Abstract
Syntenin is a PDZ protein that binds the cytoplasmic C-terminal FYA motif of the syndecans. Syntenin is widely expressed. In cell fractionation experiments, syntenin partitions between the cytosol and microsomes. Immunofluorescence microscopy localizes endogenous and epitope-tagged syntenin to cell adhesion sites, microfilaments, and the nucleus. Syntenin is composed of at least three domains. Both PDZ domains of syntenin are necessary to target reporter tags to the plasma membrane. The addition of a segment of 10 amino acids from the N-terminal domain of syntenin to these PDZ domains increases the localization of the tags to stress fibers and induces the formation of long, branching plasma membrane extensions. The addition of the complete N-terminal region, in contrast, reduces the localization of the tags to plasma membrane/adhesion sites and stress fibers, and reduces the morphotypical effects. Recombinant domains of syntenin with the highest plasma membrane localization display the lowest nuclear localization. Syndecan-1, E-cadherin, β-catenin, and α-catenin colocalize with syntenin at cell-cell contacts in epithelial cells, and coimmunoprecipitate with syntenin from extracts of these cells. These results suggest a role for syntenin in the composition of adherens junctions and the regulation of plasma membrane dynamics, and imply a potential role for syntenin in nuclear processes.
INTRODUCTION
Adherent cells express large amounts of heparan sulfate at their surfaces. This glycosaminoglycan modulates the actions of a large number of extracellular soluble and insoluble ligands. Heparan sulfate thus controls a wide variety of cellular processes, from cell adhesion to growth factor signaling (reviewed by Bernfield et al., 1999; Lander and Selleck, 2000). Two major families of heparan sulfate proteoglycan account for most of the cell surface-associated heparan sulfate: the glypicans and the syndecans. The syndecans are type I membrane proteins, with small but very characteristic cytoplasmic domains that remained near invariant throughout evolution. It is now clear that these highly conserved domains bind to a number of cytoplasmic and cytoskeletal proteins (reviewed by Zimmermann and David, 1999).
One of these proteins is syntenin. Syntenin was originally identified as a syndecan-binding protein in yeast two-hybrid screens by using the cytoplasmic domains of the syndecans as bait (Grootjans et al., 1997). Besides syndecans, syntenin also binds to other membrane proteins with structurally related C termini (Torres et al., 1998; Fernández-Larrea et al., 1999; Lin et al., 1999; Grootjans et al., 2000). Syntenin is composed of at least three separate structural domains: an N-terminal domain and a tandem of PDZ domains. The last 24 residues may constitute a separate fourth domain (Figure 1). The protein binds to the cytoplasmic domains of all four vertebrate syndecans, in yeast two-hybrid screens, surface plasmon resonance experiments, and ligand-overlay blotting assays. Site-directed mutagenesis and deletion experiments identified the FYA C-terminal amino acid sequence as the binding determinant in the syndecans (consistent with a PDZ domain-mediated interaction), and the tandem of PDZ domains as the binding site(s) in syntenin. Recombinant enhanced green fluorescent protein (EGFP)-syntenin fusion proteins decorate the plasma membrane where they colocalize with overexpressed syndecans. Cells that overexpress EGFP-syntenin show numerous cell surface extensions, suggesting effects of syntenin on cytoskeleton-membrane organization. From this we proposed that syntenin may function as an adaptor that couples syndecans (and possibly other proteins) to cytoskeletal proteins or cytosolic downstream signal effectors (Grootjans et al., 1997). Here, we analyze the expression and the subcellular distribution of endogenous syntenin. Using epitope-tagged deletion mutants of syntenin, we map the domains required for subcellular targeting and for effects on plasma membrane dynamics. Finally, we document the molecular association of endogenous syntenin with syndecan-1 and the E-cadherin/catenin complex.
Figure 1.
Alignment of the human, mouse, and rat syntenin domains. The M92-G102 region indicated in gray is proposed to play a role in signaling to the cytoskeleton (see text).
MATERIALS AND METHODS
Northern Blotting
Membranes containing lanes with poly(A)+ RNA from multiple human tissues were obtained from Clontech (Palo Alto, CA). Hybridization was performed using Expresshyb solution (Clontech) according to the manufacturer's specifications. The 32P probes were obtained by random priming. The syntenin probe corresponded to the full-length coding region (897 bp) plus part of the 3′ untranslated region (615 bp). Dehybridization included two washes with 2.0% SSC, 0.05% SDS (5 min at room temperature [RT], 30 min at 60°C) and a high stringency wash with 0.1% SSC, 0.1% SDS (30 min at 65°C).
Database Screening and cDNA Cloning
The Expressed Sequence Tags (EST)-database dbEST (http://www.ncbi.nlm.nih.gov/dbEST/) was screened for sequences related to human syntenin. Both mouse and rat syntenin-like ESTs were identified. The corresponding cDNA clones were obtained from the IMAGE Consortium (http://www-bio.llnl.gov/bbrp/image/image.html) through Research Genetics (Huntsville, AL). These clones, ID 466079 (mouse) and ID 304102 (rat), were completely sequenced. The mouse sequence was identical to the sequence deposited in the GenBank database under the accession number AF003693. The rat sequence was deposited under the accession number AF248548.
Anti-Syntenin Antibodies
BALB/c mice were immunized with 6His-syntenin-myc produced in bacteria and purified on Ni2+-nitrilotriacetic acid-agarose (Qiagen, Chatsworth, CA). Immunization schemes and the protocols for cell fusion and hybridoma selection were as described (De Boeck et al., 1987). Five clones were selected, based on the antibody binding profiles obtained in BIAcore: 1C8, 9C10, 4D12, 4F6, and 9E7. Epitope mapping and competition experiments were performed by Western blotting and BIAcore, using glutathione S-transferase (GST)–syntenin deletion mutants. The monoclonal antibodies (mAbs) 1C8 and 9C10 recognize the same or overlapping epitopes, whereas the other three antibodies react with three additional different epitopes, all localized in the N-terminal domain of syntenin. All mAbs react with syntenin in Western blotting and immunoprecipitation experiments, but none proved suitable for immunocytochemistry.
Two rabbits were immunized with recombinant syntenin, prepared from GST–syntenin with Factor Xa. Anti-syntenin polyclonal antibodies were isolated from the sera of these animals, by affinity purification on a syntenin–Sepharose 4B column (Amersham Pharmacia Biotech, Piscataway, NJ). Recombinant syntenin used for affinity purification was produced in the IMPACT T7 system, according to the protocol provided by the supplier (New England Biolabs, Boston, MA). No cross-reactivity with GST was observed after affinity purification. Western blotting experiments, using various GST–syntenin deletion mutants, indicated both antisera were directed against the N-terminal domain of syntenin. The antiserum from one of these animals proved suitable for immunocytochemistry.
Other Antibodies or Reagents
Anti-Pan cadherin rabbit serum was a generous gift from Dr. M. Mareel (Noëet al., 2001). Anti-β-catenin serum was obtained from Sigma, St. Louis, MO (#C2206). Anti-β-catenin and anti-α-catenin mouse mAbs were obtained from Transduction Laboratories, Lexington, KY (#C19220 and #C21620). Anti-vinculin mouse mAbs were obtained from Sigma (#V9131). Texas Red-X–phalloidin was obtained from Molecular Probes, Eugene, OR (#T7471). Anti-syndecan-1 and -3 mouse mAb (2E9) were described in Lories et al. (1989). Anti-c-Myc mAb (9E10) was obtained from Santa Cruz Biotechnology, Santa Cruz, CA (#sc-40).
In Vitro Transcribed-Translated Syntenin and Western Blotting
Human syntenin was produced by in vitro transcription-translation of cDNA encoding full-length syntenin [BssHII-XbaI fragment of the IMAGE clone 33203, cloned in the EcoRV-XbaI restriction sites of pcDNA3.1/Zeo(+)], using the TNT T7 Quick Coupled Transcription Translation System (Promega, Madison, WI).
Cells were rinsed with phosphate-buffered saline (PBS), scraped, and pelleted. The pellet was resuspended in sample buffer (0.05 M Tris-HCl pH 6.8, 2% SDS, 0.01% bromophenol blue, 10% glycerol) and boiled for 5 min. A 1-μl aliquot of the in vitro transcription-translation reaction mixture and SDS extracts from 1 × 106 cells were fractionated in SDS-PAGE. After electrotransfer to HYBOND-C-SUPER membranes, the blots were incubated with the biotinylated 4D12 anti-syntenin mouse mAb (5 μg/ml). After incubation with Streptavidin-horseradish peroxidase (Amersham Pharmacia Biotech), the signals were visualized with enhanced chemiluminescence Western blotting detection reagent (Amersham Pharmacia Biotech).
Subcellular Fractionation
Cells were rinsed in PBS, scraped, and pelleted. The cell pellet was resuspended in cold sucrose buffer (0.25 M sucrose, 5 mM Tris-HCl pH 7.4, 2 mM EDTA, 2 mM phenylmethylsulfonyl fluoride [PMSF], 2 μg/ml benzamidine, 2 μg/ml leupeptin, 2 μg/ml aprotinin, 1 μg/ml pepstatin). Cells were homogenized by 10 consecutive passages of the cell suspension through a ball-bearing cell cracker (EMBL-Heidelberg, Heidelberg, Germany, clearance 0.010 mm). After removal of unbroken cells, nuclei, and other large debris by low-speed centrifugation (600 × g), the cytosol fraction (C) was separated from the microsome fraction (M) by centrifugation for 2 h at 100,000 × g. The pellet was resuspended in sucrose buffer, readjusting the volume of the microsome fraction to that of the cytosol fraction before fractionation in SDS-PAGE. For the extractions, the microsome pellet was resuspended in Tris-buffered saline (TBS; 20 mM Tris-HCl pH 7.4, 150 mM NaCl, 2.7 mM KCl) at protein concentration of 1 mg/ml. One aliquot of this suspension was freeze-thawed five times in ethanol-dry ice to exclude entrapment in the microsomes. Another aliquot was incubated for 1 h at RT in 1 M hydroxylamine, pH 7, to exclude palmitoylation. Other aliquots were extracted with 0.5 M NaCl, 0.1 M Na2CO3, 1% saponin or 1% Triton X-100 1%, for 30 min at 4°C. After the different extraction procedures, the samples were centrifuged at 100,000 × g to separate the extractable fractions (SN) from the nonextractable fractions (P). After resuspension of the pellets in TBS and readjustment of the sizes of the fractions, all fractions were analyzed by Western blotting. The blots were incubated with affinity-purified rabbit anti-syntenin polyclonal antibodies (1 μg/ml), mouse anti-syntenin mAb (4D12, 20 μg/ml), or anti-PAN-cadherin rabbit serum (1/1000) and corresponding secondary goat antibodies, coupled to horseradish peroxidase (Bio-Rad, Richmond, CA).
Cell Culture and Transfections
Except for the normal human lung fibroblasts (NHF), all cells originated from American Type Culture Collection (Manassas, VA). All cells were routinely grown in DMEM/F12 medium (Life Technologies, Gaithersburg, MD) supplemented with 10% fetal bovine serum (HyClone, Logan, UT). For microscopic analysis cells were plated on noncoated eight-well chamber slides (Nalge Nunc International, Rochester, NY), except for the data in Figure 4, I and J, where the wells were coated with fibronectin (Humphries, 1998). For transient expression assays, the cells were transfected the day after plating using the LIPOFECTAMINE PLUS reagent (Life Technologies), according to the recommendations of the manufacturer.
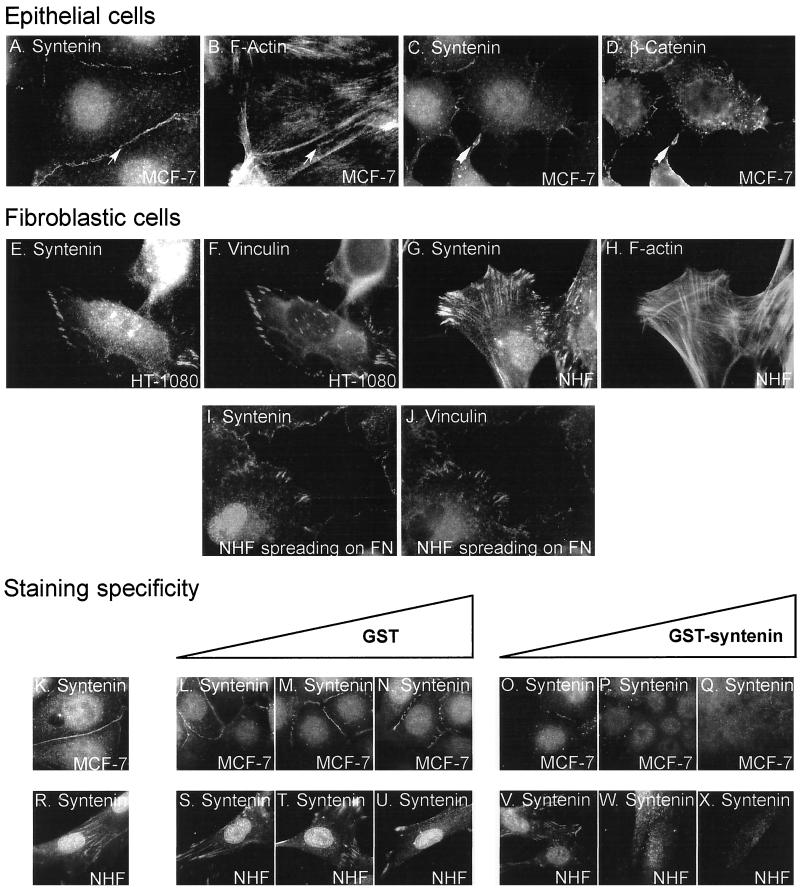
Figure 4.
Subcellular localization of endogenous syntenin. Fluorescence optical micrographs of cells cultured overnight on noncoated chamber slides (A–H and K–X) or allowed to spread for 15 min on fibronectin-coated chamber slides (I and J). The cells were stained with affinity-purified rabbit anti-syntenin polyclonal antibodies (A, C, E, G, and I) and costained with phalloidin (B and H) or with mouse mAbs specific for β-catenin (D) or vinculin (F and J). Fine arrows pinpoint colocalization of syntenin and F-actin in large cell-cell contacts, large arrows pinpoint colocalization of syntenin and β-catenin in nascent cell-cell contacts. The specificity of the stainings was addressed in competition experiments. In these experiments the cells were stained with affinity-purified rabbit polyclonal anti-syntenin antibodies (3 μg/ml) that were incubated overnight in the presence of increasing concentration of GST (0.3 μg/ml [L and S]; 3 μg/ml [M and T]; 30 μg/ml [N and U]) or GST-syntenin (0.3 μg/ml [O and V]; 3 μg/ml [P and W]; 30 μg/ml [Q and X]), or in the absence of any recombinant protein as control (K and R).
Eukaryotic Expression Vectors for Syntenin and Syntenin Domains
Various cDNAs, encoding syntenin or syntenin domains, were cloned in pEGFP-C1/C3 (Clontech) to obtain EGFP fusion proteins, or in pcDNA3.1/Zeo(+) (Invitrogen, San Diego, CA) to obtain Myc-tagged (EQKLISEEDL) proteins. These constructs and their coordinates are represented in Figure 5. Sequencing certified all expression vectors.
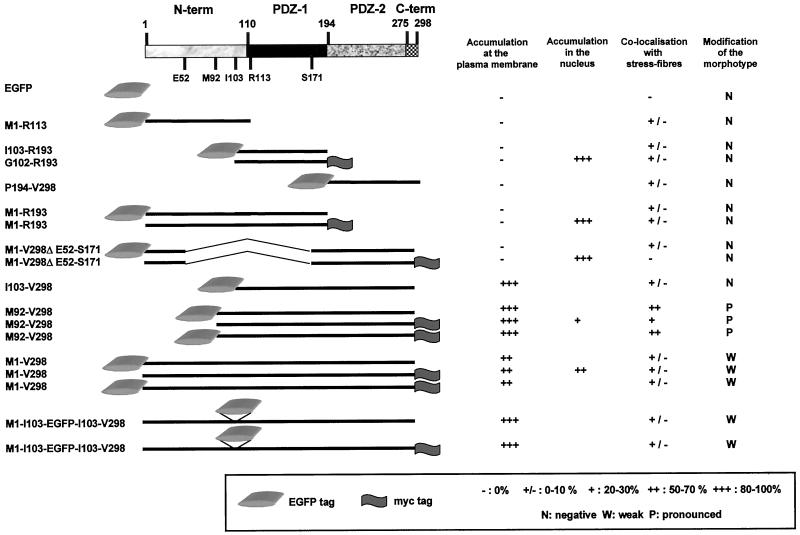
Figure 5.
Summary of the subcellular localizations and morphotypic effects of the different recombinant domains of syntenin in transient transfection experiments. The domains were tagged with EGFP and/or Myc. A schematic representation of the different syntenin domains that were overexpressed, and the coordinates of the corresponding amino acids, is given on the left. The results are represented as the percentage of transfected cells where the overexpressed proteins were enriched in the subcompartment under scrutiny, the sum of all expressing cells taken as 100%. Results presented for the accumulation at the plasma membrane were measured in MCF-7 cells, where the plasma membranes were visualized by biotinylation and Streptavidin–Texas-Red staining. Results for accumulation in the nucleus were also obtained in MCF-7 cells. Enrichment along stress fibers was measured in CHO-K1 cells, in the transfectants where the stress fibers were nicely visualized by phalloidin staining. A modification of the morphotype was observed in several cell types (see text).
Fixation and Immunostaining
Nontransfected cells were fixed the day after plating, with the exception of the cells spreading on fibronectin that were fixed 15 min after plating. Transfected and sham-transfected cells were fixed the day after transfection. Where indicated, transfected cells were washed with PBS and incubated (40 min, 4°C) with a solution of 0.5 μg/ml sulfo-NHS-Biotin (Pierce, Rockford, IL) followed (30 min, 4°C) by Streptavidin–Texas Red (Molecular Probes), before fixation. Otherwise, the cells were washed with PBS and immediately fixed with 3.7% formaldehyde or 4% paraformaldehyde. Where required, the cells were permeabilized with 0.1% Triton X-100 in PBS, incubated with first antibody and/or Texas Red-X–phalloidin, for 1 h at RT, followed by goat anti-mouse or anti-rabbit IgG conjugated to fluorescein isothiocyanate (Nordic Immunology, Tilburg, The Netherlands) or Texas-Red (Molecular Probes). For competition experiments, the GST fusion proteins were prepared as described in Grootjans et al. (1997) and preincubated with the first antibody as described in Figure 4, K–X.
Microscopic Analysis
The signals were examined by digital imaging fluorescence microscopy by using a cooled charge-coupled camera (Photometrics, Tucson, AZ) or by confocal microscopy (MRC-1024 Laser Scanning Confocal Imaging System; Bio-Rad). The length of the membrane protrusions was assessed by using The Lasersharp MRC-1024 software (Bio-Rad, Microscopy Division).
Immunoprecipitations
Syndecan-1 Coimmunoprecipitation Experiment.
Madin-Darby canine kidney (MDCK) cells were incubated for 30 min with 600 μM Dithio-bis[succinimidylpropionate] (DSP) reducible cross-linker (Pierce) at 37°C. The reaction was terminated by two washes with 20 mM Tris-HCl pH 7.4, 100 mM NaCl. The cells were extracted with 50 mM 2-(N-morpholino)ethanesulfonic acid pH 6.8, 70 mM NaCl, 1 mM EGTA, 1 mM MgCl2, 1% NP-40, 2 mM PMSF, 2 μg/ml benzamidine, 2 μg/ml leupeptin, 2 μg/ml aprotinin, 1 μg/ml pepstatin. The extract of cells, collected by scraping, was cleared by centrifugation for 10 min at 12,000 rpm in an Eppendorf tabletop centrifuge. Supernatant containing 2 mg of total protein was used for each immunoprecipitation. Before immunoprecipitation, each sample was preabsorbed on 50 μl of protein A-Sepharose CL-4B, for 1 h at 4°C under gentle agitation. For immmunoprecipitation, preabsorbed extract was mixed with a fresh sample of 50 μl of protein A-Sepharose CL-4B, 10 μg of anti-syntenin (4F6), or 12 μg of anti-syndecan-1 and -3 (2E9) mouse mAb, and 5 μg of rabbit anti-mouse immunoglobulin. After overnight incubation at 4°C under gentle agitation, the beads were collected, washed, and treated with heparitinase and chondroitinase ABC (Lories et al., 1989) or left untreated. Immunoprecipitated material was released from the beads by boiling for 5 min in sample buffer supplemented with 5% β-mercaptoethanol, and fractionated in SDS-PAGE. After transfer, the upper part of the blot was stained with anti-syndecan-1 and -3 mouse mAb 2E9 (10 μg/ml), and the lower part with 20 μg/ml anti-syntenin mouse mAb (4D12).
E-Cadherin/β-Catenin/α-Catenin Coimmunoprecipitation Experiment.
Total MDCK or Michigan Cancer Foundation (MCF)-7 cells or microsomes from MDCK cells (for syntenin detection), were extracted for 20 min at 4°C with PBS pH 7.4 containing 1% NP-40, 1% Triton X-100, 0.9 mM CaCl2, 0.334 mM MgCl2, 1.72 mM PMSF, 21 μM leupeptin, 10 μg/ml pepstatin. Microsomes used for immunoprecipitation experiments were prepared as described above, except for the presence of 0.9 mM CaCl2 and 0.334 mM MgCl2 in the buffer. After extraction, immunoprecipitations were performed as described for syndecan-1 coimmunoprecipitations except that rabbit preimmune serum (10 μl), or affinity-purified rabbit anti-syntenin polyclonal antibodies (4.5 μg), or anti-β-catenin rabbit serum (2 μl) were used. Western blottings were performed with anti-PAN-cadherin rabbit serum (1/1000), anti-α-catenin mAb (1/1000), anti-β-catenin mouse mAb (1/2000), anti-syntenin mouse mAb 4D12 (20 μg/ml) for MCF-7 extracts, or a mix of anti-syntenin mAbs 4F6 (5 μg/ml), 4D12 (20 μg/ml), and 1C8 (10 μg/ml) for MDCK extracts.
RESULTS
Syntenin Is Widely Expressed and Highly Conserved in Mammalia
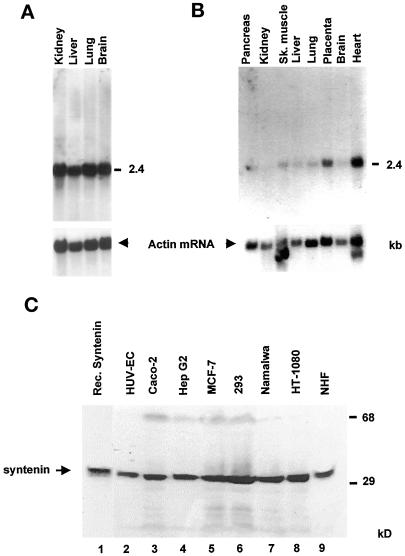
To analyze the expression pattern of syntenin, we performed Northern blotting experiments. A single signal, corresponding to a mRNA of ∼2.4 kb, was detected in all fetal and adult human tissues analyzed. High syntenin expressions were noted in fetal kidney, liver, lung, and brain (Figure 2A). In adult human tissues the message was abundant in heart and placenta. Much lower expression levels were found in all other tissues (Figure 2B). By screening the EST database for syntenin-related cDNA sequences, we also identified clones for mouse and rat syntenin. The sequencing of the open reading frames of these clones and the deduced amino acid sequences indicated that both mouse and rat syntenin are highly similar to human syntenin (Figure 1). The rodent and human protein sequences are nearly completely identical at the level of the first PDZ domain (PDZ-1) and at the level of the C terminus of the protein that might compose a separate fourth domain. The rodent PDZ-2 domains are nearly identical to each other, and 90% similar to human PDZ-2. The N-terminal domain is the most divergent between species. The human domain is 81 and 77% identical to, respectively, the mouse and rat domains.
Figure 2.
Expression of syntenin. Poly(A)+ RNA blots of human fetal (A) and adult (B) tissues hybridized with a syntenin probe, and with a β-actin probe as control, show that syntenin mRNA (2.4 kb) is widely distributed. (C) Western blot analyses with anti-syntenin mAb (4D12) of total cell extracts from human cell lines of different tissue origins (lanes 2–9) yield a positive signal around 30 kDa that comigrates with in vitro translated syntenin (lane 1).
To explore the expression and subcellular localization of syntenin at the protein level, we raised and characterized mouse mAbs and affinity-purified rabbit polyclonal antibodies against human syntenin (see MATERIALS AND METHODS). All antibodies recognize epitopes in the first 92 amino acids of human syntenin (our unpublished results), suggesting a weak immunogenicity of the PDZ domains. Some of these antibodies showed species cross-reactivity.
We then used these antibodies in Western blot analyses of total cell extracts from human cell lines of different tissue origins. All cell extracts yielded a positive signal around 30 kDa (Figure 2C, lanes 2–9) that comigrated with the signal obtained for in vitro translated human syntenin (Figure 2C, lane 1). A similar 30-kDa signal was also obtained for MDCK cells (see below). We conclude that syntenin is widely expressed in tissues and cultured cell lines.
Syntenin Partitions between the Cytosol and Microsomes
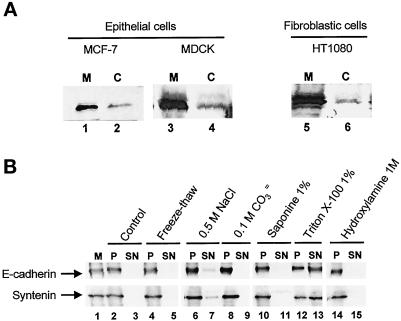
To obtain initial indications about possible syntenin associations and functions, we assessed the subcellular distribution of syntenin in different cell types. In all cells, of either epithelial or fibroblastic origin, syntenin distributed between the cytosolic (C) and the microsome (M) fraction. Syntenin was always abundant in microsomes (Figure 3A). To assess the strength of the syntenin association with microsomes, M fractions were extracted by different treatments (Figure 3B). Surprisingly, high carbonate (Figure 3B, lanes 8 and 9) or high salt (Figure 3B, lanes 6 and 7) concentrations failed to release syntenin from microsomes, suggesting that the association could not be explained solely by a PDZ interaction with transmembrane syndecans. Indeed, high-salt treatment disrupts the syntenin–syndecan interaction in vitro (Grootjans et al., 1997). Detergents such as saponin, Triton X-100 (Figure 3B, lanes 10–13), and octylglucoside (our unpublished results) also failed to completely release syntenin from microsomes. The behavior of syntenin in these experiments was similar to that of E-cadherin, a transmembrane protein (Figure 3B, lanes 1–15) that also associates with detergent-insoluble fractions (Hinck et al., 1994). β-Catenin, one of the cytoplasmic ligands of E-cadherin, displayed a more labile association with microsomes than syntenin (our unpublished results). An integral membrane association of syntenin is not predicted from its protein sequence. Syntenin does not contain a consensus sequence for prenylation or myristoylation, and treatment of microsomes with hydroxylamine 1 M for 1 h at RT failed to release any syntenin (Figure 3B, lanes 14 and 15). We thus exclude that lipidation could explain the strong association of syntenin with microsomes. Because several detergent treatments, even in combination, could not completely release syntenin (our unpublished results), we concluded that at least part of syntenin would be associated with the cytoskeleton. The experiments described below are in agreement with this interpretation.
Figure 3.
Cytosolic and microsomal syntenin. (A) Western blots of microsome (M) and cytosol (C) fractions from MCF-7, MDCK, and HT1080 cells with anti-syntenin antibodies (lanes 1 and 2, mAb 4D12; lanes 3–6, rabbit pAbs). (B) Western blots with anti-syntenin antibodies or anti-PAN-cadherin antibodies of microsomes from MCF-7 cells resuspended in TBS (M, lane 1), and of the microsome nonextractable (P) or extractable (SN) fraction by different treatments as indicated (lanes 2–15).
Endogenous Syntenin Is Enriched at Cell-Cell Contacts and Focal Adhesions, Colocalizes with Stress Fibers, and Is Concentrated in the Nucleus
To address the subcellular distribution of endogenous syntenin, affinity-purified rabbit anti-syntenin polyclonal antibodies were used in immunofluorescence microscopy on cell lines of different origins.
In cells of epithelial origin, the antibodies strongly stained the areas of cell-cell contact. This signal colocalized with that for F-actin (Figure 4, A and B), syndecan-1, and β-catenin (Figure 7A). Syntenin, as well as β-catenin, was enriched in thin plasma membrane extensions that made contact between cells at low cell density (Figure 4, C and D), suggesting syntenin is already enriched in nascent cell-cell contacts.
Figure 7.
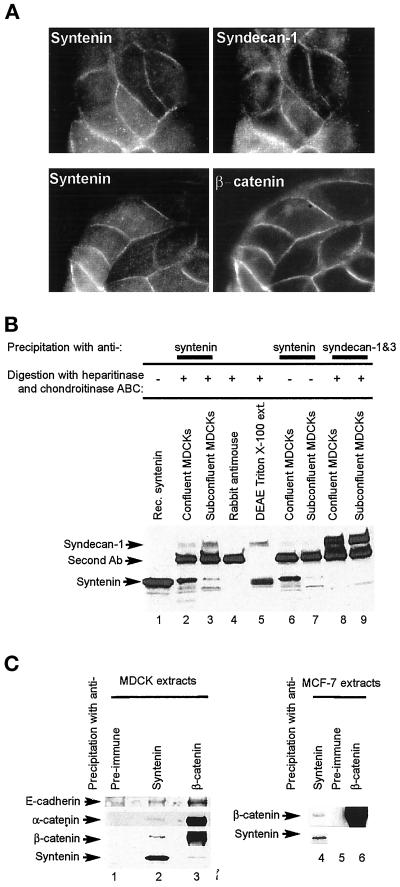
Syndecan-1 and the E-cadherin/β-catenin/α-catenin complex colocalize in cell-cell contacts and coimmunoprecipitate with syntenin from epithelial cell extracts. (A) Colocalization of syntenin with syndecan-1 and β-catenin. Fluorescence optical micrographs of MDCK cells stained with affinity-purified rabbit anti-syntenin polyclonal antibodies (left) and costained with mouse mAbs for syndecan-1 and 3 (upper right) or β-catenin (lower right). (B) Coimmunoprecipitation of syndecan-1 with syntenin. Cross-linked protein extracts from confluent (lanes 2, 4, 6, and 8) or subconfluent (lanes 3, 7, and 9) MDCK cells were immunoprecipitated with mouse anti-syntenin (lanes 2, 3, 6, and 7) or anti-syndecan (lanes 8 and 9) mAbs and rabbit antimouse polyclonal antibodies (lanes 2–4 and 6–9). In vitro transcribed-translated syntenin (lane 1), proteoglycans purified from Triton X-100 extracts of MDCK cells by chromatography over DEAE (lane 5), and the immunoprecipitated proteins were digested (lanes 2–5, 8, and 9) or not digested (lanes 6 and 7) with heparitinase and chondroitinase ABC. Samples were Western blotted for syndecan-1 and -3 (upper part) or syntenin (lower part). The band specifically stained with the 2E9 anti-syndecan antibody migrates at a position that is characteristic for syndecan-1, which is consistent with the fact that syndecan-1 is, and syndecan-3 is not expressed in MDCK cells. (C) Syntenin and members of the E-cadherin adhesion complex coimmunoprecipitate. Noncross-linked protein extracts from MDCK (lanes 1–3) or MCF-7 (lanes 4–6) cells were immunoprecipitated with preimmune serum (lanes 1 and 5), affinity-purified anti-syntenin polyclonal (lanes 2 and 4), or anti-β-catenin serum (lanes 3 and 6) and Western blotted for the proteins indicated.
In all cells of fibroblastic origin tested, the antibodies strongly stained areas of cell-substratum contact that corresponded to peripheral focal adhesions, also enriched in vinculin (Figure 4, E and F). This staining often extended along actin stress fibers (Figure 4, G and H). Staining of the focal adhesions was already noted after 15 min of spreading of the cells on fibronectin (Figure 4, I and J), indicating the enrichment of syntenin in these structures is an early event. Interestingly, in several cell types, we also observed a staining of the nucleus (Figure 4, A, G, and I). Western blot analyses of nuclear extracts confirmed the presence of syntenin in the nucleus (our unpublished results).
The specificities of all these stainings were validated in competition experiments. In epithelial cells, the cell-cell contact and nuclear stainings (Figure 4K) were blocked by incubation with recombinant GST-syntenin (Figure 4, O–Q), but not with recombinant GST (Figure 4, L–N). In fibroblasts, the focal adhesion, stress fiber, and nuclear stainings (Figure 4R) were blocked by recombinant GST–syntenin (Figure 4, V–X), but not by recombinant GST (Figure 4, S–U).
These results suggest that syntenin may be a multifunctional protein, with a role in the organization of cell-cell and cell-matrix adhesions, microfilaments, and also in the nucleus. In further experiments we attempted to relate these findings to the multidomain structure of the protein. Syntenin can indeed be divided into at least three structural domains (Figure 1): the N-terminal domain (amino acids 1–109), which does not show homology to any known structural motif; a first PDZ domain (PDZ-1) (amino acids 110–193); and a second PDZ domain (PDZ-2) (amino acids 194–274). The last 24 residues (amino acids 275–298) may constitute a separate fourth domain. This fourth domain is dispensable for the in vitro syndecan–syntenin interaction (Grootjans et al., 2000) and was not considered separately here. To confirm the endogenous distributions observed in immunofluorescence microscopy, and to map the functional domains of syntenin that were involved in these localizations, we prepared a series of eukaryotic expression vectors where different syntenin domains were fused to Myc or EGFP tags (Figure 5). We used two different modes of tagging, as we suspected that the nature and the position of the tag by itself could influence the behavior of the protein. The different mutants were analyzed in transient transfection experiments.
Paired PDZ Domains Are Required for Plasma Membrane Localization in Epithelial Cells
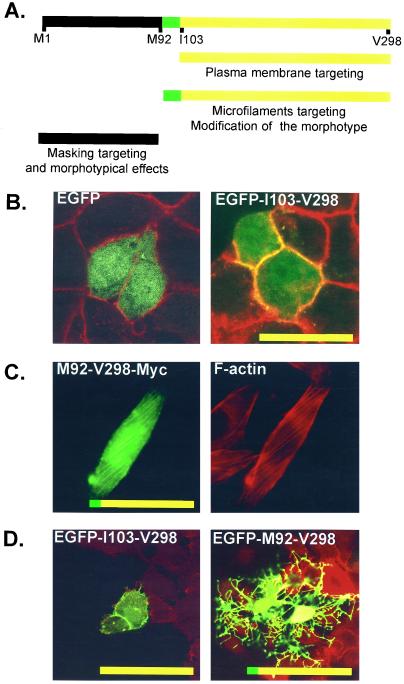
In epithelial cell lines, tagged syntenin was enriched in cell-cell contacts, where antibodies also detected endogenous syntenin. To identify the domains of syntenin involved in the plasma membrane/cell-cell contact targeting, we transfected MCF-7 epithelial cells with the different domain-expression vectors and calculated the percentage of transfected cells where the overexpressed protein was enriched at the plasma membrane, as revealed by confocal microscopy. The recombinant proteins that contained the I103-V298 fragment, and thus the paired PDZ domains, were concentrated at cell-cell contacts (Figure 5; compare also EGFP with EGFP-I103-V298 in Figure 6B). Yet, in high expressing cells, the membrane accumulation of the recombinant protein was not limited to contact-forming regions. Recombinant proteins containing solely the N-terminal domain and/or single PDZ domains were never concentrated at the plasma membrane (Figure 5). The nature of the tag made no difference. We concluded that PDZ-1 and PDZ-2 need to be paired for the plasma membrane localization of syntenin and that the N-terminal domain is not required for this localization. Yet, we observed that recombinant M92-V298 and I103-V298 localized to plasma membranes in a larger proportion of cells than a full-length syntenin with an external Myc or EGFP tag (Figure 5). This implies that the N-terminal domain has some negative influence on this membrane localization. Furthermore, recombinant full-length syntenin with an internal EGFP tag (separating the N-terminal domain from the PDZ tandem) was more localized to the plasma membrane than full-length syntenins with external tags (Figure 5). This could indicate that the negative effect of the N-terminal domain on plasma membrane localization is mostly sterical in nature.
Figure 6.
Functional regions identified in syntenin. (A) The I103-V298 fragment targets the protein to the plasma membrane and preferentially to cell-cell contacts in low expressing cells. The M92-V298 fragment displays strong coalignment with microfilaments and is able to modify the morphology of the cells. The M1-Y91 fragment partially masks the domains required for targeting and morphotypical effects (see text for more comments). (B) Micrographs of confocal images of MCF-7 cells transiently transfected with expression vectors for EGFP (left) or EGFP-I103-V298 (right) syntenin domain (green). The red signals correspond to plasma membranes outlined by biotinylation and Streptavidin–Texas-Red. EGFP does not localize to plasma membranes/cell-cell contacts, whereas EGFP fused to I103-V298 (containing both PDZ domains) does localize to these sites (yellow). (C) Micrographs of optical images of CHO-K1 cells transiently transfected with an expression vector M92-V298-Myc syntenin domain. The overexpressed protein is visualized in green and F-actin in red (staining with phalloidin). M92-V298 fused to Myc coaligns with stress fibers. Morphotypical alterations are not apparent because they are out of the focal plane of the image. (D) Micrographs of confocal images of MCF-7 cells transiently transfected with expression vectors for EGFP-I103-V298 (left) or EGFP-M92-V298 (right) syntenin domain (green). The red color corresponds to F-actin staining with phalloidin. EGFP fused to I103-V298 localizes to plasma membranes/cell-cell contacts but does not affect the morphology of the cells, whereas EGFP fused to M92-V298 has strong morphotypic effects.
Nuclear Enrichment of Syntenin Is Inversely Correlated with Plasma Membrane Localization
MCF-7 cells were also analyzed for the accumulation of the overexpressed proteins in the nucleus. As assessed by fluorescence microscopy, none of the overexpressed syntenins or syntenin domains was completely excluded from the nuclear compartment, including the largest of these constructs. Because EGFP itself concentrates in the nucleus, we only evaluated the nuclear enrichments of the Myc-tagged recombinant proteins. We observed that the syntenin domains that showed the highest affinity for the plasma membrane were the ones that displayed the lowest affinity for the nuclear compartment (Figure 5), but we could not identify a domain that would be responsible for the nuclear enrichment of syntenin.
Colocalization of Syntenin with Stress Fibers Increases Drastically When Syntenin Is Truncated for its First 91 Amino Acids
Tagged syntenin was also enriched at the plasma membrane of transiently transfected fibroblastic cells, with a similar requirement for both PDZ domains as observed in epithelial cells. However, so far, we were not able to detect any concentration of the overexpressed syntenin or syntenin domains (made as described in Figure 5) in focal adhesions. Simultaneous stainings for vinculin excluded that the overexpressions prevented the formation of focal adhesions (our unpublished results). Where the overexpressed syntenin domain did not cross-react with our anti-syntenin antibodies, we also excluded that endogenous syntenin would be removed from focal adhesions in the transfected cells. Thus, we concluded that none of the overexpressed proteins fulfills the requirements for focal adhesion localization.
Tagged-syntenin was partially localized along the stress fibers of these fibroblastic transfectants, as does endogenous syntenin. Chinese hamster ovary (CHO) cells, which form multiple stress fibers, were selected for mapping the domains responsible for this association. The association was scored in transfected cells where the stress fibers were clearly visualized with phalloidin (Figure 5). Except for EGFP and M1-V298ΔE52-S171 (Myc-tagged) transfectants (no colocalization at all), the overexpressed proteins were always present on stress fibers, at least in a small percentage (0–10%) of cells. Of all the constructs, M92-V298 showed the clearest colocalization with stress fibers (up to 70% of the CHO transfectants that expressed this domain and developed stress fibers). Figure 6C shows a representative experiment where CHO cells expressed M92-V298 tagged with Myc. As nearly all fusion proteins displayed at least some degree of enrichment along stress fibers, we conclude that multiple domains of syntenin can associate with microfilaments. Because the combination of the two PDZ domains and the flanking part of the N-terminal domain colocalized more significantly with stress fibers than separate domains, we conclude that a combination of interactions is responsible for a strong association with these filaments. Because the full-length forms of syntenin showed much less propensity to associate with stress fibers than a protein that was truncated for its first 91 amino acids (Figure 5), we again conclude that the M1-Y91 region may have a masking/modulating effect on other syntenin domains.
Overexpression of N-truncated Syntenin Enhances the Formation of Plasma Membrane Extensions
Overexpression of syntenin induces the formation of novel plasma membrane structures in all cell systems tested so far, including HT1080, CHO, MCF-7, MDCK, and NIH3T3 cells. Yet, the manifestation of the morphotypical modifications in terms of severity and form (ruffles, lamillipodia, fine extensions, neurite-like structures) varies from cell type to cell type. Transfected cells that are trypsinized and replated on fibronectin for 30 min already show an alteration of the morphotype, indicating that the overexpressed protein affects plasma membrane dynamics at early stages of cell spreading (our unpublished results). To map the syntenin domains responsible for this effect on membranes, we compared the effects of the transient expressions of the different tagged syntenin domains, initially in MCF-7 cells, subsequently in other cell types. The modification of the morphotype of the cells was most pronounced in the transfectants containing the M92-V298 segment of syntenin (Figure 5). MCF7 cells overexpressing high levels of this segment developed spectacular branching extensions (Figure 6D, right). Cells transfected with EGFP, with the syntenin domains that did not localize to the plasma membrane, or with I103-V298 that strongly localizes to the plasma membrane (Figures 5 and 6D, left) did not display such morphological alterations. To objectivate this phenomenon, we determined the mean value of the lengths of the extensions induced by I103-V298 and compared this with the mean value of the lengths of the extensions induced by M92-V298, two constructs that localize to, and outline membranes, to similar extents. We used a population of transfectants displaying five or more extensions for this measurement. In subconfluent, as well as in confluent MCF-7 cells, the plasma membrane extensions in I103-V298 transfectants were on average three times smaller than in M92-V298 transfectants. According to the Student's t test (p < 0.05), this difference was significant. Because the presence of a small sequence of 10 amino acids (M92-G102, indicated in gray in Figure 1) determines the elongation of the extensions, we propose that both PDZ domains cooperate in targeting syntenin to the plasma membrane, and that the M92-G102 segment of the N-terminal domain represents a region for further signaling to the cytoskeleton. Clearly, full-length syntenin did not alter the morphotype of the cells as strongly as the M92-V298 segment (Figure 5). Thus, the M1-Y91 segment may partially mask the effects of the M92-G102 segment on plasma membrane extensions. Although the effects on cell morphology were most pronounced and spectacular in MCF7 cells and least pronounced in CHO cells, basically, similar conclusions were reached in HT-1080, MDCK, and NIH3T3 (our unpublished results).
Syndecan-1 and the E-Cadherin/β-Catenin/α-Catenin Complex Are Physically Associated with Syntenin in Cells of Epithelial Origin
To document a potential role for syndecans in the localization of syntenin at adherens junctions, we performed coimmunoprecipitation experiments on extracts from MDCK cells. We selected these cells because they are strongly polarized and display strong endogenous expression and colocalization of syndecan-1 and syntenin at sites of cell-cell contact (Figure 7A). Immunoprecipitates obtained with anti-syntenin mAbs contained a band that depended on heparitinase-chondroitinase digestion of the precipitate and that reacted with an antisyndecan-1 mAb (compare Figure 7B, lanes 6 and 7 with lanes 2 and 3). This band comigrated with the syndecan-1 signal present in a purified fraction of digested proteoglycans from MDCK cells (Figure 7B, lane 5), and with the enzyme-digested anti-syndecan-1 immunoprecipitates from the same extracts (Figure 7B, lanes 8 and 9). This band was not observed when MDCK cell extracts were solely immunoprecipitated with rabbit anti-mouse polyclonal antibodies and digested with heparitinase and chondroitinase ABC (Figure 7B, lane 4). On the other hand, we were unable to coimmunoprecipitate syntenin, from the same material, with the anti-syndecan-1 mAb. This could be due to the fact that the mAb we need to use for the syndecan-1 immunoprecipitation from MDCK cells, which is directed against the conserved cytoplasmic domain, recognizes an epitope close to the syntenin-binding site. We also noticed a preferential coimmunoprecipitation of syndecan and syntenin from subconfluent cells (compare the syntenin-syndecan-1 ratios in Figure 7B, lanes 2 and 3).
Not only syndecan-1, but also β-catenin (Figure 7A), E-cadherin, and α-catenin (our unpublished results) colocalize with syntenin at adherens junctions. We therefore also looked for a possible physical link between syntenin and the E-cadherin/β-catenin/α-catenin complex. Western blotting of anti-syntenin and anti-β-catenin immunoprecipitates indicated that a fraction of these proteins indeed specifically immunoprecipitates with syntenin, both from MDCK and MCF-7 extracts (Figure 7C, lanes 1–6). One can note that the E-cadherin/β-catenin ratios in the syntenin immunoprecipitates from both these cells were clearly higher than the E-cadherin/β-catenin ratios measured in β-catenin immunoprecipitates from the same cells. We conclude that a syntenin-syndecan-1 complex forms at cell-cell contacts under natural conditions and is somehow linked to the E-cadherin-catenin complex.
DISCUSSION
We originally identified syntenin as a protein that binds directly to the cytoplasmic domain of the syndecan family of heparan sulfate proteoglycans, in yeast two-hybrid screens and in vitro (Grootjans et al., 1997). The present data indicate that syntenin is strongly associated with membranes; localizes to cell-adhesion sites, microfilaments, and the nucleus; and physiologically interacts with syndecan-1. The expression pattern of syntenin and many of the subcellular localizations of syntenin makes sense, for what is known of the biology of the syndecans (Bernfield et al., 1992, 1999). Like syntenin, syndecans are widely distributed (Kim et al., 1994). Syndecan-4 is concentrated in focal adhesions (Woods and Couchman, 1994; Baciu and Goetinck, 1995; Woods and Couchman, 1998; Couchman and Woods, 1999; Saoncella et al., 1999). Upon ligation, syndecans can be induced to coalign with microfilaments (Martinho et al., 1996; Carey, 1997). Syndecan-1 is enriched in cell-cell contacts and has been incriminated in the maintenance of the differentiated epithelial phenotype, and it has been proposed that it does so by affecting the organization of the actin cytoskeleton and the expression of E-cadherin (Kato et al., 1995; Leppäet al., 1996; Sun et al., 1998).
Plasma Membrane Localization of Syntenin
Syntenin is a PDZ protein and many of the proteins that contain PDZ domains are associated with membranes, suggesting that these domains are involved in either organizing transmembrane protein complexes at plasma membranes or recruiting proteins from the cytosol (Fanning and Anderson, 1998). The paired PDZ domains of syntenin are necessary for the in vitro interaction with syndecans (Grootjans et al., 1997), neurexin (Grootjans et al., 2000), and B-ephrins (Torres et al., 1998; Lin et al., 1999). This implies that the interaction of syntenin with these forms of bait may depend on cooperative PDZ bindings, an interpretation that is supported by further mutagenesis and domain-swapping experiments (Grootjans et al., 2000). Because paired PDZ domains are also necessary for the association of recombinant syntenin with the plasma membrane (Figures 5 and 6A), it is tempting to propose that cooperative PDZ interactions are responsible for the membrane localization of syntenin. In epithelial cells syndecan-1 is a good candidate for bringing syntenin to the membrane, because the endogenous syntenin colocalizes with syndecan-1 in cell-cell contacts (Figure 7A) and coprecipitates this protein (Figure 7B). Despite this consistency, the association of syntenin with microsomes is too strong to be solely explained by classical PDZ interactions with syndecans (Figure 3B). Indeed, in vitro, this binding is salt-sensitive. The strong association of syntenin with microsomes could possibly be the result of an interaction with syndecan-1 via its PDZ domains, combined to an interaction with the cortical actin cytoskeleton, possibly via the E-cadherin/β-catenin/α-catenin adhesion complex that also colocalizes and coimmunoprecipitates with syntenin (Figure 7, A and C). We are currently looking for a possible direct interaction of syntenin with E-cadherin, β-catenin, α-catenin, and other components of the adhesion zipper (Vasioukhin et al., 2000). In that context it is interesting to note that the heterotrimeric LIN-2, LIN-7, LIN-10 PDZ protein complex also forms a complex with cadherin and β-catenin in epithelia and neurons in Caenorhabditis elegans, and that this association is mediated by direct binding of LIN-7 to the C-terminal PDZ target sequence of β-catenin (Perego et al., 2000). So far we tend to exclude that syntenin would directly adapt to β-catenin. The mammalian homolog of LIN-2 is CASK. CASK and syntenin are two PDZ proteins that bind to syndecans in a probably mutually exclusive way (Cohen et al., 1998; Hsueh et al., 1998; Hsueh and Sheng, 1999; Grootjans et al., 2000). So far we have no evidence, but can also not exclude, that syntenin would also be part of a similar multimeric complex. If syntenin proves to be part of a molecular link between syndecan-1 and E-cadherin/catenins, what could then be the role of syntenin in cell-cell adhesion? Interestingly, together with E-cadherin/catenins, syntenin is already concentrated in filopodia that establish the initial contacts between epithelial cells (Figure 4, C and D). The syndecan-1–syntenin interaction could be important in the formation rather than the maintenance of cell-cell contacts as coimmunoprecipitation of syndecan-1 with syntenin appears enhanced in subconfluent cells (Figure 7C).
Effects on Plasma Membrane Dynamics
Overexpression of the M92-V298 fragment of syntenin has also a strong effect on cell morphology, inducing the formation of long cell projections that suggest interference with actin dynamics at the plasma membrane (Figure 6,A and D, right). Because the I103-V298 fragment does not affect the morphotype but is similarly enriched at the plasma membrane, the identification of proteins binding to the M92-G102 segment could be crucial for the understanding of these effects. So far our attempts to identify such proteins have failed. Yet, in preliminary further experiments we observed that the morphotype due to the overexpression of the M92-V298 fragment could be reversed by overexpression of constitutively active RhoA mutants (our unpublished results). The Rho family of GTPases have emerged as crucial regulators of cadherin-dependent adhesion (Kaibuchi et al., 1999; Evers et al., 2000). Further investigation of this pathway will hopefully help elucidating the role of syntenin in plasma membrane dynamics.
Our results also suggest that syntenin possesses partially cryptic domains and may exist as a dormant form that needs to be activated, reminiscent of the model that is proposed for ezrin-radixin-moesin proteins (Bretscher, 1999). Unfolding of the N-terminal domain might for example be modulated by post-translational modification and be a prerequisite for membrane recruitment and/or effects on plasma membrane dynamics. Further structural studies of syntenin are needed to support or refute these interpretations. Nuclear Functions?
The nuclear enrichment of syntenin suggests some nuclear function. Because the nuclear enrichments of the recombinant syntenin and syntenin domains are inversely correlated with their plasma membrane localizations (Figure 5), one could imagine that syndecans sequester syntenin to the plasma membrane and subtract it from a nuclear pool. It is tempting to speculate that when syndecans are down-regulated, syntenin may move to the nucleus, where it could have transcriptional activities. Although a transcriptional activity for syntenin remains to be explored, such a scheme has recently been described for CASK/LIN-2. Through its guanylate kinase-like domain, CASK acts as coactivator of the transcription factor Tbr-1, and overexpression of syndecans impairs the nuclear enrichment and thus the transcriptional activity of CASK (Hsueh et al., 2000). Whether such a scheme could explain the correlation between syndecan-1 expression and E-cadherin expression (see above) remains to be explored.
Other Functions
Syntenin was also identified as a major structural component of the early apical recycling endosomes (Fialka et al., 1999) and as a functional component of the early secretory pathway (Fernández-Larrea et al., 1999). The conclusions of the first study are based on proteome studies of cell fractions isolated from highly polarized MDCK cells grown on permeable filter supports, and on the evidence that overexpressed N-terminally myc-tagged proteins localize to the plasma membranes and to an intracellular vesicular compartment of these cells. It could be that a pool of syntenin is indeed present in the early endosome fraction and that we missed it because it represents a relatively minor fraction of the syntenin in MDCK cells in our short term cultures on glass substrata. The conclusions of the second study, which localized syntenin primarily to a perinuclear area, are based on immunohistochemical evidence, using polyclonal anti-syntenin antibodies different from ours and possibly directed against a different part of the protein. Indeed, all our immunological tools are specific for the N-terminal part of syntenin, which may not be similarly accessible at all localizations. Although our staining results for endogenous syntenin are not immediately suggestive, we do not exclude a role for syntenin in vesicular traffic. Some minor localizations of the epitope-tagged syntenins, on large vacuoles or plasma membrane invaginations and on smaller intracellular structures, observed in our transfection experiments are more consistent with this idea. All this would suggest a role for syntenin at the now more evident cross-sections of the pathways that control vesicular trafficking, cytoskeletal dynamics, cell adhesion, and cell polarity.
ACKNOWLEDGMENTS
We thank Dr. M. Mareel (Ghent University Hospital, Belgium) for providing anti-PAN-cadherin serum. We are grateful to W. Annaert for confocal laser scanning microscopy facilities. This work was supported by the Fonds voor Wetenschappelijk Onderzoek-Vlaanderen, the Geconcerteerde Onderzoeksacties 1996–2000, the Interuniversity Network for Fundamental Research sponsored by the Belgian Government, and the Flanders Interuniversity Institute for Biotechnology. P.Z. is a postdoctoral fellow and J.G. a predoctoral fellow of the Fonds voor Wetenschappelijk Onderzoek-Vlaanderen.
Abbreviations used:
- EGFP
enhanced green fluorescent protein
- GST
glutathione S-transferase.
REFERENCES
- Baciu PC, Goetinck PF. Protein kinase C regulates the recruitment of syndecan-4 into focal contacts. Mol Biol Cell. 1995;6:1503–1513. doi: 10.1091/mbc.6.11.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernfield M, Götte M, Woo Park P, Reizes O, Fitzgerald ML, Lincecum J, Zako M. Functions of cell-surface heparan sulfate proteoglycans. Annu Rev Biochem. 1999;68:729–777. doi: 10.1146/annurev.biochem.68.1.729. [DOI] [PubMed] [Google Scholar]
- Bernfield M, Kokenyesi R, Kato M, Hinkes MT, Spring J, Gallo RL, Lose EJ. Biology of the syndecans. Annu Rev Biochem. 1992;8:365–393. doi: 10.1146/annurev.cb.08.110192.002053. [DOI] [PubMed] [Google Scholar]
- Bretscher A. Regulation of cortical structure by the ezrin-radixin-moesin protein family. Curr Opin Cell Biol. 1999;11:109–116. doi: 10.1016/s0955-0674(99)80013-1. [DOI] [PubMed] [Google Scholar]
- Carey DJ. Syndecans. Multifunctional cell-surface co-receptors. Biochem J. 1997;327:1–16. doi: 10.1042/bj3270001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AR, Wood DF, Marfatia SM, Walther Z, Chishti AH, Anderson JM. Human CASK/LIN-2 binds syndecan-2 and protein 4.1 and localizes to the basolateral membrane of epithelial cells. J Cell Biol. 1998;142:129–138. doi: 10.1083/jcb.142.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couchman JR, Woods A. Syndecan-4 and integrins: combinatorial signaling in cell adhesion. J Cell Sci. 1999;112:3415–3420. doi: 10.1242/jcs.112.20.3415. [DOI] [PubMed] [Google Scholar]
- De Boeck H, Lories V, David G, Cassiman JJ, Van den Berghe H. Identification of a 64 kDa heparan sulfate proteoglycan core protein from lung fibroblast plasma membranes with a monoclonal antibody. Biochem J. 1987;247:765–771. doi: 10.1042/bj2470765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evers EE, Zondag GC, Malliri A, Price LS, ten Klooster JP, van der Kammen RA, Collard JG. Rho family proteins in cell adhesion and cell migration. Eur J Cancer. 2000;36:1269–1274. doi: 10.1016/s0959-8049(00)00091-5. [DOI] [PubMed] [Google Scholar]
- Fanning AS, Anderson JM. PDZ domains and the formation of protein networks at the plasma membrane. Curr Top Microbiol Immunol. 1998;228:209–233. doi: 10.1007/978-3-642-80481-6_9. [DOI] [PubMed] [Google Scholar]
- Fernández-Larrea J, Merlos-Suárez A, Urena JM, Baselga J, Arribas J. A role for a PDZ protein in the early secretory pathway for the targeting of proTGF-α to the cell surface. Mol Cell. 1999;3:423–433. doi: 10.1016/s1097-2765(00)80470-0. [DOI] [PubMed] [Google Scholar]
- Fialka I, Steinlein P, Ahorn H, Böck G, Burbelo PD, Haberfellner M, Lottspeich F, Paiha K, Pasquali C, Huber LA. Identification of syntenin as a protein of the apical early endocytic compartment in Madin-Darby canine kidney cells. J Biol Chem. 1999;274:26233–26239. doi: 10.1074/jbc.274.37.26233. [DOI] [PubMed] [Google Scholar]
- Grootjans JJ, Reekmans G, Ceulemans H, David G. Syntenin-syndecan binding requires syndecan-synteny and the cooperation of both PDZ domains of syntenin. J Biol Chem. 2000;275:19933–19941. doi: 10.1074/jbc.M002459200. [DOI] [PubMed] [Google Scholar]
- Grootjans JJ, Zimmermann P, Reekmans G, Smets A, Degeest G, Dürr J, David G. Syntenin, a PDZ protein that binds syndecan cytoplasmic domains. Proc Natl Acad Sci USA. 1997;94:13683–13688. doi: 10.1073/pnas.94.25.13683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinck L, Näthke IS, Papkoff J, Nelson WJ. Dynamics of cadherin/catenin complex formation: novel protein interactions and pathways of complex assembly. J Cell Biol. 1994;125:1327–1340. doi: 10.1083/jcb.125.6.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsueh YP, Sheng M. Regulated expression and subcellular localization of syndecan heparan sulfate proteoglycans and the syndecan-binding protein CASK/Lin-2 during rat brain development. J Neurosci. 1999;19:7415–7425. doi: 10.1523/JNEUROSCI.19-17-07415.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsueh YP, Wang TF, Yang FC, Sheng M. Nuclear translocation and transcription regulation by the membrane-associated guanylate kinase CASK/Lin-2. Nature. 2000;404:298–302. doi: 10.1038/35005118. [DOI] [PubMed] [Google Scholar]
- Hsueh YP, Yang FC, Kharazia V, Naisbitt S, Cohen AR, Weinberg RJ, Sheng M. Direct interaction of CASK/LIN-2 and syndecan heparan sulfate proteoglycan and their overlapping distribution in neuronal synapses. J Cell Biol. 1998;142:139–151. doi: 10.1083/jcb.142.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries MJ. Cell-substrate adhesion assays. In: Bonifacino JS, Dasso M, Harford JB, Lippincott-Schwartz J, Yamada K, editors. Current Protocols in Cell Biology. New York: John Wiley & Sons; 1998. pp. 9.1.1–9.1.11. [Google Scholar]
- Kaibuchi K, Kuroda S, Fukata M, Nakagawa M. Regulation of cadherin-mediated cell-cell adhesion by the Rho family GTPases. Curr Opin Cell Biol. 1999;11:591–596. doi: 10.1016/s0955-0674(99)00014-9. [DOI] [PubMed] [Google Scholar]
- Kato M, Saunders S, Nguyen H, Bernfield M. Loss of cell surface syndecan-1 causes epithelia to transform into anchorage-independent mesenchyme-like cells. Mol Biol Cell. 1995;6:559–576. doi: 10.1091/mbc.6.5.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CW, Goldberger OA, Gallo RL, Bernfield M. Members of the syndecan family of heparan sulfate proteoglycans are expressed in distinct cell-, tissue-, and development-specific pattern. Mol Biol Cell. 1994;5:797–805. doi: 10.1091/mbc.5.7.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander AD, Selleck SB. The elusive functions of proteoglycans: in vivo veritas. J Cell Biol. 2000;148:227–232. doi: 10.1083/jcb.148.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppä S, Vleminckx K, Van Roy F, Jalkanen M. Syndecan-1 expression in mammary epithelial tumor cells is E-cadherin-dependent. J Cell Sci. 1996;109:1393–1403. doi: 10.1242/jcs.109.6.1393. [DOI] [PubMed] [Google Scholar]
- Lin D, Gish GD, Songyang Z, Pawson T. The carboxyl terminus of B class ephrins constitutes a PDZ domain binding motif. J Biol Chem. 1999;274:3726–3733. doi: 10.1074/jbc.274.6.3726. [DOI] [PubMed] [Google Scholar]
- Lories V, Cassiman JJ, Van den Berghe H, David G. Multiple distinct membrane heparan sulfate proteoglycans in human lung fibroblasts. J Cell Biol. 1989;264:7009–7016. [PubMed] [Google Scholar]
- Martinho RG, Castel S, Urena J, Fernandez-Borja M, Makiya R, Oliecrona G, Reina M, Alonso A, Vilaro S. Ligand binding to heparan sulfate proteoglycans induces their aggregation and distribution along actin cytoskeleton. Mol Biol Cell. 1996;7:1771–1788. doi: 10.1091/mbc.7.11.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noë, V., Fingleton, B., Jacobs, K., Crawford, H.C., Vermeulen, S., Steelant, W., Bayneel, E., Matrisian, L.M. and Mareel, M. (2000). Release of an invasion promotor E-cadherin fragment by motrilysin and stromelysin. J. Cell Sci. 774, (In press). [DOI] [PubMed]
- Perego C, Vanconi C, Massari S, Longhi R, Pietrini G. Mammalian LIN-7 PDZ proteins associate with β-catenin at the cell-cell junctions of epithelia and neurons. EMBO J. 2000;19:3978–3878. doi: 10.1093/emboj/19.15.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saoncella S, Echtermeyer F, Denhez F, Nowlen JK, Mosher DF, Robinson SD, Hynes RO, Goetinck PF. Syndecan-4 signals cooperatively with integrins in a Rho-dependent manner in the assembly of focal adhesions and actin stress fibers. Proc Natl Acad Sci USA. 1999;96:2805–2810. doi: 10.1073/pnas.96.6.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D, McAlmon KR, Davies JA, Bernfield M, Hay ED. Simultaneous loss of expression of syndecan-1 and E-cadherin in the embryonic palate during epithelial-mesenchymal transformation. Int J Dev Biol. 1998;42:733–736. [PubMed] [Google Scholar]
- Torres R, Firestein BL, Dong H, Staudinger J, Olson EN, Huganir RL, Bredt DS, Gale NW, Yancopoulos GD. PDZ proteins bind, cluster, and synaptically colocalise with Eph receptors and their ephrin ligands. Neuron. 1998;21:1453–1463. doi: 10.1016/s0896-6273(00)80663-7. [DOI] [PubMed] [Google Scholar]
- Vasioukhin V, Bauer C, Yin M, Fuchs E. Directed actin polymerization is the driving force for epithelial cell-cell adhesion. Cell. 2000;100:209–219. doi: 10.1016/s0092-8674(00)81559-7. [DOI] [PubMed] [Google Scholar]
- Woods A, Couchman JR. Syndecan-4 heparan sulfate proteoglycan is a selectively enriched and widespread focal adhesion component. Mol Biol Cell. 1994;5:183–192. doi: 10.1091/mbc.5.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods A, Couchman JR. Syndecans: synergistic activators of cell adhesion. Trends Cell Biol. 1998;8:189–192. doi: 10.1016/s0962-8924(98)01244-6. [DOI] [PubMed] [Google Scholar]
- Zimmermann P, David G. The syndecans, tuners of transmembrane signaling. FASEB J. 1999;13:S91–S100. doi: 10.1096/fasebj.13.9001.s91. [DOI] [PubMed] [Google Scholar]