Abstract
Massive upper gastrointestinal bleeding due to malignancy is relatively uncommon and the duodenum is the least frequently involved site. Duodenal metastasis is rare in renal cell carcinoma (RCC) and early detection, especially in case of a solitary mass, helps in planning further therapy. We report a case of intractable upper gastrointestinal bleeding from metastatic RCC to the duodenum. The patient presented with melena and anemia, 13 years after nephrectomy for RCC. On esophagogastroduodenoscopy, a submucosal mass was noted in the duodenum, biopsies of which revealed metastatic RCC. In conclusion, metastasis from RCC should be considered in nephrectomized patients presenting with gastrointestinal symptoms and a complete evaluation, especially endoscopic examination followed by biopsy, is suggested.
Key Words: Duodenum, Renal cell carcinoma, Pancreaticoduodenectomy, Metastases, Gastrointestinal bleeding
Introduction
Renal cell carcinoma (RCC) has a potential to metastasize to almost any site. In descending order of frequency, the most common sites of metastasis are the lung, lymph nodes, liver, bones, adrenal glands, kidneys, brain, heart, spleen, intestine, and skin [1]. It can involve any part of the bowel and accounts for 7.1% of all metastatic tumors to the small intestine [2]. Duodenal metastasis from RCC is very uncommon and only few cases have been described in the literature (table 1). Also duodenal metastasis generally occurs when there is widespread nodal and visceral involvement and evidence of metastatic disease elsewhere in the body. Commonly, renal cell metastases present many years after initial treatment, with recurrences reported up to 17.5 years after initial surgery [3]. Most cases of duodenal metastasis from RCC present with upper gastrointestinal bleeding or obstructive symptoms, and sequelae may include anemia, melena, fatigue, and early satiety. Several treatments of solitary RCC metastasis have been reported. These include a variety of surgical and interventional therapy options that have been shown to provide effective survival benefits. Here we report on a patient with solitary duodenal metastasis who presented with gastrointestinal bleeding 13 years after nephrectomy.
Table 1.
Previously reported cases of solitary renal cell carcinoma metastatic to the duodenum/ampulla
| Authors | Year | Age/sex | Duration post nephrectomy (years) | Location of metastasis | Presenting symptoms | Treatment | Survival |
|---|---|---|---|---|---|---|---|
| Rustagi et al. (current) | 2011 | 66/M | 13 | duodenum | GI bleeding, fatigue, weight loss | embolization and PPPD | 2 weeks |
| Adamo et al. [4] | 2008 | 86/F | 13 | duodenum | anemia, early satiety | classic Whipple | 7 months |
| Bhatia et al. [10] | 2006 | 50/M | 1 | duodenum | jaundice, abdominal mass | diagnostic only | – |
| Arroyo et al. [24] | 2005 | 75/F | 13 | duodenum | – | – | – |
| Arroyo et al. [24] | 2005 | 52/M | 2 | duodenum | – | – | 5 months |
| Loualidi et al. [6] | 2004 | 76/M | 5 | duodenum | GI bleeding | palliative radiotherapy | – |
| Pavlakis et al. [7] | 2004 | 65/M | 2 | duodenum | obstruction | intestinal resection | 9 months |
| Sawh et al. [25] | 2002 | 53/M | 6 | duodenum | GI bleeding | duodenectomy and embolization | 4 years |
| Nabi et al. [26] | 2001 | 40/M | 4 | duodenum | obstruction with bilious vomiting, abdominal pain | gastrojejunostomy | 7 days |
| Hashimoto et al. [27] | 2001 | 57/M | 11 | duodenum | GI bleeding | PPPD | – |
| Sohn et al. [14] | 2001 | −/− | 6 | ampulla | – | classic Whipple | 22 months |
| Le Borgne et al. [13] | 2000 | 48/M | 13 | duodenum | GI bleeding | classic Whipple | 53 months |
| Le Borgne et al. [13] | 2000 | 72/F | 7 | duodenum | GI bleeding | classic Whipple | 18 months |
| Ohmura et al. [20] | 2000 | 62/M | 5 | duodenum | obstruction | embolization and local resection | – |
| Janzen et al. [3] | 1998 | 75/M | 17 | ampulla | GI bleeding | duodenectomy, total pancreatectomy | – |
| Toh & Hale [11] | 1996 | 59/F | 10 | duodenum | obstruction, abdominal pain, anemia | duodenectomy, mass excision | – |
| Gastaca Mateo et al. [28] | 1996 | 48/M | 8 | duodenum | anemia, fatigue, weight loss | duodenectomy | 3 years |
| Leslie et al. [29] | 1996 | 78/F | 10 | ampulla | GI bleeding, weight loss, abdominal discomfort, pruritus | PPPD | 30 months |
| Leslie et al. [29] | 1996 | 53/M | 8 | ampulla | GI bleeding, weight loss | PPPD | 78 months |
| Venu et al. [30] | 1991 | 64/M | 11 | ampulla | GI bleeding, fatigue | – | – |
| Robertson & Gertler [31] | 1990 | 70/M | 13 | ampulla | GI bleeding | classic Whipple | – |
| Lynch-Nyhan et al. [17] | 1987 | 16/M | 1 | duodenum | GI bleeding | embolization | 6 months |
| Lynch-Nyhan et al. [17] | 1987 | 61/M | 6 | duodenum | jaundice | embolization | – |
| Lynch-Nyhan et al. [17] | 1987 | 67/M | 2 | duodenum | GI bleeding | – | – |
| McNichols et al. [32] | 1981 | 52/M | 10 | duodenum | malabsorption | diagnostic only | – |
| Heymann & Vieta [9] | 1978 | 64/M | 8 | duodenum | GI bleeding | complex procedure | 3 weeks |
| Tolia & Whitmore [33] | 1975 | –/M | 16 | duodenum | – | – | 5 months |
| Lawson et al. [34] | 1966 | 69/F | 0 | duodenum | GI bleeding, anemia | classic Whipple | 8 months |
PPPD = Pylorus-preserving pancreaticoduodenectomy.
Case Report
A 66-year-old male presented with progressively worsening shortness of breath, fatigue and generalized weakness for the last 3 weeks. He reported black tarry stools for about 3–4 days prior to admission. He also complained of loss of appetite and 15 pounds unintentional weight loss over the last few weeks. He denied nausea, vomiting, or abdominal pain. There was no history of recent use of nonsteroidal antiinflammatory drugs. His past medical history was significant for hypertension, bilateral RCC status post right nephrectomy and partial left nephrectomy done 13 years earlier, ileocolectomy for perforated cecal diverticulitis, and prostate cancer treated with radiation. He had a 50 pack year history of smoking, but denied any alcohol use. On physical exam, he was orthostatic and appeared pale. Pertinent physical findings included melanotic stools. Abdominal examination was unremarkable. No signs of chronic liver disease were noted.
Laboratory investigations on admission were significant for microcytic hypochromic anemia with hemoglobin 5 g/dl, hematocrit 16.8%, MCV 72 fl and MCH 21.7 pg/l. Liver enzymes were within normal range. Esophagogastroduodenoscopy showed an actively bleeding, 4 cm irregular, polypoid, ulcerative mass in the second portion of the duodenum, adjacent to but not involving the papilla (fig. 1). Biopsies were obtained from this mass, following which the patient started having severe bleeding from the lesion. Endoscopic interventions to control the bleeding were unsuccessful. Visceral angiography was done which demonstrated no extravasation or pseudoaneurysm in the duodenal vascular distribution or pancreaticoduodenal arcade. However, empiric angiographic embolization of the gastroduodenal artery was performed. Despite these endoscopic and radiologic embolization attempts, bleeding continued, requiring another endoscopic procedure when the lesion was treated with argon plasma coagulation with successful control of the bleeding. The patient received a total transfusion of 11 units of packed red blood cells during his hospital course. Histopathology of the biopsies from the duodenal mass during endoscopy revealed metastatic RCC.
Fig. 1.
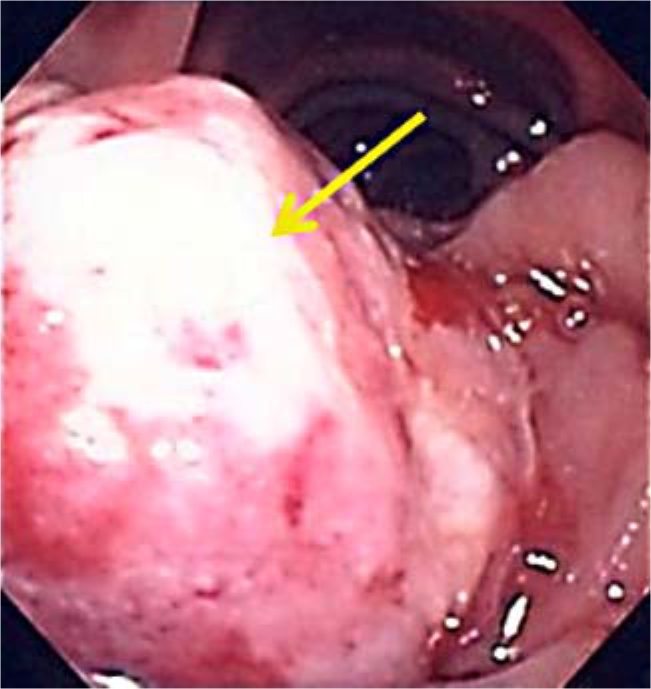
Endoscopic image showing a 4 cm polypoidal mass in the second part of the duodenum. This mass was actively bleeding and appeared irregular, ulcerative and friable giving it a ‘malignant appearance’.
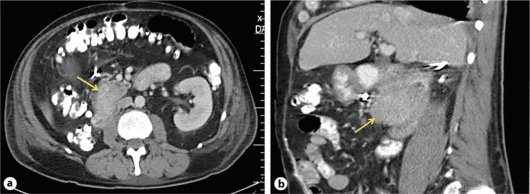
Further investigations included a computed tomography scan of the abdomen which showed an ill-defined mass measuring 5.4 × 3.3 cm at the junction of the second and third portion of the duodenum, adjacent to but not involving the head of the pancreas (fig. 2). There was no evidence of liver or visceral metastasis, and the visceral vessels and lymph nodes appeared normal. Ultrasound of the abdomen showed normal pancreaticobiliary system with no common bile duct dilatation suggestive of obstruction.
Fig. 2.
Computed tomography scan of the abdomen showing a 5.4 × 3.3 cm mass at the junction of the second and third part of the duodenum: axial view (a) and sagittal view (b). The mass is noted to be adjacent but not involving the head of the pancreas. No evidence of hepatic or visceral metastasis is seen and no lymphadenopathy is noted.
Subsequently, the patient underwent exploratory laparotomy which revealed a 7 × 4 cm mass in the second portion of the duodenum. There was no evidence of malignant ascites, carcinomatosis, omental implants, or involvement of the pancreas or liver. He underwent pylorus-sparing pancreaticoduodenectomy, choledochojejunostomy, handsewn pancreaticojejunostomy and gastrojejunostomy with removal of the mass. Biopsies from the mass during endoscopy and surgical pathology were consistent with RCC. Postoperatively, the patient's hospital course was complicated by leakage of the pancreaticojejunostomy fistula and sepsis, and he died two weeks later.
Gross examination of the surgical specimen revealed a 2.0 × 1.5 × 0.8 cm tan-red polypoid, pedunculated tumor protruding into the duodenum approximately 0.5 cm away from the ampulla of Vater. The tumor appeared confined grossly to the mucosa and submucosa of the duodenum and did not appear to extend into or through the muscularis. There was no involvement of the pancreas. Eight lymph nodes were identified in the resection specimen, and all were negative for metastatic tumor with successful excision of all involved structures with clear margins and with no lymph node involvement.
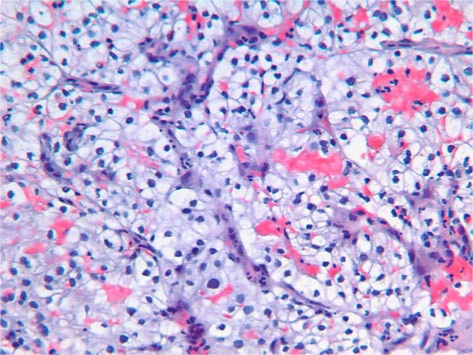
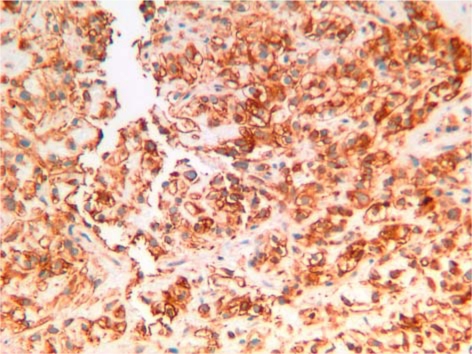
Microscopically, the tumor was composed of clear cells arranged in a trabecular and alveolar pattern (fig. 3). Immunohistochemically the tumor was positive for vimentin, CD10, AE1/AE3 and epithelial membrane antigen and negative for CK7, CK20 and PNRA (fig. 4). Histopathological features and immunostaining findings were compatible with a diagnosis of metastatic RCC of clear cell type.
Fig. 3.
Histopathology of the resected mass showing large polygonal clear cells arranged in a trabecular and alveolar pattern, yielding a diagnosis of RCC of clear cell type.
Fig. 4.
Immunohistochemical staining demonstrates clear cells positive for vimentin. Immunostaining was also positive for CD10, AE1/AE3 and epithelial membrane antigen and negative for CK7, CK20 or PNRA markers, confirming the diagnosis of RCC of clear cell type.
Discussion
Gastrointestinal tract metastases are a rare cause of massive gastrointestinal bleeding. Small bowel involvement by metastatic tumors is rare and has been reported in only 1–2% of autopsy cases [2, 4]. Common metastatic malignancies known to involve the small bowel are melanomas, lung cancer, cervical carcinomas, RCCs, thyroid carcinomas, hepatomas and Merkel cell carcinomas. RCC metastases account for 7.1% of these lesions [2]. Graham reported that only 4% of RCCs metastasized to the small intestine [5]. Solitary duodenal metastasis from RCC is exceedingly rare and most frequently involves the periampullary region or the duodenal bulb [6, 7].
We have summarized the reported cases of duodenal metastasis from RCC in table 1. We have included patients with the bulk of the gross tumor in the duodenal or ampullary regions, distinct from pancreatic involvement. The majority of patients were found to have metastasis within 1 year after nephrectomy though it could be seen even after several years [8]. The mean duration post nephrectomy to diagnosis of these solitary duodenal metastases was 7.9 ± 4.7 years (median 8 years). The range was 0 to 13 years, with one patient presenting with a synchronous metastasis. Males were more commonly affected (male:female ratio 3:1) and the incidence of metastasis increased with age [7]. Mean age at diagnosis of duodenal metastasis was 60.7 ± 14 years (median age 63 years, range 16–86 years).
The natural history of RCC is unpredictable. Disease eradication and cure are possible after nephrectomy; however, there is also the possibility of a long period of disease latency, followed by recurrence of metastatic disease at unsuspected anatomic locations. RCC can metastasize via the lymphatic or hematogenous route, as well as by peritoneal dissemination or direct invasion into adjacent anatomic structures [8]. The duodenum is an exceptionally rare site of metastasis in RCC, which is perhaps counterintuitive given its retroperitoneal proximity to the right kidney, though a majority (around 70%) occur from the right kidney.
The most common clinical presentation of gastrointestinal metastases of RCC is gastrointestinal bleeding, resulting from the invasion of intestinal vessels by the neoplastic disease and/or intestinal obstruction [7, 9]. Most of the patients with solitary duodenal RCC metastasis present with gastrointestinal bleeding (69%), anemia and fatigue, whereas others present with early satiety, bowel obstruction, abdominal pain or jaundice from biliary obstruction [4, 7, 10, 11]. Patients post nephrectomy for RCC presenting with gastrointestinal symptoms should undergo complete diagnostic work-up with both endoscopic and radiologic evaluation, for detection and evaluation of the extent of metastatic disease. On endoscopy the lesion can be seen as a submucosal mass with ulceration of the tip, multiple nodules of varying sizes or raised plaques [12]. Endoscopic biopsy of suspicious lesions provides tissue for histologic diagnosis of metastasis and helps to distinguish primary gastrointestinal malignancy from metastatic disease.
The treatment options in a case of solitary duodenal RCC metastasis depend upon the extent and location of the lesion and therapy must be individually tailored. Procedures ranging from classic pancreaticoduodenectomy (Whipple procedure) to interventional embolization have been reported (table 1). Any patient with solitary metastatic RCC to the duodenum should be considered a candidate for complete surgical excision if medically and technically feasible, both for palliation of symptoms and because it provides the opportunity for meaningful disease-free survival. Therapeutic goals include complete metastatectomy whenever surgically feasible. A curative role for pancreaticoduodenectomy in patients with solitary duodenal metastasis has been reported and has been shown to improve survival [11, 13, 14, 15, 16]. Hemostasis of gastrointestinal bleeding occurring due to metastasis or invasion of malignant tumor is hard to manage endoscopically, and data on endoscopic therapy of bleeding from these duodenal lesions are limited. In selected cases, intractable gastrointestinal bleeding can be treated with arterial embolization of tumor-supplying arteries that has been reported to control gastrointestinal bleeding effectively, but there are no long-term follow-up data [17, 18, 19]. However, embolotherapy is only palliative while the tumor develops other collateral vessels and has potential for re-bleeding [20]. Also, in these cases, the physician should keep in mind that embolization for control of hemorrhage in the small bowel carries a significant risk of bowel infarction. For disseminated malignancy, treatment is mainly supportive and palliative, in the form of palliative surgery, radiotherapy, chemotherapy or immune-stimulating agents (interleukin-2) [10, 21, 22]. Patients with metastatic disease have poor survival despite the above treatment. The average survival is about 4 months and only 10% of them survive for 1 year [23].
This case report highlights the importance of vigilance and high index of suspicion in post nephrectomy patients upon presentation of new clinical symptoms. Appropriate awareness, recognition and aggressive work-up of gastrointestinal symptoms in patients post nephrectomy for RCC are of paramount importance.
Disclosure Statement
All authors declare that there are no potential conflicts (financial, professional, or personal) relevant to this paper.
Footnotes
This is an Open Access article licensed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs 3.0 License (www.karger.com/OA-license), applicable to the online version of the article only. Distribution for non-commercial purposes only.
References
- 1.Murphy WM, Beckwith JB, Farrow GM. Atlas of Tumor Pathology, 3rd series, fascicle 11. Washington: Armed Forces Institute of Pathology; 1994. Tumors of the kidney, bladder, and related urinary structures; p. 128. [Google Scholar]
- 2.Willis RA. The Spread of Tumours in the Human Body. ed 3. London: Butterworth & Co Ltd; 1973. Secondary tumors of the intestines; pp. 209–215. [Google Scholar]
- 3.Janzen RM, Ramj AS, Flint JDA, et al. Obscure gastrointestinal bleeding from an ampullary tumor in a patient with a remote history of renal cell carcinoma: a diagnostic conundrum. Can J Gastroenterol. 1998;12:75–78. doi: 10.1155/1998/429832. [DOI] [PubMed] [Google Scholar]
- 4.Adamo R, Greaney PJ, Jr, Witkiewicz A, et al. Renal cell carcinoma metastatic to the duodenum: treatment by classic pancreaticoduodenectomy and review of the literature. J Gastrointest Surg. 2008;12:1465–1468. doi: 10.1007/s11605-007-0426-2. [DOI] [PubMed] [Google Scholar]
- 5.Graham AP. Malignancy of the kidney, survey of 195 cases. J Urol. 1947;58:10–21. doi: 10.1016/S0022-5347(17)69512-0. [DOI] [PubMed] [Google Scholar]
- 6.Loualidi A, Spooren PFMJ, Grubben MJAL, et al. Duodenal metastasis: an uncommon cause of occult small intestinal bleeding. Neth J Med. 2004;62:201–205. [PubMed] [Google Scholar]
- 7.Pavlakis GM, Sakorafas GH, Anagnostopoulos GK. Intestinal metastases from renal cell carcinoma: a rare cause of intestinal obstruction and bleeding. Mt Sinai J Med. 2004;71:127–130. [PubMed] [Google Scholar]
- 8.Chang WT, Chai CY, Lee KT. Unusual upper gastrointestinal bleeding due to late metastasis from renal cell carcinoma: a case report. Kaohsiung J Med Sci. 2004;20:137–141. doi: 10.1016/S1607-551X(09)70098-1. [DOI] [PubMed] [Google Scholar]
- 9.Heymann AD, Vieta JO. Recurrent renal carcinoma causing intestinal hemorrhage. Am J Gastroenterol. 1978;69:582–585. [PubMed] [Google Scholar]
- 10.Bhatia A, Das A, Kumar Y, Kochhar R. Renal cell carcinoma metastasizing to duodenum: a rare occurrence. Diagn Pathol. 2006;1:29. doi: 10.1186/1746-1596-1-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toh SK, Hale JE. Late presentation of a solitary metastasis of renal cell carcinoma as an obstructive duodenal mass. Postgrad Med J. 1996;72:178–179. doi: 10.1136/pgmj.72.845.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsu CC, Chen JJ, Changchein CS. Endoscopic features of metastatic tumours in upper gastrointestinal tract. Endoscopy. 1996;28:249–253. doi: 10.1055/s-2007-1005437. [DOI] [PubMed] [Google Scholar]
- 13.Le Borgne J, Partensky C, Glemain P, et al. Pancreaticoduodenectomy for metastatic ampullary and pancreatic tumors. Hepatogastroenterology. 2000;47:540–544. [PubMed] [Google Scholar]
- 14.Sohn TA, Yeo CJ, Cameron JL, et al. Renal cell carcinoma metastatic to the pancreas: results of surgical management. J Gastrointest Surg. 2001;5:346–351. doi: 10.1016/s1091-255x(01)80060-3. [DOI] [PubMed] [Google Scholar]
- 15.Yeo CJ, Cameron JL, Sohn TA, et al. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s: pathology, complications, and outcomes. Ann Surg. 1997;226:248–260. doi: 10.1097/00000658-199709000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freedman AI, Tomaszewski JE, Van Arsdalen KN. Solitary late recurrence of renal cell carcinoma presenting as duodenal ulcer. Urology. 1992;39:461–463. doi: 10.1016/0090-4295(92)90248-u. [DOI] [PubMed] [Google Scholar]
- 17.Lynch-Nyhan A, Fishman EK, Kadir S. Diagnosis and management of massive gastrointestinal bleeding owing to duodenal metastasis from renal cell carcinoma. J Urol. 1987;138:611–613. doi: 10.1016/s0022-5347(17)43275-7. [DOI] [PubMed] [Google Scholar]
- 18.Blake MA, Owens A, O'Donoghue DP, et al. Embolotherapy for massive upper gastrointestinal haemorrhage secondary to metastatic renal cell carcinoma: report of three cases. Gut. 1995;37:835–837. doi: 10.1136/gut.37.6.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gordon B, Lossef SV, Jelinger E, et al. Embolotherapy for small bowel hemorrhage from metastatic renal cell carcinoma: case report. Cardiovasc Intervent Radiol. 1991;14:311–313. doi: 10.1007/BF02578457. [DOI] [PubMed] [Google Scholar]
- 20.Ohmura Y, Ohta T, Doihara H, et al. Local recurrence of renal cell carcinoma causing massive gastrointestinal bleeding: a report of two patients who underwent surgical resection. Jpn J Clin Oncol. 2000;30:241–245. doi: 10.1093/jjco/hyd061. [DOI] [PubMed] [Google Scholar]
- 21.Mascarenhas B, Konety B, Rubin JT. Recurrent metastatic renal cell carcinoma presenting as a bleeding gastric ulcer after a complete response to high-dose interleukin-2 treatment. Urology. 2001;57:168–169. doi: 10.1016/s0090-4295(00)00877-3. [DOI] [PubMed] [Google Scholar]
- 22.Lee DS, White DE, Hurst R, et al. Patterns of relapse and response to retreatment in patients with metastatic melanoma or renal cell carcinoma who responded to interleukin-2-based immunotherapy. Cancer J Sci Am. 1998;4:86–93. [PubMed] [Google Scholar]
- 23.Yuvaraja BT, Mahantshetty U, Chamarajanagar RS, et al. Management of renal cell carcinoma with solitary metastasis. World J Surg Oncol. 2005;3:48. doi: 10.1186/1477-7819-3-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arroyo C, Palacios P, Uribe N, et al. Uncommon metastases in renal carcinoma. Gac Med Mex. 2005;141:543–546. [PubMed] [Google Scholar]
- 25.Sawh RN, Borkowski J, Broaddus R. Metastatic renal cell carcinoma presenting as a hemorrhoid. Arch Pathol Lab Med. 2002;126:856–858. doi: 10.5858/2002-126-0856-MRCCPA. [DOI] [PubMed] [Google Scholar]
- 26.Nabi G, Gandhi G, Dogra PN. Diagnosis and management of duodenal obstruction due to renal cell carcinoma. Trop Gastroenterol. 2001;22:47–49. [PubMed] [Google Scholar]
- 27.Hashimoto M, Miura M, Matruda M, et al. Concomitant duodenal and pancreatic metastases from renal cell carcinoma: report of a case. Surg Today. 2001;31:180–183. doi: 10.1007/s005950170208. [DOI] [PubMed] [Google Scholar]
- 28.Gastaca Mateo MA, Ortiz de Urbina Lopez J, Diaz Aguirregoitia J, et al. Duodenal metastasis of renal cell adenocarcinoma. Rev Esp Enferm Dig. 1996;88:361–363. [PubMed] [Google Scholar]
- 29.Leslie KA, Tsao JI, Rossi RL, et al. Metastatic renal cell carcinoma to ampulla of Vater: an unusual lesion amenable to surgical resection. Surgery. 1996;119:349–351. doi: 10.1016/s0039-6060(96)80122-x. [DOI] [PubMed] [Google Scholar]
- 30.Venu RP, Rolny P, Geenen JE, et al. Ampullary tumor caused by metastatic renal cell carcinoma. Dig Dis Sci. 1991;36:376–378. doi: 10.1007/BF01318213. [DOI] [PubMed] [Google Scholar]
- 31.Robertson GS, Gertler SL. Late presentation of metastatic renal cell carcinoma as a bleeding ampullary mass. Gastrointest Endosc. 1990;36:304–306. doi: 10.1016/s0016-5107(90)71032-2. [DOI] [PubMed] [Google Scholar]
- 32.McNichols DW, Segura JW, De Weerd JH. Renal cell carcinoma in long-term survival and late recurrence. J Urol. 1981;126:17–23. doi: 10.1016/s0022-5347(17)54359-1. [DOI] [PubMed] [Google Scholar]
- 33.Tolia BM, Whitmore WF. Solitary metastasis from renal cell carcinoma. J Urol. 1975;114:836–838. doi: 10.1016/s0022-5347(17)67155-6. [DOI] [PubMed] [Google Scholar]
- 34.Lawson LJ, Holt LP, Rooke HWP. Recurrent duodenal hemorrhage from renal cell carcinoma. Br J Urol. 1966;38:133–137. doi: 10.1111/j.1464-410x.1966.tb09690.x. [DOI] [PubMed] [Google Scholar]