Abstract
Background
High medium-term survivorship of hip resurfacing arthroplasty in young patients has led to its increased usage. To achieve high survival rates, selecting patients with appropriate proximal femoral morphology and bone quality is important. For patients with poor bone quality or abnormal morphology, the mid-head resection technique is an alternative, bone-conserving procedure but whether this technique results in acceptable complications and survival is unknown.
Questions/purposes
We therefore assessed (1) implant survivorship of a mid-head resection device during short- and medium-term followup, (2) hip function, (3) adverse radiographic features emphasizing proximal stress shielding, and (4) complications.
Methods
We retrospectively reviewed 164 patients (171 hips) who underwent reconstruction with the Birmingham Mid-Head Resection device (Smith and Nephew Orthopaedics Ltd, Warwick, UK) between 2003 and 2008. Patients were reviewed with hip outcome questionnaires, clinical examination, and radiographs. We report findings in 156 of these 171 hips with a minimum followup of 2 years (mean, 3.5 years, range, 2–7.5 years). They include three successive iterations based on the same design rationale.
Results
There were four revisions during this period, including two femoral failures, giving 3.5-year survivorships of 97.4% and 98.7% with revision or reoperation for any reason and femoral failure as the end points, respectively. No patient is currently awaiting revision. Average hip function was 98%, as assessed by Oxford hip score. Five of the 87 intermediate-iteration (V1) stems showed proximal femoral stress shielding, a phenomenon not observed in the other two iterations. Four patients had asymptomatic below-knee deep venous thrombosis and one had nonfatal pulmonary embolism, all of which resolved uneventfully.
Conclusions
The mid-head resection technique can circumvent the need for a more invasive procedure such as standard THA in patients who would benefit from a conservative arthroplasty but do not possess good femoral head bone quality or morphology.
Level of Evidence
Level IV, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
An arthroplasty device implanted in a young, active patient must not only withstand high initial demands but also last for an extended period. This makes the threat of multiple revisions in the future a real possibility. Therefore, a bone-conserving procedure such as hip resurfacing arthroplasty is theoretically advantageous in young patients. The medium-term survivorship (96%–99% at 3–10 years) of modern hip resurfacing [2, 6, 24] has led to its increasing usage [18], but results are subject to proper patient selection [4, 23] and accurate implantation. Poor bone quality and distorted femoral head and neck anatomy can jeopardize the long-term results of a hip resurfacing, with cumulative failure rates varying from 7% to 12% at 9 years [2, 5].
Extensive femoral head osteonecrosis [2, 13], cystic degeneration of the femoral head [1], and periarticular osteopenia [4, 20] are risk factors for femoral failure with resurfacing. Abnormal morphology of the proximal femur, as in slipped capital femoral epiphysis (SCFE), severe Legg-Calvé-Perthes disease (LCPD), and developmental hip dysplasia (DDH), also increase the risk of failure [2, 4, 5, 23]. In the above conditions, the only alternative to a resurfacing procedure has been a stemmed THA, until recently.
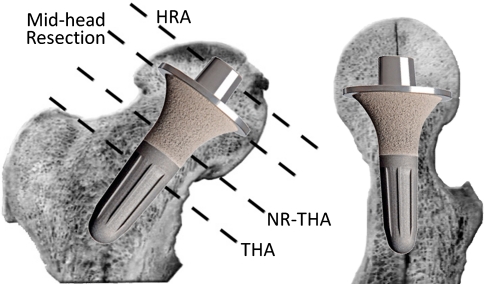
In an attempt to provide a less invasive hip arthroplasty device, several neck-retaining short stem devices were introduced [8, 10, 19]. Most of these devices, whether short or long stemmed, employ a femoral resection level at or distal to the head-neck junction (Fig. 1). Distal to the head-neck junction, the internal profile of the femur begins to widen. Therefore, to achieve secure fixation, the implants have to obtain purchase on the inner cortex of the upper femur, or the medullary canal must be filled with a large amount of metal. The site of load bearing is then predominantly distal to the neck, leading to the risk of proximal stress shielding of the neck.
Fig. 1.
An image shows the resection levels in THA and neck-retaining THA (NR-THA) at or distal to the femoral head-neck junction from where the interior of the femur widens downward. Only a MHR is at a level where a cone can be created so a conical stem can be fitted. Being distal to the resection level of a traditional hip resurfacing (HRA) eliminates the part of the femoral head where avascular and cystic changes often occur.
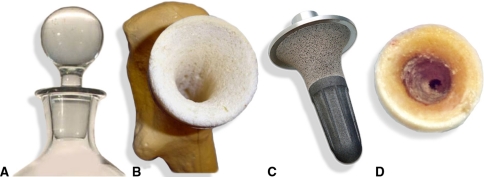
The exclusive feature of the mid-head resection (MHR) technique is its osteotomy level (Fig. 1), which runs through the middle of the femoral head. Being distal to the traditional resurfacing level, it eliminates poor-quality bone located in the proximal femoral head (osteonecrosis). The base of the femoral head, along with the neck, provides an excellent convergent conical profile (Fig. 2), which offers the perfect geometry for robust stem fixation. Continued load bearing through the head-neck segment prevents stress shielding deterioration of the neck, a feature that has bedeviled other neck-preserving THAs [9]. While this device has theoretical advantages, it has not yet been shown whether these are realized.
Fig. 2A–D.
Images show (A) the analogy of the robust fix of a bottle stopper, (B) a Sawbones® model prepared for the MHR stem, (C) a VST stem, and (D) the intraoperative appearance of the internal cone prepared in the proximal femur for the BMHR. Reprinted with permission of SLACK Inc from Daniel J, Pradhan C, Ziaee H, McMinn DJ. A clinicoradiologic study of the Birmingham Mid-Head Resection Device. Orthopedics. 2008;31(12 Suppl 2). pii: orthosupersite.com/view.asp?rID = 37186.
We therefore assessed (1) implant survivorship of a MHR device during short- and medium-term followup, (2) hip function, (3) adverse radiographic features emphasizing proximal stress shielding, and (4) complications.
Patients and Materials
We retrospectively reviewed 164 patients (171 hips) who underwent a hip arthroplasty using the MHR technique between January 2003 and December 2008. One hundred fifty-seven of these arthroplasties were unilateral procedures. The ideal candidate for this technique was a young or active patient who would benefit from a conservative arthroplasty but had poor bone quality or suboptimal proximal femoral morphology. Therefore, the indications for this device were arthritis secondary to femoral head osteonecrosis, primary or secondary osteoarthritis with severe cystic change in the femoral head, femoral morphology unsuitable for a resurfacing (as in severe SCFE and LCPD), or DDH with superolateral osteopenia in the uncovered portion of the femoral head. Besides those that apply to hip arthroplasty in general and to metal-on-metal arthroplasty in particular, we considered the following to be contraindications for this procedure: generalized osteoporosis, a relatively older, inactive patient unlikely to outlast a standard hip arthroplasty, and severe leg length discrepancy, especially if it was contributed by a site other than the arthritic hip. The primary pathologies in this cohort of hips included femoral head osteonecrosis (24), hip dysplasia (15), post-LCPD disease (8), severe SCFE (15), and others (109), including destructive type of osteoarthritis, osteoarthritis with severe cystic changes in the femoral head, and NSAID-induced destruction (Table 1). The patients’ mean age at operation was 57 years (range, 16–82 years). There were 121 men and 50 women. Their mean height, weight, and body mass index were 174 cm (range, 151–203 cm), 80 kg (range, 46–140 kg), and 26 (range, 17–42), respectively. In terms of implant survivorship, no patients were lost to followup. No patients were recalled specifically for this study; all data were obtained from medical records and radiographs obtained during these followup visits. One hundred fifty-six of these 171 hips completed a minimum followup of 2 years (mean, 3.5 years, range, 2–7.5 years).
Table 1.
Breakdown of the different diagnoses in the 171 hips included in the study
| Diagnosis | Entire cohort | Curved stem | V1 stem | VST stem |
|---|---|---|---|---|
| Femoral head AVN | 24 | 5 | 12 | 7 |
| DDH | 15 | 3 | 9 | 3 |
| Post-LCPD | 8 | 1 | 4 | 3 |
| SCFE | 15 | 0 | 9 | 6 |
| Others | 109 | 4 | 53 | 52 |
| Total with minimum 2-year followup | 156 | 13 | 87 | 56 |
| Total | 171 | 13 | 87 | 71 |
VST = visual stop technology; AVN = avascular necrosis; DDH = developmental hip dysplasia; LCPD = Legg-Calvé-Perthes disease; SCFE = slipped capital femoral epiphysis.
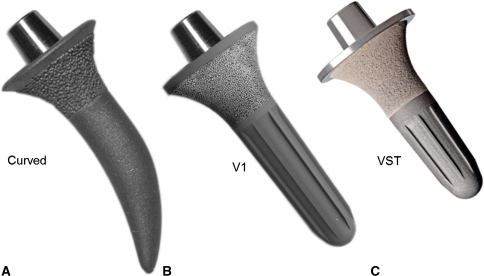
The device used was the Birmingham Mid-Head Resection (BMHR; Smith and Nephew Orthopaedics Ltd, Warwick, UK), the first 13 of which were of the curved-stem variety; the next 87 were straight V1 stems, and the last 56 were visual stop technology (VST) stems. The strategic resection level and proximal frustoconical design of the stem offered the advantages of conservative design, robust stem fixation, and avoidance of stress shielding of the femoral neck. The cup and the bearing were the same as a Birmingham Hip Resurfacing (BHR; Smith and Nephew Orthopaedics Ltd) device and the instrumentation was similar, allowing seamless transition during the operation from a BHR to a BMHR if the bone quality or morphology dictated.
The curved stem (Fig. 3) was introduced in 2003. Good early clinical, radiographic, and 2-year roentgen stereogrammetric analysis results were seen with this device, but the curved distal part of the stem required free hand rasping, which could lead to inaccurate bone cuts. Hence, the V1 BMHR, with a straight stem and distal flutes, was introduced in May 2006. Implantation was possible using rotating instruments on a guide bar. In patients with a short femoral neck, the tip of the V1 stem approached the lateral cortex of the femur, or if placed in valgus, it approached the inferomedial cortex of the femoral neck. In both positions, there was the potential for “spot welding” and proximal stress shielding of the neck. Furthermore, it was difficult to visualize complete seating due to absence of a lipped edge. Impacting beyond that carried the risk of creating a log-splitting fracture. Therefore, it was modified to the shorter VST stem with a proximal lipped edge in November 2007 (Fig. 3).
Fig. 3A–C.
Photographs of the different versions of the BMHR stem are shown. The hydroxyapatite-coated porous frustoconical portion of the stem provides good proximal fixation. Rotational stability was provided (A) initially through the curved stem and (B, C) later through the longitudinal splines in the straight stems. (C) The VST stem is shorter and has a lipped edge proximally, which serves as a guide to adequate stem seating. Reprinted with permission of SLACK Inc from Daniel J, Pradhan C, Ziaee H, McMinn DJ. A clinicoradiologic study of the Birmingham Mid-Head Resection Device. Orthopedics. 2008;31(12 Suppl 2). pii: orthosupersite.com/view.asp?rID = 37186.
The curved-stem devices were implanted between January 2003 and March 2004 as a custom-made device for each patient, since their need was not satisfactorily met by existing devices. We explained the absence of a clinical history with the stem to each patient and informed consent was obtained. We obtained Ethics Committee approval and informed consent for the straight-stem devices. In some cases, it was obvious from plain radiographs that the bone quality or femoral morphology precluded a resurfacing, and we accordingly advised patients. Where the radiographic appearances were equivocal, we informed patients the assessment would be made at the time of operation. If the bone quality was reasonable, a resurfacing was performed. If, during the procedure, the surgeon believed a resurfacing would be unsuitable, it was converted to a BMHR. We did not perform any preoperative imaging studies, other than regular plain radiographs. In patients with dysplasia, we sometimes used a CT scan to assess cup anteversion and femoral neck anteversion, but this was not needed for any patient in this series. The senior author (DJWM) performed all operations using a posterior approach [14–16].
The BMHR cup is the same as the BHR cup, and their implantations use the same technique [12, 14, 16], which is similar to implanting an uncemented THA cup. We used a 2-mm underream in most patients, unless the bone was very sclerotic (1 mm). The head size that matched the cup determined the stem size, although it was possible to use a stem one size above or below. A central position was preferred for the stem ensuring it did not reach too close to the lateral cortex of the femur or the calcar femorale. The correct stem filled approximately half of the diameter of the femoral neck. If the selected stem did not meet these criteria, we chose a smaller stem. We were careful to preserve the soft tissue over the femoral neck and practised negative suction venting of the femur.
In patients with no leg length or offset discrepancy, we used the medial head-neck junction as the point of reference when measuring the osteotomy level. In patients with leg length or offset discrepancies, it was possible to lengthen the femoral neck by displacing the osteotomy level as far proximally as the proximal surface of the femoral head, creating a femoral neck from the exposed distal part of the head. In such cases, we delayed unaided weightbearing until corticalization of the exposed cancellous bone in the recreated neck had occurred.
One patient received a bilateral curved stem device, three had bilateral V1 stems, one had bilateral VST stems, and two had a V1 on one hip and a VST on the other. The median cup, stem, and femoral head sizes used were 54 mm (range, 46–64 mm), Size 3 (range, 1–5), and 48 mm (range, 40–58 mm), respectively.
We administered antibiotic and thromboprophylaxis as described in earlier publications [3, 6]. Mobilization started on the first postoperative day, with 50% partial weightbearing with two elbow crutches for a month. This was followed by full weightbearing with two elbow crutches for another month before using a walking stick (cane) for a further month and then unaided walking. For a short period, beginning September 2006, we were confident in allowing full weightbearing with elbow crutches from the first postoperative day. However, this led to two early femoral neck fractures in April 2007, when the patients failed to use their crutches. We then reverted back to the original regime as described. Other standard hip precautions were also followed as for a resurfacing [6]. We discharged patients 4 to 6 days after the operation.
We had a regular system for clinical and radiographic followup at 2 months, 1 year, 2 years, and 5 years after the operation. At each followup, we examined the patients and obtained an Oxford hip score [7] and recorded major and minor and local complications as they occurred [21].
Radiographic assessment at each visit included an AP plain radiograph of the pelvis, including both hips and a crosstable horizontal beam lateral view of the index hip(s). Patients from overseas forwarded a progress report and digital radiographs at the same followup points. We contacted patients who could not attend the review or forward their radiographs by telephone to confirm implant survivorship and current hip function. We have current radiographic assessment of 128 of the 156 hips, with a minimum followup of 2 years. The remaining patients were unable to attend the relevant followup appointments or send radiographs taken locally. Radiographic assessment for lucent lines, osteolysis, spot welding, component loosening, and migration was performed by a single observer (HZ), blinded to the outcome, using the same criteria as those used in earlier reports on resurfacings [1, 2]. The interteardrop line was used as the reference for cup inclination. We used the femoral component diameter for correction of radiographic magnification in the measurement of neck thinning. The distance between the ischial tuberosity and a fixed point on the lesser trochanter was used to measure leg length and compared to the contralateral side.
We considered reoperation or revision failure. We analyzed implant survival with Kaplan-Meier plots using revision of either component or reoperation for any reason as the end point and computed 95% confidence intervals.
Results
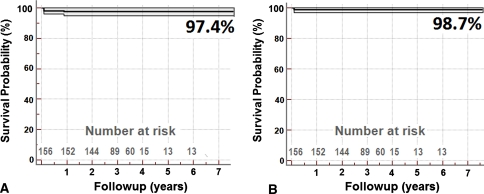
Survivorship at 3.5 years was 97.4% with revision or reoperation for any reason as the end point and 98.7% with femoral failure as the end point (Fig. 4). There were no revisions/reoperations in the 15 hips that did not reach minimum 2-year followup. Four patients had revisions, two burst fractures of the femoral head and neck, one infection, and one cup loosening. One fracture occurred in a man 4 weeks after surgery, and the second occurred in a woman 5 days postoperatively. Both underwent surgery during the brief period when we had been allowing full weightbearing with crutches immediately after operation (September 2006 to April 2007). Furthermore, they had not been using the elbow crutches in the prescribed manner when they sustained the fractures. After femoral component revision, their Oxford hip scores were 12 (100%) and 19 (85%) in the man and woman, respectively. Cup loosening occurred in a 40-year-old obese man with posttraumatic osteonecrosis. The primary etiology was hip fracture/dislocation sustained in a flying accident. We had used a dysplasia cup with supplementary screws. The cup loosened and the screws broke 2 years after his procedure, requiring cup revision. Intraoperative specimens grew coagulase-negative staphylococcus and needed systemic antibiotic treatment. One case of deep infection 2 months postoperatively required two-stage revision. No other patient is currently awaiting revision.
Fig. 4A–B.
Graphs show Kaplan-Meier survival analysis with the MHR technique, all ages and diagnoses included, with (A) failure of either component as the end point and (B) femoral failure as the end point. Light lines represent 95% confidence intervals.
Mean Oxford score at latest followup was 13 (range, 12–31; the best possible score being 12 and the worst 60). Considering 100% as perfect hip function, a score of 13 represents 98% hip function. The mean Oxford score for patients with the curved stems was 14 (range, 12–31) (96%), for V1 was 13 (12–22), and for VST was 13 (12–16). No patient complained of hip or thigh pain at rest. However, these scores cannot differentiate between symptoms arising from the operated hip or another joint.
Five patients with the V1 stem had relative proximal stress shielding and distal spot welding (Fig. 5). We did not observe this phenomenon in the curved (Fig. 6) or VST stem (Fig. 7). Mean cup inclination was 39º (range, 29°–48°). One patient had a radiolucent line in Zone 1 of the stem and another showed tilt of the stem into mild varus, but both were asymptomatic and functioning normally. We saw no acetabular lucent lines or loosening. No patient had radiographic signs of osteolysis. The MHR technique made it possible to equalize limb length in patients with severe LCPD (Fig. 7) and SCFE. One patient with a preoperative limb length discrepancy of 60 mm was left with a residual clinically apparent shortening and radiographic limb length discrepancy of 28 mm. Eighteen other patients with a limb length discrepancy of greater than 10 mm each (mean, 17.8 mm) had a mean gain of 9 mm. This improvement, coupled with improved hip flexibility, allowed equalization of apparent leg length in all these patients (Table 2).
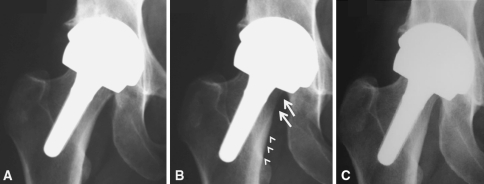
Fig. 5A–C.
Radiographs show a V1 stem placed in valgus (A) at 2 months (B) resulting at 1 year in spot welding of its distal half (arrowheads) and stress shielding of the proximal neck (arrows). (C) The 2-year radiograph shows no subsequent deterioration, suggesting the femur has remodeled in adaptation to the new loading regimen.
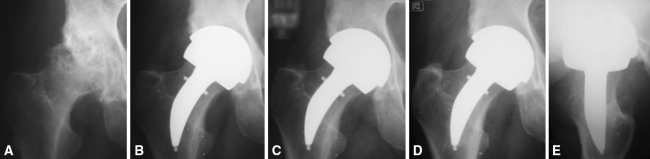
Fig. 6A–E.
A radiographic series of a patient with cystic change in the femoral head treated with a curved stem is shown: (A) preoperative; (B) 2 months postoperative; (C) 1 year postoperative; and (D) AP and (E) lateral 5 years postoperative. There are no radiographic adverse features and no evidence of proximal stress shielding. Reprinted with permission of SLACK Inc from Daniel J, Pradhan C, Ziaee H, McMinn DJ. A clinicoradiologic study of the Birmingham Mid-Head Resection Device. Orthopedics. 2008;31(12 Suppl 2). pii: orthosupersite.com/view.asp?rID = 37186.
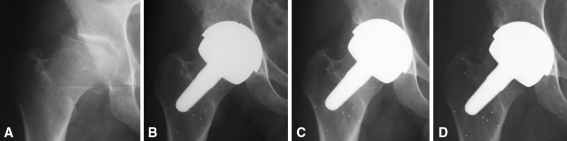
Fig. 7A–D.
Radiographs of a 40-year-old patient with post-LCPD arthritis and a foreshortened femoral neck treated with a VST stem are shown: (A) preoperative; (B) 2 months postoperative; (C) 6 months postoperative; and (D) 1 year postoperative. To equalize leg length and offset, the femoral neck was lengthened to the desired extent by performing the resection more proximally and carving the distal part of the femoral head into neck. The stem is placed in the neutral position and shows no sign of early proximal stress shielding.
Table 2.
Radiographic findings at followup
| Type of stem | Number of current radiographs | Femoral position | Heterotopic ossification (Brooker Grades I–IV) | Femoral neck thinning | Radiolucent lines | Stem tilt/migration/loosening | Stress shielding | Osteolysis |
|---|---|---|---|---|---|---|---|---|
| Curved stem | 13 | All neutral | I (2) | 1 (<10%) | 0 | 0 | 0 | 0 |
| V1 stem | 67 | Valgus placement in 7 | I (3), II (1), III (1) | 4 (<10%), 1 (>10%) |
1 (Stem Zone 1; 1 mm) | 0 | 5 | 0 |
| VST stem | 48 | All neutral | I (3), II (1) | 2 (<10%) | 0 | 1 | 0 | 0 |
VST = visual stop technology.
In addition to the complications noted above, on routine ultrasound scanning of all patients performed before discharge, four had asymptomatic below-knee deep venous thrombosis (DVT): two on the operated side and two contralateral. On subsequent serial ultrasound scanning, the thrombi resolved spontaneously within 3 weeks. One patient with a history of pulmonary embolism (PE) developed a calf DVT and symptomatic PE 3 weeks after his MHR procedure and needed anticoagulation. Patients without a history of thromboembolism had no PE or above-knee DVT (Table 3). We observed no patients with nerve palsies or wound dehiscence.
Table 3.
List of complications in the operated hips (n = 171)
| Complication | Number |
|---|---|
| Systemic major complications | |
| 90-day mortality | 0 |
| Cerebrovascular accident | 0 |
| Short-term memory loss/confusion (recovered fully) | 1 |
| Minor complications | |
| Dural leak (healed without further intervention) | 1 |
| Cardiac arrhythmia (transient) | 1 |
| Blood transfusion | 6 |
| Transient urinary symptoms (incontinence, frequency) | 2 |
| Diarrhea (all negative for Clostridium difficile) | 3 |
| Flare up of gout | 1 |
| Local complications | |
| Wound dehiscence | 0 |
| Vascular injury | 0 |
| Symptomatic DVT | 0 |
| Symptomatic pulmonary emboli | 1 |
| Asymptomatic below-knee DVT (detected by ultrasound) | 4 |
| Foot drop | 0 |
| Trochanteric bursitis | 3 |
| Hemarthrosis in the knee | 1 |
| Heel ulcer (healed in 4 weeks) | 1 |
DVT = deep venous thrombosis.
Discussion
Patients with a primary diagnosis of osteonecrosis and severe cystic change are prone to femoral failure after hip resurfacing arthroplasty [2, 22]. In these and other patients with abnormal morphology, a device that is less reliant on the integrity of the femoral head is needed. A bone-conserving, proximal load-bearing, short-stem device that does not invade the medullary canal of the femur is one alternative offered by the BMHR. We assessed (1) implant survivorship of the BMHR during short- and medium-term followup, (2) hip function, (3) adverse radiographic features emphasizing proximal stress shielding, and (4) complications.
We acknowledge limitations to our study. First, this is a retrospective study of a nonrandomized cohort of patients with no matched control group. The difficulty is that this is a cohort for whom the demand is not met by existing devices and there is no equivalent patient/device combination to compare it to. Their relative young age makes them less suited for a hip arthroplasty, and their poor bone quality makes them unsuitable for a resurfacing arthroplasty. Second, the followup is short compared to the time the device is expected to last in young patients. There are insufficient numbers at 7 years’ followup, while at the mean followup point of 3.5 years, the 60 hips at risk allowed short-term survivorship analysis with confidence. Third, two successive changes to the design make this a mixed group. However, the mid-head osteotomy level and the frustoconical design of the proximal part of the stem, which form the foundational basis of the MHR design, are tested in this study. These have remained unchanged through these iterations.
The early complication rate with this device is similar to other hip arthroplasties [3, 6, 17, 21]. The functional outcome with the MHR technique, as assessed by a mean Oxford hip score of 13, is excellent. The good functional recovery and absence of hip or thigh pain in this series are comparable to results of other conservative arthroplasty devices [2, 6, 19].
There were two femoral failures in this series, giving a survivorship from femoral failure of 98.7% and an overall survivorship of 97.4% at a mean followup of 3.5 years. This compares well with several other similar series (Table 4) and with the 7-year overall survivorship of the BHRs, which stands at 98% [17]. Amstutz et al. [1] reported an overall survivorship of 94.4% at 4 years with their single-surgeon series using the Conserve® Plus. Their survivorship for patients with a high risk index was 89%. All the patients in the present cohort were deemed high risk and would have otherwise needed a THA in view of avascular, weak, or cystic bone or abnormal morphology. Morrey et al. [19] reported an overall revision rate of 11.9% (19 of 159) at a longer followup (mean, 6.2 years) with an uncemented THA using the Mayo® stem.
Table 4.
Results of less invasive hip devices in the literature
| Study | Device* | Number of hips | Mean age (years) | Maximum followup (years) | Mean followup (years) | Radiographic stem loosening/migration (%) | Osteolysis (%) | Femoral revision rate (%) | Infection rate (%) | Overall revision rate (%) | Overall implant survivorship (followup) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Amstutz et al. [1] | Conserve Plus | 400 | 48.2 | 6 | 3.5 | 1.75 | 0 | 2.5 | 0.3 | 3 | 94% (4 years) |
| McMinn et al. [17] | BHR | 3014 | 53.6 | 12 | 7 | 0 | 0.03 | 1.2 | 0.5 | 1.9 | 98% (7 years) |
| Ender et al. [8] | ESKA CUT | 123 | 53 | 7 | 5 | 4.1 | 0 | 10.6 | 1.6 | 85% (5 years) | |
| Morrey et al. [19] | Mayo stem | 162 | 50.9 | 13 | 6.2 | 7.4 | 6 | 3.7 | 0.6 | 11.7 | |
| McMinn et al. | BMHR | 171 | 57 | 7 | 3.5 | 1 | 0 | 1.3 | 0.6 | 2.6 | 97% (3.5 years) |
*Devices included: Conserve® Plus (Wright Medical Technology, Inc, Arlington, TN); Birmingham Hip Resurfacing (BHR; Smith and Nephew Orthopaedics Ltd, Warwick, UK); ESKA CUT (ESKA Implants, Lübeck Germany); Mayo® stem (Zimmer, Inc, Warsaw, IN); Birmingham Mid-Head Resection (BMHR; Smith and Nephew Orthopaedics Ltd, Warwick, UK).
Radiographic assessment showed no osteolysis, or aseptic loosening, in the present series. It could be argued a reason for this is our short followup. Morrey et al. [19] had a 6% osteolysis incidence, but their series had a longer followup, and they rightly point out that the use of a conservative implant does not protect against debris-related problems. Osteolysis, aseptic component loosening [11], and pseudotumors are influenced by bearing debris. Since the BMHR employs the same bearing as the BHR, it is reasonable to expect the medium-term debris-related effects of the BMHR to be similar to those of well-implanted BHRs, which was 0.3% at 12 years in our series [17]. We had no failures due to pseudotumors in this series.
Morrey et al. [19] also reported stem subsidence in 7% and lucent lines or evidence of neocortex in Stem Zone 3 or 6 in 35% of their hips. In our series, spot welding and potential proximal stress shielding were seen in 7.5% of V1 stems only. This was because, in patients with a short femoral neck, the lower part of the stem approached the lateral femoral cortex, or if placed in valgus, it approached the inferomedial cortex of the neck. However, the process appears to have stabilized, rather than progressively deteriorate (Fig. 5). When the VST with a lipped edge was introduced, the stem length was also shortened, and the stress shielding problem did not occur.
In summary, our early observations indicate the MHR technique is a reliable conservative hip arthroplasty device with the following advantages: (1) compared to a resurfacing, it is less dependent on the integrity of the proximal femoral head when bone is unavailable due to distortion from conditions such as SCFE or LCPD or is rendered unreliable due to osteonecrosis, cystic change, or NSAID damage; (2) it is a proximal-loading stem and prevents stress shielding when performed as recommended; and (3) it is more versatile than a resurfacing in the management of limb length and offset discrepancies. The MHR technique is a reliable conservative option for young, active patients at high risk of failure with a resurfacing due to poor bone quality or deformity in the femoral head.
Acknowledgments
The authors thank Messrs Tim Band, Roger Ashton, and Tom Pynsent for their expert help in the design and development of the BMHR device.
Footnotes
One of the authors (DJWM) is a paid consultant to Smith and Nephew Orthopaedics Ltd (Warwick, UK). The institution of the authors (JD, CP, HZ, DJWM) receives royalties and funding from Smith and Nephew Orthopaedics Ltd.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Amstutz HC, Beaulé PE, Dorey FJ, Le Duff MJ, Campbell PA, Gruen TA. Metal-on-metal hybrid surface arthroplasty: two to six-year follow-up study. J Bone Joint Surg Am. 2004;86:28–39. [PubMed] [Google Scholar]
- 2.Daniel J, McBryde C, Pradhan C, Ziaee H. Results of Birmingham Hip Resurfacing in different diagnoses. In: McMinn DJ, editor. Modern Hip Resurfacing. London, UK: Springer; 2009. pp. 357–370. [Google Scholar]
- 3.Daniel J, Pradhan A, Pradhan C, Ziaee H, Moss M, Freeman J, McMinn DJ. Multimodal thromboprophylaxis following primary hip arthroplasty: the role of adjuvant intermittent pneumatic calf compression. J Bone Joint Surg Br. 2008;90:562–569. doi: 10.1302/0301-620X.90B5.19744. [DOI] [PubMed] [Google Scholar]
- 4.Daniel J, Pradhan C, Ziaee H. Patient selection and timing of operation. In: McMinn DJ, editor. Modern Hip Resurfacing. London, UK: Springer; 2009. pp. 163–167. [Google Scholar]
- 5.Daniel J, Pradhan C, Ziaee H, McMinn DJ. Management of complex anatomy. In: McMinn DJ, editor. Modern Hip Resurfacing. London, UK: Springer; 2009. pp. 333–348. [Google Scholar]
- 6.Daniel J, Pynsent PB, McMinn DJ. Metal-on-metal resurfacing of the hip in patients under the age of 55 years with osteoarthritis. J Bone Joint Surg Br. 2004;86:177–184. doi: 10.1302/0301-620X.86B2.14600. [DOI] [PubMed] [Google Scholar]
- 7.Dawson J, Fitzpatrick R, Carr A, Murray D. Questionnaire on the perceptions of patients about total hip replacement. J Bone Joint Surg Br. 1996;78:185–190. [PubMed] [Google Scholar]
- 8.Ender SA, Machner A, Pap G, Hubbe J, Grashoff H, Neumann HW. Cementless CUT femoral neck prosthesis: increased rate of aseptic loosening after 5 years. Acta Orthop. 2007;78:616–621. doi: 10.1080/17453670710014301. [DOI] [PubMed] [Google Scholar]
- 9.Hayaishi Y, Miki H, Nishii T, Hananouchi T, Yoshikawa H, Sugano N. Proximal femoral bone mineral density after resurfacing total hip arthroplasty and after standard stem-type cementless total hip arthroplasty, both having similar neck preservation and the same articulation type. J Arthroplasty. 2007;22:1208–1213. doi: 10.1016/j.arth.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 10.Learmonth ID. Conservative stems in total hip replacement. Hip Int. 2009;19:195–200. doi: 10.1177/112070000901900301. [DOI] [PubMed] [Google Scholar]
- 11.McMinn D, Daniel J. History and modern concepts in surface replacement. Proc Inst Mech Eng H. 2006;220:239–251. doi: 10.1243/095441105X68944. [DOI] [PubMed] [Google Scholar]
- 12.McMinn DJ. Acetabular preparation and insertion of the standard Birmingham Hip Resurfacing cup. In: McMinn DJ, editor. Modern Hip Resurfacing. London UK: Springer; 2009. pp. 223–264. [Google Scholar]
- 13.McMinn DJ, Daniel J, Pradhan C, Ziaee H. Avascular necrosis in the young patient: a trilogy of arthroplasty options. Orthopedics. 2005;28:945–947. doi: 10.3928/0147-7447-20050901-19. [DOI] [PubMed] [Google Scholar]
- 14.McMinn DJ, Daniel J, Pynsent PB, Pradhan C. Mini-incision resurfacing arthroplasty of hip through the posterior approach. Clin Orthop Relat Res. 2005;441:91–98. doi: 10.1097/01.blo.0000192034.37049.83. [DOI] [PubMed] [Google Scholar]
- 15.McMinn DJ, Daniel J, Ziaee H, Pradhan C. Mid-Head Resection Technique for complex deformity: European experience. Tech Orthop. 2010;25:33–38. doi: 10.1097/BTO.0b013e3181d2a969. [DOI] [Google Scholar]
- 16.McMinn DJ, Daniel J, Ziaee H, Pradhan C. Posterior surgical approach for hip resurfacing arthroplasty. Tech Orthop. 2010;25:56–66. doi: 10.1097/BTO.0b013e3181d2a987. [DOI] [Google Scholar]
- 17.McMinn DJ. Daniel J, Ziaee H, Pradhan C. Hip resurfacing. In: Bentley G, ed. European Instructional Lectures. EFORT II. Volume 10. London, UK: Springer; 2010:133–142.
- 18.Mont MA, Schmalzried TP, Zywiel MG, McGrath MS, Seyler TM. Perceptions concerning hip resurfacing from attendees at the Second Annual U.S. Comprehensive Course on Total Hip Resurfacing Arthroplasty. Bull NYU Hosp Jt Dis. 2009;67:102–107. [PubMed] [Google Scholar]
- 19.Morrey BF, Adams RA, Kessler M. A conservative femoral replacement for total hip arthroplasty: a prospective study. J Bone Joint Surg Br. 2000;82:952–958. doi: 10.1302/0301-620X.82B7.10420. [DOI] [PubMed] [Google Scholar]
- 20.Nunley RM, Della Valle CJ, Barrack RL. Is patient selection important for hip resurfacing? Clin Orthop Relat Res. 2009;467:56–65. doi: 10.1007/s11999-008-0558-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pradhan C, Daniel J, Ziaee H. Complications and revisions of the Birmingham Hip Resurfacing. In: McMinn DJ, editor. Modern Hip Resurfacing. London, UK: Springer; 2009. pp. 371–384. [Google Scholar]
- 22.Schmalzried TP. Total resurfacing for osteonecrosis of the hip. Clin Orthop Relat Res. 2004;429:151–156. doi: 10.1097/01.blo.0000150319.35347.2d. [DOI] [PubMed] [Google Scholar]
- 23.Schmalzried TP, Silva M, Rosa MA, Choi ES, Fowble VA. Optimizing patient selection and outcomes with total hip resurfacing. Clin Orthop Relat Res. 2005;441:200–204. doi: 10.1097/01.blo.0000192354.76792.bb. [DOI] [PubMed] [Google Scholar]
- 24.Treacy RB, McBryde CW, Pynsent PB. Birmingham Hip Resurfacing arthroplasty: a minimum follow-up of five years. J Bone Joint Surg Br. 2005;87:167–170. doi: 10.1302/0301-620X.87B2.15030. [DOI] [PubMed] [Google Scholar]