Abstract
Human T cells genetically modified to express chimeric antigen receptors (CARs) specific to the B cell tumor antigen CD19 can successfully eradicate systemic human CD19+ tumors in immunocompromised SCID-Beige mice. However, in the clinical setting, CD4+ CD25hi T regulatory cells (Tregs) present within the tumor microenvironment are potent suppressors of tumor-targeted effector T cells. In order to assess the impact of Tregs on CAR-modified T cells in the SCID-Beige xenotransplant model, we isolated, genetically targeted and expanded natural T regulatory cells (nTregs). In vitro, nTregs, modified to express CD19 targeted CARs efficiently inhibited the proliferation of activated human T cells, as well as the capacity of CD19-targeted 19-28z+ effector T cells to lyse CD19+ Raji tumor cells. Intravenous infusion of CD19-targeted nTregs into SCID-Beige mice with systemic Raji tumors traffic to sites of tumor and recapitulate a clinically relevant hostile tumor microenvironment. Anti-tumor efficacy of subsequently infused 19-28z+ effector T cells was fully abrogated as assessed by long-term survival of treated mice. Optimal suppression by genetically targeted nTregs was dependent on nTreg to effector T cell ratios and in vivo nTreg activation. Prior infusion of cyclophosphamide in the setting of this nTreg-mediated hostile microenvironment was able to restore the anti-tumor activity of subsequently infused 19-28z+ effector T cells through the eradication of tumor targeted nTregs. These findings have significant implications on the design of future clinical trials utilizing CAR-based adoptive T cell therapies of cancer.
Introduction
T cells may be genetically targeted to tumor antigens through the expression of chimeric antigen receptors (CARs) transduced using gammaretroviral vectors(1). We have previously demonstrated that human T cells genetically modified to express a CD19-targeted CAR successfully eradicate established systemic human CD19+ B cell tumor cell lines in immune suppressed SCID-Beige mice(2). However, despite promising preclinical in vivo studies(2-5), results from initial clinical trials utilizing CAR-modified T cells have to date been disappointing(6-8).
A potential etiology of treatment failure in the clinical setting may be the suppression of targeted T cells by a hostile tumor microenvironment infiltrated with CD4+ CD25hi regulatory T cells (Tregs) and myeloid derived suppressor cells (MDSCs), as well as tumor expression of inhibitory ligands (PD-L1) and cytokines (TGF-β and IL-10)(9-11). This hostile tumor microenvironment is largely unaddressed in pre-clinical models utilizing immune compromised mice. To address this limitation, we sought to investigate the impact of Tregs, a potent endogenous suppressive element of the immune system, on the anti-tumor activity of adoptively transferred CAR modified T cells in a previously established SCID-Beige mouse tumor model.
Natural Tregs (nTregs) are CD4+ T cells derived from the thymus and defined by a CD4+ CD25+ CD127- Foxp3+ phenotype. Natural Tregs have been found to facilitate suppression of autoimmune T cell responses and maintenance of peripheral tolerance(12-14), represent approximately 5-10% of peripheral CD4+ T cells in both mice and humans(13, 15), and express high levels of cytotoxic T lymphocyte associated antigen 4 (CTLA-4), glucocorticoid-induced TNFR-related protein (GITR), CD39, and CD73(16-18). Patients with cancer, including B cell malignancies, have elevated numbers of Tregs in the peripheral blood and within the tumor microenvironment(19-21). Furthermore, in a variety of cancers, increased numbers of Tregs portends a poor prognosis(19, 22). Although the mechanism of suppression by Tregs appears to be multifactorial(23), it is clear that the presence of Tregs within the tumor microenvironment could markedly hinder the anti-tumor efficacy of adoptively transferred tumor targeted effector T cells(24).
Many studies have been published implicating Tregs as the cause of failed anti-tumor immune responses using clinical correlates, Treg depleting strategies(22, 25), and systemic lymphodepletion(26, 27). Recently, investigators have developed protocols to readily isolate(28), stimulate, and expand enriched Treg populations for pre-clinical experimental purposes(29, 30).
In this report, we investigate the in vivo impact of nTregs on CD19 targeted CAR+ T cell therapy in a previously established xenotransplant SCID-Beige tumor model of Burkitt lymphoma (2, 3) by recapitulating a clinically relevant tumor microenvironment hostile to effector T cell function through the infusion of CD19-targeted nTregs. Systemic injection of targeted nTregs into SCID-Beige mice bearing established systemic Raji tumors prior to infusion of CD19-targeted CAR+ effector T cells wholly abolished effector T cell anti-tumor benefit while prior treatment with cyclophosphamide effectively reversed in vivo nTreg-mediated suppression of CD19-targeted CAR+ effector T cells. Taken together, our data support the hypothesis that tumor specific nTregs may significantly compromise the anti-tumor efficacy of CAR-modified tumor-targeted effector T cells in the clinical setting and may, in part, explain the modest clinical outcomes reported in previously published clinical trials utilizing adoptively transferred CAR-modified T cells(6-8).
Materials and Methods
Cell lines and T cells
The Raji tumor cell line was cultured in RPMI 1640 (Life Technologies, Carlsbad, CA) supplemented with 10% heat-inactivated FCS, nonessential amino acids, HEPES buffer, pyruvate, and BME (Life Technologies, Carlsbad, CA). T cells were cultured in RPMI 1640 (Life Technologies) supplemented with 10% heat-inactivated FCS supplemented with 20IU IL-2/mL (R&D Systems, Minneapolis, MN). PG-13 and gpg29 retroviral producer cell lines were cultured in DMEM (Life Technologies) supplemented with 10% FCS, and NIH-3T3 artificial antigen-presenting cells (AAPC), were cultured in DMEM supplemented with 10% heat-inactivated donor calf serum. All media were supplemented with 2 mmol/L L-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin (Life Technologies).
Isolation of CD4+ CD25- effector T cells and CD4+ CD25+ nTregs
Peripheral blood from healthy donors, obtained under institutional review board (IRB)-approved protocol 95-054, was fractionated in BD Vacutainer CPT tubes (BD Medical, Sandy, UT), to isolate peripheral blood mononuclear cells (PBMCs). CD4+ CD25- responder T cells and CD4+ CD25+ nTregs were isolated from PBMCs using the CD4+ CD25+ Regulatory T Cell Isolation Kit (Dynal brand; Invitrogen, Carlsbad, CA).
Retroviral genetic modification of T cells
Generation of retroviral producer PG-13 cell lines and gene transfer into effector T cells have been previously described(3, 31). For nTreg retroviral gene transfer, isolated nTregs were activated with Dynal CD3/CD28 Human Treg Expander magnetic beads (Invitrogen, Carlsbad, CA), cultured in RPMI media supplemented with IL-2 500IU and Rapamycin 100ng/ml (Sigma, St. Louis, MO) for 48 hours(30, 32), and similarly transduced.
Expansion of CAR+ T cells
CAR+ effector T cells were expanded ex vivo on NIH-3T3 derived AAPCs as described previously(3). CAR+ nTregs were expanded with either Dynal CD3/CD28 Human Treg Expander beads (Invitrogen), or with AAPCs in RPMI medium supplemented with IL-2 and Rapamycin.
In vitro nTreg proliferation and suppression assay
5 ×105 T effector cells were labeled with 5 μM carboxyfluorescein diacetate succinimidyl ester (CFSE) (Invitrogen) and cultured simultaneously with titrated numbers of purified autologous CAR+ CD4+ CD25+ Foxp3+ nTregs in 24-well tissue culture plates (Costar, Corning, NY)(33). T cell co-cultures were stimulated with Dynabeads CD3/CD28 T Cell Expander beads (Invitrogen) at a bead-to-responder T cell ratio of 1:1 in the absence of exogenous IL-2 and proliferation was assessed by FACS at 72 hours.
Cytokine detection assays
Cytokine levels in tissue culture supernatant as well as serum were assessed using the multiplex Human Cytokine Detection System (Millipore Corp. Billerica MA) in conjunction with the Luminex IS100 system and IS 2.2 software (Luminex Corp. Austin TX).
In vitro cytotoxicity assay
19-28z+ effector T cells were co-cultured with Raji cells in RPMI media at 1:1 ratio with or without equal numbers of 19z1+ nTregs for 24 hours. Tumor lysis was subsequently assessed by FACS to detect residual CD19+ tumor cells. The GranToxiLux® (OncoImmunin, Inc, Gaithersburg, MD) cytotoxicity assay was performed per manufacturer's instructions.
In vivo analyses of Treg function
We inoculated 8- to 12-week-old FOX CHASE C.B-17 (SCID-Beige) mice (Taconic, Hudson, NY) with Raji tumor cells by tail vein injection or subcutaneously as indicated. In the systemic tumor model, mice were injected by tail vein (i.v.) with 5 × 105 Raji tumor cells on day 1, and on day 5 were treated with a single i.v. infusion of 1 × 107 CAR+ nTregs, followed by a single i.v. infusion of 1 × 107 CAR+ effector T cells on day 6. For cyclophosphamide experiments, mice were injected by tail vein with 5 × 105 Raji tumor cells on day 1, on day 5 were treated with a single i.v. infusion of 1 × 107 CAR+ Tregs, followed by intraperitoneal (i.p.) injection of 100mg/kg cyclophosphamide on day 6, and on day 7 injected with an i.v. dose of 1 × 107 CAR+ effector T cells. Mice were sacrificed when disease became clinically evident. All in vivo studies were done in the context of an Institutional Animal Care and Use Committee (IACUC) approved protocol (#00-05-065).
Bioluminescent imaging
For in vivo imaging of Raji tumor cells we utilized Raji tumor cells modified to express GFP-FFLuc (Clontech Laboratories, Mountain View, CA). Imaging of nTregs was performed using nTregs modified with the previously described 19z1 IRES extGLuc bicistronic retroviral vector(34). Tumor and T cells were imaged using the Xenogen IVIS Imaging System (Xenogen, Alameda, CA) (34).
Immunohistochemistry staining
Mouse bone marrow samples were fixed in 10% buffered formalin phosphate (Fisher Scientific, Pittsburgh PA). All tissues were processed by routine methods and embedded in paraffin wax. Five-micrometer sections were stained with H&E (Poly Scientific, Bay Shore NY). Human T cells in the paraffin-embedded mouse tissues were detected using rabbit polyclonal sera specific to human CD3 (DakoCytomation, Carpinteria CA).
Flow cytometry
We performed FACS with a FACScan cytometer with FlowJo software (Tree Star, Ashland, OR), using PE-labeled CAR-specific monoclonal antibody (12D11, MSKCC monoclonal antibody core facility), FITC-labeled human CD4 specific antibody (S3.5, Caltag), CD8 specific antibody (3B5, Caltag), CD62L specific antibody (DREG 56, BD Pharmingen), PE-labeled human CD25 specific antibody (CD25.3G10, Caltag), and APC-labeled human CD19 specific antibody (SJ25-C, Caltag). Foxp3 expression was assessed using the Human Regulatory T cell Staining Kit (eBioscience).
Statistical Analysis
Statistical analysis utilizing the GraphPad Prism software (GraphPad Software, San Diego, CA) were done using log-rank analyses for survival and the Student's t test and Wilcoxon rank sum test for line and bar graph comparisons.
Results
Natural Tregs are efficiently modified to express CARs by retroviral transduction
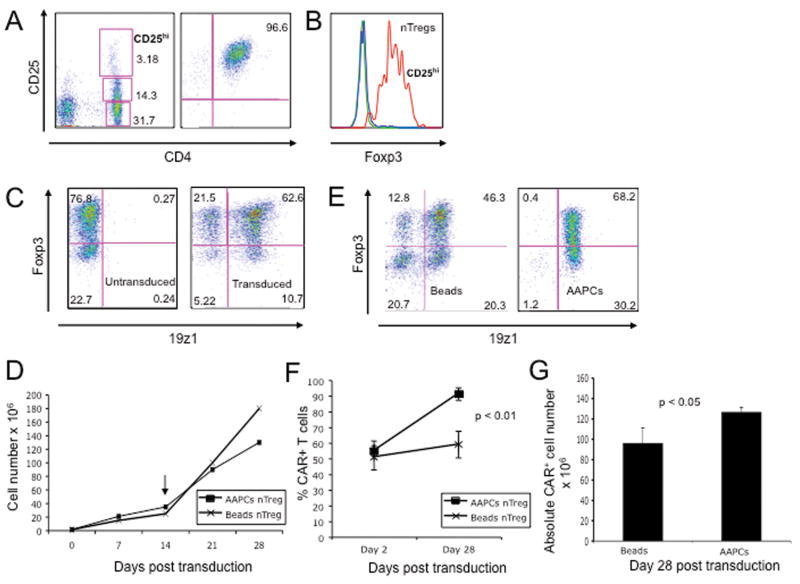
To assess whether human nTregs can be genetically manipulated despite their anergic nature and difficulty to maintain in culture(30, 35), we isolated nTregs using immunomagnetic sorting for the CD4+ CD25hi population from PBMCs consistently achieving CD4+ T cell populations that were >95% CD25hi, 70-90% Foxp3+ (Fig. 1A-B), CD62Lhi and CD127- (data not shown). CD3/CD28 bead activated nTregs were subsequently transduced with CD19-targeted CARs using retroviral supernatants, routinely resulting in >60% gene transfer (Fig 1C).
Figure 1. Efficient transduction and expansion of CAR+ nTregs.
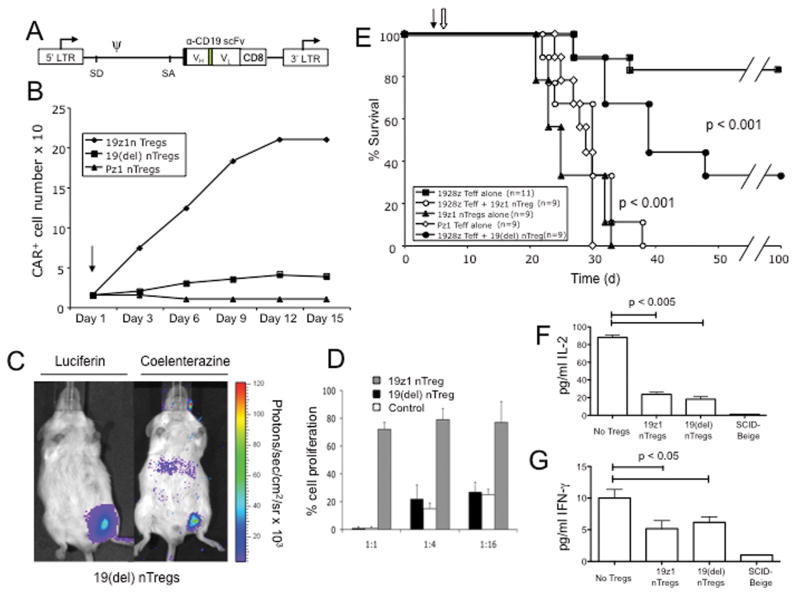
(A) FACS analysis of nTregs isolated from peripheral blood utilizing the Dynal Regulatory T Cell Isolation Kit (Invitrogen). (B) FACS analyses of isolated nTregs as assessed by intracellular staining for Foxp3 expression. Blue line represents isotype control, green line represents CD4+ CD25- non-Treg control and red line represents nTregs. (C) FACS analyses for Foxp3 and 19z1 expression of isolated nTregs at day 7 following isolation and 19z1 CAR retroviral gene transfer. Similar gene transfer was obtained for nTregs transduced with the 19-28z and Pz1 control CARs (data not shown). (D) nTreg expanded following activation with Dynal CD3/CD28 Human Treg Expander beads (day 0), transduction with the 19z1 CAR, and restimulation (arrow, day 14) with either Expander beads or 3T3(hCD19/CD80) AAPCs. Cell counts, normalized to total cell number, show similar expansion between the AAPC and CD3/CD28 bead activated groups. (E) Foxp3 expression is largely retained following restimulation with either Dynal CD3/CD28 Human Treg Expander beads or 3T3(hCD19/CD80) AAPCs, with the latter population demonstrating enhanced CAR expression. (F) Percentage of expanded T cells expressing the CAR is increased following stimulation on 3T3(hCD19/CD80) AAPCs, but stable following stimulation with Dynal CD3/CD28 Human Treg Expander beads (p < 0.01). (G) Absolute numbers of 19z1+ Tregs is increased following expansion on 3T3(hCD19/CD80) AAPCs when compared to expansion with Dynal CD3/CD28 Human Treg Expander beads.
We next compared CAR+ nTreg expansion in the context of high dose IL-2 and rapamycin, either by co-culture on NIH-3T3 AAPCs (3T3(CD19/CD80))(2) or through the addition of Dynal CD3/CD28 Human Treg Expander beads. CAR+ nTregs proliferated equally well under either condition with largely retained Foxp3 expression (Fig. 1D-E) while only expansion of CAR+ nTregs by co-culture on 3T3(CD19/CD80) AAPCs (2) enriched the CAR+ nTreg fraction (Fig. 1E-F) resulting in a significantly increased absolute number of CAR+ nTregs (Fig. 1G). However, due to contamination by persistent 3T3 fibroblasts in AAPC expanded nTreg populations, we utilized nTregs generated by CD3/CD28 Human Treg Expander beads for further studies.
Expanded genetically modified nTregs inhibit naïve T cell proliferation and CAR+ effector T cell cytotoxicity in vitro
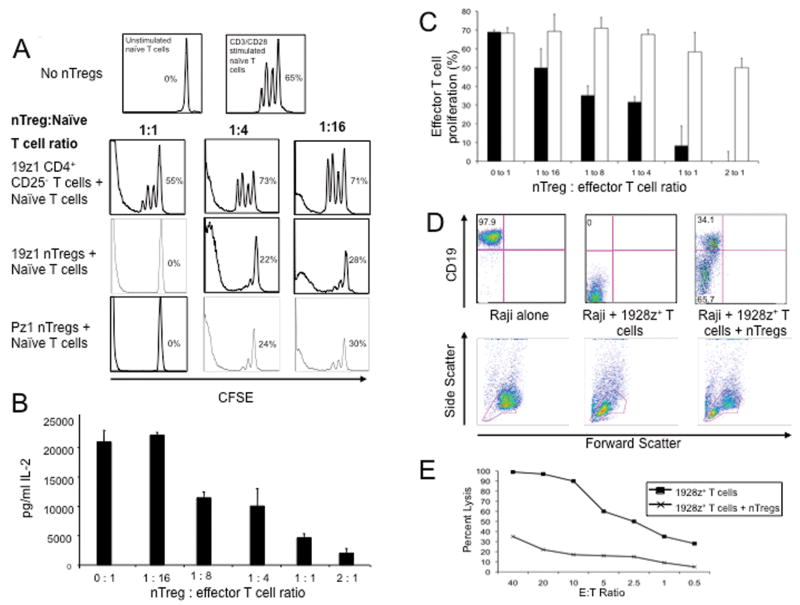
We next co-cultured activated CFSE-labeled naïve T cells with varying numbers of 19z1+ nTregs, and as controls, nTregs transduced with the irrelevant Pz1 CAR specific to the prostate specific membrane antigen (31), and control 19z1+ non-Treg CD4+ T cells. 19z1+ and Pz1+ nTregs, but not 19z1+ non-Treg control T cells, induced a potent inhibition of non-specific T cell expansion even at low nTreg to effector T cell ratios (Fig. 2A). Consistent with our CFSE studies, IL-2 levels in nTreg co-cultures decreased in a dose dependent manner (Fig. 2B), a finding similar to results published elsewhere(35, 36). Co-culture of CFSE-labeled 19-28z+ T cells with titrated numbers of 19z1+ nTregs or control 19z1+ non-Tregs demonstrated that only 19z1+ nTregs suppressed 19-28z+ effector T cell expansion (Fig. 2C).
Figure 2. CAR+ nTregs inhibit expansion of activated naïve T cells, and cytotoxicity of 19-28z+ effector T cells.
(A) CSFE-labeled naïve T cells co-cultured with 19z1+ nTregs, Pz1+ nTregs, or 19z1+ Foxp3- CD4+ CD25- T cells at titrated effector to suppressor ratios were activated with CD3/CD28 Human T cell Expander beads. CFSE+ T cells were analyzed via flow cytometry on day 3 post activation. Percent proliferation was calculated using FlowJo software. 19z1+ and Pz1+ nTregs, in contrast to control non-Tregs, efficiently suppressed proliferation of naïve T cells. (B) 19z1+ nTregs inhibit secretion of IL-2 by activated naïve T cells in a dose dependent manner, as assessed by Luminex-based analyses of tissue culture supernatants at 24 hours post co-culture. (C) CFSE-labeled 19-28z+ effector T cells co-cultured with 19z1+ nTregs (solid bars) or Foxp3- CD4+ CD25- T cells at varying effector to suppressor ratios were activated on 3T3(hCD19/CD80) AAPCs for 3 days. 19z1+ nTregs, but not control non-Tregs, suppressed proliferation of 19-28z+ effector T cells. (D) 19z1+ nTregs inhibit 19-28z+ effector T cell cytotoxicity. CD19+ Raji tumor cells were co-cultured at a 1:1:1 ratio with 19-28z+ effector T cells and 19z1+ nTregs. At 24 hours, persistence of Raji tumor cells was assessed by FACS with corresponding FSC/SSC plots provided. Persistence of Raji tumor cells is evident when 19-28z+ effector T cells were co-cultured with 19z1+ nTregs, in contrast to coculture with 19-28z+ effector T cells in the absence of nTregs. (E) Standardized cytotoxicity assay of CD19+ Raji tumor cells co-cultured with 19-28z+ effector T cells alone or 19-28z+ effector T cells with 19z1+ nTregs (at a 1:1 ratio) at various effector to target (E:T) ratios. E:T ratios represent the ratio of 19-28z+ effector T cells to target Raji tumor cells.
To assess the role of 19z1+ nTregs on effector T cell cytotoxicity, 19-28z+ effector T cells were co-cultured with 19z1+ nTregs at a 1:1 ratio for 24 hours followed by the addition of target CD19+ Raji tumor cells at a 1:1 ratio with effector 19-28z+ T cells. We found that 19z1+ nTregs inhibited killing of Raji tumor cells by 19-28z+ effector T cells (Fig. 2D). As expected, 19z1+ nTregs co-cultured with CD19+ Raji tumors alone failed to eradicate Raji tumor cells consistent with the notion that CD19-targeted nTregs lack cytotoxic potential (data not shown). Similar to the above findings, nTregs markedly abrogated the lysis of Raji tumor cells by 19-28z+ effector T cells in a standard cytotoxicity assay (Fig. 2E).
19z1+ nTregs traffic to CD19+ Raji tumors
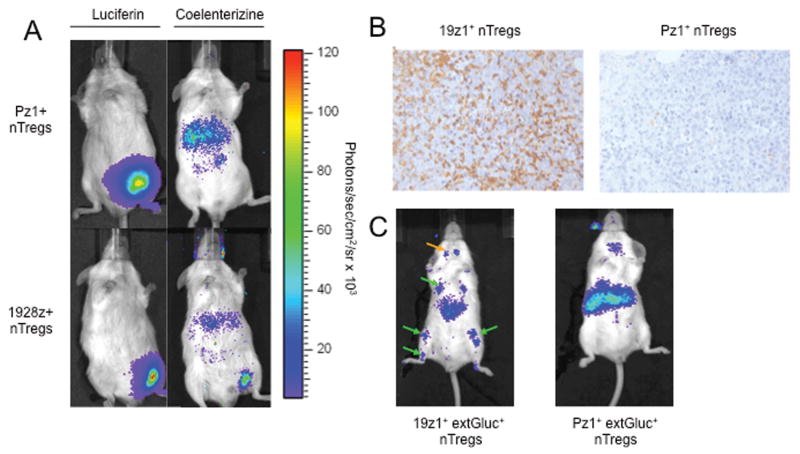
In order to assess whether CAR-modified nTregs efficiently traffic to Raji tumors in SCID-Beige mice, we employed dual bioluminescent imaging (BLI) enabling simultaneous imaging of both tumor cells and T cells within the same animal(34). SCID-Beige mice previously injected subcutaneously with Raji(GFP-FFLuc) tumor cells underwent bioluminescent imaging to verify detectable tumor (Figure 3A). Subsequently, mice were infused i.v. with either 19z1+ or Pz1+ nTregs further modified to express extGLuc bioluminescent enzyme (extGLuc+ nTregs). BLI at 24 hours following nTreg infusion demonstrated 19z1+ extGLuc+ nTreg but not Pz1+ extGLuc+ nTreg signal localized to the Raji tumor (Fig. 3A). Immunohistochemistry studies confirmed the presence of 19z1+ extGLuc+, but not Pz1+ extGLuc+ nTregs within the Raji tumors (Fig. 3B). Similar results were obtained in mice bearing systemic Raji tumors, which primarily infiltrate the bone marrow and lymphnodes, demonstrating specific localization of 19z1+ extGLuc+ nTregs but not Pz1+ extGLuc+ nTregs to these sites at 24 hours (Fig. 3C). These findings indicate that by 24 hours, 19z1+ nTregs successfully traffic to CD19+ tumors, recapitulating a hostile tumor microenvironment which may be seen in the clinical setting.
Figure 3. CD19-targeted CAR+ nTregs traffic to CD19+ Raji tumor cells in vivo.
(A) Dual bioluminescent imaging of Raji tumor and CAR+ nTregs show trafficking of 19z1+ nTregs, but not Pz1+ nTregs, to subcutaneous Raji tumors at 24 hours. SCID-Beige mice were injected subcutaneously with Raji(GFP-FFLuc) cells and 10 days later, with 19z1+ extGLuc+ or control Pz1+ extGLuc+ nTregs. Tumor cells were imaged using the FFLuc specific luciferin substrate, while T cells were imaged using the GLuc specific coelenterazine substrate. (B) Immunohistochemistry staining with an anti-human CD3 antibody confirms the presence of 19z1+ nTregs, but not Pz1+ nTregs, within Raji tumor microenvironment. (C) Bioluminescent imaging CAR+ nTregs show trafficking of 19z1+ nTregs, but not Pz1+ nTregs, to systemic Raji tumors at 24 hours, with predicted signal in the bone marrow of the femurs, tibia, and humeri (green arrows) of 19z1+ extGluc+ nTreg infused mice, as well as infiltration of these nTregs into the submandibular lymphnodes (orange arrow).
Tumor infiltrating 19z1+ nTregs inhibit eradication of systemic CD19+ Raji tumors by 19-28z+ effector T cells
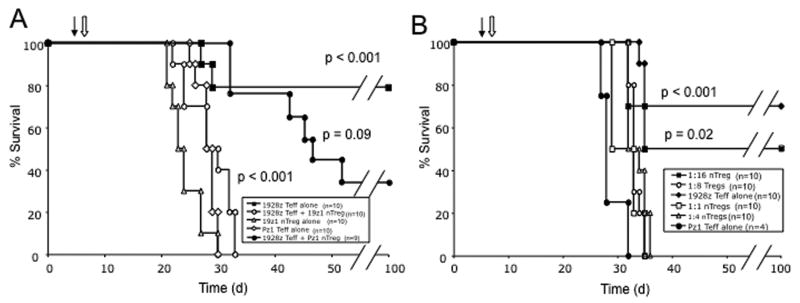
We have previously demonstrated that CD19-targeted effector T cells successfully eradicate systemic Raji tumors in SCID-Beige mice as assessed by long term survival (2, 3). In SCID-Beige mice, Raji tumors have a primary tropism for the bone marrow and untreated mice reliably develop hind-limb paralysis at 3-5 weeks as a consequence of spinal cord compression by tumor expanding from vertebral bodies (2). To determine whether CD19-targeted nTregs within the tumor microenvironment could inhibit successful tumor eradication in this model, mice were injected systemically with Raji tumor cells on day 1, with 19z1+ nTregs on day 5, and with 19-28z+ effector T cells at a nTreg to T effector ratio of 1:1 on day 6. In all in vivo experiments, >80% of infused CAR+ effector T cell populations retained a central memory phenotype (CD62Lhi CCR7+) and consisted of 50-65% CD8+ and 35-50% CD4+ T cells as assessed by FACS prior to infusion (data not shown). Prior infusion of 19z1+ nTregs, in contrast to Pz1+ nTregs, wholly abolished any anti-tumor effect by subsequently infused 19-28z+ effector T cells as assessed by overall survival (Fig 4A). To confirm the in vivo presence of Tregs, we further assessed the nTreg to effector T cell ratio in the bone marrow of mice infused with 19z1+ nTregs followed by 19-28z+ effector T cells by FACS analysis at 24 hours following effector T cell infusion, demonstrating a 1:1 nTreg to effector T cell ratio (Fig. 6B).
Figure 4. CD19-targeted nTregs within the Raji tumor microenvironment suppress 19-28z+ effector T cell function in vivo.
(A) SCID-Beige mice were injected i.v. with Raji tumor cells on day 0, followed by CAR+ nTregs on day 5 (filled arrow) and CAR+ effector T cells on day 6 (open arrow). 19z1+ nTregs fully abrogated eradication of systemic Raji tumors by 19-28z+ effector T cells as assessed by survival over time when compared to mice treated with 19-28z+ effector T cells alone (p < 0.001). Pz1+ nTregs did not demonstrate significant suppression (p = 0.09 when compared to the 19-28z+ effector T cells alone cohort; p < 0.001 compared to the 19z1+ nTreg plus 19-28z+ effector T cells cohort). Data represents combined results from 2 independent experiments. (B) 19z1+ nTregs inhibited effector 19-28z+ T cells in a dose dependent manner with infused nTreg to effector T cell ratios of 1:1, 1:4, and 1:8 resulting in no long term surviving mice (all with p < 0.001, compared to 19-28z Teff alone cohort) while a 1:16 nTreg to effector T cell ratio allowed for a 50% long-term survival of treated mice (p = 0.02, compared to Pz1+ Teff treated control cohort). Survival of the 1:16 nTreg to effector T cell treated cohort was statistically similar to the 19-28z Teff alone control cohort (p = 0.3). Similar results were obtained in tumor bearing mice following prior infusion with 19-28z+ nTregs (data not shown). d: days since Raji tumor cell injection.
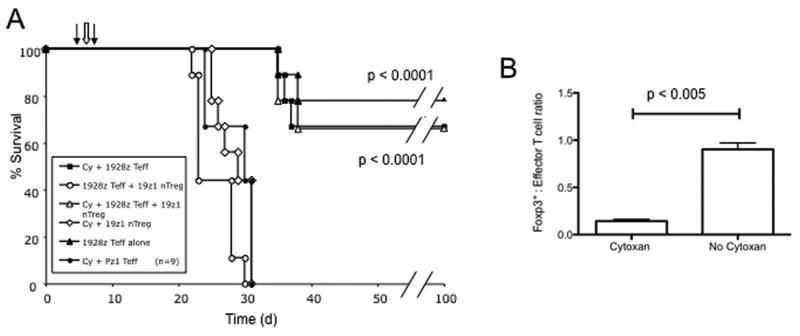
Figure 6. Cyclophosphamide lymphodepletion following 19z1+ nTreg infusion, enhances 19-28z+ effector T cell tumor cell eradication, altering the nTreg to effector T cell ratio within the tumor microenvironment.
(A) SCID-Beige were mice injected i.v. with Raji tumor cells on day 0, followed by 19z1+ nTregs on day 5 (left filled arrow), i.p. injection of cyclophosphamide (100mg/kg) on day 6 (open arrow), and 19-28z or Pz1+ effector T cells on day 7 (right black arrow). Long term survival comparable to no-nTreg controls was observed in mice injected with 19z1+ nTregs followed by cyclophosphamide therapy and 19-28z+ effector T cell infusion which was significantly superior to similarly treated mice without prior lymphodepletion (p < 0.0001). Data represents combined results from 2 independent experiments. (B) Foxp3+ nTreg to effector cell ratios were assessed in tumor-involved tissues (bone marrow) by FACS at 24 hours following 19-28z+ effector T cell infusion. Prior cyclophosphamide therapy significantly altered the nTreg to effector T cell ratios in favor of the 19-28z+ effector T cells (0.14 with versus 0.9 without lymphodepletion, p < 0.005). Data represent the average from 2 independent experiments each with cohorts of 3 mice per treatment group.
To assess in vivo potency of nTreg effector suppression, we titrated the 19z1+ nTreg to effector 19-28z+ T cell ratio in Raji tumor bearing SCID-Beige mice. We observed that the 19z1+ nTregs were able to fully suppress the in vivo anti-tumor efficacy of effector 19-28z+ T cells, as assessed by survival, at a nTreg to effector T cell ratio as low as 1:8, but found recovery of effector T cell anti-tumor efficacy at a 1:16 nTreg to effector T cell ratio (Fig. 4B). Similar results were obtained when this experiment was conducted using 19-28z+ nTregs (data not shown).
Optimal suppression of 19-28z+ effector T cells requires nTreg activation within the tumor microenvironment
We next generated a CD19-targeted CAR lacking the ζ chain signaling domain termed 19(del) (Fig 5A). 19(del)+ nTregs failed to expand on 3T3(CD19/CD80) AAPCs verifying the lack of T cell activating signaling by the ζ chain-deleted 19(del) CAR (Fig 5B). However, despite loss of CAR signaling, 19(del)+ nTregs retained the capacity to traffic to CD19+ Raji tumors in vivo (Fig 5C) and the ability to potently suppress both naïve T cell proliferation (Fig 5D) and 19-28z effector T cell cytotoxicity in vitro (data not shown).
Figure 5. Optimal in vivo nTreg suppression is dependent on activation of nTregs within the tumor microenvironment.
(A) Schematic of the 19(del) CAR. Black box, CD8 leader sequence; green box, (Gly3Ser)4 linker; LTR, long terminal repeat; SD, splice donor; SA, splice acceptor; arrows, start of transcription. (B) 19(del)+ nTregs, like control Pz1+ nTregs, but in contrast to 19z1+ nTregs, fail to expand following co-culture on 3T3(hCD19/CD80) AAPCs consistent with a lack of T cell activation mediated through the 19(del) CAR. (C) 19(del)+ extGLuc + nTregs retain the ability to traffic to Raji tumor in vivo. SCID-Beige mice bearing palpable Raji(GFP-FFLuc) tumors were injected i.v. with 19(del)+ extGLuc+ T cells. Mice were imaged at 24 hours post nTreg infusion. (D) 19(del) + nTregs retain the ability to inhibit expansion of activated naïve T cells. CFSE-labeled naïve T cells co-cultured with 19(del) + Tregs, 19z1+ Tregs, or 19z1+ CD4+ CD25- non-Treg control T cells at varying effector to suppressor ratios were activated with Dynabeads CD3/CD28 Human T cell Expander beads. Both 19(del)+ nTregs and 19z1+ nTregs suppressed proliferation of naïve T cells when compared to the non-Treg control co-cultures. (E) Infusion of Raji tumor bearing mice with 19(del)+ nTregs partially inhibited antitumor efficacy of 19-28z+ effector T cells as assessed by long-term survival. Survival of mice previously infused with 19(del)+ nTregs, when compared to mice treated with 19-28z+ effector T cells alone, was significantly lower (p < 0.001) but significantly improved when compared to mice previously treated with 19z1+ nTregs (p < 0.001). Data represents combined results from 2 independent experiments. Closed arrow: nTreg infusion; open arrow, effector 19-28z+ T cell infusion; d, days since Raji tumor cell injection. At 24 hours following 19-28z+ effector T cell infusion, prior infusion with either 19z1+ or 19(del)+ nTregs significantly reduced serum levels of IL-2 (F) (p < 0.005), and IFNγ (G) (p < 0.05), when compared to mice treated with 19-28z+ effector T cells alone. Figure 5F and 5G represent the combined data from 2 independent experiments with cohorts of 3 mice in each experiment.
In order to assess the in vivo inhibitory capacity of 19(del)+ nTregs, SCID-Beige mice were injected i.v. with Raji tumor cells on day 1, injected with either 19z1+ or 19(del)+ nTregs on day 5, followed by injection of 19-28z+ effector T cells, at a 1:1 nTreg to effector T cell ratio, on day 6. As expected, 19z1+ nTregs conferred full suppression of 19-28z+ effector T cells, while 19(del)+ Tregs conferred only partial suppression (Fig. 5E). These findings suggest that optimal suppression requires both nTreg localization to and activation within the tumor microenvironment.
To further define the mechanism whereby 19(del)+ nTregs mediated partial inhibition of 19-28z+ T cell effector function, we next measured serum levels of human IL-2 and IFNγ as well as the inhibitory human cytokines TGF-β and IL-10 in treated mice. Human TGF-β and IL-10 levels were consistently low to undetectable in all treated cohorts (data not shown), while human IL-2 and IFNγ levels were significantly and equally decreased in both 19z1 and 19(del)+ nTreg infused cohorts (Figs. 5F-G). This finding is consistent with sequestration of IL-2 by nTregs, a previously described mechanism of nTreg mediated T cell suppression(35, 36), and inhibition of effector T cell activation as assessed by IFNγ levels. These findings were independent of nTreg activation status, and may explain, in part, the observed partial inhibition mediated by 19(del)+ nTregs.
Cyclophosphamide lymphodepletion eradicates 19z1+ nTregs and restores anti-tumor efficacy of 19-28z+ effector T cells
Lymphodepleting pre-conditioning regimens can enhance the anti-tumor efficacy of adoptively transferred cytotoxic T cells which may be mediated in part through the eradication of Tregs in the host (26, 27). In particular, cyclophosphamide chemotherapy been shown to effectively eliminate Tregs(37-39). To this end, we next investigated whether cyclophosphamide therapy (100mg/kg) following infusion of 19z1+ nTregs can abrogate effector T cell suppression by nTregs. SCID-Beige mice bearing systemic Raji tumors were injected with 19z1+ nTregs on day 5 following tumor cell infusion. On day 6, 24 hours prior to treatment with 19-28z+ T cells, mice were injected i.p. with cyclophosphamide. In contrast to mice infused with 19z1+ nTregs followed by 19-28z+ effector T cells with no long-term survival, mice infused sequentially with 19z1+ nTregs, i.p. cyclophosphamide, and 19-28z+ effector T cells demonstrated a prolonged long-term survival (67%) which compares favorably to mice treated with 19-28z+ effector T cells alone (78%) (Fig. 6A). Furthermore, these studies demonstrated Raji tumors to be largely refractory to cyclophosphamide treatment at this dose level as evidenced by the poor survival of the cyclophosphamide followed by Pz1+ effector T cell treated control cohort (Fig. 6A).
FACS analyses to assess the 19z1+ nTreg to 19-28z+ effector T cell ratios in the bone marrow of cyclophosphamide treated mice at 24 hours post 19-28z+ effector T cell infusion demonstrated a 0.14 nTreg to effector T cell ratio, in contrast to a 0.9 nTreg to effector T cell ratio seen in non-lymphodepleted mice (Fig. 6B). These data verify cyclophosphamide depletion of endogenous nTregs.
Discussion
The generation of tumor targeted T cells for adoptive therapy may be insufficient to achieve significant anti-tumor responses in the clinical setting. Specifically, the tumor itself may foster an environment capable of impairing targeted effector T cell function. Specifically, Tregs, which are relevant in both the clinic and in immune-competent animal models of disease(40, 41), are absent in the xenograft tumor models previously used to study modified human T cells in vivo.
Herein we report that isolated human nTregs from healthy donors may be efficiently transduced to express CARs and subsequently expanded in vitro. While other groups have examined the effects of induced Tregs (iTregs) toward similar ends (42), we opted to isolate and expand natural Tregs due to concerns regarding the stability of FOXP3 expression in iTregs (43). The resulting tumor targeted nTregs successfully traffic to tumor in SCID-Beige mice recapitulating a hostile tumor microenvironment seen in the clinical setting. Natural Treg infiltrated tumors were markedly resistant to eradication by CAR-modified effector T cells even at low (1:8) nTreg to effector T cell ratios, while tumor resistance to effector T cell eradication was no longer apparent at a ratio of 1:16, consistent with a dose dependent nTreg mediated suppression. This dose dependent nature of inhibition, evidence of persistence of tumor targeted nTregs within the tumors, and the inability of non-tumor targeted control Pz1+ nTregs to inhibit 19-28z+ effector T cells even at a 1:1 ratio, all support the notion that the suppression of tumor targeted effector T cells is specifically dependent upon the presence of these nTregs within the tumor microenvironment.
While our in vitro data suggest that 19z1+ Tregs are capable of inhibiting both CAR mediated cytotoxicity and proliferation, which of these factors predominates in vivo in this tumor model is currently unclear. However, our prior studies suggest that proliferation or persistence does not play a dominant role in this tumor model, as multiple infusions of 19-28z+ T cells are needed to achieve optimal anti-tumor efficacy in a pre B cell ALL tumor model (3). These data are consistent with limited in vivo persistence and proliferation of the CAR modified cells in SCID-Beige mice favoring inhibited effector T cell cytotoxicity by co-localized nTregs as the primary mechanism of suppression observed in our studies.
The mechanism of in vivo nTreg suppression in our model further appears to be dependent, in part, on the activation status of nTregs at the tumor site since nTregs modified to express the 19(del) CAR retained a statistically significant ability to suppress in vivo effector T cell function when compared to mice treated with 19-28z+ effector T cells alone, but demonstrated statistically inferior suppression when compared to mice pre-treated with 19z1+ nTregs (Fig. 5E). While both sets of CAR modified nTregs were previously activated using CD3/28 beads and IL-2, only the 19z1+ nTregs receive additional intratumoral activation via a functional CAR. This phenomenon is consistent with prior literature reporting the requirement for activation to induce Treg mediated suppression(36) although activation and suppression can be separated temporally (44). The mechanism of this retained but attenuated suppression may be partially mediated by direct contact of the nTreg with effector T cells since the 19(del)+ nTregs successfully traffic to the tumor(35). Additionally, we found that both 19z1+ and 19(del)+ nTreg infused mice demonstrated equal levels of IL-2 reduction in serum suggesting that 19(del)+ nTregs may further inhibit effector T cell function through a retained ability to sequester of IL-2, a known mechanism of Treg mediated suppression(23).
Our data is consistent with the notion that tumor infiltrating Tregs may represent a significant obstacle to the successful application of this adoptive cell therapy. One relatively direct method of overcoming this obstacle would be to eliminate Tregs prior to CAR modified T cell infusion. While several therapeutic options exist for this purpose, including anti-CD25 antibodies and immunotoxins (45), we chose cyclophosphamide due to its established ability to eliminate Tregs in the context of an anti-tumor response (46, 47) as well as the applicability of this approach to ongoing Phase I clinical trials at our center using autologous 19-28z modified T cells to treat patients with relapsed or refractory B cell malignances. In our studies, prior lymphodepletion with cyclophosphamide before adoptive cell therapy of SCID-Beige mice bearing systemic established Raji tumors infiltrated with 19z1+ nTregs abrogated the suppression of subsequently infused 19-28z+ effector T cells by significantly lowering the nTreg to effector T cell ratios from 0.90 to 0.14 within the tumor microenvironment (Fig. 6B). These data are furthermore consistent with our observed in vivo dose dependent nTreg mediated suppression of effector T cells (Fig. 4B).
In conclusion, these data validate concerns that a hostile tumor microenvironment may markedly compromise CAR-modified effector T cell anti-tumor efficacy in the clinical setting. Further our data supports the incorporation of lymphodepleting chemotherapy prior to infusion of CAR-modified tumor targeted T cells in the modification of ongoing clinical trials and the design of future clinical trials.
Acknowledgments
Supported by CA138738, CA95152, CA059350, CA08748, CA86438, CA096945, CA094060, The Alliance for Cancer Gene Therapy, Damon Runyon Clinical Investigator Award (RJB), The Annual Terry Fox Run for Cancer Research (New York, NY) organized by the Canada Club of New York, Kate's Team, Mr. William H. Goodwin and Mrs. Alice Goodwin and the Commonwealth Cancer Foundation for Research and the Experimental Therapeutics Center of MSKCC, and the Geoffrey Beene Cancer Foundation. E.H, is a Howard Hughes Medical Institute award recipient.
References
- 1.Sadelain M, Riviere I, Brentjens R. Targeting tumours with genetically enhanced T lymphocytes. Nat Rev Cancer. 2003;3:35–45. doi: 10.1038/nrc971. [DOI] [PubMed] [Google Scholar]
- 2.Brentjens RJ, Latouche JB, Santos E, et al. Eradication of systemic B-cell tumors by genetically targeted human T lymphocytes co-stimulated by CD80 and interleukin-15. Nat Med. 2003;9:279–86. doi: 10.1038/nm827. [DOI] [PubMed] [Google Scholar]
- 3.Brentjens RJ, Santos E, Nikhamin Y, et al. Genetically targeted T cells eradicate systemic acute lymphoblastic leukemia xenografts. Clin Cancer Res. 2007;13:5426–35. doi: 10.1158/1078-0432.CCR-07-0674. [DOI] [PubMed] [Google Scholar]
- 4.Cheadle EJ, Gilham DE, Hawkins RE. The combination of cyclophosphamide and human T cells genetically engineered to target CD19 can eradicate established B-cell lymphoma. Br J Haematol. 2008;142:65–8. doi: 10.1111/j.1365-2141.2008.07145.x. [DOI] [PubMed] [Google Scholar]
- 5.James SE, Orgun NN, Tedder TF, et al. Antibody-mediated B-cell depletion before adoptive immunotherapy with T cells expressing CD20-specific chimeric T-cell receptors facilitates eradication of leukemia in immunocompetent mice. Blood. 2009;114:5454–63. doi: 10.1182/blood-2009-08-232967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morgan RA, Dudley ME, Wunderlich JR, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–9. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kershaw MH, Westwood JA, Parker LL, et al. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin Cancer Res. 2006;12:6106–15. doi: 10.1158/1078-0432.CCR-06-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Till BG, Jensen MC, Wang J, et al. Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells. Blood. 2008;112:2261–71. doi: 10.1182/blood-2007-12-128843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Movahedi K, Guilliams M, Van den Bossche J, et al. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood. 2008;111:4233–44. doi: 10.1182/blood-2007-07-099226. [DOI] [PubMed] [Google Scholar]
- 10.Gajewski TF, Meng Y, Blank C, et al. Immune resistance orchestrated by the tumor microenvironment. Immunol Rev. 2006;213:131–45. doi: 10.1111/j.1600-065X.2006.00442.x. [DOI] [PubMed] [Google Scholar]
- 11.Hoechst B, Ormandy LA, Ballmaier M, et al. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cells. Gastroenterology. 2008;135:234–43. doi: 10.1053/j.gastro.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 12.Liu W, Putnam AL, Xu-Yu Z, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701–11. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–52. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 14.Suri-Payer E, Amar AZ, Thornton AM, Shevach EM. CD4+CD25+ T cells inhibit both the induction and effector function of autoreactive T cells and represent a unique lineage of immunoregulatory cells. J Immunol. 1998;160:1212–8. [PubMed] [Google Scholar]
- 15.Gavin M, Rudensky A. Control of immune homeostasis by naturally arising regulatory CD4+ T cells. Curr Opin Immunol. 2003;15:690–6. doi: 10.1016/j.coi.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 16.Deaglio S, Dwyer KM, Gao W, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–65. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McHugh RS, Whitters MJ, Piccirillo CA, et al. CD4(+)CD25(+) immunoregulatory T cells: gene expression analysis reveals a functional role for the glucocorticoid-induced TNF receptor. Immunity. 2002;16:311–23. doi: 10.1016/s1074-7613(02)00280-7. [DOI] [PubMed] [Google Scholar]
- 18.Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J Exp Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beyer M, Schultze JL. Regulatory T cells in cancer. Blood. 2006;108:804–11. doi: 10.1182/blood-2006-02-002774. [DOI] [PubMed] [Google Scholar]
- 20.Wolf AM, Wolf D, Steurer M, Gastl G, Gunsilius E, Grubeck-Loebenstein B. Increase of regulatory T cells in the peripheral blood of cancer patients. Clin Cancer Res. 2003;9:606–12. [PubMed] [Google Scholar]
- 21.Yang ZZ, Novak AJ, Ziesmer SC, Witzig TE, Ansell SM. Attenuation of CD8(+) T-cell function by CD4(+)CD25(+) regulatory T cells in B-cell non-Hodgkin's lymphoma. Cancer Res. 2006;66:10145–52. doi: 10.1158/0008-5472.CAN-06-1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Curtin JF, Candolfi M, Fakhouri TM, et al. Treg depletion inhibits efficacy of cancer immunotherapy: implications for clinical trials. PLoS ONE. 2008;3:e1983. doi: 10.1371/journal.pone.0001983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miyara M, Sakaguchi S. Natural regulatory T cells: mechanisms of suppression. Trends Mol Med. 2007;13:108–16. doi: 10.1016/j.molmed.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 24.June CH. Adoptive T cell therapy for cancer in the clinic. J Clin Invest. 2007;117:1466–76. doi: 10.1172/JCI32446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rasku MA, Clem AL, Telang S, et al. Transient T cell depletion causes regression of melanoma metastases. J Transl Med. 2008;6:12. doi: 10.1186/1479-5876-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dudley ME, Wunderlich JR, Yang JC, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346–57. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muranski P, Boni A, Wrzesinski C, et al. Increased intensity lymphodepletion and adoptive immunotherapy--how far can we go? Nat Clin Pract Oncol. 2006;3:668–81. doi: 10.1038/ncponc0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wichlan DG, Roddam PL, Eldridge P, Handgretinger R, Riberdy JM. Efficient and reproducible large-scale isolation of human CD4+ CD25+ regulatory T cells with potent suppressor activity. J Immunol Methods. 2006;315:27–36. doi: 10.1016/j.jim.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 29.Battaglia M, Stabilini A, Migliavacca B, Horejs-Hoeck J, Kaupper T, Roncarolo MG. Rapamycin promotes expansion of functional CD4+CD25+FOXP3+ regulatory T cells of both healthy subjects and type 1 diabetic patients. J Immunol. 2006;177:8338–47. doi: 10.4049/jimmunol.177.12.8338. [DOI] [PubMed] [Google Scholar]
- 30.Strauss L, Whiteside TL, Knights A, Bergmann C, Knuth A, Zippelius A. Selective survival of naturally occurring human CD4+CD25+Foxp3+ regulatory T cells cultured with rapamycin. J Immunol. 2007;178:320–9. doi: 10.4049/jimmunol.178.1.320. [DOI] [PubMed] [Google Scholar]
- 31.Gong MC, Latouche JB, Krause A, Heston WDW, Bander NH, Sadelain M. Cancer Patient T Cells Genetically Targeted to Prostate-Specific Membrane Antigen Specifically Lyse Prostate Cancer Cells and Release Cytokines in Response to Prostate-Specific Membrane Antigen. Neoplasia. 1999;1:123–7. doi: 10.1038/sj.neo.7900018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Battaglia M, Stabilini A, Roncarolo MG. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood. 2005;105:4743–8. doi: 10.1182/blood-2004-10-3932. [DOI] [PubMed] [Google Scholar]
- 33.Oberg HH, Wesch D, Lenke J, Kabelitz D. An optimized method for the functional analysis of human regulatory T cells. Scand J Immunol. 2006;64:353–60. doi: 10.1111/j.1365-3083.2006.01825.x. [DOI] [PubMed] [Google Scholar]
- 34.Santos EB, Yeh R, Lee J, et al. Sensitive in vivo imaging of T cells using a membrane-bound Gaussia princeps luciferase. Nat Med. 2009;15:338–44. doi: 10.1038/nm.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takahashi T, Kuniyasu Y, Toda M, et al. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int Immunol. 1998;10:1969–80. doi: 10.1093/intimm/10.12.1969. [DOI] [PubMed] [Google Scholar]
- 36.Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188:287–96. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matsushita N, Pilon-Thomas SA, Martin LM, Riker AI. Comparative methodologies of regulatory T cell depletion in a murine melanoma model. J Immunol Methods. 2008;333:167–79. doi: 10.1016/j.jim.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.North RJ. Cyclophosphamide-facilitated adoptive immunotherapy of an established tumor depends on elimination of tumor-induced suppressor T cells. J Exp Med. 1982;155:1063–74. doi: 10.1084/jem.155.4.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berd D, Mastrangelo MJ. Effect of low dose cyclophosphamide on the immune system of cancer patients: depletion of CD4+, 2H4+ suppressor-inducer T-cells. Cancer Res. 1988;48:1671–5. [PubMed] [Google Scholar]
- 40.Beyer M, Kochanek M, Darabi K, Popov A, Jensen M, Endl E, et al. Reduced frequencies and suppressive function of CD4+ CD25hi regulatory T cells in patients with chronic lymphocytic leukemia after therapy with fludarabine. Blood. 2005;106:2018–2025. doi: 10.1182/blood-2005-02-0642. [DOI] [PubMed] [Google Scholar]
- 41.Serafini P, Mgebroff S, Noonan K, Borrello I. Myeloid-derived suppressor cells promote cross-tolerance in B-cell lymphoma by expanding regulatory T cells. Cancer Res. 2008;68:5439–49. doi: 10.1158/0008-5472.CAN-07-6621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Loskog A, Giandomenico V, Rossig C, Pule M, Dotti G, Brenner MK. Addition of the CD28 signaling domain to chimeric T-cell receptors enhances chimeric T-cell resistance to T regulatory cells. Leukemia. 2006;20:1819–28. doi: 10.1038/sj.leu.2404366. [DOI] [PubMed] [Google Scholar]
- 43.Koenecke C, Czeloth N, Bubke A, Schmitz S, Kissenpfennig A, Malissen B, Huehn J, Ganser A, Förster R, Prinz I. Alloantigen-specific de novo-induced Foxp3+ Treg revert in vivo and do not protect from experimental GVHD. Eur J Immunol. 2009;39:3091–6. doi: 10.1002/eji.200939432. [DOI] [PubMed] [Google Scholar]
- 44.Thornton AM, Piccirillo CA, Shevach EM. Activation requirements for the induction of CD4+CD25+ T cell suppressor function. Eur J Immunol. 2004;34:366–76. doi: 10.1002/eji.200324455. [DOI] [PubMed] [Google Scholar]
- 45.Koenecke C, Ukena SN, Ganser A, Franzke A. Regulatory T cells as therapeutic target in Hodgkin's lymphoma. Expert Opin Ther Targets. 2008;12:769–82. doi: 10.1517/14728222.12.6.769. [DOI] [PubMed] [Google Scholar]
- 46.Ghiringhelli F, Larmonier N, Schmitt E, Parcellier A, Cathelin D, Garrido C, Chauffert B, Solary E, Bonnotte B, Martin F. CD4+CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur J Immunol. 2004;34:336–44. doi: 10.1002/eji.200324181. [DOI] [PubMed] [Google Scholar]
- 47.Roux S, Apetoh L, Chalmin F, Ladoire S, Mignot G, Puig PE, Lauvau G, Zitvogel L, Martin F, Chauffert B, Yagita H, Solary E, Ghiringhelli F. CD4+CD25+ Tregs control the TRAIL-dependent cytotoxicity of tumor-infiltrating DCs in rodent models of colon cancer. J Clin Invest. 2008;118:3751–61. doi: 10.1172/JCI35890. [DOI] [PMC free article] [PubMed] [Google Scholar]