Summary
When chaperoning tumour antigens, glucose-regulated protein 170 (GRP170) is capable of inducing effective antitumour immune responses. In the present study, we determined whether such immunoadjuvant properties of GRP170 also involve the ability to induce ‘danger signals’ through interaction with APC. We prepared recombinant GRP170 in the baculovirus expression system with low endotoxin concentration at which LPS did not have any effect on dendritic cells (DC). We showed that GRP170 binds DC in a receptor-mediated fashion and induces DC to upregulate the expression of MHC class II, CD86 and CD40 molecules, and to secrete pro-inflammatory cytokines. GRP170 also induced expression of CD40 molecules in a B16F10 cell line, whereas LPS failed to do so. These findings show that GRP170 acts as a danger signal through its interaction with DC, regardless of its endotoxin component.
Keywords: APC, danger signal, glucose-regulated protein 170, heat shock protein, innate immunity
Introduction
Stress proteins are molecular chaperones that are involved in inhibition of protein aggregation. Molecular chaperones also participate in numerous normal cellular processes such as protein folding, transport and assembly.1,2 The cellular functions of chaperones are essential to all living organisms from prokaryotes to man. Glucose-regulated protein (GRP) 170 is an important stress protein/molecular chaperone resident in the endoplasmic reticulum that is induced by stress conditions such as hypoxia, ischaemia, glucose deprivation, reductive reagents, anoxia and interference in calcium homeostasis. Immunization with tumour-derived GRP170 can elicit tumour-specific CD8+ T-cell responses and also significantly reduce pulmonary metastatic disease.3,4 During the past several years, studies have been carried out to understand the mechanism of interaction of GRP, gp96 in particular, with the immune system. These studies indicated that the GRP94/gp96 can interact with APC and result in maturation of dendritic cells (DC).5–7 These reports emphasize the immunoadjuvant properties of GRP94/gp96 to induce both innate and adaptive immune responses.8,9 Induction of innate immune responses is an intrinsic property of stress proteins when they interact with APC, whereas adaptive immune responses can be generated when stress proteins chaperone antigens. These two immunological properties of stress proteins are interrelated.
Certain stress proteins have been reported to function as ‘danger signals’ alerting the immune system by induction of innate immune responses, that is, secretion of pro-inflammatory cytokines as well as upregulation of certain surface molecules on APC.5–7,10–12 Generation of such danger signals would in turn result in the induction of adaptive immune responses against the antigens associated with stress proteins. Several studies have supported that activation of lymphocytes and induction of immune responses could occur as soon as the immune system senses a danger signal.13–15 Stress proteins were also reported to enhance immunogenicity of apoptotic tumour cells leading to antitumour immune responses.16–18 We have reported that tumour-derived GRP170 can induce antitumour immune responses.3,4 Endotoxin contamination in stress proteins argues that LPS may be responsible for the ability of stress proteins to induce innate immune responses. These controversial reports as to the endotoxin issue have been discussed in a recent review.19 Low endotoxin stress proteins prepared in a nonbacterial expression system are highly reliable when their function is compared with that of LPS with a comparable endotoxin component. Controversial results have been reported when polymixin B was used to remove LPS or to abrogate LPS activity. Although polymixin B inhibits the endotoxin activity of stress protein preparations in some studies, it only partially inhibits the endotoxin activity in other studies.20,21 Therefore, polymixin B may not be a totally dependable control for endotoxin effect in stress proteins or in other studies. In the present study we have used new controls such as low endotoxin GRP170, irrelevant insect cell protein (luciferase) and tumour cell line B16F10 melanoma. We did not detect expression of toll-like receptors (TLR) 2 or 4 in the B16F10 tumour line. Therefore, this cell line was used as a control to determine whether immunoadjuvant properties of GRP170 may be due to the endotoxin component of GRP170.
Materials and methods
Proteins
Mouse recombinant GRP170 was made using the pBacPAK. His vector (Clontech Laboratories, Palo Alto, CA, USA). This vector-carrying GRP170 gene is cotransfected with BacPAK6 viral DNA into Sf21 insect cells using a BacPAK Baculovirus Expression System Kit (Clontech Laboratories), followed by amplification of the recombinant virus, purification of GRP170 protein using nickel-chelated-nitrilotriacetic acid-agarose (Qiagen, Valentia, CA, USA) and dialysis of protein using endotoxin-free buffer. When 2 μg of GRP170 was examined by coomassie blue, no secondary bands were visible. Protein concentration was determined using BCA protein assay kit (Pierce, Rockford, IL, USA). Endotoxin level was less than 25 endotoxin units (EU)/mg as determined by a Limulus amoebocyte lysate kit as per the manufacturer's instruction (BioWhittaker, Walkersville, MD, USA). LPS (Sigma, St Louis, MO, USA) at 0.1–1 ng/mL concentrations, containing approximately 1–8 EU, did not have any function while interacting with DC (data not shown). Luciferase extraction from firefly (Sigma) was used as a control insect cell protein for GRP170.
FITC labelling protein
The FITC conjugation kit was purchased from Sigma. FITC labelling was carried out as per the manufacturer's protocol.
Mouse bone marrow-derived DC
Bone marrow cells were isolated from the femurs of C57BL/6 mice. Red cells were lysed by incubation of the cells in Tris-NH4Cl at room temperature for 5 min. The remaining cells were incubated for 1 h at 4°C with a combination of monoclonal antibodies purified from supernatants of B hybridomas GK1.5 (anti-CD4) 2.43 (anti-CD8) and anti-B220 Abs (BD Pharmingen, Palo Alto, CA, USA). Each antibody was present at 20 μg/106 bone marrow cells. The cells were pelleted and resuspended for 1 h at 37°C in complement (Accurate, Westbury, NY, USA) diluted 1:10 in RPMI-1640. Cells were cultured in Petri dishes overnight in RPMI-1640 supplemented with 10% heat-inactivated fetal bovine serum (FBS), 50 mmol/L 2-ME, 20 mmol/L L-glutamine, 200 IU penicillin and 100 μg streptomycin, and then pulsed every 3 days with 10–20 ng/mL GM-CSF (Immunex, Seattle, WA, USA). After 7 days of expansion, DC were used for maturation studies. These DC were of CD11b+ and CD11c+ characteristics. Animal care and use were in accordance with institutional guidelines.
In vitro binding assay
Bone marrow-derived DC (106 cells) were pulsed with several concentrations of GRP170 to determine the optimal concentration where saturation occurs. Ovalbumin (OVA) was used as control protein to determine whether binding of GRP170 was specific. In competition studies, DC were pulsed with FITC-GRP170 (100 μg/mL) in the presence or absence of fourfold molar excess of GRP170 or OVA at 4°C for 20 min followed by washing and fixing and flow cytometry analysis. OVA was used as control to determine whether competition of GRP170 with FITC-GRP170 was specific.
Tumour cells
The B16F10 melanoma cell line was maintained in the culture medium, RPMI-1640 supplemented with 10% FBS.
In vitro signalling studies
Immature DC were plated at 106 cells/well per mL RPMI-1640 supplemented with 10% FBS and pulsed with GRP170 (200 μg/mL), LPS (0.1 μg/mL), or luciferase (70 μg/mL). At these concentrations, endotoxin components of the proteins were calculated to be 5, 800 and 3 EU, respectively. Control wells pulsed with medium only. Plates were incubated at 37°C in an atmosphere of 5% CO2 for 22 h. Supernatants were collected and subjected to multiplex cytokine assay using bead-based xMAP technology. Cells were also detached gently using a cell scraper and subjected to flow cytometry-based analysis to determine the expression of surface molecules.
Flow cytometry
All the procedures for antibody staining of cells was conducted on ice, and washing steps were carried out with a PBS–0.1% sodium azide solution to avoid internalization/recycling of the receptors in DC. FITC- or phycoerythrin (PE)-conjugated antimouse Ig, CD11c, CD11b, I-Ab, CD40 and CD86 antibodies (BD PharMingen, San Diego, CA, USA) were used at 0.5–1 μg/200 μL per 2–5 × 105 cells. Antimouse CD16/CD32 antibody (0.5 μg/200 μL per 2–5 × 105 cells; BD PharMingen, San Diego, CA, USA) was used before the specific antibody staining to block the cell surface Fc receptors. Cells were finally washed and fixed with 1% ultrapure formaldehyde and read by FACScan within 24 h. Data are presented as the mean fluorescence intensity (MFI) of triplicate experiments. Melanoma cell line, B16F10, was used as control to compare the ability of GRP170 and LPS in the induction of CD40 expression.
Statistical analysis
Results were analyzed using the Student's t-test. A value of P < 0.05 was considered significant.
Results
GRP170 binds bone marrow-derived DC in a receptor-mediated fashion
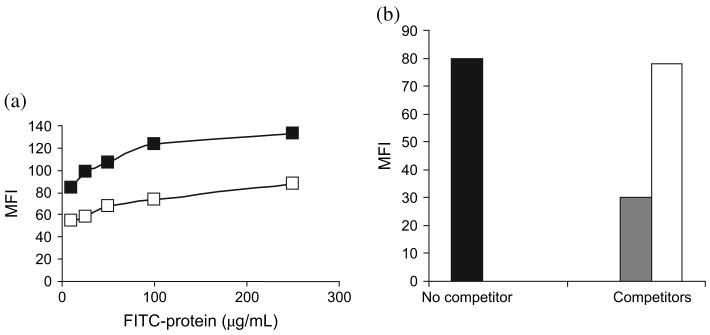
To determine whether GRP170 binds DC in a receptor-dependent fashion, binding and competition studies were carried out. FITC-conjugated GRP170 appeared to bind DC in a saturable manner where it showed maximum binding at 100 μg/mL, whereas OVA as a control protein did not bind DC appreciably (Fig. 1a). Weak binding of OVA to the cells was predictable because of the ability of OVA to bind glycoproteins. However, OVA failed to compete with FITC-GRP170, whereas nonconjugated GRP170 was able to compete with FITC-GRP170 when used at fourfold higher concentration over FITC-GRP170 (Fig. 1b). Using BSA as another control protein also showed the specificity of GRP170 surface binding to DC (data not shown).
Figure 1.
Glucose-regulated protein 170 (GRP170) binds dendritic cells (DC) in a receptor-mediated fashion. (a) Bone marrow-derived DC (106 cells) were pulsed with several concentrations of GRP170 (■) or OVA (□). (b) In competition studies, DC were pulsed with FITC-GRP170 (100 μg/mL) in the presence or absence of fourfold molar excess of GRP170 or OVA at 4°C for 20 min. MFI, mean fluorescence intensity; ■, FITC-GRP170;
 , FITC-GRP170 + excess GRP170; □, FITC-GRP170 + excess OVA.
, FITC-GRP170 + excess GRP170; □, FITC-GRP170 + excess OVA.
GRP170 induces maturation of DC and enhances their antigen presentation capability
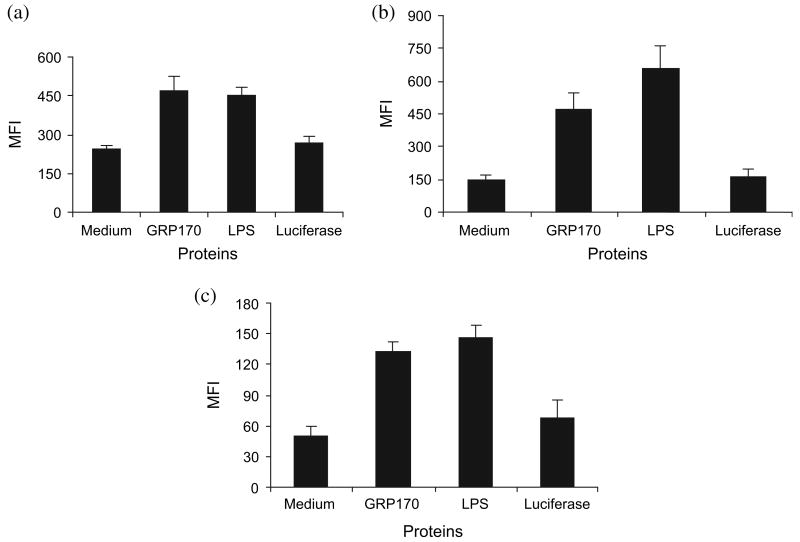
To examine one of the possible mechanisms by which GRP170 induces adaptive immune responses, we evaluated expression of surface molecules on DC upon stimulation with GRP170 in vitro. We used an insect cell-derived protein, luciferase, as a control because GRP170 was prepared in baculovirus expression system using insect cells. Stimulation of mouse bone marrow-derived DC with GRP170 for 22 h resulted in upregulation of surface expression of MHC class II (P = 0.01), CD40 (P = 0.003) and CD86 (P = 0.001) molecules (Fig. 2). LPS showed similar effects as GRP170 on maturation of DC. Luciferase, when used at the same molar ratio as GRP170, did not show any effect. Recombinant HER-2/neu protein (intracellular domain) expressed in Escherichia coli,22 as an additional control, did not induce maturation of DC (data not shown). All the cells used for expression of GRP170 were routinely tested for mycoplasma contamination to make sure there was no such contamination.
Figure 2.
Glucose-regulated protein 170 (GRP170) upregulates expression of (a) MHC class II, (b) CD86 and (c) CD40 molecules in dendritic cells (DC). Bone marrow-derived DC (106/mL), derived from C57BL/6 mice, were cultured with mouse recombinant GRP170 (200 μg/mL), LPS (0.1 μg/mL), or luciferase at the same molar ratio as GRP170 (70 μg/mL) in RPMI-1640 supplemented with 10% FBS for 22 h followed by blocking of the Fc receptors using rat antimouse CD16/CD32 antibody. Cells were stained with FITC-conjugated rat antimouse antibodies and subjected to flow cytometry analysis. Isotype control antibody (1 μg/200 μL) was used as control and revealed <12 mean fluorescence intensity (MFI). Data are presented as MFI of triplicate experiments.
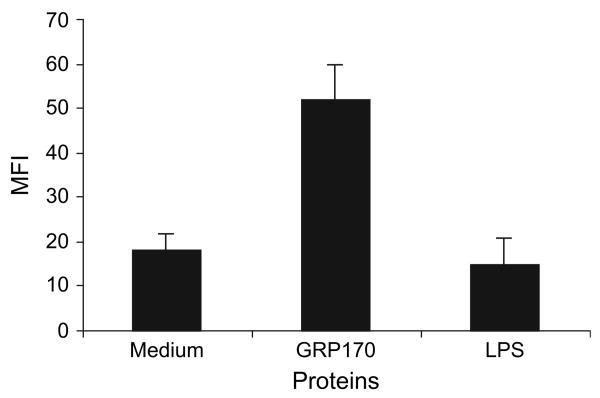
To determine whether GRP170 and LPS may have similar effects on the B16F10 melanoma cell line, we evaluated expression of CD40 in B16F10 on in vitro stimulation with these proteins. Unlike LPS, GRP170 resulted in elevation of CD40 expression (Fig. 3).
Figure 3.
Glucose-regulated protein 170 (GRP170) augments expression of CD40 molecules in the B16F10 melanoma cell line. B16F10 (106/mL) was cultured in the presence or absence of recombinant GRP170 (200 μg/mL) or LPS (0.1 μg/mL) in RPMI-1640 (37°C, 5% CO2) for 22 h. Tumour cells were stained with FITC-conjugated rat antimouse CD40 antibody. Cells were then subjected to flow cytometry analysis after fixing with 1% formaldehyde. Isotype control antibody (1 μg/200 μL) was used as control and revealed <12 mean fluorescence intensity (MFI). Data are presented as MFI of triplicate experiments.
GRP170 induces secretion of pro-inflammatory cytokines by DC
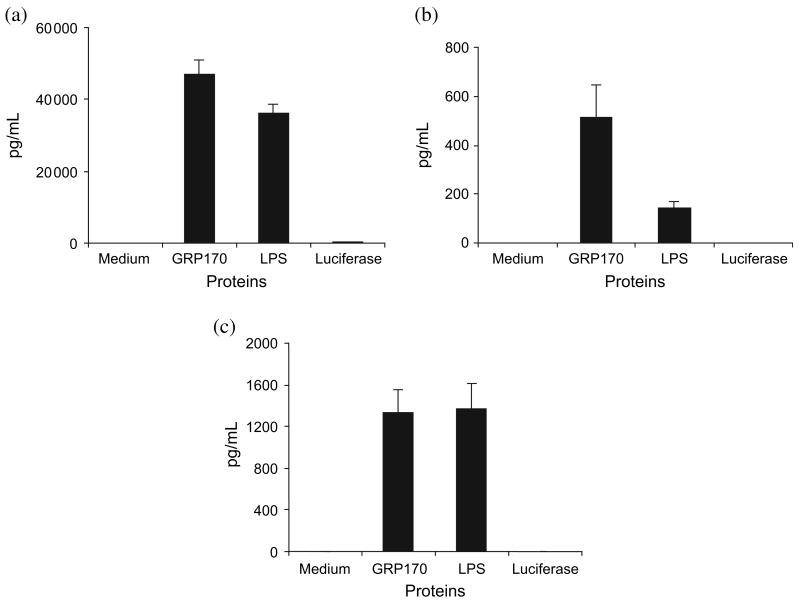
To determine whether GRP170 may induce danger signals on interaction with DC, we evaluated secretion of pro-inflammatory cytokines by DC in the presence or absence of GRP170. Multiplex cytokine assay showed that GRP170 and LPS equally induced secretion of IL-6 and TNF-α in DC (Fig. 4a,b). However, GRP170 induced significantly higher IL-12 (P = 0.02) than that induced by LPS (Fig. 4c). Luciferase did not show any significant effect on secretion of these cytokines.
Figure 4.
Glucose-regulated protein 170 (GRP170) induces dendritic cells (DC) to secrete pro-inflammatory cytokines. Mouse bone marrow-derived DC (106/mL) were cultured in the presence or absence of recombinant GRP170 (200 μg/mL), LPS (0.1 μg/mL), or a control protein luciferase (70 μg/mL) for 22 h. Supernatants were collected and subjected to multiplex cytokine assay using the cytokine-specific antibody-coated beads (anti-IL-6, anti-IL-12 and anti-TNF-α), biotinylated detection antibodies (anti-IL6, anti-IL12 and anti-TNF-α), and phycoerythrin (PE)-avidin. Data are presented as the mean of triplicate experiments. (a) IL-6, (b) IL-12, (c) TNF-α.
Discussion
In the present study, we showed that GRP170 can induce danger signals, as evidenced by the induction of secretion of pro-inflammatory cytokines, while interacting with DC. Low endotoxin GRP170 was used at a concentration less than 25 EU/mg, whereas a control protein, luciferase, at similar endotoxin levels failed to induce DC maturation, indicating that interaction of GRP170 preparation with DC was not due to possible contamination with insect cell proteins. In addition, when 2 μg of GRP170 was examined by coomassie blue, no secondary bands were visible. LPS induced maturation of DC at the concentration of 0.1 μg/mL where its endotoxin concentration was 800 EU per sample, as evaluated by a Limulus amoebocyte lysate kit. Furthermore, GRP170 showed distinct function in the expression of CD40 molecule in B16F10 melanoma cells, whereas LPS failed to show similar effects. GRP170 may enhance antigen presentation efficacy of DC by upregulation of MHC class II, CD86 and CD40 molecules.
Pro-inflammatory cytokines are secreted upon infection or injury to alert the immune system. DC secrete these cytokines upon activation, thereby alerting lymphocytes against their surface antigens that they took up by vaccination. We showed that GRP170, similar to LPS, induced secretion of IL-6 and TNF-α by DC. However, GRP170 appeared to be a stronger stimulator of IL-12 secretion compared with LPS. This, again, confirms that GRP170 can induce danger signals regardless of its endotoxin components. Similarities in the interaction of GRP170 and LPS with DC may suggest that both proteins use similar receptors, TLR2 and/or TLR4, for the signal transduction in DC.23 However, the differences between the two proteins in the induction of IL-12 secretion in DC and also in their interaction with B16F10 tumour cells, lacking TLR2 and TLR4, may indicate that GRP170 binds to other yet to be identified surface receptors than TLR. B16F10 tumour cells engineered to secrete GRP170 are also capable of stimulating the production of TNF-α by both DC and macrophages, whereas parental B16F10 tumour cells did not (Wang et al., unpubl. obs.). TLR-9 is an intracellular receptor by which CpG activates DC. Whether GRP170 may bind TLR-9 remains to be determined. GRP are molecular chaperones that efficiently interact with other proteins. Therefore, they are not restricted to interaction with specific cell surface receptors. Furthermore, these findings rule out contribution of endotoxin in GRP170 function while interacting with tumour cells.
It has been reported that IL-12 with immunoregulatory functions bridges innate and antigen-specific adaptive immunity.24 It has recently been reported that IL-12 induces activation of T cells even in the presence of CD4+ CD25+ regulatory T cells (T reg).25 Solid tumours including melanoma are under hypoxic conditions and overexpress stress proteins, GRP in particular. Tumours may also release their endogenous GRP on necrosis and these tumour-derived GRP may generate danger signals alerting the immune system against the tumour.26 However, these GRP may not be abundant enough to induce effective antitumour immunity. Tumour cells engineered to secrete GRP94/gp96 can induce antitumour immunity, although the quantity of extracellular GRP in this case may be significantly greater than might be obtained by in situ cell death and release of intracellular components.27
The present study suggests that GRP170 appears to be a multifunctional adjuvant inducing antitumour immune responses against its associated antigen by (i) maturation of DC that result in an efficient antigen presentation, (ii) interaction with B16F10 to upregulate CD40 molecules that may induce tumour apoptosis and (iii) interaction with DC for the induction of danger signals.
Acknowledgments
This work was supported by Public Health Service grants CA99326 and CA104757, and the Susan G. Komen Grant BCTR0504184. We thank Melanie Murawaski for her assistance, and Earl A. Timm and Aileen Cinquino (all from Roswell Park Cancer Institute) for their assistance and advice in carrying out the multiplex cytokine assay. We gratefully acknowledge the support of VCU Massey Cancer Centre and the Commonwealth Foundation for Cancer Research.
References
- 1.Lindquist S, Craig EA. The heat-shock proteins. Annu Rev Genet. 1988;22:631–77. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- 2.Clarke AR. Molecular chaperones in protein folding and translocation. Curr Opin Struct Biol. 1996;6:43–50. doi: 10.1016/s0959-440x(96)80093-5. [DOI] [PubMed] [Google Scholar]
- 3.Wang XY, Kazim L, Repasky EA, Subjeck JR. Characterization of heat shock protein 110 and glucose-regulated protein 170 as cancer vaccines and the effect of fever-range hyperthermia on vaccine activity. J Immunol. 2001;166:490–97. doi: 10.4049/jimmunol.166.1.490. [DOI] [PubMed] [Google Scholar]
- 4.Wang XY, Kazim L, Repasky EA, Subjeck JR. Immunization with tumour-derived ER chaperone grp170 elicits tumour-specific CD8+ T-cell responses and reduces pulmonary metastatic disease. Int J Cancer. 2003;105:226–31. doi: 10.1002/ijc.11058. [DOI] [PubMed] [Google Scholar]
- 5.Binder RJ, Anderson KM, Basu S, Srivastava PK. Cutting edge: heat shock protein gp96 induces maturation and migration of CD11c+ cells in vivo. J Immunol. 2000;165:6029–35. doi: 10.4049/jimmunol.165.11.6029. [DOI] [PubMed] [Google Scholar]
- 6.Zheng H, Dai J, Stoilova D, Li Z. Cell surface targeting of heat shock protein gp96 induces dendritic cell maturation and antitumor immunity. J Immunol. 2001;167:6731–5. doi: 10.4049/jimmunol.167.12.6731. [DOI] [PubMed] [Google Scholar]
- 7.Vabulas RM, Braedel S, Hilf N, et al. The endoplasmic reticulum-resident heat shock protein Gp96 activates dendritic cells via the Toll-like receptor 2/4 pathway. J Biol Chem. 2002;277:20, 847–53. doi: 10.1074/jbc.M200425200. [DOI] [PubMed] [Google Scholar]
- 8.Anderson KM, Srivastava PK. Heat, heat shock, heat shock proteins and death: a central link in innate and adaptive immune responses. Immunol Lett. 2000;74:35–9. doi: 10.1016/s0165-2478(00)00246-7. [DOI] [PubMed] [Google Scholar]
- 9.Massa C, Melani C, Colombo MP. Chaperon and adjuvant activity of hsp70: different natural killer requirement for cross-priming of chaperoned and bystander antigens. Cancer Res. 2005;65:7942–9. doi: 10.1158/0008-5472.CAN-05-0377. [DOI] [PubMed] [Google Scholar]
- 10.Bethke K, Staib F, Distler M, et al. Different efficiency of heat shock proteins (HSP) to activate human monocytes and dendritic cells: superiority of HSP60. J Immunol. 2002;169:6141–8. doi: 10.4049/jimmunol.169.11.6141. [DOI] [PubMed] [Google Scholar]
- 11.Zeng Y, Feng H, Graner MW, Katsanis E. Tumour-derived, chaperone-rich cell lysate activates dendritic cells and elicits potent antitumor immunity. Blood. 2003;101:4485–91. doi: 10.1182/blood-2002-10-3108. [DOI] [PubMed] [Google Scholar]
- 12.Manjili MH, Park J, Facciponte JG, Subjeck JR. HSP110 induces “danger signals” upon interaction with antigen presenting cells and mouse mammary carcinoma. Immunobiology. 2005;210:295–303. doi: 10.1016/j.imbio.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 13.McLellan AD, Brocker EB, Kampgen E. Dendritic cell activation by danger and antigen-specific T-cell signalling. Exp Dermatol. 2000;9:313–22. doi: 10.1034/j.1600-0625.2000.009005313.x. [DOI] [PubMed] [Google Scholar]
- 14.Gallucci S, Matzinger P. Danger signals: SOS to the immune system. Curr Opin Immunol. 2001;13:114–19. doi: 10.1016/s0952-7915(00)00191-6. [DOI] [PubMed] [Google Scholar]
- 15.Campisi J, Leem TH, Fleshner M. Stress-induced extracellular Hsp72 is a functionally significant danger signal to the immune system. Cell Stress Chaperones. 2003;8:272–86. doi: 10.1379/1466-1268(2003)008<0272:sehiaf>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feng H, Zeng Y, Whitesell L, Katsanis E. Stressed apoptotic tumour cells express heat shock proteins and elicit tumour-specific immunity. Blood. 2001;97:3505–512. doi: 10.1182/blood.v97.11.3505. [DOI] [PubMed] [Google Scholar]
- 17.Feng H, Zeng Y, Graner MW, Katsanis E. Stressed apoptotic tumour cells stimulate dendritic cells and induce specific cytotoxic T cells. Blood. 2002;100:4108–115. doi: 10.1182/blood-2002-05-1389. [DOI] [PubMed] [Google Scholar]
- 18.Feng H, Zeng Y, Graner MW, Likhacheva A, Katsanis E. Exogenous stress proteins enhance the immunogenicity of apoptotic tumour cells and stimulate antitumor immunity. Blood. 2003;101:245–52. doi: 10.1182/blood-2002-05-1580. [DOI] [PubMed] [Google Scholar]
- 19.Manjili MH, Wang XY, MacDonald IJ, et al. Cancer immunotherapy and heat-shock proteins: promises and challenges. Expert Opin Biol Ther. 2004;4:363–73. doi: 10.1517/14712598.4.3.363. [DOI] [PubMed] [Google Scholar]
- 20.Bethke K, Staib F, Distler M, et al. Different efficiency of heat shock proteins (HSP) to activate human monocytes and dendritic cells: superiority of HSP60. J Immunol. 2002;169:6141–8. doi: 10.4049/jimmunol.169.11.6141. [DOI] [PubMed] [Google Scholar]
- 21.Gao B, Tsan MF. Endotoxin contamination in recombinant human heat shock protein 70 (Hsp70) preparation is responsible for the induction of tumour necrosis factor alpha release by murine macrophages. J Biol Chem. 2003;278:174–9. doi: 10.1074/jbc.M208742200. [DOI] [PubMed] [Google Scholar]
- 22.Manjili MH, Henderson R, Wang XY, et al. Development of a recombinant HSP110-HER-2/neu vaccine using the chaperoning properties of HSP110. Cancer Res. 2002;62:1737–42. [PubMed] [Google Scholar]
- 23.Asea A, Rehli M, Kabingu E, et al. Novel signal transduction pathway utilized by extracellular HSP70: role of toll-like receptor (TLR) 2 and TLR4. J Biol Chem. 2002;277:15, 028–34. doi: 10.1074/jbc.M200497200. [DOI] [PubMed] [Google Scholar]
- 24.Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–76. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 25.King IL, Segal BM. Cutting edge: IL-12 induces CD4+CD25–T cell activation in the presence of T regulatory cells. J Immunol. 2005;175:641–5. doi: 10.4049/jimmunol.175.2.641. [DOI] [PubMed] [Google Scholar]
- 26.Todryk SM, Gough MJ, Pockley AG. Facets of heat shock protein 70 show immunotherapeutic potential. Immunology. 2003;110:1–9. doi: 10.1046/j.1365-2567.2003.01725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baker-LePain JC, Sarzotti M, Fields TA, Li CY, Nicchitta CV. GRP94 (gp96) and GRP94 N-terminal geldanamycin binding domain elicit tissue nonrestricted tumour suppression. J Exp Med. 2002;196:1447–59. doi: 10.1084/jem.20020436. [DOI] [PMC free article] [PubMed] [Google Scholar]