Abstract
We investigated the anti-tumor effect of peritumoral resveratrol in combination with immunotherapy in vivo in neuroblastoma-bearing mice. Subcutaneous NXS2 tumors were induced in A/J mice. On day 10, some mice received 15 mcg of intravenous immunocytokine for 5 days, mice received 20 mg of peritumoral resveratrol twice a week (starting on day 12) for a total of 5 injections, and a separate group received a combination of both regimens. Tumor progression and survival were assessed every 3–4 days. Blood and primary tumor tissue samples were collected on day 20 for Complete Blood Count and CD45 immunohistochemistry and histology, respectively. The primary tumor regressed in all mice receiving peritumoral resveratrol. Most of these mice receiving peritumoral resveratrol alone developed metastatic tumors and recurrence of the primary tumor after cessation of therapy. When resveratrol and immunocytokine regimens were combined, 61% of the mice receiving this combination therapy resolved their primary tumors and survived without developing metastatic tumors, compared to 15 and 13% receiving resveratrol or immunocytokine alone, respectively. None of the therapeutic regimes prevented lymphocyte infiltration or affected the complete blood count. Greater necrosis was observed microscopically in tumors from mice receiving the combination therapy. These results demonstrate that the combination therapy of peritumoral resveratrol plus intravenous immunocytokine provides better anti-tumor effects in this model than either therapy alone.
Keywords: Resveratrol, Immunocytokine, Anti-GD2 antibody, Neuroblastoma, Hu14.18-IL2
Introduction
Resveratrol (RV), a natural compound found in red wine, some fruits, and nuts, has shown anti-tumor activity in concentrations and regimens that appear to show minimal toxicity to normal cells [1]. RV slows tumor growth and, depending on the dose and route of administration, can also lead to the regression of local tumors [1, 2]. The anti-tumor action of RV has been tested in mice bearing different types of malignant tumors such as breast, lung, liver, melanoma, and neuroblastoma [3–6]. This study on the anti-tumor activity of RV combined with immunotherapy is focused on neuroblastoma because GD2+ mouse NXS2 neuroblastoma has been a useful model of cancer immunotherapy with hu14.18-IL2 immunocytokine (IC) in our preclinical studies [7, 8].
Neuroblastoma (NB) is the most common pediatric extracranial solid tumor. Current therapies against NB are able to induce remissions or significant responses in most patients but unfortunately they do not prevent recurrence of the disease in the majority of patients with high risk factors [9]. Also the ability to add additional chemotherapy agents is limited due to the cumulative toxicity to normal cells [10, 11]. Our group is involved in the development of a novel immunotherapy based on a fusion protein composed of a humanized IgG mAb linked to interleukin-2 (IL-2), known as the hu14.18-IL2 immunocytokine (IC) [8]. The mAb component of this IC recognizes GD2, a disialoganglioside expressed with relatively little heterogeneity and at high density on the cell surface of tumors of neuroectodermal origin such as melanoma and NB. The proposed mechanisms of action of this IC involve recruitment of IL-2 receptor- and Fc receptor-positive immune cells to the tumor microenvironment where it facilitates tumor-specific cell destruction mainly via antibody-dependent cell-mediated cytotoxicity (ADCC) executed by NK cells [12]. When this IC is injected i.v. in tumor-bearing mice, it has anti-tumor activity against small subcutaneous tumors [13, 14] and is able to prevent or reduce the development of metastatic tumors induced experimentally or arising spontaneously [7].
Studies on combinations of RV with chemotherapeutic agents suggest that RV, in addition to being a tumor growth inhibitor, may also enhance the anti-tumor effect of other agents [15–18]. RV improved the anti-tumor activity of 5-FU when given to mice carrying lung tumors [18]. The strategy of using RV in combination with other cancer therapeutics may hold a clinical promise. In this study, we aimed to determine whether RV can act synergistically with IC to result in enhanced anti-tumor effects than observed with either agent alone.
The anti-tumor response induced by the hu14.18-IL2 IC is dependent on functional NK cells. RV can cause concentration-dependent immunosuppression in vitro to certain components of the immune system, while systemic in vivo immunosuppression has not been reproducibly observed after RV treatment in certain mouse models [19–22]. This discrepancy may be due to the different doses used in vitro and the level of RV achieved in vivo following systemic administration. The concentrations exerting anti-tumor and immunosuppressive activity in vitro are about 25- to 50-fold higher than the peak plasma levels of RV [~1 micromolar (mcM)] after systemic administration [1]. In separate in vitro studies, concentrations of RV ≥ 25 mcM inhibited cell proliferation, blocked DNA synthesis, and induced G1 phase arrest in tumor and immune cells. In addition, RV at 12–50 mcM inhibits antibody-dependent cell-mediated cytotoxicity (ADCC) of tumor cells facilitated by the IC. In contrast, 1–10 mcM RV had no inhibitory effects in these proliferative and ADCC assays.1 In vivo studies using systemic RV regimens as anti-tumor therapy showed that RV can slow tumor growth but does not induce tumor regression [1, 2]. The in vivo activity of RV may be limited by amount or dose of RV actually reaching the tumor. One way of increasing the RV content in the tumor environment is by directly injecting RV into the tissues surrounding the tumor. Ginkel et al. previously used this approach by injecting 5 mg RV peritumorally (p.t.) to xenograft models of human neuroblastoma [1]. This route of RV administration was able to provide a robust anti-tumor effect, achieving tumor regression without causing toxicity to the mice. In this report, we confirm and extend these findings by demonstrating temporal regression of subcutaneous NXS2 murine neuroblastoma in syngeneic mice after local RV treatment. Moreover, we show that a combination of RV with IC immunotherapy prevents tumor recurrence and metastases in the majority of the treated mice.
Here, we report on (1) the action of RV administered by direct injection locally into the tissues surrounding a subcutaneous NXS2 murine neuroblastoma tumor; RV was given as single-agent therapy and in combination with systemic IC; (2) the effect of local RV on circulating leukocytes as well as the infiltration of CD45+ cells to these tumors; (3) the level of RV in the serum of mice receiving local RV; and (4) the influence of RV in vitro on GD2 expression.
Materials and methods
Cells, media, and reagents
NXS2, a GD2-positive murine neuroblastoma cell line, was provided by Dr. R. Reisfeld (Scripps Research Institute, La Jolla, CA). Cells were cultured in high-glucose Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% fetal calf serum, 100 units/ml of penicillin, 100 ug/ml streptomycin and 2 nM l-glutamine. Media and supplements were purchased from Fisher scientific, Pittsburgh, PA. Cultures were maintained at 37°C, 5% CO2. Resveratrol was obtained from Cayman laboratories, Dallas, TX (provided by Dr. A. Polans). The IC was obtained from EMD-Lexigen-Research Center (Billerica, MA). As the concentrations of RV used in this study are not soluble in aqueous solvents, the organic solvent dimethyl sulfoxide (DMSO) was used. RV diluted in DMSO has been used in in vivo studies by others without any reported toxicity to rodents [1].
Mice
Six- to eight-week-old female A/J mice were obtained from Jackson Laboratories (Bar Harbor, Maine). All animals were housed in the University of WI AAALAC–approved facilities and handled according to the NIH and UW-Madison Research Animal Resource Center guidelines.
Resveratrol plus IC combination in vivo study
NXS2 tumors were induced in A/J mice (n = 86) by injecting 2 × 106 cells in 100 mcL PBS subcutaneously (s.c) on the left flank proximal to the spleen. This is designated as the “primary” tumor. On day 10, the average tumor size was between 60 and 70 mm3, and mice were randomized into 4 groups. One group of mice (n = 23) received 15 mcg of IC intravenously (i.v.) in 100 mcL on days 10 through 14. Two other groups of mice (n = 20 each) received a total of 5 p.t. injections of vehicle DMSO or 20 mg of resveratrol/injection in 100 mcL. These injections were given on days 12, 16, 19, 22, and 26. The 20-mg dose was chosen because it showed the best anti-tumor activity on NXS2 upon initial in vivo screening compared to other doses (data not shown). The remaining group (n = 23) received combination treatment of IC and RV by the same schedule. Tumors were measured every 3–4 days. Tumor volume was determined using the formula: V = (width × length × width/2) = mm3. Mice were observed for survival for 100 days, or until their primary tumor reached the size required by our animal facility for euthanasia (15 mm in any dimension). In this experiment, 2 mice were randomly selected from each treatment group, sacrificed after the third RV injection, and blood and tumor were collected, as previously described [1]. Blood was sent to a commercial laboratory (Marshfield’s laboratories, Marshfield WI) for complete blood count (CBC) and differential count. Tumor tissue was used for immunohistochemistry (see below).
Metastasis assessment
Metastatic disease was assessed by mouse necropsy and visual inspection of lymph nodes, lungs, and organs in the peritoneum at the time of sacrifice due to progressive disease or when a mouse was found dead. The comparison of metastatic tumor development between groups was not performed, because mice presenting exponential tumor progression (e.g., DMSO-treated mice) had to be sacrificed due to their tumor reaching the allowed maximum size before gross metastases were evident.
Immunohistochemistry
On day 20 (24 hrs after the third injection of RV), tumors were excised and placed in disposable base molds (Fisher Scientific, Pittsburgh, PA) containing tissue freezing medium (O. C. T.). Tumors were processed and stained by the Experimental Pathology Shared Services of the Carbone Cancer Center at the University of Wisconsin (Madison, WI). O. C. T.-embedded tissue sections were cut at 5 μm on the cryostat, mounted on slides, fixed in acetone for 10 min at 4°C, and then air dried. Slides were washed three times with PBS. Nonspecific binding was blocked with 10% goat serum in PBS for 1 h, and endogenous peroxidase was blocked with 0.3% hydrogen peroxide in methanol for 10 min. Endogenous biotin was blocked with 0.001% avidin in PBS for ten minutes and the avidin quenched with 0.001% biotin in PBS for 10 min, both at 25°C. The slides were then incubated with CD45 (Ly-5) rat monoclonal primary antibody (eBioscience, San Diego, CA, 14-0451-85, clone 30F11) at a 1:160 dilution in PBS with 1% goat serum for 1 h at 37°C. After washing with PBS, the sections were incubated with biotinylated goat anti-rat (Vector Laboratories, Burlingame, CA) at 1:200 in PBS for 1 hour at room temperature. Slides were washed in PBS and then incubated 30 min at room temperature with Vectastain ABC Elite (Vector Laboratories, Burlingame CA). Following three washes in PBS, slides were developed with DAB (3, 3-diaminobenzidine) (Vector Laboratories, Burlingame CA), counterstained with Mayer’s hematoxylin, dehydrated and cover slipped with permount. For Hematoxylin and Eosin (H&E) staining, a modified version of the routine Mayer’s staining protocol [23] was used. Slides containing dried frozen sections were fixed in 100% EtOH for 2 min followed by a rinse in tap H2O for 2 min. Sections were stained with Mayer’s Hematoxylin, for 15 min, and then the excess of stain was washed off under running tap H2O for 20 min. Eighty percent EtOH was applied for 1 min, and sections were counterstained with Eosin (Surgipath, Richmond, IL) for 30 s. The slides were then dipped 3 times in 95% EtOH and once in 100% EtOH for 15 s each. Then, 100% EtOH was applied to sections for 2 min. Xylene was applied 3 times over sections for 2 min each. Finally, coverslips were placed over slides with Micromount (Surgipath, Richmond, IL). All reagents and chemicals were obtained from Sigma, St. Louis, MO, unless otherwise noted. Slides were blindly photographed by one of the authors using an AxioPhot microscope (Zeiss, Thornwood, New York).
RV concentration in mouse serum
A/J mice (n = 4) with subcutaneous NXS2 tumors received a peritumoral injection of 20 mg RV. Blood was collected 20 min (n = 2) or 24 h (n = 2) post-injection. The concentration of RV in the serum was determined as previously described [1].
Detection of GD2 levels by flow cytometry
NXS2 cells (1 × 106) were seeded in 12-well plates (n = 4), in duplicate wells, and incubated at 37°C and 5% CO2 for 3 days with DMSO, 50, 10, or 1 mcM RV. At the end of the incubation period, cells were washed with PBS and 0.5 × 106 cells were incubated with 1.4 mcg of hu14.18-IL2-FITC for 1 h at 4°C. Cells were then washed with PBS and stained with propidium iodine. Twenty thousand viable events were collected using a BD FACSCalibur (San Diego, CA). Data were analyzed using FlowJo (Ashland, OR). MFI ratio was calculated by dividing the geometric mean of the sample by the geometric mean of the isotype control immunocytokine.
Statistical analyses
In order to avoid underestimation of tumor volume due to sacrificed mice, tumor volume among treatment groups was analyzed when ≥75% of mice remain alive in each group. These were compared using nonparametric Wilcoxon rank sum test. The survival function of time to death or euthanasia was estimated using the Kaplan–Meier method and compared among treatment groups using logrank test. Bonferonni multiple adjustments were applied when significance was obtained with the above-mentioned tests. GD2 level in MFI ratio was compared using one-way analysis of variance (ANOVA). When ANOVA yielded significance, pairwise comparisons using two-tailed t-test were conducted between two groups with no adjustments were made for multiple comparisons.
Results
Combination of RV and IC enhanced anti-tumor activity and improved tumor-free survival
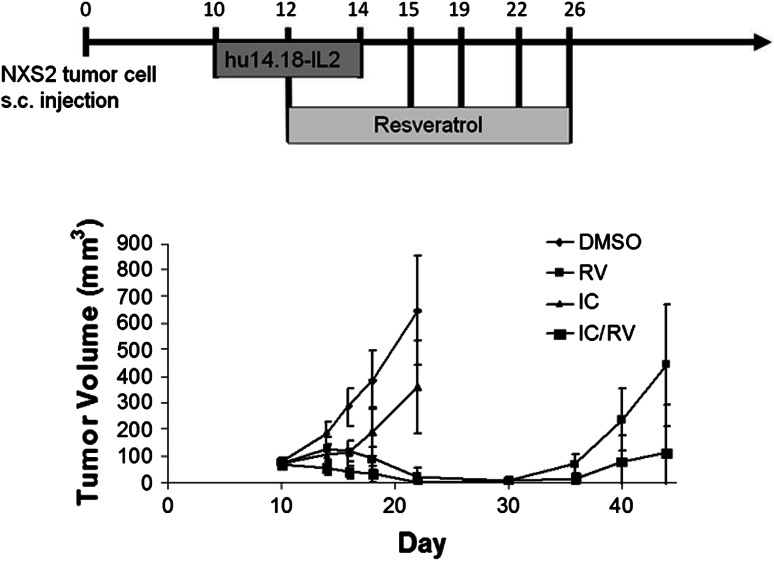
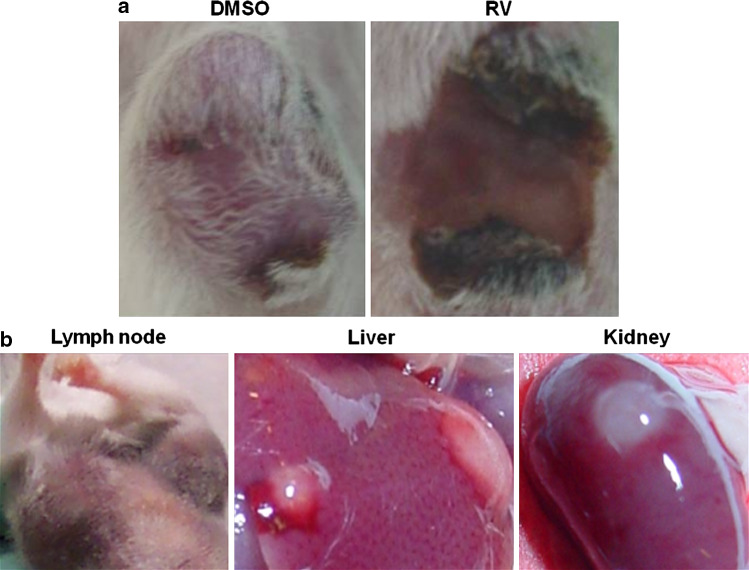
The anti-tumor effects of RV or IC alone and the combination of the two were assessed. Mice were treated with the control vehicle, with DMSO, or with 20 mg RV given p.t. or with IC given i.v. The combined treatment was IC with RV. The tumors in the mice treated with vehicle DMSO progressed and grew in an exponential fashion (Fig. 1). The majority of the tumors in mice receiving IC i.v. alone stabilized and did not progress during the time of treatment; however, following completion of the i.v. IC treatment, 20 out of 23 tumors progressed. Conversely, all tumors treated with RV regressed. However, 14 of 20 primary tumors recurred approximately 1 week after cessation of RV treatment. In addition, in 11 of these 20 mice, grossly evident metastatic tumors developed in one or more of the following organs: lymph nodes, liver, or kidney (Fig. 2b). Mice also developed metastatic tumors in the lungs and ovaries. For the group receiving the combined IC/RV treatment, the primary tumors all regressed as in the RV-alone group (Fig. 2a); however, unlike the RV-alone group, only 5 of 23 mice receiving the IC/RV combination showed primary tumor recurrence following cessation of treatment and only one of these 5 mice developed grossly evident metastases.
Fig. 1.
NXS2 tumor growth in mice receiving IC/RV combination therapy. Mice carrying GD2+ NXS2 tumors were treated with DMSO p.t. (n = 20), 20 mg RV p.t. (n = 20), IC i.v. (n = 23), or IC/RV in combination (n = 23). Tumors treated with DMSO grew in an exponential fashion. In contrast, tumors treated with RV regressed, but animals developed metastatic tumors and in some animals the primary tumor recurred after cessation of therapy. IC induced anti-tumor activity initially, but eventually tumors progressed. Data shown are tumor volume measurements of the primary tumors at the indicated times. Bars represent the standard deviation. The distribution of tumor volume for the RV group differs from the IC/RV group (P = 0.004 on day 44)
Fig. 2.
Representative pictures of primary and metastatic tumors after treatment. Representative pictures of an NXS2 primary tumor from a DMSO-treated and RV-treated mouse (a) and of organs where metastatic tumors were observed after microscopic examination and (b) after treatment completion
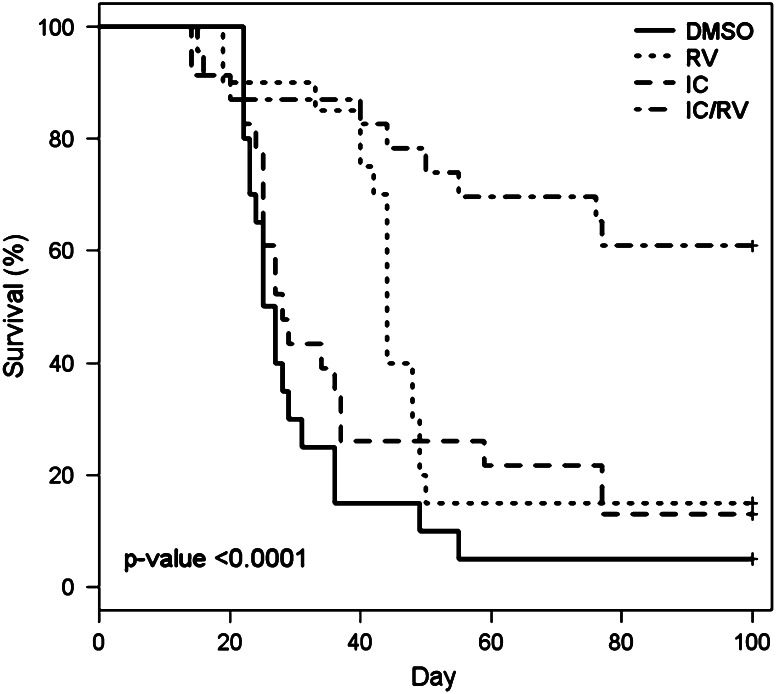
Survival of mice was assessed for 100 days after initial tumor induction (Fig. 3). Sixty-one percent of the mice in the combination therapy were alive with no detectable tumor on day 100, compared to 15 and 13% in both RV- and IC-alone groups, respectively. Overall, the group treated with the combination therapy showed a statistically greater survival (survival with no tumor requiring euthanasia) than that of all other therapy groups. The results from Figs. 1 and 3 indicate that RV in combination with IC provides an enhanced anti-tumor activity above that seen by treating with either agent alone. These data are combined from 3 independent experiments which showed similar results.
Fig. 3.
Survival of mice receiving IC/RV combination therapy. NXS2 tumor-bearing mice were treated with DMSO p.t. (n = 20), 20 mg RV p.t. (n = 20), IC i.v. (n = 23), or IC/RV in combination (n = 23). Mice were observed for survival for 100 days or until their primary tumor reached the size limit (15 mm in any dimension) requiring euthanasia. Sixty-one percent of the mice receiving IC/RV combination survived to day 100 with no detectable tumors compared to 15 and 13% of the mice in the RV-alone or the IC-alone group, respectively. There was a significant difference in overall survival among the four treatment groups (P < 0.0001). Pairwise comparisons of survival distribution over time: DMSO versus RV: P = 0.005, DMSO versus IC: P = 0.19, DMSO versus IC/RV: P < 0.0001, IC/RV versus IC: P = 0.0003, IC/RV versus RV: P = 0.001, RV versus IC: P = 0.16
Influence of RV on leukocyte counts and tumor infiltration
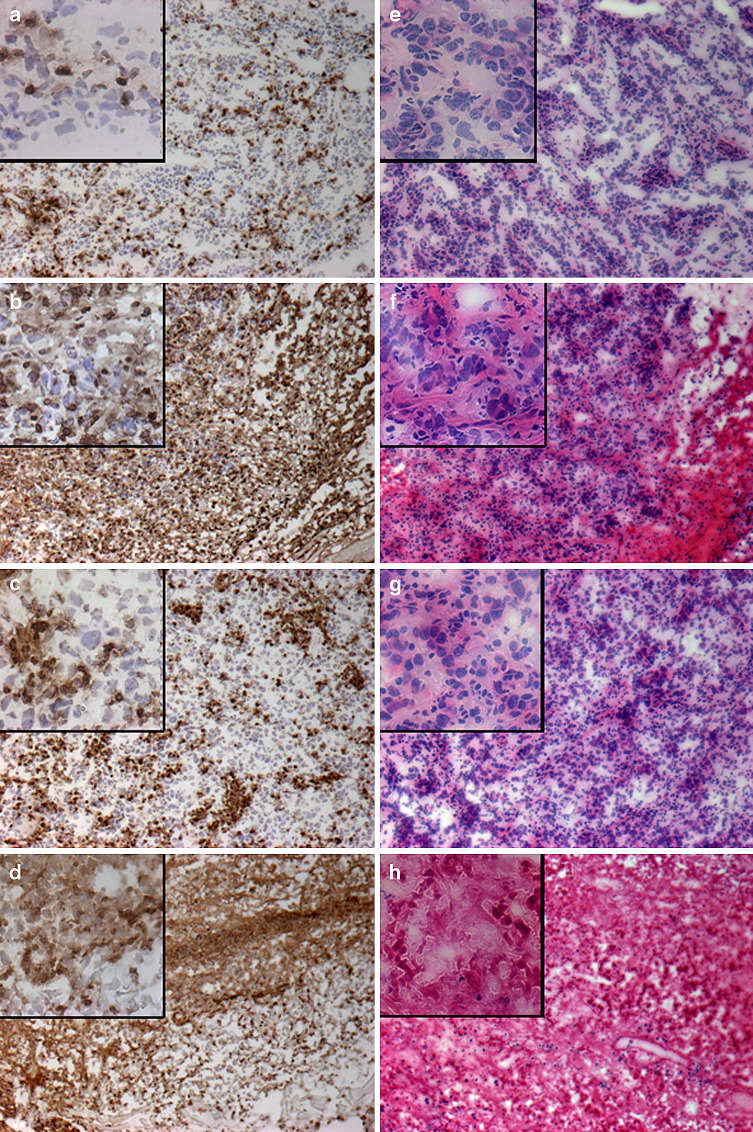
Next, we asked whether RV treatment affected immune cells. The 20-mg p.t. RV regimen was not found to markedly affect the circulating leukocyte population in treated mice; as comparable white blood cell numbers were obtained from all animals using 2 representative mice from each treatment group on day 20 just after the third RV injection (data not shown). Peritumoral treatment with RV alone did not reduce, but seemed to increase the infiltration of leukocytes into the tumor microenvironment. This was seen in the CD45 staining for infiltrating leukocytes (Fig. 4a, b). The H&E staining revealed that tumors obtained from mice treated with RV alone or in combination with IC showed areas of significant tumor necrosis (Fig. 4f, h) when compared to tissue from tumors treated with DMSO or IC alone (Fig. 4e, g). The tumor tissue destruction was more prominent in tumors from mice receiving combination therapy (Fig. 4h) than RV alone (Fig. 4f). These combined results of the blood counts and histology suggest that the peritumoral injections of RV caused tumor tissue damage without interfering with leukocyte infiltration into the tumor or changing the leukocytes in the circulation. Furthermore, the combination of RV and IC appeared to induce more tumor damage than RV alone.
Fig. 4.
Immunohistochemistry of tumors from the IC/RV combination study. Tumor tissue was obtained on day 20 from mice treated with p.t. DMSO (a, e), p.t. 20 mg RV (b, f), IC i.v. (c, g) or IC/RV in combination (d, h). Tissue was stained with anti-CD45 (a–d) and H&E (e–f). Pictures were taken on a bright field at 10X, and the inserts on each picture are at 40X. Representative fields are shown
Concentration of RV in mouse serum after peritumoral administration
The concentration of free or unmetabolized RV in the serum of mice receiving 20 mg RV by peritumoral injection was determined in the serum obtained following a single injection. At 20 min, the serum RV level was 24 mcM, and at 24 h, the RV level had fallen to 2.8 mcM. The RV level in serum 20 min after administration is in the range of concentrations known to be immunosuppressive in vitro1 [22]; however, in our in vivo studies, this concentration did not affect the number of circulating lymphocytes and CD45+ cell infiltration to the tumor. These results show that the amount of RV available after local administration is higher than after systemic administration (~1 mcM), resulting in tumor cell death at higher concentrations [1].
RV induces increased levels of GD2 in tumor cells in vitro
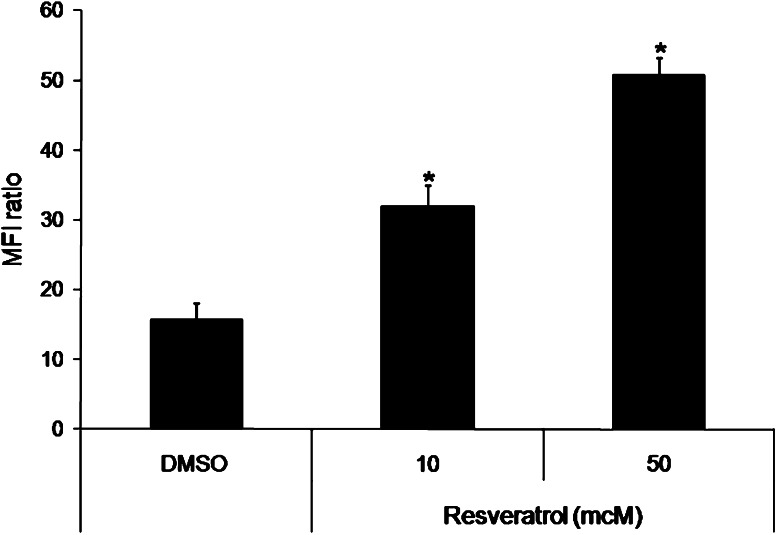
To determine a possible mechanism of the increased anti-tumor effect of RV with IC (Fig. 1), we asked whether RV would upregulate the density of GD2 in tumor cells. To test this, NXS2 tumor cells were incubated with 50 and 10 mcM RV for 3 days. On day 3, the GD2 content on the cells was assessed by flow cytometry using the IC directly conjugated with FITC.
After incubation of the NXS2 tumor cells with 50 mcM RV, the GD2 density was significantly higher than the GD2 density seen on cells incubated with the DMSO control (Fig. 5). Cells incubated with RV at 10 mcM RV also showed an increase in GD2 when compared with the GD2 expression on cells cultured with the DMSO control. In a separate experiment, media alone and 1 mcM RV did not modulate GD2 levels (data not shown). These results indicate that RV induces a concentration-dependent GD2 increase and this increase is seen at 50 mcM RV, a concentration previously shown to stop proliferation of NXS2 cells in vitro.1
Fig. 5.
GD2 levels in tumor cells incubated with RV. NXS2 cells were incubated with DMSO, 50 mcM, or 10 mcM RV for 3 days. On day 3, cells were harvested and analyzed by flow cytometry for GD2 expression. Cells incubated with RV for 3 days showed an increase in GD2 compared to DMSO in a concentration-dependent manner. Bars represent the standard deviation. *P < 0.001 versus DMSO
Discussion
Treatment of mice bearing NXS2 tumors with peritumoral injections of 20 mg RV induced tumor regression; however, the animals developed late local recurrence at the primary tumor site as well as metastatic tumors after cessation of RV treatment. We sought to identify an agent to be used in combination with RV that would prevent the development of these recurrent and metastatic tumors. IC therapy, given i.v., lowers the metastatic tumor incidence in mice bearing NXS2 tumors and can be effective against small local tumors [7]. This study was designed to determine whether the addition of IC to p.t. treatment with RV could improve survival in NXS2 tumor-bearing animals. NXS2 tumor-bearing mice received RV alone or in combination with IC. The combination of IC plus RV resulted in long-term survival of 61% of the mice. Ginkel et al. demonstrated in a previous study [1] that p.t. RV-treated tumors have significant tumor destruction when examined histologically and compared to tumors treated with DMSO, the RV vehicle. DMSO-treated tumors appeared histologically similar to untreated tumors [1]. We obtained similar results in our NXS2 tumor model, as tumors treated with RV alone or in combination appeared to have marked tumor necrosis. These results indicate that RV causes significant tumor tissue damage in vivo and that there is an additional benefit in using the p.t. RV in combination with i.v. IC. As DMSO is not an optimal solvent for clinical administration, other vehicles are being pursued as a vehicle for p.t. RV for subsequent preclinical studies (Polans A. Unpublished).
Resveratrol as a local p.t. treatment induced a robust anti-tumor effect over a period of 2 weeks. However, during this period of time, some tumor cells may have spread and established undetectable or micro-metastatic disease in different tissues, accounting for local and distant recurrences in these mice. Thus, this tumor debulking regimen of local RV provides a scenario for combining with systemic IC immunotherapy. Thus, our results with leukocyte blood counts and histological evaluation of CD45+ cells in s.c. tumors suggest that RV treatment has no effect on systemic leukocyte numbers, but induces local leukocyte infiltration. The IC consists of a tumor-specific anti-GD2 antibody linked to two molecules of IL-2. The IL-2 component of the IC induces the activation of lymphocytes through their IL-2 receptors. These may include antigen-specific T cells and NK cells. One of the anti-tumor mechanisms of the IC is ADCC performed mainly by NK cells [7, 12]. Initiating systemic treatment with the IC prior to giving RV may initiate priming of the lymphocytes so that tumor cells that spread systemically before or during local RV treatment are controlled by the activated lymphocytes via ADCC. These disseminated tumor cells are thereby destroyed and unable to establish metastatic sites of tumor. In addition, our in vitro data suggest that RV treatment may increase the level of GD2 on the surface of the tumor cells (Fig. 5). RV was shown to induce ceramide accumulation on tumor cell lines [24–26]. Ceramide is a sphingolipid metabolized to gangliosides including GD2. The modulation of GD2 in our cell line by RV may be via ceramide accumulation in vitro, and this may be one pathway whereby RV causes tumor death as ceramide has been previously implicated in cell death [27, 28]. In vivo p.t. RV (20 mg) treatment may induce accumulation of ceramide in NXS2 tumors and consequently increase the levels of GD2 on the surface of the tumor cells. Thus, peritumoral RV in addition to causing direct tumor cell death might also render the tumor cell more susceptible to recognition by the anti-GD2 IC and subsequent ADCC due to increased GD2 expression. However, the potential for RV to induce augmented in vivo GD2 expression has not yet been studied.
In summary, RV in combination with IC provided an enhanced anti-tumor treatment in this preclinical model as it lowered the tumor burden and increased survival.
Acknowledgments
We would like to acknowledge Ruth Sullivan, Joseph Hardin, and Jane Weeks for their help with histology and immunohistochemistry. This work was supported by R01-CA-32685-25, CA87025, GM67386, UL1RR025011, P30-CA14520 and grants from the Midwest Athletes for Childhood Cancer Fund, the Crawdaddy Foundation, The Evan Dunbar Foundation, Abbie’s Fund, the Super Jake Foundation and Matthews Retina Research Foundation.
Footnotes
Brenda L. Soto, Jacquelyn A. Hank, Soesiawati R. Darjatmoko, Arthur S. Polans, Eric M. Yanke, Alexander L. Rakhmilevich, Songwong Seo, KyungMann Kim, Ralph A. Reisfeld, Stephen D. Gillies, and Paul M. Sondel. Anti-tumor and immunomodulatory activity of resveratrol in vitro and its potential for future combination cancer immunotherapy. Submitted manuscript, 2010.
References
- 1.van Ginkel PR, Sareen D, Subramanian L, et al. Resveratrol inhibits tumor growth of human neuroblastoma and mediates apoptosis by directly targeting mitochondria. Clin Cancer Res. 2007;13:5162–5169. doi: 10.1158/1078-0432.CCR-07-0347. [DOI] [PubMed] [Google Scholar]
- 2.Chen Y, Tseng SH, Lai HS, Chen WJ. Resveratrol-induced cellular apoptosis and cell cycle arrest in neuroblastoma cells and antitumor effects on neuroblastoma in mice. Surgery. 2004;136:57–66. doi: 10.1016/j.surg.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 3.Busquets S, Ametller E, Fuster G, et al. Resveratrol, a natural diphenol, reduces metastatic growth in an experimental cancer model. Cancer Lett. 2007;245:144–148. doi: 10.1016/j.canlet.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 4.Garvin S, Ollinger K, Dabrosin C. Resveratrol induces apoptosis and inhibits angiogenesis in human breast cancer xenografts in vivo. Cancer Lett. 2006;23:113–122. doi: 10.1016/j.canlet.2005.01.031. [DOI] [PubMed] [Google Scholar]
- 5.Kimura Y, Okuda H. Resveratrol isolated from Polygonum cuspidatum root prevents tumor growth and metastasis to lung and tumor-induced neovascularization in Lewis lung carcinoma-bearing mice. J Nutr. 2001;131:1844–1849. doi: 10.1093/jn/131.6.1844. [DOI] [PubMed] [Google Scholar]
- 6.Liu HS, Pan CE, Yang W, Liu XM. Antitumor and immunomodulatory activity of resveratrol on experimentally implanted tumor of H22 in Balb/c mice. World J Gastroenterol. 2003;9:1474–1476. doi: 10.3748/wjg.v9.i7.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neal ZC, Yang JC, Rakhmilevich AL, et al. Enhanced activity of hu14.18-IL2 immunocytokine against murine NXS2 neuroblastoma when combined with interleukin 2 therapy. Clin Cancer Res. 2004;10:4839–4847. doi: 10.1158/1078-0432.CCR-03-0799. [DOI] [PubMed] [Google Scholar]
- 8.Yamane BH, Hank JA, Albertini MR, Sondel PM. The development of antibody-IL-2 based immunotherapy with hu14.18-IL2 (EMD-273063) in melanoma and neuroblastoma. Expert Opin Investig Drugs. 2009;18:991–1000. doi: 10.1517/13543780903048911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laverdière C, Liu Q, Yasui Y, et al. Long-term outcomes in survivors of neuroblastoma: a report from the childhood cancer survivor study. J Natl Cancer Inst. 2009;101:1131–1140. doi: 10.1093/jnci/djp230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peters GJ, van der Vijgh WJ. Protection of normal tissues from the cytotoxic effects of chemotherapy and radiation by amifostine (WR-2721): preclinical aspects. Eur J Cancer. 1995;31A(Suppl 1):S1–S7. doi: 10.1016/0959-8049(95)00145-9. [DOI] [PubMed] [Google Scholar]
- 11.Siebler T, Shalet SM, Robson H. Effects of chemotherapy on bone metabolism and skeletal growth. Horm Res. 2002;58(Suppl 1):80–85. doi: 10.1159/000064769. [DOI] [PubMed] [Google Scholar]
- 12.Hank JA, Robinson RR, Surfus J, et al. Augmentation of antibody dependent cell mediated cytotoxicity following in vivo therapy with recombinant Interleukin-2. Cancer Res. 1990;50:5234–5239. [PubMed] [Google Scholar]
- 13.Johnson EE, Lum HD, Rakhmilevich AL, et al. Intratumoral immunocytokine treatment results in enhanced antitumor effects. Cancer Immunol Immunother. 2008;57:1891–1902. doi: 10.1007/s00262-008-0519-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neal ZC, Sondel PM, Bates MK, Gillies SD, Herweijer H. Flt3-L gene therapy enhances immunocytokine-mediated antitumor effects and induces long-term memory. Cancer Immunol Immunother. 2007;56:1765–1774. doi: 10.1007/s00262-007-0320-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fuggetta MP, D’Atri S, Lanzilli G, et al. In vitro antitumour activity of resveratrol in human melanoma cells sensitive or resistant to temozolomide. Melanoma Res. 2004;14:189–196. doi: 10.1097/01.cmr.0000130007.54508.b2. [DOI] [PubMed] [Google Scholar]
- 16.Kubota T, Uemura Y, Kobayashi M, Taguchi H. Combined effects of resveratrol and paclitaxel on lung cancer cells. Anticancer Res. 2003;23:4039–4046. [PubMed] [Google Scholar]
- 17.Nicolini G, Rigolio R, Miloso M, Bertelli AA, Tredici G. Anti-apoptotic effect of trans-resveratrol on paclitaxel-induced apoptosis in the human neuroblastoma SH-SY5Y cell line. Neurosci Lett. 2001;302:41–44. doi: 10.1016/S0304-3940(01)01654-8. [DOI] [PubMed] [Google Scholar]
- 18.Wu SL, Sun ZJ, Yu L, Meng KW, Qin XL, Pan CE. Effect of resveratrol in combination with 5-FU on murine liver cancer. Pharmacol Res. 2004;10:3048–3052. doi: 10.3748/wjg.v10.i20.3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boscolo P, del Signore A, Sabbioni E, et al. Effects of resveratrol on lymphocyte proliferation and cytokine release. Ann Clin Lab Sci. 2003;33:226–231. [PubMed] [Google Scholar]
- 20.Falchetti R, Fuggetta MP, Lanzilli G, Tricarico M, Ravagnan G. Effects of resveratrol on human immune cell function. Life Sci. 2001;70:81–96. doi: 10.1016/S0024-3205(01)01367-4. [DOI] [PubMed] [Google Scholar]
- 21.Feng YH, Zhou WL, Wu QL, Li XY, Zhao WM, Zou JP. Low dose of resveratrol enhanced immune response of mice. Acta Pharmacol Sin. 2002;23:893–897. [PubMed] [Google Scholar]
- 22.Gao X, Deeb D, Media J, et al. Immunomodulatory activity of resveratrol: discrepant in vitro and in vivo immunological effects. Biochem Pharamacol. 2003;66:2427–2435. doi: 10.1016/j.bcp.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 23.Prophet EB, Mills B, Arrington JB, Sobin, LH Laboratory Methods in Histotechnology. Armed Froces Intitutes of Pathology, Washington, pp 53–55
- 24.Dolfini E, Roncoroni L, Dogliotti E, et al. Resveratrol impairs the formation of MDA-MB-231 multicellular tumor spheroids concomitant with ceramide accumulation. Cancer Lett. 2007;249:143–147. doi: 10.1016/j.canlet.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 25.Signorelli P, Munoz-Olaya JM, Gagliostro V, Casas J, Ghidoni R, Fabriàs G. Dihydroceramide intracellular increase in response to resveratrol treatment mediates autophagy in gastric cancer cells. Cancer Lett. 2009;282:238–243. doi: 10.1016/j.canlet.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 26.Ulrich S, Huwiler A, Loitsch S, Schmidt H, Stein JM. De novo ceramide biosynthesis is associated with resveratrol-induced inhibition of ornithine decarboxylase activity. Biochem Pharmacol. 2007;74:281–289. doi: 10.1016/j.bcp.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 27.Minutolo F, Sala G, Bagnacani A, et al. Synthesis of a resveratrol analogue with high ceramide-mediated proapoptotic activity on human breast cancer cells. J Med Chem. 2005;48:6783–6786. doi: 10.1021/jm050528k. [DOI] [PubMed] [Google Scholar]
- 28.Scarlatti F, Sala G, Ricci C, et al. Resveratrol sensitization of DU145 prostate cancer cells to ionizing radiation is associated to ceramide increase. Cancer Lett. 2007;253:124–130. doi: 10.1016/j.canlet.2007.01.014. [DOI] [PubMed] [Google Scholar]