Abstract
Background
Total ankle arthroplasty (TAA) implantation is increasing, as the potential for pain relief and restoration of function and risks are compared with those for ankle fusion. A previous analysis with a simple decision tree suggested TAA was cost-effective compared with ankle fusion. However, reevaluation is warranted with the availability of newer, more costly implants and longer-term patient followup data.
Questions/purposes
Considering all direct medical costs regardless of the payer, we determined if TAA remains a cost-effective alternative to ankle fusion when updated evidence is considered.
Patients and Methods
Using a Markov model, we evaluated expected costs and quality-adjusted life years (QALY) for a 60-year-old hypothetical cohort with end-stage ankle arthritis treated with either TAA or ankle fusion. Costs were estimated from 2007 diagnosis-related group (DRG) and current procedural terminology (CPT) codes for each procedure. Rates were extracted from the literature. The incremental cost-effectiveness ratio (ICER), a measure of added cost divided by QALY gained for TAA relative to ankle fusion, was estimated. To identify factors affecting the value of TAA, sensitivity analyses were performed on all variables.
Results
TAA costs $20,200 more than ankle fusion and resulted in 1.7 additional QALY, with an ICER of $11,800/QALY gained. Few variables in the sensitivity analyses resulted in TAA no longer being cost-effective.
Conclusion
Despite more costly implants and longer followup, TAA remains a cost-effective alternative to ankle fusion in a 60-year-old cohort with end-stage ankle arthritis.
Level of Evidence
Level II, economic and decision analyses. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Ankle arthritis has an incidence of 1984 per 100,000 patients [3], which is eight times less frequent than knee or hip arthritis in clinical practice [4]. The physical and mental impairments for patients with ankle arthritis are equivalent to that of hip arthritis and other severely disabling medical problems [10, 21, 23]. Patients for whom nonoperative management fails may be offered surgery. Ankle fusion reduces pain at the cost of loss of ankle motion, altered gait kinematics [19, 27], and accelerated arthritis in surrounding joints [5]. Patients also have a 3% to 10% risk of nonunion [13, 20]. TAA in contrast offers greater ankle ROM, which may improve gait kinematics [19], decrease the stress on other joints, and potentially lead to less arthritis in surrounding joints. However, TAA is more expensive and has its own complications including: implant loosening, ankle instability, osteolysis, higher infection rates, and higher reoperation rates [13, 17, 20, 25].
A decision analysis is valuable in weighing two alternatives against each other by systematically assigning probabilities, costs, and utilities that are estimates of quality of life to treatment alternatives based on reported evidence-based data. The question of whether TAA is a cost-effective alternative to ankle fusion is an ideal question for decision analysis, as there is some uncertainty regarding rates of TAA revision and development of ipsilateral arthritis. The Panel on Cost-effectiveness in Health and Medicine created by the United States Public Health Service [11] has established methodologic guidelines to frame a cost-effectiveness study question, identify outcomes, estimate costs, and test uncertainty [11] that were adhered to in this study.
The current study is a special type of cost-effectiveness study termed cost-utility analysis. The effectiveness of each procedure or health state is measured in utility units that incorporate subjective measures of wellness or quality of life [11]. Each health state in the model is assigned a utility value along a continuum of 1.0 equaling perfect health and 0.0 equaling death based on quality of well-being index scores [9, 10, 23, 24, 26]. The utility for each health state then is multiplied by the number of years a patient experiences that health state and is reported in QALY. A QALY is the recommended effectiveness measure for cost-effectiveness analyses of health interventions [11]. SooHoo and Kominski performed a simple decision analysis on this topic, showing TAA would be cost-effective compared with an ankle fusion if the arthroplasty survived 10 years [24]. Since that analysis, nonrandomized cohort studies of intermediate outcomes for TAA have been published [8, 14–16, 20, 22, 25, 28]. Furthermore, a more sophisticated Markov decision analysis can assess yearly changes in TAA revision rates and complications during a patient’s lifetime. Therefore, reevaluation of the comparison is warranted using a Markov decision analysis with updated data.
We determined if TAA is cost-effective compared with ankle fusion in a hypothetical 60-year-old cohort. Secondarily, we used sensitivity analyses to identify when the value of TAA exceeded $100,000/QALY gained, a commonly used ‘cost-effectiveness’ benchmark [18]. The sensitivity analyses focused on (1) TAA and ankle fusion revision rates; (2) the risk of perioperative complications or risk of future ipsilateral arthritis for each procedure; (3) procedure costs; (4) patient age; and (5) the health outcome utilities (QALY) gained.
Materials and Methods
We created a hypothetical 60-year-old cohort with end-stage ankle arthritis for whom nonoperative management had failed. The cohort age and demographics reflect the mean age and demographics among participants in the largest TAA and ankle fusion studies [20, 28]. The TAA study had approximately 50% females and 50% males [20]. The clinical pathway modeled began with patients undergoing a TAA or ankle fusion. Then a Markov cost-effectiveness decision model was created to analyze health outcomes and direct medical costs. Patients who survived surgery were followed annually, transitioning between health states until they died or reached 100 years of age [11] (Fig. 1). Each health state was assigned a utility [9] value to represent quality of life in QALY and a cost measured in 2007 US dollars. The model was constructed using decision-analysis software (TreeAge Pro 2009; TreeAge Software, Inc; Williamstown, MA, USA). When the model was run, TAA and ankle fusion arms of the tree were compared based on the number of years patients spent in each health state with the associated QALY and costs. Costs and utilities were discounted at a rate of 3% per year (range, 0%–5% per year in sensitivity analyses) [11].
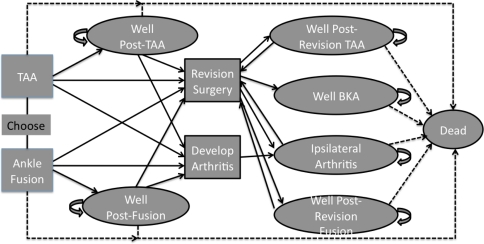
Fig. 1.
Individuals initially undergo either TAA or ankle fusion. As the model progresses patients enter different health states, represented by the ovals in the diagram, based on reported rates in the literature. A certain percentage of patients will die after the initial surgery, represented by the dashed arrow to the Dead state. Individuals who survive the initial surgery begin in either the Well Post-TAA or Well Post-Fusion health state. Patients may remain in either of these two health states for several years, represented by the larger curved arrows. Eventually patients either will die and move to the Dead state, or need a revision surgery or have arthritis develop and transition to Revision Surgery or Develop Arthritis. After revision surgery, patients either will enter the Well Post-Revision TAA or Well Post-Revision Fusion health state. A small percentage of patients having revision TAA and revision ankle fusions also will undergo a BKA and enter the Well BKA health state. Patients who have additional areas of arthritis develop either will enter the Ipsilateral Arthritis health state or undergo revision surgery. Patients might remain in each of the health states (ie, Well Post-TAA, Well Post-Fusion, Well Post-Revision TAA, Well BKA) for several years, again represented by the large curved arrows. The dashed lines show age-specific transitions from each health state to death based on US life-table mortality rates.
Several assumptions were made in model construction: (1) age-specific mortality rates for patients who survive surgery follow 2004 US life table norms [2]; (2) perioperative mortality after any ankle surgery was estimated at 0.02% [28]; (3) patients who need a revision TAA will undergo one revision surgery and then have conversion surgery to an ankle fusion or have below-knee amputation (BKA), based on the prevalence of TAAs that progress to BKA as reported in the literature [13, 25]; (4) patients who have ankle fusion will undergo one revision and possibly one fusion/BKA [13, 25], then have no additional surgeries until death, however, patients who have ankle fusion still could have progressive ipsilateral arthritis; and (5) if patients undergo fusion after TAA or revision fusion, all ipsilateral arthritic joints will be fused simultaneously, leading to improved QALY for individuals in the fusion compared with the arthritis states.
Model parameters for revision and complication rates were obtained from published studies (Table 1). Ankle fusion revision rates were estimated from data reported by SooHoo et al. [25]. The Scandinavian Total Ankle Replacement (STAR; Small Bone Innovations, Morrisville, PA, USA) prosthesis, a mobile-bearing design, was used for modeling TAA survivorship. Three different fixed-bearing implants and one newer mobile-bearing implant are approved for use in the United States. Some evidence suggests greater longevity in STAR mobile-bearing prostheses over standard fixed-bearing designs [16, 20, 28]. Furthermore, the study used for the reference case has the largest reported series of TAAs (200 patients), with some of the longest followup data published (range, 5–13 years), and greater than 80% survivorship [28]. This study was used to estimate annual revision rates for Years 1 to 6 [28]. For Year 7 and onward, revision rates were estimated at 0.021 based on the average observed revision rates from Years 6 to 11 (Table 2) [28]. There are currently no 15- to 20-year followup studies. We tested TAA yearly survival rates from 2% to 20% in a sensitivity analysis using published rates [1, 8, 13–16, 20, 22, 25].
Table 1.
Model parameters from literature review and pooled results
| Description | Value | Range | References |
|---|---|---|---|
| Probability of death | |||
| Baseline probability death for age, gender, race | 2004 US life tables | 2004 US life tables | 2 |
| Probability of death within 30 days of ankle surgery | 0.02% | – | 28 |
| Probability of major short-term complications | |||
| TAA | 20% | 5%–25% | 16, 20, 25, 28 |
| TAA revision | 40% | – | Estimated |
| Ankle fusion | 7% | 0.5%–10% | 13, 20, 25 |
| Revision ankle fusion | 14% | – | Estimated |
| Yearly rates – ipsilateral arthritis | |||
| After TAA | 1.5% | 0%–6% | 16, 25, 28 |
| After ankle fusion | 4% | 0%–6% | 5, 25 |
| Yearly rates – revision surgery | |||
| TAA failure | see Table 2 | 2%–20% | 1, 8, 13–16, 20, 22, 25, 28 |
| TAA revision failure | see Table 2 | – | Estimated |
| Ankle fusion failure | 1% | 1%–10% | 25 |
| Yearly rates – BKA | |||
| BKA after revision TAA | 0.2% | 0.2%–1% | 13, 25 |
| BKA after revision fusion | 1% | 0.2%–5% | 13, 24 |
TAA = total ankle arthroplasty; BKA = below-knee amputation.
Table 2.
Annual probability of total ankle arthroplasty
| Years | Rate of implant failure requiring revision | Rate of revision TAA implant failure requiring revision |
|---|---|---|
| 1 | 0.03 | 0.06 |
| 2 | 0.021 | 0.042 |
| 3 | 0.005 | 0.01 |
| 4 | 0 | 0 |
| 5 | 0.012 | 0.024 |
| 6 | 0.024 | 0.048 |
| 7 and beyond | 0.021 | 0.042 |
TAA = total ankle arthroplasty.
Data on the occurrence and rate of short-term complications were obtained from SooHoo et al. [25] for ankle fusions and Saltzman et al. [20] for the STAR TAA based on 2 years of followup. The complications included were: postoperative infections, major wound problems, nontraumatic bone fractures, implant problems, or osteolysis [20, 25]. Other reports of complication rates between 5% and 25% for TAA [1, 13, 16] and 0.5% to 10% for ankle fusion [13, 20, 25] were used to establish ranges for sensitivity analyses.
Coester et al. reported the rate of radiographic and clinical arthritis progressing in the ipsilateral hindfoot and forefoot after ankle fusion is 100% at 22 years of followup [5]. That calculates to an average progression of arthritis of 4.5% per year, which is consistent with 2.8% of ankle fusion patients underwent subtalar fusion within 5 years [25]. The rate of progressing ipsilateral arthritis after TAA (based on various implants) is 0.7% to 3% per year [25, 28]. In theory, this is attributable to increased motion of the ankle leading to decreased stress being placed on surrounding foot joints. Young patients after ankle fusion or TAA will experience consequences of lower utilities from progressive ipsilateral foot arthritis. The progressive arthritis rate is not well defined in the literature; and therefore is an important variable for sensitivity analyses.
The costs of TAA, ankle fusion, and ankle revision surgeries were estimated from Medicare 2007 payment schedules (Table 3). The total reimbursement was the sum of the DRG code payment, professional charges based on 2007 CPT codes, and implant costs. The latter were estimated at $200 for the two screws needed for an ankle fusion and $9000 for TAA implants [7]. The surgeon fees, anesthesiologist fees, hospital charges, and physical therapy charges are assumed to be equivalent as operative time and length of hospital stay are similar between the two procedures, and therefore do not need to be added to the total costs in this model. A yearly TAA followup office charge was added to the TAA costs but not to ankle fusion costs as life-long followup is not necessary for patients who have fusion. The costs of short-term complications for TAA and ankle fusion were estimated at $2000, considering rehospitalization and treatment costs.
Table 3.
Variables used in the model with associated utilities
| Variables | Utilities | Reference | Costs |
|---|---|---|---|
| Total ankle arthroplasty | 0.9 | 24 | $19,650 |
| Revision total ankle arthroplasty | 0.8 | 24 | $16,230 |
| Primary ankle fusion | 0.8 | 24 | $6580 |
| Revision ankle fusion | 0.75 | Estimated | $6580 |
| Below-knee amputation | 0.6 | Estimated | $11,180 |
| Ipsilateral foot arthritis | 0.7 | 24 | – |
| Short-term toll complication | −0.5 | Estimated | $2000 |
| Short-term toll total ankle arthroplasty | −0.125 | Estimated | – |
| Short-term toll ankle fusion and below-knee amputation | −0.25 | Estimated | – |
All health states were assigned utilities ranging from 0.0 (representing death) to 1.0 (representing perfect health) based on reported quality of well-being index scores (Table 3) [9, 10, 23, 24, 26]. Utilities estimated by SooHoo and Kominski [24] were used (Table 3). Arthritis of the lower extremity was assigned a utility near 0.7 from SF-36 surveys, similar to values of other lower extremity impairments [10, 26]. TAA was given a utility of 0.9 [24], which is higher than that of ankle fusion (0.8), as studies suggest improved functional outcomes after TAA, including gait and ROM [6, 19, 20, 27]. A recent study reported equivalent utilities of 0.73 one year postoperatively for patients who had TAA and ankle fusion [23]. These values were used in a sensitivity analysis, but not in the primary analysis because the study was not a randomized controlled trial. The study included an older TAA group compared with the ankle fusion group, and had a 50% attrition rate [23]. No differences in utilities based on race or gender have been established; therefore, the utilities were not stratified by gender or race.
Short-term negative postoperative experiences (eg, pain, limited mobility) were accounted for with acute procedure tools (ie, ‘disutilities’). The disutility of having a TAA was −0.125 owing to patients wearing a cast for 6 weeks (1.5 months divided by 12 months). The disutility of ankle fusion or BKA was −0.25 owing to the 3 months of nonweightbearing and limited mobility (3 months divided by 12 months). Revision arthroplasty and revision fusion also were assigned disutilities of −0.125 and −0.25, respectively, as the healing time for revision surgery is similar to that for primary procedures (Table 3).
To determine if TAA is cost-effective compared with ankle fusions, we calculated the ICER by dividing the difference in costs by the difference in utilities (QALY) between TAA and ankle fusion. This ratio reflects how much the improved quality of life of TAA compares with the less expensive ankle fusion. This ICER calculation then can be used to compare TAA with different medial therapies across all specialties. One-way sensitivity analyses were performed by changing the range of values of one variable while keeping all other variables static. These analyses determine when the cost per QALY gained for TAA relative to ankle fusion surgery exceeded the willingness-to-pay threshold of $100,000/QALY [18]. From these analyses, the importance of the uncertainty present in the literature and which variables will change the conclusions of the model can be determined. Sensitivity analyses were performed on the revision surgery rate, risk of perioperative complications or risk of future ipsilateral arthritis for each procedure, procedure costs, patient age, and health utilities for each procedure.
Results
TAA remained cost-effective compared with ankle fusion in a 60-year-old hypothetical cohort. TAA costs $28,000 compared with $7900 for ankle fusion, which is an incremental cost of $20,200. TAA had higher average utility of 14.4 QALY compared with 12.7 QALY for ankle fusion, which is an average utility gain of 1.7 QALY. When the incremental cost was divided by the average utility gained, TAA had an ICER of $11,800/QALY gained (Table 4). The discount rate had minimal impact on the value of TAA, with ICERs ranging from $9600 to $13,300 per QALY gained as the annual discount rate varied from 0% to 5% per year.
Table 4.
Results of cost-effectiveness analysis
| Procedure | Average cost | Incremental cost ($) | Average utility gained (QALY) | Incremental effectiveness (QALY) | ICER ($/QALY) |
|---|---|---|---|---|---|
| Ankle fusion | $7900 | 12.7 | |||
| TAA | $28,000 | $20,200 | 14.4 | 1.70 | $11,800 |
TAA = total ankle arthroplasty; QALY = quality adjusted life-years; $ = US dollars.
Incremental cost = increased cost of TAA over ankle fusion; Incremental effectiveness = increased utility of TAA over ankle fusion; ICER = incremental cost-effectiveness ratio.
Most variables tested in sensitivity analyses did not change the cost-effectiveness of TAA compared with ankle fusion. Sensitivity analyses revealed that TAA, fusion, or revision surgery failure rates did not influence the analysis. Nor did short-term complication and ipsilateral arthritis rates of TAA or ankle fusion change the model. Ankle fusions would need to cost greater than $30,000 for TAA to cost less and provide improved utilities compared with ankle fusion. If TAA costs more than $170,000, the $100,000/QALY cost-effectiveness threshold is reached and TAA is no longer a cost-effective alternative. TAA was more cost-effective for 30-year-old patients (ICER = $9600/QALY) compared with 80-year-old patients (ICER = $18,000/QALY).
In sensitivity analyses, varying the utilities of TAA and ankle fusion did affect the model. If the TAA utility was 0.05 lower than the ankle fusion utility, the TAA ICER exceeded the $100,000/QALY cost-effectiveness threshold. If the TAA utility were 1, the TAA ICER would be as low as $6900. If the utility after ankle fusion was one-tenth lower than the current value of 0.8, the cost-effectiveness of TAA would improve to $7300/QALY. In contrast, if the ankle fusion utility increased by one-tenth, the ICER for TAA would increase to $30,600/QALY. TAA remained a cost-effective strategy under both scenarios. The $100,000/QALY threshold is exceeded by TAA only when the utility for an ankle fusion is greater than 0.95. In another scenario, when the utilities of TAA and ankle fusion were both made 0.73, TAA remained cost-effective at $71,300/QALY. Under this scenario, only when the utility of an ankle fusion was greater than 0.74 or the utility of TAA was less than 0.72, did the TAA ICER exceed $100,000/QALY.
Discussion
End-stage ankle arthritis leads to decreases in physical and mental quality of life [10, 21, 23]. The traditional surgery for ankle arthritis is ankle fusion. These patients may have improvements in pain but lose ankle motion, have progressive hindfoot and midfoot arthritis develop [5], and lose normal gait patterns [19, 27]. TAA has the benefits of maintaining ankle motion but the risks of implant failures, wound infections, and need for complicated revision surgery [13, 17, 20, 25]. A previous analysis with a simple decision tree suggested TAA was cost-effective compared with ankle fusion [24]. The purposes of the current study were to reevaluate if TAA remains cost-effective compared with ankle fusion with more costly implants and longer-term followup, and to determine which are the most important variables contributing to the cost-effectiveness of TAA based on sensitivity analyses.
Although we have outlined many assumptions of our model, we acknowledge the following limitations. First, Markov decision analyses are dependent on the strength of the data used for the model. Although Markov analyses are superior to simple decision analyses, which cannot account for changing variables across time, we still are limited by the deficiencies in the literature. There are no prospective studies with similar cohorts comparing the two procedures on which to base model assumptions. A multicentered, randomized, controlled or case-controlled trial on TAA versus ankle fusions may provide data to update the current deficiencies, including the probabilities of short-term complications, ipsilateral foot arthritis, revision surgery after 11 years, and postoperative utilities. Second, we estimated costs for this model. The reference case was set at an age of 60 years; however, the costs were estimated from a gross-costing methodology from the Medicare perspective. As TAA implants cost between $8700 and $9500 [7] and an ankle fusion implant costs $200 for two screws per procedure, the implant cost should be a major contribution to the hospital costs, but are not reflected in the reimbursement rate of $10,651 for the longer, more technically demanding TAA compared with $6577 for an ankle fusion. Therefore, the implant costs were added to the reimbursement costs to determine the net costs in the model. More precise estimates of costs could be found with microcosting techniques [11]. Third, our analysis included only direct medical costs and did not include measures of indirect costs such as loss of work productivity and caregivers time, which are difficult to estimate. Fourth, the utilities were estimated from a previous cost-effectiveness analysis [24] and may overestimate the health state achieved by TAA [23]. However, we found in the sensitivity analysis that TAA remains cost-effective when the utilities of TAA and ankle fusion are equivalent. Only if an ankle fusion has a higher utility than TAA would TAA no longer be considered cost-effective. Finally, the cost-effectiveness threshold of $100,000 per QALY has been set as the current standard in the literature [18] and is helpful in comparing different medical therapies. However, this threshold is arbitrary and the orthopaedic community may in the future set a different threshold to eliminate procedures that are too costly per utility gained.
TAA is a cost-effective alternative to ankle fusion in a 60-year-old cohort. The average cost for TAA was higher, but there was improved QALY gained compared with ankle fusions. SooHoo and Kominski performed a cost-effectiveness analysis using a simple decision tree rather than a Markov model [24]. The two models had a similar ICER for the respective reference cases: their $18,000/QALY [24] compared with $11,800/QALY in our study. However, a Markov model is more precise for this decision as the rate of implant failure changes, the rate of ipsilateral foot arthritis increases, and patients may require several revisions during a lifetime. We also used long-term followup data for TAA [28] that were not available to SooHoo and Kominski in 2004. We updated the costs from their 1998 estimate to a more current 2007 estimation.
Most variables tested in the sensitivity analyses did not change the cost-effectiveness analysis. Even if future literature were to show widely different rates than currently reported, TAA will remain a cost-effective alternative to ankle fusions. The yearly TAA implant failure rate, risk of perioperative complications, and future ipsilateral arthritis for each procedure did not influence the model. A wide range of TAA survivor curves (70%–90% 5-year survival) have been reported [1, 8, 13–16, 20, 22, 25, 28], and it is unknown how fast ipsilateral arthritis develops. The costs of TAA and ankle fusion affected the model only if the costs were increased to implausible amounts. TAA was cost-effective for patients between 30 to 80 years old. Although, TAA is more cost-effective for younger populations who can benefit for a longer time with improved utilities, optimal candidates for TAA may be older, thin patients with low-activity demands [12].
The utilities of TAA and ankle fusion did influence the model in sensitivity analyses. If the TAA utility was 0.05 QALY lower than the ankle fusion utility or if the ankle fusion utility was 0.15 QALY higher than TAA, then TAA was no longer a cost-effective alternative. A recent study estimated from 1-year postoperative SF-36 scores that patients with ankle arthritis after TAA and ankle fusion have similar utilities of 0.73 QALY [23]. When these data were used in the Markov model, TAA remained cost-effective at $71,300/QALY. Therefore, TAA must have an equivalent or improved utility compared with ankle fusion to remain cost-effective.
In this analysis, we assessed the trade-offs among risk of progressive arthritis in surrounding joints, perioperative complications, risk of revision surgeries, age of the patient, costs, and utilities. Costs and utilities were the major cost-effectiveness drivers that we identified in sensitivity analyses. Physicians traditionally decide which patients meet the indications for surgery. In the future, American policymakers, insurance companies, or hospital administrators may decide which health services are cost-effective and which services have too high a risk-benefit ratio to be offered. This study supports TAA as a cost-effective alternative to ankle fusion.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
No IRB approval was necessary as the study did not include human subjects or animal data.
References
- 1.Anderson T, Montgomery F, Carlsson A. Uncemented STAR total ankle prostheses: three to eight-year follow-up of fifty-one consecutive ankles. J Bone Joint Surg Am. 2003;85:1321–1329. [PubMed] [Google Scholar]
- 2.Arias E. United States life tables, 2004. Natl Vital Stat Rep. 2007;56:1–39. [PubMed] [Google Scholar]
- 3.Brown TD, Johnston RC, Saltzman CL, Marsh JL, Buckwalter JA. Posttraumatic osteoarthritis: a first estimate of incidence, prevalence, and burden of disease. J Orthop Trauma. 2006;20:739–744. doi: 10.1097/01.bot.0000246468.80635.ef. [DOI] [PubMed] [Google Scholar]
- 4.Buckwalter JA, Saltzman C, Brown T. The impact of osteoarthritis: implications for research. Clin Orthop Relat Res. 2004;427(suppl):S6–15. doi: 10.1097/01.blo.0000143938.30681.9d. [DOI] [PubMed] [Google Scholar]
- 5.Coester LM, Saltzman CL, Leupold J, Pontarelli W. Long-term results following ankle arthrodesis for post-traumatic arthritis. J Bone Joint Surg Am. 2001;83:219–228. doi: 10.2106/00004623-200102000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Doets HC, Middelkoop M, Houdijk H, Nelissen RG, Veeger HE. Gait analysis after successful mobile bearing total ankle replacement. Foot Ankle Int. 2007;28:313–322. doi: 10.3113/FAI.2007.0313. [DOI] [PubMed] [Google Scholar]
- 7.Eisner W. FDA ortho panel recommends STAR ankle PMA approval: with elephant in the room. Orthopedics. 2009;3:9–12. [Google Scholar]
- 8.Fevang BT, Lie SA, Havelin LI, Brun JG, Skredderstuen A, Furnes O. 257 ankle arthroplasties performed in Norway between 1994 and 2005. Acta Orthop. 2007;78:575–583. doi: 10.1080/17453670710014257. [DOI] [PubMed] [Google Scholar]
- 9.Fryback DG, Dasbach EJ, Klein R, Klein BE, Dorn N, Peterson K, Martin PA. The Beaver Dam Health Outcomes Study: initial catalog of health-state quality factors. Med Decis Making. 1993;13:89–102. doi: 10.1177/0272989X9301300202. [DOI] [PubMed] [Google Scholar]
- 10.Glazebrook M, Daniels T, Younger A, Foote CJ, Penner M, Wing K, Lau J, Leighton R, Dunbar M. Comparison of health-related quality of life between patients with end-stage ankle and hip arthrosis. J Bone Joint Surg Am. 2008;90:499–505. doi: 10.2106/JBJS.F.01299. [DOI] [PubMed] [Google Scholar]
- 11.Gold MR, Siegel JE, Russell LB, Weinstein MC. Cost-Effectiveness in Health and Medicine. New York, NY: Oxford University Press; 1996. [Google Scholar]
- 12.Guyer AJ, Richardson EG. Current concepts review: total ankle arthroplasty. Foot Ankle Int. 2008;29:256–264. doi: 10.3113/FAI.2008.0256. [DOI] [PubMed] [Google Scholar]
- 13.Haddad SL, Coetzee JC, Estok R, Fahrbach K, Banel D, Nalysnyk L. Intermediate and long-term outcomes of total ankle arthroplasty and ankle arthrodesis: a systematic review of the literature. J Bone Joint Surg Am. 2007;89:1899–1905. doi: 10.2106/JBJS.F.01149. [DOI] [PubMed] [Google Scholar]
- 14.Henricson A, Skoog A, Carlsson A. The Swedish Ankle Arthroplasty Register: an analysis of 531 arthroplasties between 1993 and 2005. Acta Orthop. 2007;78:569–574. doi: 10.1080/17453670710014248. [DOI] [PubMed] [Google Scholar]
- 15.Hosman AH, Mason RB, Hobbs T, Rothwell AG. A New Zealand national joint registry review of 202 total ankle replacements followed for up to 6 years. Acta Orthop. 2007;78:584–591. doi: 10.1080/17453670710014266. [DOI] [PubMed] [Google Scholar]
- 16.Karantana A, Hobson S, Dhar S. The Scandinavian total ankle replacement: survivorship at 5 and 8 years comparable to other series. Clin Orthop Relat Res. 2009;468:951–957. doi: 10.1007/s11999-009-0971-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knecht SI, Estin M, Callaghan JJ, Zimmerman MB, Alliman KJ, Alvine FG, Saltzman CL. The Agility total ankle arthroplasty: seven to sixteen-year follow-up. J Bone Joint Surg Am. 2004;86:1161–1171. [PubMed] [Google Scholar]
- 18.Laupacis A, Feeny D, Detsky AS, Tugwell PX. How attractive does a new technology have to be to warrant adoption and utilization? Tentative guidelines from using clinical and economic evaluations. CMAJ. 1992;146:473–481. [PMC free article] [PubMed] [Google Scholar]
- 19.Piriou P, Culpan P, Mullins M, Cardon JN, Pozzi D, Judet T. Ankle replacement versus arthrodesis: a comparative gait analysis study. Foot Ankle Int. 2008;29:3–9. doi: 10.3113/FAI.2008.0003. [DOI] [PubMed] [Google Scholar]
- 20.Saltzman CL, Mann RA, Ahrens JE, Amendola A, Anderson RB, Berlet GC, Brodsky JW, Chou LB, Clanton TO, Deland JT, DeOrio JK, Horton GA, Lee TH, Mann JA, Nunley JA, Thordarson DB, Walling AK, Wapner KL, Coughlin MJ. Prospective controlled trial of STAR total ankle replacement versus ankle fusion: initial results. Foot Ankle Int. 2009;30:579–596. doi: 10.3113/FAI.2009.0579. [DOI] [PubMed] [Google Scholar]
- 21.Saltzman CL, Zimmerman MB, O’Rourke M, Brown TD, Buckwalter JA, Johnston R. Impact of comorbidities on the measurement of health in patients with ankle osteoarthritis. J Bone Joint Surg Am. 2006;88:2366–2372. doi: 10.2106/JBJS.F.00295. [DOI] [PubMed] [Google Scholar]
- 22.Skytta ET, Koivu H, Eskelinen A, Ikavalko M, Paavolainen P, Remes V. Total ankle replacement: a population-based study of 515 cases from the Finnish Arthroplasty Register. Acta Orthop. 2010;81:114–118. doi: 10.3109/17453671003685459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Slobogean GP, Younger A, Apostle KL, Marra CA, Wing K, Penner M, Daniels T, Glazebrook M. Preference-based quality of life of end-stage ankle arthritis treated with arthroplasty or arthrodesis. Foot Ankle Int. 2010;31:563–566. doi: 10.3113/FAI.2010.0563. [DOI] [PubMed] [Google Scholar]
- 24.SooHoo NF, Kominski G. Cost-effectiveness analysis of total ankle arthroplasty. J Bone Joint Surg Am. 2004;86:2446–2455. doi: 10.2106/00004623-200411000-00014. [DOI] [PubMed] [Google Scholar]
- 25.SooHoo NF, Zingmond DS, Ko CY. Comparison of reoperation rates following ankle arthrodesis and total ankle arthroplasty. J Bone Joint Surg Am. 2007;89:2143–2149. doi: 10.2106/JBJS.F.01611. [DOI] [PubMed] [Google Scholar]
- 26.Tengs TO, Wallace A. One thousand health-related quality-of-life estimates. Med Care. 2000;38:583–637. doi: 10.1097/00005650-200006000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Thomas R, Daniels TR, Parker K. Gait analysis and functional outcomes following ankle arthrodesis for isolated ankle arthritis. J Bone Joint Surg Am. 2006;88:526–535. doi: 10.2106/JBJS.E.00521. [DOI] [PubMed] [Google Scholar]
- 28.Wood PL, Prem H, Sutton C. Total ankle replacement: medium-term results in 200 Scandinavian total ankle replacements. J Bone Joint Surg Br. 2008;90:605–609. doi: 10.2106/JBJS.G.00685. [DOI] [PubMed] [Google Scholar]