after hip resurfacing.
Therefore, we sought to confirm whether the following correlated with whole blood metal ion concentrations: (1) age, gender, preoperative severity of osteoarthritis, and postoperative Harris hip scores or UCLA activity scores; (2) acetabular inclination and acetabular version, femoral stem-shaft and the relative alignment of the femoral stem; and (3) femoral head size.
Patients and Methods
We retrospectively assessed all 91 patients who underwent unilateral metal-on-metal hip resurfacing between 2004 and 2006 with a minimum of 2 years followup. We included patients 18 years and older with no other metallic implants or occupational exposure to metal ions. We excluded patients with any systemic illness known to affect the concentration of metal ions such as chronic renal failure, women of childbearing age, and patients with known metal allergies. The mean age of the patients at the time of surgery was 53 years (range, 38–73 years) and included 74 men and 17 women (Table 1). The preoperative diagnosis was osteoarthritis in all but one patient who presented with arthritis subsequent to developmental dysplasia of the hip. Preoperative osteoarthritis was graded according to the Tönnis classification [29]. The median severity of preoperative osteoarthritis was 2 (range, 1–3). All 91 patients had complete radiographic and metal ion data at a minimum of 24 months (mean, 37 months; range, 24–55 months) after unilateral hip resurfacing. We obtained Institutional Review Board and informed consent for the purpose of this study.
Table 1.
Patient characteristics: preoperative and postoperative
| Patient characteristics | Results |
|---|---|
| Number of patients (men:women) | 91 (74:17) |
| Mean age, years (range) | 53 (38–73) |
| Mean followup, months (range) | 37 (24–55) |
| Diagnosis | |
| Osteoarthritis | 90 |
| Developmental dysplasia of the hip | 1 |
| Median preoperative Tönnis score (range) | 2 (1–3) |
| Median preoperative functional outcome scores | |
| Harris hip score (range) | 46 (20–67) |
| UCLA (range) | 4 (2–10) |
| Median postoperative functional outcome scores at latest followup | |
| Harris hip score (range) | 94 (40–99) |
| UCLA (range) | 8 (2–10) |
| Median femoral head size mm (range) | 53 (46–59) |
| Mean acetabular inclination angle degrees (range) | 48 (36–60) |
| Mean acetabular version angle degrees (range) | 13 (−8–40) |
| Mean femoral stem-shaft angle (range) | 143 (128–155) |
| Mean femoral stem varus-valgus alignment degrees (range) | 8 (−17–17) |
We used a single device (the Articular Surface Replacement, DePuy, Warsaw, IN) and were all implanted through a posterior approach by one surgeon (JA). The patient was placed in the lateral decubitus position and the skin incision was made centered over the greater trochanter approximately 10 to 15 cm in length. The short external rotators were removed using cautery followed by a T-shaped posterior capsular incision. The femoral head was then dislocated from the acetabulum. The implant was placed, which consisted of a noncemented monoblock acetabular component and a cemented femoral head. The median femoral head diameter was 53 mm (range, 43–59 mm). The hip was taken through a ROM and assessed for stability. The posterior capsule was then closed and the short external rotators were repaired using transosseous sutures. Each patient was allowed to fully weightbear right after surgery and followed individual physiotherapy.
All patients were followed in the clinic at 6 weeks postoperatively followed by a visit at 3 months and subsequently on a yearly basis. Each visit included clinical and radiographic assessment. The clinical outcomes were measured using the Harris hip score (HHS) [14] and the UCLA activity score [33]. The median preoperative HHS and UCLA scores were 46 and 4, respectively. Radiographs taken consisted of an AP pelvis and AP and crosstable lateral of the operated hip.
One of us (SGB) who was not one of the treating surgeons measured various radiographic parameters on standard AP pelvis and crosstable lateral radiographs. The acetabular inclination and the femoral stem-shaft angle were measured from the AP projection [25]. The acetabular inclination was measured as an angle between the horizontal interteardrop line and the axis of the opening of the acetabular component. This measurement is a reliable method to determine inclination [28]. The alignment of the femoral component in the coronal plane was calculated by subtracting the preoperative neck-shaft angle from the postoperative stem-shaft angle, a positive value indicating relative valgus alignment, whereas a negative value represents varus insertion of the femoral component. The lateral radiograph was used to determine the version of the acetabular component and the orientation of the femoral stem relative to the femoral neck. The acetabular version was assessed from a crosstable lateral radiograph by subtracting the position of the acetabular cup from a line drawn perpendicular to the radiographic table. We considered a femoral stem that was angulated 10° or greater from the main axis of the neck either anterior or posterior, whereas a femoral implant positioned within 10° of the neck axis was designated as neutral [1]. The patients’ mean radiographic parameters were calculated (Table 1).
We measured the levels of Co, Cr, and Mo ions from whole blood using a previously described technique to minimize metal ion contamination [2, 5]. The whole blood from each patient was initially collected using Sarstedt Monovette tubes with 21-gauge needles (Sarstedt, Montreal, Quebec, Canada) and stored at −80°C. Ions were analyzed using inductively coupled plasma-mass spectrometry (SCIEX Elan 6100 DRC ICP-MS system; PerkinElmer Instruments, Norwalk, CT).
We established two femoral head size groups (< 53 mm and > 53 mm). The inclination angles were subdivided into three groups: < 40°, 40°–50°, and > 50°. Given that metal ion data were distributed asymmetrically, we determined the correlation coefficient between the ion data and the clinical (age, head size, HHS, and UCLA scores) and radiographic (stem shaft, inclination, version, and valgus angles) parameters using Spearman’s rank correlation test. This test is a nonparametric measure of statistical dependence between two variables and is also resistant to outliers. For comparison of metal ions between genders and for the preoperative degree of osteoarthritis, the Kruskal-Wallis test was used. The Kruskal-Wallis test is a nonparametric equivalent of a one-way analysis of variance (ANOVA); it is essentially calculated as a regular ANOVA but is resistant to outliers because it uses the ranks of the data. Statistics were performed using StatView (SAS Institute, Cary, NC).
Results
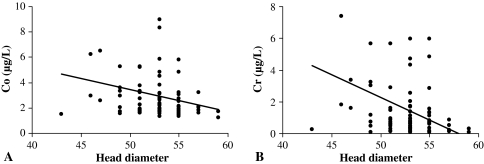
We observed a positive correlation (r = 0.232; p = 0.027) between age and Co levels indicating the older the patient, the more circulating levels of Co. However, there was no effect of age on Cr and Mo levels (Table 2). Men on average had lower (p = 0.009) levels of Cr than females, although there was no difference between gender and levels of Co and Mo (Table 3). We found no association between the degree of preoperative osteoarthritis and metal ion levels (Table 3). We identified a negative correlation between the size of the femoral implant and the levels of Co (r = –0.220; p = 0.036) and Cr (r = −0.349; p = 0.0006), but none with Mo levels (Fig. 1A–B). We found that patients with head sizes < 53 mm had higher Cr levels than patients with head sizes > 53 mm (p = 0.019). When we assessed femoral head size in men and women separately, we observed no difference between the two groups. A negative correlation was observed between the postoperative HHS score and Co levels (r = –0.257; p = 0.014), although there was no correlation with Cr and Mo levels. There was a similar negative correlation between the postoperative UCLA score and Co (r = –0.333; p = 0.001) and Cr levels (r = –0.231; p = 0.027); however, no correlation existed for Mo levels (Table 2). The median postoperative Co, Cr, and Mo levels were 2.100, 0.690, and 1.650 μg/L, respectively, at the last followup.
Table 2.
Clinical parameters
| Parameter | Ion | Correlation | p Value |
|---|---|---|---|
| Age | Co | 0.232 | 0.027 |
| Cr | 0.124 | 0.24 | |
| Mo | 0.053 | 0.62 | |
| Head diameter | Co | −0.220 | 0.036 |
| Cr | −0.349 | 0.0006 | |
| Mo | −0.087 | 0.41 | |
| Harris Hip Score | Co | −0.257 | 0.014 |
| Cr | −0.200 | 0.057 | |
| Mo | −0.145 | 0.17 | |
| UCLA | Co | −0.333 | 0.001 |
| Cr | −0.231 | 0.027 | |
| Mo | −0.045 | 0.67 |
Co = cobalt; Cr = chromium; Mo = molybdenum.
Table 3.
Gender and Tönnis score
| Gender | Co (µg/L) | Cr (µg/L) | Mo (µg/L) | Tönnis | Co (µg/L) | Cr (µg/L) | Mo (µg/L) |
|---|---|---|---|---|---|---|---|
| Male | 2.04 | 0.62 | 1.65 | 2 | 2.02 | 0.62 | 1.70 |
| Female | 2.84 | 1.82 | 1.70 | 3 | 2.10 | 0.60 | 1.67 |
| p Value | 0.068 | 0.009 | 0.83 | p Value | 0.27 | 0.27 | 0.99 |
Co = cobalt; Cr = chromium; Mo = molybdenum.
Fig. 1A–B.
Effect of head diameter on (A) cobalt (Co) and (B) chromium (Cr) levels. Note the negative correlation with increasing size.
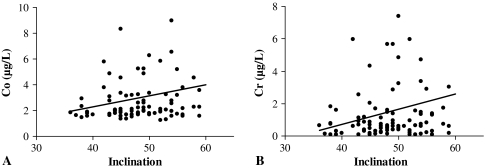
There was a positive correlation between inclination and levels of Co (r = 0.218; p = 0.037) and Cr (r = 0.228; p = 0.030) (Fig. 2A–B). We found no correlation between ion levels in patients with the three groups of inclination angle when analyzing each group individually; however, there was trend for higher Co and Cr throughout each group as the inclination angles increased. We observed no correlations between acetabular version angle and metal ions (Table 4).
Fig. 2A-B.
Effect of acetabular inclination on (A) cobalt (Co) and (B) chromium (Cr) levels. Note the positive correlation with increasing inclination.
Table 4.
Radiographic parameters
| Parameter | Ion | Correlation | p Value |
|---|---|---|---|
| Stem shaft | Co | 0.063 | 0.56 |
| Cr | 0.081 | 0.45 | |
| Mo | −0.045 | 0.67 | |
| Inclination | Co | 0.218 | 0.037 |
| Cr | 0.228 | 0.030 | |
| Mo | −0.114 | 0.28 | |
| Valgus | Co | 0.050 | 0.64 |
| Cr | 0.033 | 0.76 | |
| Mo | 0.081 | 0.45 | |
| Version | Co | 0.036 | 0.76 |
| Cr | 0.100 | 0.38 | |
| Mo | 0.081 | 0.48 |
Co = cobalt; Cr = chromium; Mo = molybdenum.
No radiographic femoral component parameter influenced metal ion levels (Table 4).
Discussion
Metal-on-metal hip implants cause persistent elevations in circulating metal ions. Although the long-term clinical effects of elevated metal ion levels are unclear, they have been associated with adverse lymphocytic reactions, chromosomal aberrations, and cellular toxicity [11, 13, 18, 22, 27, 31]. Therefore, it is important to identify patient and surgical factors that cause an increase in metal ion levels in whole blood given the increasing use of metal bearings in young patients undergoing resurfacing [6, 12]. Whole blood more closely approximates systemic exposure to metal ions compared with serum [8]. Therefore, we sought to confirm whether (1) age, gender, preoperative severity of osteoarthritis, and postoperative Harris hip scores or UCLA activity scores; (2) acetabular inclination and acetabular version, femoral stem-shaft and the relative alignment of the femoral stem; and (3) femoral head size correlated with whole blood metal ion concentrations.
There are several limitations to our study. First, the majority of our patients were men (74 patients) with only 17 women. This may account for the positive correlation between female gender and elevated Cr ions. In general, smaller femoral components were inserted in female patients, which likely contributed to the increase in Cr ion level [23, 24]. The median head size in women was 49 mm, whereas that in men was 53 mm. This finding is similar to that previously published in which Co and Cr levels were higher in women, although this was explained by a smaller femoral implant in women [10]. Second, when we analyzed the ion levels in the head size of women and men separately and the three acetabular inclination angles, we found no correlation. This could be explained by the reduced number of patients in each group. Third, the radiographic parameters were measured by a single observer and, therefore, we were unable to calculate inter- or intrarater precision. Lastly, we also arbitrarily classified the femoral stem position on the lateral radiograph as anterior, neutral, or posterior depending on whether the stem deviated from the main neck axis by 10° or greater. We chose to use these criteria as previously described by Amstutz et al. because it was difficult to consistently define the main axis of the femoral neck objectively on the lateral radiograph [1].
We found smaller diameter head sizes in hip resurfacing were associated with higher levels of Co and Cr. Langton et al. reported similar findings and found that femoral components 53 mm or greater resulted in lower ion levels compared with femoral heads 51 mm or less [23] (Table 5). They believed larger bearings cause a thicker fluid film and a reduction in diametric clearance, thus decreasing wear and metal ion release. We also found that patients with head sizes < 53 mm had higher Cr levels than patients with head sizes > 53 mm (p = 0.019), but no difference in Co and Mo levels. The group with head sizes < 53 mm contained fewer patients and was likely underpowered which could explain the absence of a statistically significant difference for Co.
Table 5.
Review of recent literature on variables affecting whole blood metal ion concentrations after hip resurfacing
| Authors | Type of implant (number of patients) | Cup inclination | Cup version | Femoral head size | Functional outcome scores |
|---|---|---|---|---|---|
| Daniel et al. (2009) [7] | BHR (26) | No relationship between ion levels and inclination | NA | NA | No relationship between ion levels and levels of activity |
| De Haan et al. (2008) [10] | BHR (155), Conserve plus (50), ASR (8), Durom resurfacing system (1) | 55° | NA | NA | NA |
| Co = 9.8 μg/L | |||||
| Cr = 9.7 μg/L | |||||
| < 55° | |||||
| Co = 2.4 μg/L | |||||
| Cr = 3.6 μg/L | |||||
| Hart et al. (2008) [15] | BHR (26) | 50° | NA | NA | NA |
| Co = 4.45 ppb | |||||
| Cr = 4.30 ppb | |||||
| < 50° | |||||
| Co = 1.60 ppb | |||||
| Cr = 1.88 ppb | |||||
| *Khan et al. (2008) [21] | BHR (15), Cormet 2000 hip resurfacing (6) | R2 = 0.47 (p = 0.032) | NA | NA | NA |
| *Langton et al. (2008) [23] | ASR (76) | Co = 0.399 (p < 0.001) | Co = 0.177 (p = 0.131) | Co = −0.306 (p = 0.008) | UCLA score Cr Small heads = −0.190 (p = 0.844) |
| Cr = 0.297 (p = 0.011) | Cr = 0.132 (p = 0.258) | Cr = −0.307 (p = 0.007) | Large heads = −0.361 (p = 0.154) | ||
| *Langton et al. (2009) [24] | ASR (90) | Co = 0.417 (p < 0.001) | Co = 0.308 (p = 0.006) | Co = −0.248 (p = 0.024) | UCLA score Co = −0.368 (p = 0.001) |
| Cr = 0.312 (p = 0.005) | Cr = 0.250 (p = 0.026) | Cr = −0.283 (p = 0.010) | Cr = −0.325 (p = 0.104) | ||
| BHR (70) | Co = −0.009 (p = 0.949) | Co = 0.285 (p = 0.037) | Co = −0.173 (p = 0.178) | UCLA score Co = 0.234 (p = 0.239) | |
| Cr = −0.048 (p = 0.731) | Cr = 0.115 (p = 0.406) | Cr = −0265 (p = 0.038) | Cr = 0.109 (p = 0.585) | ||
| *Vendittoli et al. (2007) [30] | Durom resurfacing system (64) | Inclination angle independent predictor of [Co] at 1 year | NA | Head size inversely proportional to Cr R2 = 0.089 (p = 0.018) Co was not significant | No influence on Co and Cr |
| *Desy et al. [current article] | ASR (91) | Co = 0.218 (p = 0.037) | Co = 0.036 (p = 0.76) | Co = −0.220 (p = 0.036) | HHS Co = −0.257 (p = 0.014) |
| Cr = −0.20 (p = 0.057) | |||||
| Cr = 0.228 (p = 0.030) | Cr = 0.100 (p = 0.38) | Cr = −0.349 (p = 0.0006) | UCLA score Co = −0.333 (p = 0.001) | ||
| Cr = −0.231 (p = 0.027) |
* Metal ion data expressed as correlation coefficients; BHR = Birmingham hip resurfacing; ASR = articular surface replacement; Co = cobalt; Cr = chromium; NA = not available.
The cause of lower metal ion levels seen in patients with higher functional outcome scores is unknown. The Co levels were negatively correlated with both the HHS and UCLA scores, whereas lower Cr levels were associated with a higher UCLA score. A recent study also showed a negative correlation between Co levels and the UCLA score, although no difference was observed with Cr [24] (Table 5). Another study by De Haan et al. reported no relationship between metal ion levels and patient activity as measured with the UCLA score [10]. Given that there was a negative correlation between HHS and UCLA scores along with Co and Cr levels, this could indicate that patients who do better clinically generate less metal ions or have the ability to better excrete circulating ions. It is also possible that more accurately positioned implants may result in greater patient satisfaction and functional outcome scores as well as lower metal ions.
When we assessed the alignment of the acetabular component and its effect on metal ion levels, we found that only the acetabular inclination angle correlated with metal ion levels. A higher inclination angle resulted in greater Co and Cr release. Hart et al. also found inclination greater than 50° resulted in higher metal ion levels, although only three patients in their study actually had an acetabular component that was inserted with an abduction angle greater than 50° [15] (Table 5). Another study reported a higher concentration of metal ions with inclination greater than 55° [10]. However, these authors analyzed the means instead of the medians, which was influenced by a greater number of outliers in the group with a steep acetabular cup. Langton et al. also showed higher levels of Co and Cr with a larger inclination angle, but this effect was seen only in implants with a smaller head diameter [23, 24]. Furthermore, when we further divided our cohort into separate groups of < 40°, 40°–50°, and > 50°, we found no correlation within each group. This is likely explained by the reduced number of patients within each group. However, each group did demonstrate a trend toward increasing metal ion levels as the inclination angle increased in each group. We observed no effect of version angle on metal ion levels. This is in contrast to another study [24] that found a positive correlation between ion levels and acetabular anteversion less than 10° and greater than 20°. This relationship was only observed with smaller component sizes (51 mm or less), whereas version had no effect on larger heads.
In two studies Langton et al. [23, 24] found no correlation between the stem-shaft angle and metal ion levels. Our data using the position of the femoral stem on the AP and lateral radiographs confirm their findings.
Our data confirm various clinical and radiographic parameters are associated with higher levels of circulating Co and Cr in whole blood. Of the clinical factors studied, it appears that patients with lower HHS and UCLA scores and patients with smaller femoral head sizes are each associated with higher metal ion levels. Gender and age have an effect on Cr and Co levels, respectively, although these results should be interpreted cautiously. Larger inclination angles are also associated with higher metal ion levels but the version angle did not have any effect on Co, Cr, or Mo levels. Similarly, it appears the position of the femoral component does not influence metal ion levels. By better identifying the parameters that influence Co and Cr levels, metal ion release may be decreased after metal-on-metal hip resurfacing along with its associated deleterious effects.
Acknowledgments
We thank Maricar Alminia and Laura Desrosiers for their help in the administration of the questionnaires, recording the clinical data, and collecting the blood samples.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at the Division of Orthopaedic Surgery, McGill University, SMBD-Jewish General Hospital, Montreal, Quebec, Canada.
References
- 1.Amstutz HC, Beaule PE, Dorey FJ, Duff MJ, Campbell PA, Gruen TA. Metal-on-metal hybrid surface arthroplasty: two to six-year follow-up study. J Bone Joint Surg Am. 2004;86:28–39. [PubMed] [Google Scholar]
- 2.Antoniou J, Zukor DJ, Mwale F, Minarik W, Petit A, Huk OL. Metal ion levels in the blood of patients after hip resurfacing: a comparison between twenty-eight and thirty-six-millimeter-head metal-on-metal prostheses. J Bone Joint Surg Am. 2008;90(Suppl 3):142–148. doi: 10.2106/JBJS.H.00442. [DOI] [PubMed] [Google Scholar]
- 3.Back DL, Young DA, Shimmin AJ. How do serum cobalt and chromium levels change after metal-on-metal hip resurfacing? Clin Orthop Relat Res. 2005;438:177–181. doi: 10.1097/01.blo.0000166901.84323.5d. [DOI] [PubMed] [Google Scholar]
- 4.Campbell P, Beaule PE, Ebramzadeh E, LeDuff M, Smet K, Lu Z, Amstutz HC. The John Charnley Award: a study of implant failure in metal-on-metal surface arthroplasties. Clin Orthop Relat Res. 2006;453:35–46. doi: 10.1097/01.blo.0000238777.34939.82. [DOI] [PubMed] [Google Scholar]
- 5.Case CP, Ellis L, Turner JC, Fairman B. Development of a routine method for the determination of trace metals in whole blood by magnetic sector inductively coupled plasma mass spectrometry with particular relevance to patients with total hip and knee arthroplasty. Clin Chem. 2001;47:275–280. [PubMed] [Google Scholar]
- 6.Daniel J, Pynsent PB, McMinn DJ. Metal-on-metal resurfacing of the hip in patients under the age of 55 years with osteoarthritis. J Bone Joint Surg Br. 2004;86:177–184. doi: 10.1302/0301-620X.86B2.14600. [DOI] [PubMed] [Google Scholar]
- 7.Daniel J, Ziaee H, Pradhan C, McMinn DJ. Six-year results of a prospective study of metal ion levels in young patients with metal-on-metal hip resurfacings. J Bone Joint Surg Br. 2009;91:176–179. doi: 10.1302/0301-620X.91B2.21654. [DOI] [PubMed] [Google Scholar]
- 8.Daniel J, Ziaee H, Pynsent PB, McMinn DJ. The validity of serum levels as a surrogate measure of systemic exposure to metal ions in hip replacement. J Bone Joint Surg Br. 2007;89:736–741. doi: 10.1302/0301-620X.89B6.18141. [DOI] [PubMed] [Google Scholar]
- 9.Davies AP, Sood A, Lewis AC, Newson R, Learmonth ID, Case CP. Metal-specific differences in levels of DNA damage caused by synovial fluid recovered at revision arthroplasty. J Bone Joint Surg Br. 2005;87:1439–1444. doi: 10.2106/JBJS.C.00949. [DOI] [PubMed] [Google Scholar]
- 10.Haan R, Pattyn C, Gill HS, Murray DW, Campbell PA, Smet K. Correlation between inclination of the acetabular component and metal ion levels in metal-on-metal hip resurfacing replacement. J Bone Joint Surg Br. 2008;90:1291–1297. doi: 10.1302/0301-620X.90B10.20533. [DOI] [PubMed] [Google Scholar]
- 11.Gawkrodger DJ. Metal sensitivities and orthopaedic implants revisited: the potential for metal allergy with the new metal-on-metal joint prostheses. Br J Dermatol. 2003;148:1089–1093. doi: 10.1046/j.1365-2133.2003.05404.x. [DOI] [PubMed] [Google Scholar]
- 12.Graves SE, Davidson D, Ingerson L, Ryan P, Griffith EC, McDermott BF, McElroy HJ, Pratt NL. The Australian Orthopaedic Association National Joint Replacement Registry. Med J Aust. 2004;180:S31–S34. doi: 10.5694/j.1326-5377.2004.tb05911.x. [DOI] [PubMed] [Google Scholar]
- 13.Hallab NJ, Anderson S, Caicedo M, Skipor A, Campbell P, Jacobs JJ. Immune responses correlate with serum-metal in metal-on-metal hip arthroplasty. J Arthroplasty. 2004;19:88–93. doi: 10.1016/j.arth.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 14.Harris WH. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. J Bone Joint Surg Am. 1969;51:737–755. [PubMed] [Google Scholar]
- 15.Hart AJ, Buddhdev P, Winship P, Faria N, Powell JJ, Skinner JA. Cup inclination angle of greater than 50 degrees increases whole blood concentrations of cobalt and chromium ions after metal-on-metal hip resurfacing. Hip Int. 2008;18:212–219. doi: 10.1177/112070000801800304. [DOI] [PubMed] [Google Scholar]
- 16.Hart AJ, Hester T, Sinclair K, Powell JJ, Goodship AE, Pele L, Fersht NL, Skinner J. The association between metal ions from hip resurfacing and reduced T-cell counts. J Bone Joint Surg Br. 2006;88:449–454. doi: 10.2106/JBJS.E.01077. [DOI] [PubMed] [Google Scholar]
- 17.Hart AJ, Skinner JA, Winship P, Faria N, Kulinskaya E, Webster D, Muirhead-Allwood S, Aldam CH, Anwar H, Powell JJ. Circulating levels of cobalt and chromium from metal-on-metal hip replacement are associated with CD8 + T-cell lymphopenia. J Bone Joint Surg Br. 2009;91:835–842. doi: 10.1302/0301-620X.91B6.21844. [DOI] [PubMed] [Google Scholar]
- 18.Haynes DR, Rogers SD, Hay S, Pearcy MJ, Howie DW. The differences in toxicity and release of bone-resorbing mediators induced by titanium and cobalt-chromium-alloy wear particles. J Bone Joint Surg Am. 1993;75:825–834. doi: 10.2106/00004623-199306000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Heisel C, Streich N, Krachler M, Jakubowitz E, Kretzer JP. Characterization of the running-in period in total hip resurfacing arthroplasty: an in vivo and in vitro metal ion analysis. J Bone Joint Surg Am. 2008;90(Suppl 3):125–133. doi: 10.2106/JBJS.H.00437. [DOI] [PubMed] [Google Scholar]
- 20.Huk OL, Catelas I, Mwale F, Antoniou J, Zukor DJ, Petit A. Induction of apoptosis and necrosis by metal ions in vitro. J Arthroplasty. 2004;19:84–87. doi: 10.1016/j.arth.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 21.Khan M, Kuiper JH, Richardson JB. The exercise-related rise in plasma cobalt levels after metal-on-metal hip resurfacing arthroplasty. J Bone Joint Surg Br. 2008;90:1152–1157. doi: 10.1302/0301-620X.90B9.20243. [DOI] [PubMed] [Google Scholar]
- 22.Ladon D, Doherty A, Newson R, Turner J, Bhamra M, Case CP. Changes in metal levels and chromosome aberrations in the peripheral blood of patients after metal-on-metal hip arthroplasty. J Arthroplasty. 2004;19:78–83. doi: 10.1016/j.arth.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 23.Langton DJ, Jameson SS, Joyce TJ, Webb J, Nargol AV. The effect of component size and orientation on the concentrations of metal ions after resurfacing arthroplasty of the hip. J Bone Joint Surg Br. 2008;90:1143–1151. doi: 10.1302/0301-620X.90B9.20785. [DOI] [PubMed] [Google Scholar]
- 24.Langton DJ, Sprowson AP, Joyce TJ, Reed M, Carluke I, Partington P, Nargol AV. Blood metal ion concentrations after hip resurfacing arthroplasty: a comparative study of articular surface replacement and Birmingham Hip Resurfacing arthroplasties. J Bone Joint Surg Br. 2009;91:1287–1295. doi: 10.1302/0301-620X.91B10.22308. [DOI] [PubMed] [Google Scholar]
- 25.Massin P, Schmidt L, Engh CA. Evaluation of cementless acetabular component migration. An experimental study. J Arthroplasty. 1989;4:245–251. doi: 10.1016/S0883-5403(89)80020-8. [DOI] [PubMed] [Google Scholar]
- 26.Pandit H, Glyn-Jones S, McLardy-Smith P, Gundle R, Whitwell D, Gibbons CL, Ostlere S, Athanasou N, Gill HS, Murray DW. Pseudotumours associated with metal-on-metal hip resurfacings. J Bone Joint Surg Br. 2008;90:847–851. doi: 10.1302/0301-620X.90B7.20213. [DOI] [PubMed] [Google Scholar]
- 27.Rae T. A study on the effects of particulate metals of orthopaedic interest on murine macrophages in vitro. J Bone Joint Surg Br. 1975;57:444–450. [PubMed] [Google Scholar]
- 28.Tannast M, Langlotz U, Siebenrock KA, Wiese M, Bernsmann K, Langlotz F. Anatomic referencing of cup orientation in total hip arthroplasty. Clin Orthop Relat Res. 2005;436:144–150. doi: 10.1097/01.blo.0000157657.22894.29. [DOI] [PubMed] [Google Scholar]
- 29.Tonnis D, Heinecke A. Acetabular and femoral anteversion: relationship with osteoarthritis of the hip. J Bone Joint Surg Am. 1999;81:1747–1770. doi: 10.2106/00004623-199912000-00014. [DOI] [PubMed] [Google Scholar]
- 30.Vendittoli PA, Mottard S, Roy AG, Dupont C, Lavigne M. Chromium and cobalt ion release following the Durom high carbon content, forged metal-on-metal surface replacement of the hip. J Bone Joint Surg Br. 2007;89:441–448. doi: 10.1302/0301-620X.89B4.18054. [DOI] [PubMed] [Google Scholar]
- 31.Willert HG, Buchhorn GH, Fayyazi A, Flury R, Windler M, Koster G, Lohmann CH. Metal-on-metal bearings and hypersensitivity in patients with artificial hip joints. A clinical and histomorphological study. J Bone Joint Surg Am. 2005;87:28–36. doi: 10.2106/JBJS.A.02039pp. [DOI] [PubMed] [Google Scholar]
- 32.Witzleb WC, Hanisch U, Ziegler J, Guenther KP. In vivo wear rate of the Birmingham Hip Resurfacing arthroplasty. A review of 10 retrieved components. J Arthroplasty. 2009;24:951–956. doi: 10.1016/j.arth.2008.06.022. [DOI] [PubMed] [Google Scholar]
- 33.Zahiri CA, Schmalzried TP, Szuszczewicz ES, Amstutz HC. Assessing activity in joint replacement patients. J Arthroplasty. 1998;13:890–895. doi: 10.1016/S0883-5403(98)90195-4. [DOI] [PubMed] [Google Scholar]