Abstract
Background
Muscle atrophy impacts almost every patient seen for orthopaedic conditions. Unfortunately, no effective treatment is available to date. Matrix metalloproteinases (MMPs), especially MMP-2, are involved in skeletal muscle atrophy. MMP-2 null mice reportedly have substantially reduced muscle atrophy after tendon transection compared with wild-type mice, suggesting MMP-2 plays an important role in muscle atrophy. Although the exact mechanisms remain unknown, a newly-discovered intracellular form of MMP-2 suggests a possible novel mechanism of MMP-2 digesting muscle matrix during muscle atrophy. I propose a new pharmacologic treatment for muscle atrophy using selective MMP-2 inhibitors.
Questions/Hypothesis
I hypothesize: (1) intracellular MMP-2 plays an important role during muscle atrophy by digesting intramuscular matrix; (2) AP-1 and NFAT signal transduction pathways are responsible for expression and activation of the intracellular MMP-2 during muscle atrophy; and (3) specific MMP-2 inhibitors can serve as a novel pharmacologic strategy in treating disuse-induced muscle atrophy.
Method of Study
Expression and activity of extracellular and intracellular MMP-2 will be determined in a mouse tendon transection model. The role of AP-1 and NFAT signal transduction pathways in MMP-2 transcriptional regulation in muscle atrophy will be determined using chromatin-immunoprecipitation (ChIP) and small interfering RNA (siRNA). I also will test the feasibility of treating muscle atrophy using selective MMP-2 inhibitors.
Significance
Understanding the signaling transduction pathway of extracellular and intracellular MMP-2 expression during muscle atrophy may lead to novel treatments for muscle atrophy that preserve the normal physiologic function of MMP-2.
Hypothesis
I hypothesize: (1) intracellular MMP-2 plays an important role during muscle atrophy by digesting intramuscular matrix; (2) AP-1 and NFAT signal transduction pathways are responsible for expression and activation of intracellular MMP-2 during muscle atrophy; and (3) specific MMP-2 inhibitors can serve as a novel pharmacologic strategy in treating disuse-induced muscle atrophy.
Background
Atrophy is a harmful consequence of skeletal muscles deprived of normal activity and often leads to major difficulties with mobility and other activities of daily living [2, 7, 10]. Decreased muscle protein synthesis and increased muscle degradation are believed to be responsible for the loss of muscle mass in muscle atrophy. Multiple proteases, including the ubiquitin-proteasome system, lysosomal proteases, and calcium-dependent calpains, are involved in skeletal muscle atrophy. Studies have indicated that MMPs also are involved in this common muscle disorder. A recent study suggests an intracellular MMP-2 form can digest intracellular matrix under certain pathologic circumstances, such as oxidative stress in the cardiac muscles [4]. I have observed increased expression of MMP-2, MMP-9 [8], and MMP-13 (unpublished data) during muscle atrophy after tendon transection. However, substantially reduced muscle atrophy was observed only in MMP-2 knockout mice, but not in the MMP-9 knockout mice [5] and MMP-13 knockout mice (unpublished data). These findings suggest MMP-2 plays a specific critical role during muscle atrophy.
MMP-2 is tightly regulated at transcription, translation, and posttranslation levels in myocytes. Tissue inhibitors of metalloproteinases (TIMPs), especially TIMP-2, play an important role in regulating the activity of MMP-2. TIMP-2 plays a complicated role in regulating the activity of MMP-2. It binds to MMP-2 and inactivates it. However, it also facilitates binding of MMP-2 to MMP-14 (MMP-2 activator), thus activating MMP-2 [9]. An alternative transcription of MMP-2 gene results in the pathologic intracellular form of MMP-2 during oxidative injury in cardiac muscles (Oral communication, David Lovett MD, University of California – San Francisco. August 10, 2010). This intracellular MMP-2 remains in myocytes and digests various intracellular substrates, including troponin I in cardiac muscle [1].
Proposed Program
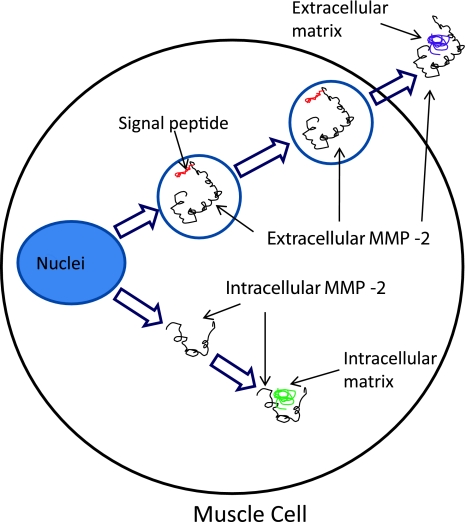
I will examine the expression and activity of extracellular and intercellular MMP-2 in muscle atrophy. RT-PCR, Western-blot, and zymography will be used to study the expression of both MMP-2 isoforms in atrophic muscle. Furthermore, immunohistochemistry and electron microscopy will be used, if necessary, to detect subcellular distribution of intracellular MMP-2 in atrophic muscle (Fig. 1).
Fig. 1.
A schematic shows extracellular and intracellular MMP-2 in muscle atrophy. Normal transcription of MMP-2 generates extracellular MMP-2 with a signal peptide, which directs it to the extracellular matrix. An alternative transcription mechanism generates an intracellular form of MMP-2 without the signal peptide. The intracellular MMP-2 remains inside the muscle cell and results in intracellular matrix degradation of the muscle.
First, two-dimensional electrophoresis and pull-down assay will be used to identify intracellular and extracellular substrates of MMP-2 during muscle atrophy. MMP-2 knockout mice will be used to study the functional role of MMP-2 during muscle atrophy. In this study the role of MMP-2 in the activation of the NFκB pathway in muscle atrophy will be studied by comparing wild-type and MMP-2 knockout mice.
The transcription regulation and corresponding signal transduction pathways responsible for intracellular MMP-2 during muscle atrophy will be investigated. I will study the role of AP-1 and NFAT signal transduction pathways in MMP-2 transcriptional regulation in muscle atrophy using chromatin-immunoprecipitation (ChIP) and small interfering RNA (siRNA).
I recently reported reduced muscle atrophy after 2 weeks of treatment with selective MMP-2 inhibitor [6]. No notable side effects were observed in the MMP-2 inhibitor-treated animals in this study. In the current proposed study, the role of selective and nonselective MMP-2 inhibitors will be investigated in treating muscle atrophy in a mouse tendon transection model.
Limitations
Inhibiting MMP-2 activity with a MMP-2 inhibitor may cause undesired side effects by blocking normal physiologic functions of MMP-2, such as wound healing and tissue remodeling in patients with muscle atrophy. However, this problem may be avoided by limiting application of MMP-2 inhibitors in a relatively short period in patients before physical rehabilitation or other measurements can be applied (eg, during casting period). Furthermore, observations from the proposed studies may help distinguish a signal transduction pathway that is responsible only for intracellular MMP-2 expression, thus helping to design selective treatments that target this pathologic intracellular MMP-2 isoform.
Next Steps
The next steps of this study will focus on examining the role of MMP-2 inhibitors in treating muscle atrophy through clinical trials. In bench studies, the feasibility of treating muscle atrophy will be tested by blocking the expression and activation of the pathologic intracellular MMP-2.
Numerous synthesized MMP inhibitors have been in clinical trials since the 1980s. To date, at least 58 clinical trials for nine different MMP inhibitors have been conducted. Many of these MMP inhibitors have in vivo MMP-2 inhibitory activities [3]. Although various MMP inhibitors have been tested for treatment of cancers and other diseases, to date none has been tested for treatment of skeletal muscle atrophy. A clinical trial testing the role of MMP-2 inhibitor in treating muscle atrophy will be initiated.
The molecular mechanisms of MMP-2 expression and activation during muscle atrophy will be studied, and the specific signal transduction pathways responsible for extracellular and intracellular MMP-2s will be identified. The feasibility of blocking intracellular MMP-2 expression by blocking specific signal transduction pathways will be tested.
Vision of the Future
In the next decade, I expect MMP-2 inhibitors will become routine treatment for patients with muscle atrophy, at least when physical rehabilitation or other measurements are not applicable. Hopefully this research on the functional role of MMP-2 in muscle atrophy will lead to more basic and clinical research of this unique and interesting MMP family member. MMP-2 has many functions beyond the extracellular matrix which need to be revealed.
Acknowledgments
I thank the ORS Grant Writing Workshop for the education provided. I appreciate my mentors, Drs Hubert T. Kim and David H. Lovett (SFVAMC and UCSF), for their intensive mentorship. I also thank the Orthopaedic Research & Education Foundation and the Illinois Bone and Joint Institute for research support.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Footnotes
The author certifies that he has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
References
- 1.Bergman MR, Teerlink JR, Mahimkar R, Li L, Zhu BQ, Nguyen A, Dahi S, Karliner JS, Lovett DH. Cardiac matrix metalloproteinase-2 expression independently induces marked ventricular remodeling and systolic dysfunction. Am J Physiol Heart Circ Physiol. 2007;292:H1847–H1860. doi: 10.1152/ajpheart.00434.2006. [DOI] [PubMed] [Google Scholar]
- 2.Booth FW, Seider MJ. Recovery of skeletal muscle after 3 months of hindlimb immobilization in rats. J Appl Physiol. 1979;47:435–439. doi: 10.1152/jappl.1979.47.2.435. [DOI] [PubMed] [Google Scholar]
- 3.Flingleton B. MMP inhibitor clinical trials—the past, present, and future. In: Edwards D, Høyer-Hansen G, Blasi F, Sloane BF, editors. The Cancer Degradome. New York, NY: Springer Inc; 2008. pp. 759–785. [Google Scholar]
- 4.Kandasamy AD, Chow AK, Ali MA, Schulz R. Matrix metalloproteinase-2 and myocardial oxidative stress injury: beyond the matrix. Cardiovascular Res. 2010;85:413–423. doi: 10.1093/cvr/cvp268. [DOI] [PubMed] [Google Scholar]
- 5.Liu X, Lee DJ, Skittone LK, Natsuhara K, Kim HT. Role of gelatinases in disuse-induced skeletal muscle atrophy. Muscle Nerve. 2010;41:174–178. doi: 10.1002/mus.21463. [DOI] [PubMed] [Google Scholar]
- 6.Liu X, Manzano G, Kim HT. Effect of MMP-2 Inhibitor on Skeletal Muscle Atrophy after Tendon Rupture. 56th Annual Meeting of the Orthopaedic Research Society. New Orleans, LA, 2010.
- 7.Meada H, Kimmel DB, Raab DM, Lane NE. Musculoskeletal recovery following hindlimb immobilization in adult female rats. Bone. 1993;14:153–159. doi: 10.1016/8756-3282(93)90242-3. [DOI] [PubMed] [Google Scholar]
- 8.Skittone LK, Liu X, Tseng A, Kim HT. Matrix metalloproteinase-2 expression and promoter/enhancer activity in skeletal muscle atrophy. J Orthop Res. 2008;26:357–363. doi: 10.1002/jor.20513. [DOI] [PubMed] [Google Scholar]
- 9.Yu AE, Murphy AN, Stetler-Stevenson WG. 72-kDa gelatinase (gelatinase A): structure, activation, regulation, and substrates specificity. In: Parks WC, Mecham RP, editors. Matrix Metalloproteinases. San Diego, CA: Academic Press Inc; 1998. pp. 85–113. [Google Scholar]
- 10.Zarzhevsky N, Carmeli E, Fuchs D, Coleman R, Stein H, Reznick AZ. Recovery of muscles of old rats after hindlimb immobilisation by external fixation is impaired compared with those of young rats. Exp Gerontol. 2001;36:125–140. doi: 10.1016/S0531-5565(00)00189-3. [DOI] [PubMed] [Google Scholar]