Abstract
Kawasaki disease is an acute febrile disease predominantly seen in young children. We report a case of Kawasaki disease in a 32-year-old pregnant woman. She developed a generalized erythematous skin rash accompanied by high fever. Bilateral conjunctival congestion, tender cervical lymphadenopathy, an edematous lower lip and peripheral edema followed by desquamation were observed. She was successfully treated with aspirin and intravenous gammaglobulin (1 g/kg/day). Her course was not complicated by coronary artery aneurysm and she delivered a healthy baby. To the best of our knowledge, this is the first case of Kawasaki disease in a pregnant woman. We suggest that Kawasaki disease should be included in the differential diagnosis of a generalized, erythematous skin rash accompanied by high fever in adults.
Key Words: Kawasaki disease, Pregnancy, TNF-α, NF-κB, Intravenous immunoglobulin
Introduction
Kawasaki disease (KD) was originally described by a Japanese pediatrician, Dr. Tomisaku Kawasaki, in 1967. It is an acute febrile illness occurring predominantly in young children. Here, we describe a case of KD in a 32-year-old pregnant woman.
Case Report
Our patient was a 32-year-old Japanese woman at 17 weeks of gestation, not multigravida, and without complications. She had presented with a headache and a low-grade fever. Her local obstetrician had diagnosed her with a common cold and had administered a 3-day course of amoxicillin. Seven days later, the patient developed a generalized skin rash accompanied by high fever (over 38°C) and was referred to the Department of Dermatology for further evaluation.
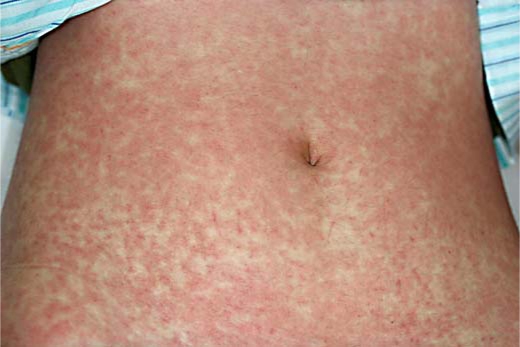
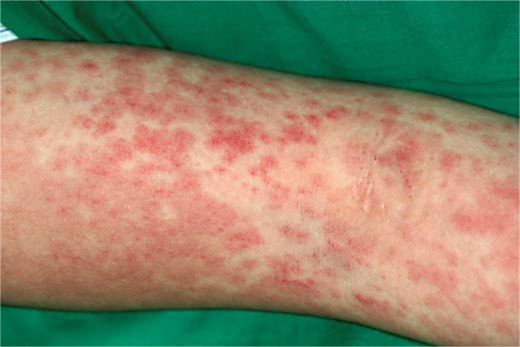
On admission, physical examination revealed erythematous macules up to 1–2 cm in size covering almost the entire body (fig. 1). Some erythematous plaques had dusky centers that resembled the iris lesion of erythema multiforme (fig. 2). The patient's temperature was 40.0°C. Her pulse was regular at 130 beats/min and blood pressure was 110/60 mm Hg. Her palms and soles were edematous. Bilateral conjunctival congestion, tender cervical lymphadenopathy, and an edematous lower lip were also noted. Examination of the lungs, abdomen and neurologic system did not reveal any abnormal findings. Electrocardiogram and transthoracic echocardiogram were normal. Laboratory investigations revealed an elevated erythrocyte sedimentation rate and C-reactive protein (54.7 mm/h and 5.66 μg/dl, respectively), leukocytosis (12.05 × 109/l), and elevated liver enzymes including aspartate aminotransferase (78 U/l) and alanine aminotransferase (102 U/l). Ferritin was elevated at 1,479.2 ng/ml. No eosinophilia was detected. Analysis of urine revealed 2+ protein, 20–30 white blood cells, and 2+ of bacteria. Blood and urine cultures did not yield growth of pathogenic microorganism. Serological tests for hepatitis B and C, measles, rubella, Epstein-Barr virus, parvovirus B19, cytomegalovirus, Streptococcus, and Mycoplasma showed no evidence of a recent infection. Antinuclear antibodies and anti-neutrophil cytoplasmic antibodies were not detected. Histopathological examination of a skin biopsy showed focal spongiosis, scattered dyskeratotic cells and hydropic degeneration of the epidermis. Perivascular mononuclear lymphocytic infiltrates and extravasation of erythrocytes without vasculitis were also noted.
Fig. 1.
Confluent, erythematous macules covering almost the entire body.
Fig. 2.
Erythematous plaques with dusky centers.
The patient had features which fulfilled the 6 diagnostic criteria of KD [1] on admission, i.e. prolonged fever, conjunctival congestion, injected lip, generalized exanthema, cervical lymphadenopathy, and swollen hands and feet. Intravenous prednisolone (40 mg/d for 2 days) and imipenem/cilastatin (1 g/d for 3 days) were started, because a bacterial infection or a drug allergy could not be completely excluded. The patient did not respond to these treatments and the high fever remained. Administration of intravenous gammaglobulin (1 g/kg/day, given once) and oral aspirin (660 mg daily) were started under the presumptive diagnosis of KD. The symptoms dramatically improved over the next 48 h, resulting in cessation of the fever and reduction of the erythematous rash, and the daily dosage of aspirin was subsequently reduced to 162 mg. After 10 days of hospitalization, the patient's fingertips began to desquamate. The clinical course and laboratory findings are summarized in fig. 3. Serial transthoracic echocardiograms showed no evidence of coronary artery aneurysm (CAA). At 40 weeks of gestation, the patient spontaneously delivered a viable infant with Apgar scores of 8 at 1 min and 9 at 5 min. Her postpartum course was uncomplicated and her child remained healthy.
Fig. 3.
Clinical course of the present case. IPM =Imipenem; CS = cilastatin.
Discussion
In 1967, based on a series of 50 Japanese children, Kawasaki described a unique disorder characterized by fever, generalized rash, conjunctival congestion, cervical lymphadenopathy, inflammation of the lips and oral cavity, and redness and swelling of the hands and feet [2]. Although this disorder was originally referred to as ‘mucocutaneous lymph node syndrome’, the condition is nowadays referred to as KD. KD predominantly affects infants and young children and rarely occurs in adults [4, 5]. In the literature, to the best of our knowledge, there have been 81 reported adult cases of classical (complete) KD [5]. Because of the lack of specific laboratory findings, the diagnosis depends on clinical criteria, which are classified as principal and other minor clinical/laboratory findings. The principal findings are: (1) fever lasting for at least 5 days; (2) peripheral extremity findings, such as peripheral edema, peripheral erythema, and desquamation; (3) a polymorphic exanthema; (4) bilateral conjunctival injection; (5) oral mucous membrane findings, such as injected pharynx, injected lips, and strawberry tongue; and (6) acute non-suppurative swelling of the cervical lymph nodes [1]. The presence of at least 5 of these principal findings establishes the diagnosis, but the presence of 4 is accepted, if the patient has evidence of CAA. Our patient had all 6 principal findings.
Because KD is rarely encountered in adolescents or adults, the diagnosis in these age groups is occasionally problematic. Differences in clinical manifestations, laboratory findings, and therapeutic response between adult and pediatric KD cases have not been fully investigated. A literature review by Jackson et al. [3] in 1994 revealed that adult patients with an age range of 18–68 years had various types of exanthema, described as a diffuse erythematous, macular, maculopapular, or scarlatiniform rash. Comparison of the clinical features of adult and pediatric KD showed that lymphadenopathy, arthralgia, and liver dysfunction occur more frequently in adults (93, 61, and 65% of adult cases, respectively) than in children (15, 24–38, and 10% of cases, respectively) [4]. On the contrary, CAAs are less common in adults (5–7%) than in children (20%). However, this may reflect the difficulty in visualizing the adult coronary vasculature using transthoracic echocardiography [4].
Although the pathogenesis of KD remains unknown, epidemiologic features and clinical presentations suggest an infectious etiology. Marked immune perturbations, including stimulation of cytokine cascade, occur during the acute phase of KD. Among the various cytokines, tumor necrosis factor-alpha (TNF-α) seems to play an essential role in the vascular injury of KD [6]. TNF-α activates nuclear factor kappa B (NF-κB), a pivotal transcription factor for the genes of proinflammatory cytokines, chemokines and adhesion molecules [7]. NF-κB is also activated by numerous stimuli such as bacterial lipopolysaccharide endotoxin, reactive oxygen species and various cytokines. Kraus et al. [8] found that serum TNF-α level is increased throughout gestation. Ross et al. [9] showed activation of NF-κB during the swine estrous cycle and specifically in the initial phase of pregnancy. This suggests that pregnancy might also affect the pathophysiology of KD. Two cases of KD in postpartum women have been reported [10, 11]. Infantile KD cases accompanied by coronary artery lesions may become pregnant after growing up as well [12, 13]. However, to the best of our knowledge, our patient is the first case with acute onset of KD in a pregnant woman in the literature.
Intravenous immunoglobulin (IVIG) and oral aspirin have been used for treatment of KD. IVIG is usually administered as a single infusion of 2 g/kg (high-dose IVIG) in children [14]. Although previous reports showed a clinical benefit in adults with KD receiving IVIG, there has been no randomized controlled study evaluating the optimal dose or timing of IVIG therapy in adults. Some clinical studies have shown that either a single dose or 2 infusions of IVIG at 1 g/kg (medium-dose IVIG) was equally effective [15]. In our case, a single infusion of medium-dose IVIG achieved a satisfactory improvement. It has been reported that anti-TNF agents are useful for KD patients who failed to respond to IVIG treatment [16].
Recently, the term ‘atypical’ or ‘incomplete’ KD has been proposed to denote cases that do not fulfill the classical criteria of KD. Recently, Gomard-Mennesson et al. [5] described 10 cases of adult KD, 6 of which did not fulfill the classical KD criteria. Therefore, KD might not always be recognized in adults. A literature review by Sève et al. [4] revealed that the clinical features and sequelae of KD are similar in adults and children, but adults have a more favorable prognosis because of a lower incidence of CAA. Our case suggests that KD needs to be considered in adults – including pregnant women – with a protracted high fever and a generalized skin rash because of the potential risk of CAA.
Footnotes
This is an Open Access article licensed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs 3.0 License (www.karger.com/OA-license), applicable to the online version of the article only. Distribution for non-commercial purposes only.
References
- 1.Ayusawa M, Sonobe T, Uemura S. Kawasaki Disease Research Committee: Revision of diagnostic guidelines for Kawasaki disease (the 5th revised edition) Pediatr Int. 2005;47:232–234. doi: 10.1111/j.1442-200x.2005.02033.x. [DOI] [PubMed] [Google Scholar]
- 2.Kawasaki T. Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children. Clinical observation of 50 patients. Jpn Allergy. 1967;16:178–222. [PubMed] [Google Scholar]
- 3.Jackson JL, Kunkel MR, Libow L, et al. Adult Kawasaki disease. Report of two cases treated with intravenous gamma globulin. Arch Intern Med. 1994;154:1398–1405. doi: 10.1001/archinte.154.12.1398. [DOI] [PubMed] [Google Scholar]
- 4.Sève P, Stanknovic K, Smail A. Adult Kawasaki disease: report of two cases and literature review. Semin Arthritis Rheum. 2005;34:785–792. doi: 10.1016/j.semarthrit.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 5.Gomard-Mennesson E, Landron C, Dauphin C. Kawasaki disease in adults: report of 10 cases. Medicine (Baltimore) 2010;89:149–158. doi: 10.1097/MD.0b013e3181df193c. [DOI] [PubMed] [Google Scholar]
- 6.Furukawa S, Matsubara T, Jujoh K. Peripheral blood monocyte/macrophages and serum tumor necrosis factor in Kawasaki disease. Clin Immunol Immunopathol. 1988;48:247–251. doi: 10.1016/0090-1229(88)90088-8. [DOI] [PubMed] [Google Scholar]
- 7.Furukawa S, Matsubara T, Ichiyama T. Regulation of proinflammatory cytokine cascade in Kawasaki disease. Nippon Rinsho. 2008;66:258–264. [PubMed] [Google Scholar]
- 8.Kraus TA, Sperling RS, Engel SM. Peripheral blood cytokine profiling during pregnancy and post-partum periods. Am J Reprod Immunol. 2010;64:411–426. doi: 10.1111/j.1600-0897.2010.00889.x. [DOI] [PubMed] [Google Scholar]
- 9.Ross JW, Ashworth MD, Mathew D. Activation of the transcription factor, nuclear factor kappa-B, during the estrous cycle and early pregnancy in the pig. Reprod Biol Endocrinol. 2010;8:39. doi: 10.1186/1477-7827-8-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fason JT, Fry YW, Smith D. Kawasaki disease in a postpartum patient. J Natl Med Assoc. 2004;96:1499–1502. [PMC free article] [PubMed] [Google Scholar]
- 11.Nakamura T, Azuma I. An adult case of Kawasaki disease in a two-day post-partum woman (in Japanese) Syusanki Igaku. 2005;35:1001–1003. [Google Scholar]
- 12.Tuda E, Ishida Y, Kawamura K. Pregnancy and delivery in patients with coronary artery lesions caused by Kawasaki disease. Heart. 2005;91:1481–1482. doi: 10.1136/hrt.2004.058842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shear R, Leduc L. Successful pregnancy following Kawasaki disease. Obstet Gynecol. 1999;94:841. doi: 10.1016/s0029-7844(99)00348-8. [DOI] [PubMed] [Google Scholar]
- 14.Newburger JW, Takahashi M, Gerber MA. Diagonosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Pediatrics. 2004;114:1708–1733. doi: 10.1542/peds.2004-2182. [DOI] [PubMed] [Google Scholar]
- 15.Han SB, Choi JW, Kim SK. The optimal dosages of gammaglobulin and aspirin in treating kawasaki disease. J Korean Pediatr Soc. 1996;39:703–711. [Google Scholar]
- 16.Burns JC, Mason WH, Hauger SB. Infliximab treatment for refractory Kawasaki syndrome. J Pediatr. 2005;146:662–667. doi: 10.1016/j.jpeds.2004.12.022. [DOI] [PubMed] [Google Scholar]