Abstract
Idiopathic pulmonary fibrosis (IPF) is a progressive disease causing unremitting extracellular matrix deposition with resultant distortion of pulmonary architecture and impaired gas exchange. β-arrestins regulate G-protein-coupled receptors through receptor desensitization while acting as signaling scaffolds that facilitate numerous effector pathways. Here we examine the role of β-arrestin1 and β-arrestin2 in the pathobiology of pulmonary fibrosis. In the bleomycin-induced mouse lung fibrosis model, loss of eitherβ-arrestin1 or β-arrestin2 results in protection from mortality, inhibition of matrix deposition, and protected lung function. Fibrosis is prevented despite preserved recruitment of inflammatory cells and fibroblast chemotaxis. However, isolated lung fibroblasts from bleomycin-treated β-arrestin null mice fail to invade extracellular matrix while displaying altered expression of genes involved in matrix production and degradation. Furthermore, knockdown of β-arrestin2 in fibroblasts from IPF patients attenuated the invasive phenotype. These data implicate β-arrestins as mediators of fibroblast invasion and development of pulmonary fibrosis, thus representing a potential target for therapeutic intervention for patients with IPF.
INTRODUCTION
Idiopathic pulmonary fibrosis (IPF) is a chronic and progressive lung disease of unknown etiology that leads to loss of lung function (1, 2). IPF is characterized by patchy subpleural parenchymal fibrosis with pathological features including accumulation of myofibroblasts, formation of fibroblast foci, distortion of pulmonary architecture, and increased collagen deposition (1, 3).
After injury to the airway epithelium, important profibrotic mediators, such as transforming growth factor-β (TGF-β) and matrix metalloproteinases (MMPs), are released by myofibroblasts and the epithelium. There is abundant evidence that TGF-β is critical for the progression of pulmonary fibrosis in mice due to its role in regulating collagen synthesis, fibroblast proliferation, apoptosis, and myofibroblast differentiation (4–6). MMPs have been implicated in extracellular matrix remodeling and basement membrane disruption, which allows fibroblasts to invade into the alveolar space where they proliferate, form foci, and produce collagen (3, 7, 8). When dysregulated, a decrease in fibroblast/myofibroblast apoptosis and increase in fibroblast/myofibroblast activity leads to thickening and stiffening of the septal walls, which reduces the ability to transport oxygen into the capillaries and ultimately results in respiratory failure.
β-arrestins are ubiquitously expressed members of the arrestin protein family. Studies show that two of the arrestin isoforms, termed β-arrestin1 and β-arrestin2, have non-redundant roles; however, some functional redundancy has been observed depending on the situation. β-arrestins are classically known to regulate G protein-coupled receptor (GPCR) signaling through receptor desensitization and internalization. More recently, β-arrestins have been shown to be important signaling scaffolds that facilitate the activation of numerous effector pathways, such as the mitogen-activated protein kinases (MAPKs) and Akt (9). Appreciation of the number of GPCR signaling pathways that are engaged through the β-arrestins has grown rapidly, and recent publications have also documented roles for the β-arrestins in signaling and/or endocytosis of other cellular receptors such as the single membrane-spanning TGFβ receptors and the non-classical seven-transmembrane receptors Frizzled and Smoothened (10, 11). As with the GPCRs, many of these molecules are shown to interact with the β-arrestins in a ligand- or stimulus-dependent fashion (9, 11). With the emerging appreciation of β-arrestins as signaling mediators downstream of numerous classes of receptors, their demonstrated roles in mediating physiological responses have been expanding rapidly.
The mechanisms that regulate the progression of pulmonary fibrosis are not well understood. Currently, no medical therapies exist to increase life expectancy or improve quality of life, and median survival from the time of diagnosis is only two to three years (12). Prevailing hypotheses suggest that IPF is an epithelial-fibroblastic disorder that results from numerous microinjuries to the alveolar epithelia that lead to an impaired fibrotic repair response. Further research is necessary to enhance our understanding of the molecular mechanisms that play an important role in the aberrant fibroblast response and re-epithelialization after injury. Due to their vital role in numerous cell signaling mechanisms, β-arrestins have been implicated in a broad range of diseases including asthma and cancer (13, 14). The underlying mechanisms for progression of these diseases, such as cell recruitment and motility, may also be important in the development of fibrotic lung disease. However, the ability of β-arrestins to regulate fibroblast activity in pathological settings has not been investigated.
To elucidate the potential role of β-arrestins in the development of pulmonary fibrosis and fibroblast regulation, we used the well-established bleomycin mouse model of pulmonary fibrosis (15, 16). Traditional histological and biochemical techniques as well as physiological measurements of lung mechanics were used to demonstrate that both β-arrestin1−/− and β-arrestin2−/− mice are protected from the excessive collagen deposition, architectural distortion, and reduced lung compliance that result after bleomycin treatment. In addition, we investigated the inflammatory response and the behavior of primary lung fibroblasts to better understand the mechanism involved in this protection. Our studies reveal an important role for β-arrestins in regulating fibroblast invasion and collagen formation in bleomycin-induced pulmonary fibrosis and suggest directions for development of novel therapeutic approaches.
RESULTS
β-arrestin1−/− and β-arrestin2−/− mice are protected from bleomycin-induced pulmonary fibrosis
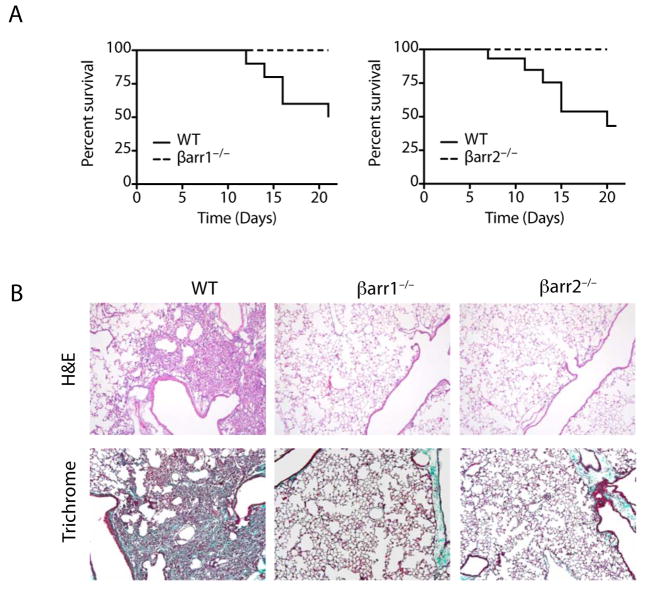
To investigate the role of β-arrestin in the progression of pulmonary fibrosis, we used mice deficient in either β-arrestin1 or β-arrestin2 in the well-established bleomycin mouse model of lung fibrosis (15, 16). Intratracheal bleomycin administration (2.5 U/kg) results in approximately 50% mortality in wild-type (WT) mice within 21 days after treatment. However, as shown in Fig. 1A, both the β-arrestin1−/− and β-arrestin2−/− mice were remarkably protected from mortality. In congruence with the survival curve data, the WT bleomycin-treated mice had severe architectural changes as well as abundant collagen deposition upon histological examination. However, the lungs from the β-arrestin1−/− and β-arrestin2−/− mice demonstrated minimal collagen deposition and a histological appearance similar to untreated mice (Fig. 1B).
Figure 1.
Loss of either β-arrestin1 or β-arrestin2 protects mice from bleomycin-induced mortality. (A) Survival curves for β-arrestin1−/−, β-arrestin2−/−, and WT mice after 2.5 U/kg bleomycin instillation. n = 10, *P < 0.05. (B) Lung sections from the surviving mice at Day 21 were stained with hematoxylin-eosin or Masson’s trichrome. Original magnification, 100×.
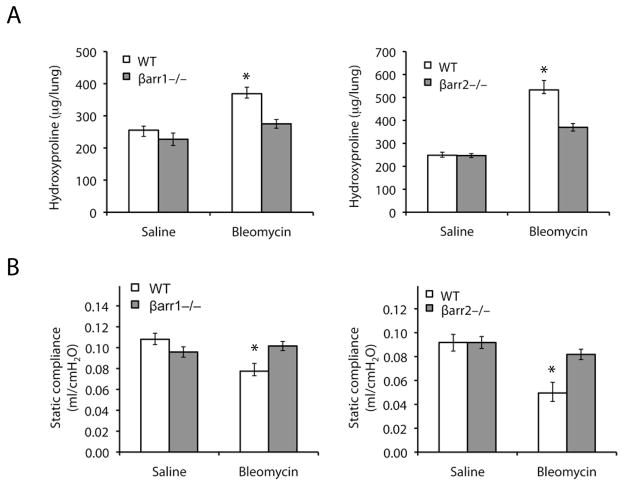
To further understand this protective mechanism, we used a lower dose of bleomycin (1.25 U/kg) that does not result in substantial mortality, which allowed us to study the entire cohort of mice at later time points. As previously described for this model, the WT mice had a significant increase in collagen deposition 21 days after bleomycin administration as quantitated biochemically by measuring hydroxyproline levels. Consistent with the lung histology results, the β-arrestin1−/− and β-arrestin2−/− mice had significantly less hydroxyproline in whole lung homogenates compared to the WT mice after bleomycin (Fig. 2A).
Figure 2.
Loss of either β-arrestin1 or β-arrestin2 protects mice from alterations in lung mechanics and excessive collagen deposition after bleomycin instillation. (A) Hydroxyproline content in the lungs of β-arrestin1−/−, β-arrestin2−/−, and WT mice 21 days after the instillation of 1.25 U/kg bleomycin. (B) Lung mechanics were measured in anesthetized, paralyzed, and mechanically ventilated mice 21 days after 1.25 U/kg bleomycin or saline administration. Static compliance was determined by fitting the Salazar-Knowles equation to pressure-volume curves. n = 10, *P < 0.05 compared to all other groups by ANOVA followed by Tukey-Kramer honestly significant difference post hoc test.
In humans, interstitial fibrosis leads to stiffening of the alveolar walls and a decrease in compliance (2). Similarly, bleomycin treatment has been shown to cause a significant decrease in static compliance in WT mice (17). Using a computer-controlled small animal ventilator, we generated pressure-volume curves and determined static compliance. As expected, WT mice had a significant decrease in static compliance values 21 days after bleomycin administration at 1.25 U/kg (Fig. 2B). Interestingly, the bleomycin-treated β-arrestin1−/− and β-arrestin2−/− mice had static compliance values similar to the saline control mice. Together with the histological and biochemical data, this suggests that the β-arrestin1−/− and β-arrestin2−/− mice are protected from the excessive collagen deposition, architectural changes, and stiffening of the lungs that normally occur after bleomycin treatment.
β-arrestin1−/− and β-arrestin2−/− mice exhibit normal recruitment of inflammatory cells after bleomycin administration
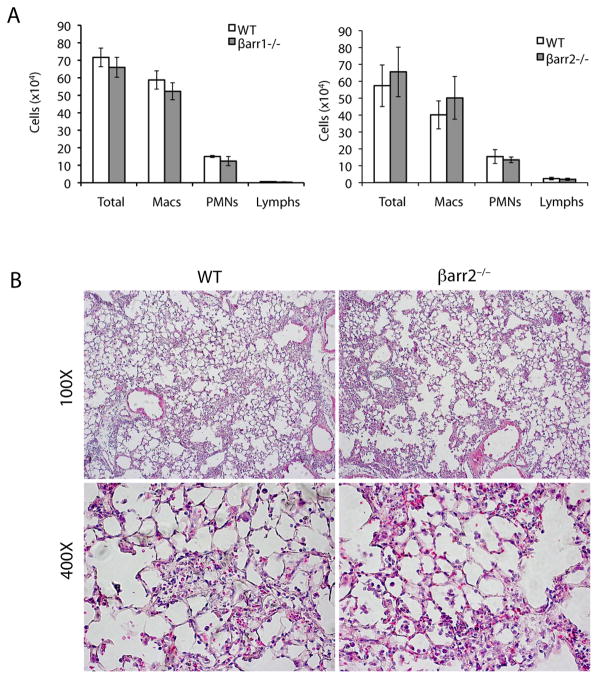
The bleomycin model of pulmonary fibrosis is characterized by an initial influx of inflammatory cells in response to injury. The magnitude of this initial inflammatory response is often correlated with enhanced fibrosis at later stages (18). To determine if loss of β-arrestins alters inflammatory cell recruitment, we collected bronchoalveolar lavage fluid (BALF) from WT, β-arrestin1−/−, and β-arrestin2−/− mice 7 days after administration of 1.25 U/kg of bleomycin. Although the β-arrestin1−/− and β-arrestin2−/− mice are protected from the fibrotic response after bleomycin administration, they had a similar number of total inflammatory cells in BALF compared to WT mice (Fig. 3A). Additionally, histological analysis of lung sections from the bleomycin-treated WT and β-arrestin2−/− mice show similar patterns of inflammatory cells within the lung parenchyma (Fig. 3B). To determine whether the composition of the inflammatory influx differed between groups, differential staining was performed to identify the leukocyte populations. Again, no differences were observed between the WT, β-arrestin1−/−, and β-arrestin2−/− mice in the ability to recruit macrophages, neutrophils, or lymphocytes (Fig. 3A). These data suggest that the inflammatory response after bleomycin treatment in β-arrestin−/− mice is preserved and that the protection from bleomycin-induced fibrosis in these animals may be due to another factor.
Figure 3.
Inflammatory cell influx is similar in the β-arrestin1−/−, β-arrestin2−/−, and WT mice. (A) Seven days after 1.25 U/kg bleomycin instillation, BALF cells were collected, and total cell counts as well as differential cell counts were determined. n = 5. (B) Lung sections from the β-arrestin2−/− and WT mice 7 days after bleomycin treatment were stained with hematoxylin-eosin. Original magnifications are indicated.
Loss of β-arrestin1 or β-arrestin2 does not alter TGF-β responsiveness in fibroblasts after bleomycin treatment
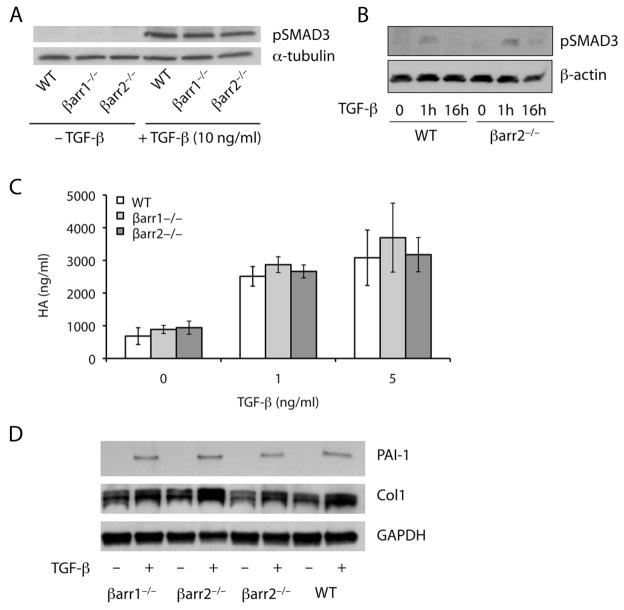
TGF-β plays a key role in the development of pulmonary fibrosis. In addition, β-arrestin2 has been shown to regulate TβRIII internalization and subsequent TGF-β signaling (19). To determine if the protection of the β-arrestin−/− mice is due to loss of TGF-β responsiveness, we isolated primary lung fibroblasts from WT, β-arrestin1−/−, andβ-arrestin2−/− mice 7 days after administration of 1.25 U/kg of bleomycin. Fibroblasts were treated with 5–10 ng/ml of TGF-β1 for 60 minutes, and total protein was isolated from the fibroblasts for Western blot analysis. As shown in Fig. 4A and 4B, the β-arrestin1−/− and β-arrestin2−/− fibroblasts had similar phospho-SMAD3 levels compared to the WT fibroblasts.
Figure 4.
Primary lung fibroblasts from β-arrestin1−/− and β-arrestin2−/− mice exhibit a normal response to TGF-β stimulation. (A–B) Fibroblasts were treated with 10 ng/ml (A) or 5 ng/ml (B) of TGF-β1 and harvested for protein after 1 (A–B) or 16 hours (B) of stimulation. Western blot analysis demonstrated a similar amount of phospho-SMAD3 in the WT, β-arrestin1−/−, and β-arrestin2−/− fibroblasts. Sample loading was verified by expression of α-tubulin or β-actin. The data are representative of three independent experiments. (C) Fibroblasts were stimulated with 1 or 5 ng/ml of TGF-β1 for 24 hours. The cell culture supernatant was removed and analyzed for HA content by ELISA. Data represent mean ± SE of four independent experiments. (D) Fibroblasts were treated with 5 ng/ml of TGF-β1 and harvested for protein after 16 hours of stimulation. Western blot analysis demonstrated a similar amount of PAI-1 and collagen I in the WT, β-arrestin1−/−, and β-arrestin2−/− fibroblasts. Sample loading was verified by expression of GAPDH.
To further investigate TGF-β signaling after loss of β-arrestins, we next examined the production of a well-known TGF-β-stimulated matrix component, the glycosaminoglycan, hyaluronan (HA). Primary lung fibroblasts from WT, β-arrestin1−/−, and β-arrestin2−/− mice were stimulated with 1–5 ng/ml of TGF-β1. After 24 hours, the media was removed and analyzed for total HA content by ELISA. Similar to the Western blot analysis of phospho-SMAD3, the fibroblasts lacking either β-arrestin1 or β-arrestin2 had an increase in HA production after TGF-β stimulation equivalent to the increase in the WT fibroblasts suggesting that the ability of fibroblasts to respond to TGF-β is not dependent on β-arrestin1 or β-arrestin2 (Fig. 4C). Additionally, we examined other downstream potentiators of TGF-β signaling that are important for the development and maintenance of pulmonary fibrosis such as PAI-1 and collagen 1. Fibroblasts were isolated from bleomycin-treated mice and then treated with 5 ng/ml of TGF-β 1 (Fig. 4D). Both PAI-1 and collagen 1 levels increased after TGF-β1 treatment in WT, β –arrestin1−/−and β –arrestin2−/− cells. These data confirm the preserved signaling downstream of TGF-β pathways in the bleomycin-induced mouse model of IPF and suggest that the prevention of pulmonary fibrosis in the absence of β-arrestin is not due to impaired responsiveness to TGF-β.
Primary lung fibroblasts from β-arrestin1−/− and β-arrestin2−/− mice migrate normally in response to BALF
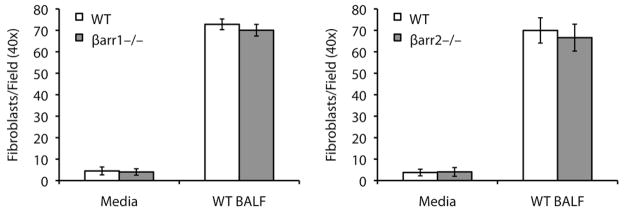
Since fibroblasts lacking β-arrestin1 or β-arrestin2 appeared to have normal TGF-β-induced signaling responses, we next hypothesized that these fibroblasts may be impaired in their ability to migrate to the site of injury. Lysophosphatidic acid, a component of BALF that increases after injury, mediates fibroblast migration by signaling through a specific G protein-coupled receptor, LPA1 (20). Loss of β-arrestins could potentially alter receptor internalization and thus cell surface expression of LPA1 or other receptors important in fibroblast chemotaxis. To investigate whether loss of β-arrestins could affect fibroblast migration we used modified Boyden chambers to measure the chemotaxis of primary lung fibroblasts towards BALF. As demonstrated in Fig. 5, the migratory ability of both the β-arrestin1−/− and β-arrestin2−/− fibroblasts in response to BALF from bleomycin-treated mice was similar to that of the WT fibroblasts. These data suggest that β-arrestins may not regulate fibroblast migration under these experimental conditions.
Figure 5.
BALF-induced fibroblast chemotaxis is similar in WT, β-arrestin1−/−, and β-arrestin2−/− mice. Primary lung fibroblasts were plated in serum-free DMEM onto modified Boyden chambers with a fibronectin-coated filter. BALF from bleomycin-treated mice was diluted in serum-free DMEM and added to the bottom chamber. After 4 hours, the number of fibroblasts that migrated to the other side of the filter was counted. Data represent mean ± SE of four independent experiments.
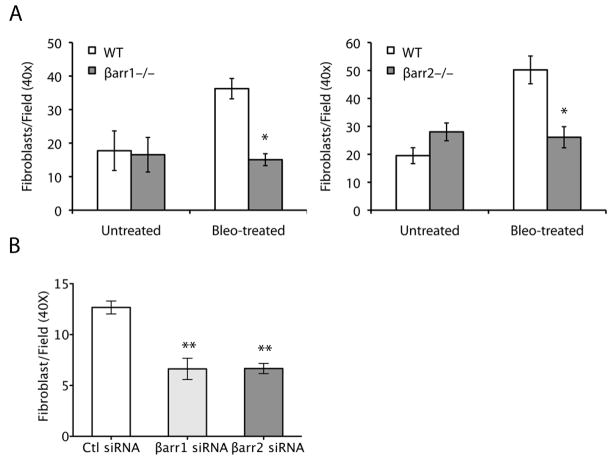
Primary lung fibroblasts from the β-arrestin1−/− and β-arrestin2−/− mice display a less invasive phenotype
The ability of fibroblasts to invade through the basement membrane and deposit excess collagen into the interstitium may be an important contributor to the progression of pulmonary fibrosis. White et al. demonstrated that, unlike normal lung fibroblasts, fibroblasts from patients with IPF spontaneously invade basement membranes (21). Consequently, we wondered whether fibroblasts that were isolated from bleomycin-treated mice would invade the basement membrane. Using the matrigel transwell assay, we found that fibroblasts from WT mice treated with bleomycin invaded the basement membrane matrix while fibroblasts from untreated mice had minimal invasive capacity. Since β-arrestin has been shown to play a role in cancer cell invasion (14), we hypothesized that the protective effect mediated by loss of β-arrestin function in mice subjected to the bleomycin-induced fibrosis model may be the result of a reduced invasive capacity of lung fibroblasts. To this end, we compared the invasive capacity of lung fibroblasts isolated from the WT, β-arrestin1−/−, and β-arrestin2−/− mice after bleomycin treatment. Interestingly, as shown in Fig. 6A, the fibroblasts from the bleomycin-treated β-arrestin1−/− and β-arrestin2−/− mice were significantly less invasive than the WT fibroblasts, and their invasive capacity was more similar to the fibroblasts from the untreated control mice. Acute knockdown of β-arrestins from isolated WT fibroblasts through transient transduction of siRNAs specific for either β-arrestin1 or β-arrestin2 (Fig. 6B) recapitulated the loss of invasiveness seen in knockout mouse fibroblasts, suggesting that removal of either β-arrestin might serve as a therapeutic intervention for pulmonary fibrosis.
Figure 6.
β-arrestin1 and β-arrestin2 are required for primary lung fibroblast invasion. (A) Primary lung fibroblasts were isolated from untreated mice and mice 7 days after bleomycin treatment. After 3 passages, the fibroblasts were loaded onto a matrigel-coated filter, and after 24 hours, the number of fibroblasts that invaded through the matrix was counted. Data represent mean ± SE of four independent experiments. *P < 0.05 compared to all other groups by the Tukey-Kramer test for multiple comparisons. (B) Primary lung fibroblasts isolated from bleomycin-treated mice were transduced with siRNA specific to β-arrestin1, β-arrestin2, or nonspecific control (Ctl). Invasive capacity of the fibroblasts was assessed with the Matrigel invasion assay. Quantitative analysis of knockdown experiments demonstrated decreased invasion after knockdown of both β-arrestin1 and β-arrestin2. **P < 0.01. The experiments were repeated three times.
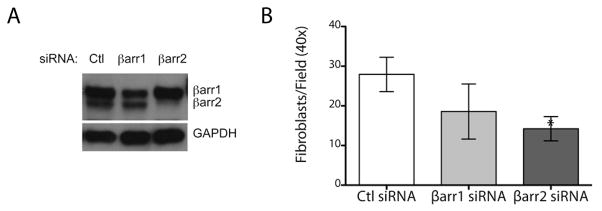
To determine if either β-arrestin1 or β-arrestin2 could contribute to the invasive phenotype of primary lung fibroblasts isolated from patients with IPF, we performed acute knockdown of β-arrestins. Knockdown of β-arrestin2 was more complete than β-arrestin1 in IPF fibroblasts (Fig. 7A) and resulted in an inhibition of fibroblast invasion in 5/5 patient samples (Fig. 7B). The effect of β-arrestin1 on IPF fibroblast invasion was less consistent and inhibition was observed in 2/4 IPF fibroblast samples resulting in a trend that did not achieve statistical significance. These data suggest that β-arrestins are important in mediating fibroblast invasion in both mouse and man although the relative contributions of β-arrestin1 and β-arrestin2 may not be identical between species. These data suggest that β-arrestins are necessary for fibroblast invasiveness during tissue injury, and the reduced invasive capacity of β-arrestin-deficient fibroblasts may indicate a possible target for therapeutic intervention.
Figure 7.
β-arrestin2 is important in mediating fibroblast invasion of human IPF fibroblasts. (A) Primary human lung fibroblasts from IPF patients were transduced with siRNA specific to β-arrestin1, β-arrestin2, or nonspecific control (Ctl) and harvested for protein after 48 hours. Western blot analysis demonstrated a significant reduction of β-arrestin2 and a less robust knockdown of β-arrestin1. Sample loading was verified by blotting of GAPDH. (B) Invasive capacity of the fibroblasts was assessed with the Matrigel invasion assay. *P < 0.05. β-arrestin2 knockdown was performed on 5 different IPF fibroblast patient samples and β-arrestin1 knockdown on 4 different samples.
Loss of β-arrestin1 or β-arrestin2 in primary lung fibroblasts results in altered expression of genes involved in matrix production, basement membrane degradation, and cell adhesion
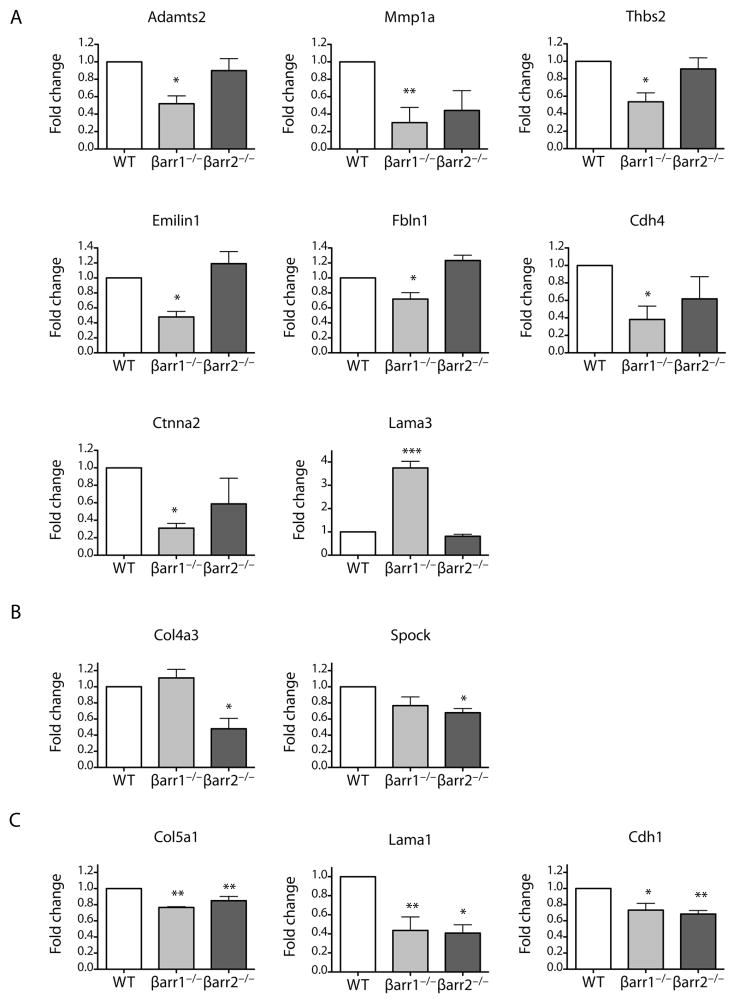
To gain additional insights into the mechanisms by which loss of the β-arrestins causes reduced fibroblast invasion and protection from bleomycin-induced pulmonary fibrosis, we used quantitative RT-PCR to analyze gene expression in fibroblasts isolated from the bleomycin-treated WT, β-arrestin1−/−, and β-arrestin2−/− mice. Our hypothesis was that the non-invasive β-arrestin1−/− and β-arrestin2−/− fibroblasts would have decreased expression of genes important in basement membrane degradation, such as the matrix metalloproteinases, or cell-matrix adhesion, such as the integrins. We analyzed 84 genes by using a targeted qRT-PCR array for extracellular matrix and adhesion proteins. In the β-arrestin1−/− fibroblasts, ten genes were significantly downregulated compared to WT fibroblasts. These genes included the following: Emilin1, Col5a1, Lama1, Cdh1, Cdh4, Fbln1, Ctnna2, Adamts2, MMP1a, and Thbs2 (Fig. 8). In the β-arrestin2−/− fibroblasts, a similar group of genes was downregulated, and the following five genes were significantly different from WT: Col5a1, Lama1, Cdh1, Col4a3, and Spock (Fig. 8). Only one gene, Lama3, was significantly upregulated in the β-arrestin1−/− fibroblasts compared to the WT fibroblasts (Fig. 8). This analysis indicates that a number of genes involved in extracellular matrix production and remodeling are altered in their expression patterns in β-arrestin−/− mice after bleomycin treatment compared to WT mice. This change in gene expression may be related to one or more signaling pathways that are compromised by the loss of β-arrestins. The decrease in activity of these pathways likely contributes to the decrease in invasion by fibroblasts from β-arrestin−/− animals after bleomycin treatment.
Figure 8.
Loss of β-arrestin1 and β-arrestin2 alters the expression of genes involved in collagen formation and invasion. Using a qRT-PCR array, 84 genes involved in adhesion and extracellular matrix production and degradation were analyzed. (A) Genes altered in β-arrestin1−/− primary fibroblasts only. (B) Genes altered in β-arrestin2−/− primary fibroblasts only. (C) Genes altered in both β-arrestin1−/− and β-arrestin2−/− primary fibroblasts. *P < 0.05, **P < 0.01, ***P < 0.001 as calculated by one-way ANOVA with Bonferoni correction.
DISCUSSION
IPF is a fatal disorder characterized by progressive fibrosis that leads to respiratory failure. The hallmark characteristics of IPF include fibroblast foci and increased collagen and extracellular matrix deposition. Although our understanding of the disease has increased over the last decade, an efficacious treatment remains elusive. Recent reviews have emphasized the importance of profibrotic mediators and alterations in fibroblast characteristics in disease pathogenesis (3). In this study, we demonstrate the importance of β-arrestins, ubiquitous mediators of cell signaling, in the development of bleomycin-induced pulmonary fibrosis. In addition, our data demonstrate a previously unidentified role for the β-arrestins in regulating certain aspects of fibroblast behavior.
Using the bleomycin mouse model of pulmonary fibrosis, we show that loss of function of either β-arrestin1 or β-arrestin2 protected mice from the excessive collagen deposition, architectural distortion, and decreased compliance that occur after bleomycin treatment. We show by physiological, biochemical, and pathological analysis that bleomycin-treated β-arrestin1−/− and β-arrestin2−/− mice were more similar to saline-treated mice than the bleomycin-treated WT mice. These findings confirm the necessity of β-arrestins for the development of bleomycin-induced pulmonary fibrosis. Furthermore, qRT-PCR analysis of primary lung fibroblasts from bleomycin-treated mice revealed decreased expression of genes involved in extracellular matrix production in the β-arrestin-deficient fibroblasts. This included genes that encode proteins important for collagen production such as collagen type IV α3, collagen type Vα1, and a disintegrin- like and metallopeptidase with thrombospondin type1 motif 2 (Adamts2). Adamts2 is a procollagen N-proteinase that processes several types of procollagen proteins to mature collagen and is also important for collagen fibril assembly in the extracellular matrix. Interestingly, Adamts2-null mice are protected from carbon tetrachloride-induced hepatic fibrosis (22).
In addition to genes involved in collagen production, the primary lung fibroblasts from knockout animals treated with bleomycin also displayed decreased expression of genes that encode extracellular glycoproteins including thrombospondin 2, emilin, and laminin-α1 in comparison to WT mice. Mice deficient in thrombospondin 2 display abnormal collagen formation, and mice deficient in emilin have defective elastic fiber formation (23, 24). Together, these data suggest an important role for β-arrestins in modulating signaling pathways that regulate collagen and extracellular matrix production and formation mediated by fibroblasts after the onset of fibrosis.
Recently, a new hypothesis has emerged that links IPF and cancer due to their similar hallmark pathological alterations such as aberrant myofibroblast proliferation and apoptosis, extracellular matrix invasion, and intracellular signaling (25). One study has challenged current paradigms by demonstrating that the fibroblast foci of IPF form an interconnected, highly complex reticulum or neoplasm (26). Interestingly, the invasion of ovarian cancer cells was shown to be dependent on β-arrestin as a scaffolding protein necessary for endothelin-induced, β-catenin signaling (14). In the present study, we demonstrated that primary fibroblasts from bleomycin-treated β-arrestin-deficient mice had a less invasive phenotype than their WT counterparts. Importantly, we were able to confirm a critical role for β-arrestins in fibroblast invasion by knocking down either β-arrestin1 or 2 in fibroblasts isolated from WT mice treated with bleomycin and thwarting the invasive phenotype. Furthermore, we provide data suggesting that β-arrestins may contribute to the invasiveness of fibroblasts isolated from human IPF patients, although more studies are needed in this area due to the heterogeneity of IPF fibroblasts.
Invasive cells must alter cell-cell and cell-extracellular matrix adhesions, degrade the extracellular matrix, and rearrange the actin cytoskeleton (27). Our qRT-PCR analysis revealed alterations in a number of genes that have been implicated in cancer invasion including MMP1a, Adamts2, Spock, Thbs2, Lama1, and Lama3. Matrix metalloprotease 1 (MMP1), a secreted enzyme that breaks down interstitial collagen, promotes the invasion of breast carcinoma and melanoma cells (28, 29). Increased expression of MMP1 has also been linked to the metastatic ability of colorectal cancer, and specific inhibition of MMP1 prevents metastasis of invasive melanomas (30). In addition, mice deficient in tissue inhibitors of metalloproteinase 3 (Timp3) have increased expression of MMP1a and enhanced pulmonary fibrosis after bleomycin treatment (31), suggesting that a decrease in MMP1a expression would have the opposite effect. Expression of Adamts2, another enzyme in the metalloprotease family, is upregulated in highly invasive mouse mammary tumors (32). Sparc/osteonectin, CWCV and kazal-like domain proteoglycan 1 (Spock1) is upregulated in prostate cancer and malignant thyroid tumors (33–35). Interestingly, Spock1 was also upregulated in mice that developed profibrotic-like changes in the lung after spaceflight (36). Thrombospondin 2 (Thbs2) is elevated in epithelial ovarian tumors and endometrial cancer samples from patients with a poor prognosis as well as at the invasive front of hepatocellular carcinomas (37–39). In addition, thrombospondin 2 has been shown to promote the invasiveness of pancreatic cancer cells (40). Interestingly, although both are basement membrane constituents, laminin-α1 (Lama1) has increased expression in glioblastomas and has been shown to promote invasion of melanoma cells while laminin-α3 (Lama3), the only upregulated gene in β-arrestin-deficient fibroblasts, is actually downregulated in both breast carcinoma and adenocarcinoma (41–44). Taken together, it seems that genes that have altered expression in β-arrestin-deficient fibroblasts are important for cellular invasion on multiple fronts and may indicate another link between IPF and metastatic cancers.
Our data suggest a previously unrecognized important role for β-arrestins in regulating fibroblast activity and collagen formation; however, we cannot rule out other potential mechanisms of β-arrestin-mediated signaling that contribute to the development of pulmonary fibrosis due to the complexity of β-arrestin signaling cascades. For example, loss of β-arrestin2 promoted re-epithelialization in a wound healing model, and the phenotype was attributed to increased CXCR2-mediated neutrophil recruitment and activity (45). In our model of bleomycin-induced injury, neutrophil recruitment, as well as macrophage and lymphocyte recruitment, was not altered in the β-arrestin1−/− and β-arrestin2−/− mice, so the mechanism appears to be different. Consistent with our finding,β-arrestin2−/− mice were shown to have a normal inflammatory cell response to LPS suggesting that these mice do not have a universal defect in inflammatory cell recruitment (13). Thus, our data do not support an impact on the inflammatory response as the protection incurred by loss of β-arrestins. In wound healing, other cell types besides neutrophils, such as keratinocytes and fibroblasts, also play vital roles. However, the ability of β-arrestins to regulate fibroblast activity during re-epithelialization was not investigated in the study by Su et al. (45). It is interesting to note that migration, and not invasion, of fibroblasts is important in wound healing, and our data demonstrate that there is no difference in the ability of fibroblasts lacking β-arrestins to migrate in response to BALF. In addition, the β-arrestin1−/− and β-arrestin2−/− fibroblasts responded normally to stimulation with TGF-β, a critical regulator of fibroblast production of extracellular matrix during wound healing (46). Together, this suggests that fibroblasts lacking β-arrestins do not have an overall impairment in motility or cytokine responsiveness even though their ability to specifically invade basement membrane is impeded.
β-arrestins function as multiprotein scaffolds to coordinate complex signal transduction networks. One of the most studied of these β-arrestin-mediated signaling pathways is the ERK/MAPK signaling axis. β-arrestin increases ERK activation through a G protein-independent pathway. However, β-arrestin-dependent ERK does not have typical nuclear functions, and specific downstream targets remain unknown although reports have suggested that cytosolic ERK may play a role in cytoskeletal rearrangement and chemotaxis (9). Interestingly, β-arrestin was shown to be a necessary scaffolding protein for activation of RAF-MEK-ERK1/2 signaling and MMP1 transcriptional activation in human bronchial epithelia, and this signaling pathway is also responsible for increased MMP1 expression in invasive melanoma cells (29, 47). In our study, the reduced expression of MMP1 as well as other genes in the fibroblasts lacking β-arrestins may also be due to a loss of β-arrestin-dependent ERK phosphorylation. However, due to the dramatic phenotype observed in the β-arrestin-deficient mice, we believe that more than one mechanism is responsible for this profound protection. As mentioned previously, β-arrestins may play a role in fibroblast invasion through activation of β-catenin signaling, as in ovarian cancer cells (14). In addition, the classical role of β-arrestins in receptor desensitization and internalization may also be important in the protection observed in the β-arrestin-deficient mice.
The data presented here demonstrate that both β-arrestin1 and β-arrestin2 play a pivotal role in the pathogenesis of bleomycin-induced pulmonary fibrosis. This suggests that both isoforms have a significant and non-redundant function in disease progression with possible mechanisms including the necessity for heterodimerization of both β-arrestins or the necessity of each in completely independent processes. There are numerous pathways that signal through β-arrestins (11), and consequently, genes that are dysregulated in fibroblasts isolated from bleomycin-treated mice could be downstream of any number of aberrant signaling pathways. Furthermore, at this early stage it can be hypothesized that β-arrestins are functioning in the same pathways redundantly, in some pathways synergistically, or actually opposing one another’s function in the pathways examined, as all these signaling permutations have been observed (9). However, the gene expression profiles for these isolated fibroblasts appear distinct in that some genes are changed in only β-arrestin1−/− animals, some genes only in β-arrestin2−/− animals, and some genes are changed similarly in both knockouts. Consequently, it seems a reasonable hypothesis that there are signaling differences between these two genotypes, though these differences have yet to be fully elucidated.
IPF is a deadly disease that currently has no known effective therapy. Recent reviews have emphasized that IPF is not only an epithelial-fibroblastic disorder but also a disease with many similarities to cancer (3, 25). Thus, novel therapeutic agents that target fibroblasts and cancer-like mechanisms, such as invasion, warrant investigation. Our studies demonstrate that loss of β-arrestins protects against the development of pulmonary fibrosis by limiting the ability of fibroblasts to invade the basement membrane and deposit excess collagen and extracellular matrix. Furthermore, we demonstrate that acute removal of either β-arrestin from diseased mouse fibroblasts and β-arrestin2 in human IPF fibroblasts causes a significant reduction in the pathologic potential of these cells. Taken together, these findings suggest that locally delivered β-arrestin inhibitors may be a potential therapeutic for IPF as well as other causes of pulmonary fibrosis.
MATERIALS AND METHODS
Animals
β-arrestin1−/− and β-arrestin2−/− mice were generated as previously described (48, 49). All experiments were carried out using 8- to 12-week-old mice that had been backcrossed onto the C57BL/6 background for at least 12 generations. All studies were conducted in accordance with NIH guidelines for the care and use of animals and with approval from the Duke University Animal Care and Use Committee.
Bleomycin administration
Under anesthesia, either 1.25 U/kg or 2.5 U/kg bleomycin dissolved in sterile PBS or sterile PBS alone as a control was administered via oropharyngeal aspiration as previously described (50).
Histology
Twenty-one days following bleomycin treatment, the lungs were inflated with 10% formalin via a tracheal cannula and removed from the thoracic cavity. Tissue was fixed overnight, embedded in paraffin, and sectioned for staining with hematoxylin-eosin and trichrome.
Lung Mechanics
Static compliance measurements were generated as previously described (17). Briefly, mice were anesthetized, tracheostomized, paralyzed, and mechanically ventilated with a computer-controlled small-animal ventilator (Scireq, Montreal, Canada). Custom designed software (Flexivent, Scireq) was used to generate pressure-volume curves and calculate static compliance using the Salazar-Knowles equation.
Hydroxyproline
After assessment of lung mechanics, the whole lung was removed and total collagen content was measured with a conventional hydroxyproline method (51).
Bronchoalveolar lavage
Whole lung lavage was performed as previously described (52). The total number of cells in the BALF was determined using a hemacytometer, and differential cell counts were performed on cytospin preparations stained with Protocol Hema3 stain set.
Fibroblast Isolation
Mouse lung fibroblasts were isolated as previously described (53). The lungs from either unchallenged mice or mice 7 days after bleomycin treatment were minced, digested twice for 30 min at 37°C in digestion buffer (DMEM with 100 μg/ml of DNase I, 1 mg/ml Type IV collagenase, and 1 mg/ml BSA), passed through a 100 μm filter, centrifuged at 1500 rpm for 10 min., and plated in DMEM supplemented with 10% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin. Fibroblasts were cultured for ~10–12 days, and experiments were performed at passage 3–5.
Human lung fibroblasts were isolated from surgical lung biopsies or lung transplant explants obtained from patients with IPF (53). The diagnosis of IPF was arrived at by standard accepted American Thoracic Society recommendations (54). The specimens were obtained under the auspices of IRB-approved protocols. The tissues were minced, and cultured in DMEM medium supplemented with 15% FBS, 100 U/ml penicillin, 100 μg/ml streptomycin, 5 μg/ml gentamicin and 0.25 μg/ml amphotericin B. The cells of passage 3 – 5 were used for invasion assays and siRNA interference assays. All experiments were approved by the Duke University Institutional Review Board and in accordance with the guidelines outlined by the board.
TGF-β Stimulation
Fibroblasts were stimulated with recombinant human TGF-β1 (R&D Systems). Hyaluronan content in the supernatant was measured using a hyaluronan-specific enzyme-linked immunosorbent assay as described (55). To isolate protein for western blot analysis, the fibroblasts were lysed in RIPA buffer (Sigma) containing phosphatase and protease inhibitor cocktails (Sigma) and centrifuged for 10 min at 10000 rpm. The supernatant was removed and stored at −80°C until further use. Protein content was measured using the BCA assay (Thermo scientific), and 50 μg of total protein per sample was separated on SDS-polyacylamide gels and transferred onto polyvinylidene difluoride membranes (Bio-Rad). Membranes were blocked with 5% BSA in TBST for 1h at room temperature. Immunoblots were probed with primary antibody to phospho-SMAD3 (Cell Signaling, #9520, 1:1000 dilution), PAI-1 (R&D Systems, #AF3828), collagen I (Abcam, #ab21286), α-tubulin (Cell Signaling, #2144, 1:2000 dilution), β-actin, or GAPDH (Cell Signaling, #14C10) at 4°C overnight followed by donkey anti-rabbit or donkey anti-goat secondary antibodies (Jackson ImmunoResearch, 1:10,000 dilution) for 50 min at room temperature.
Fibroblast Migration
Modified Boyden chambers (12-well transwell plate, Costar) were used to measure the chemotaxis of lung fibroblasts to BALF as previously described (53, 56). The BALF was from mice 5 days after administration of 5 U/kg bleomycin. Primary lung fibroblasts (0.5×105 cells) in serum-free DMEM were loaded into the top chamber, and BALF diluted in serum-free DMEM containing 0.1% BSA was loaded into the bottom chamber. After 4 hours at 37°C with 5% CO2, the fibroblasts that migrated across the fibronectin-coated filter were stained with Protocol Hema3 stain set and counted in 5 randomly chosen fields per filter from duplicate filters per sample at 400x magnification.
Fibroblast Invasion
Fibroblasts (0.5×105 cells) were loaded into the top chamber of a BioCoat Matrigel Invasion Chamber (BD Biosciences), and DMEM containing 10% FBS was loaded into the bottom chamber. After 24 hours at 37°C with 5% CO2, the filters were fixed and stained using the Protocol Hema3 stain set. Non-invading cells as well as the basement membrane matrix were removed from the upper side of the filter by gentle scrubbing with a cotton swab. The number of fibroblasts that invaded through the basement membrane was counted in 5 randomly chosen fields per filter from triplicate filters per sample at 400x magnification.
Gene expression analysis
RNA was isolated from fibroblasts using the Qiagen RNeasy kit with added DNAse purification according to manufacturer’s adapted protocol using the QiaCube purification rotor. Reverse transcription was performed using the RT2 First Strand cDNA Synthesis kit (SABiosciences), and 84 genes were assessed by RT-PCR using the Mouse Extracellular Matrix and Adhesion Molecules array (RT2 Profiler PCR Array PAMM-013; SABiosciences) according to manufacturers instructions using a MyIQ qRT-PCR machine (BioRad). For analysis, the expression level for each gene of interest (GOI) was calculated as 2−Ct followed by normalization to Hprt1, the housekeeping gene (HKG), using the formula 2− (Ct GOI - Ct HKG). Ultimately the fold change in normalized gene expression was calculated by comparing values from knockout fibroblasts purified from bleomycin-treated animals (EXP) to those purified from WT bleomycin-treated animals (CTL) according to the following formula: 2−ΔCt EXP/2−ΔCt CTL. Values were calculated for replicates of three independent experiments, and p-values were calculated using one-way ANOVA analysis with Bonferoni correction.
siRNA transfection
Fibroblasts isolated from bleomycin-treated mice were transfected with oligos targeting either β-arrestin1 (agccuucugugcugagaac) or β-arrestin2 (ggaccggaaaguguucgug), whereas lung fibroblasts isolated from IPF patients were transfected with oligos targeting either β-arrestin1 (ggaccgcaaaguguuugug) or β-arrestin2 (ccaaccucauugaauuuga), using the Transductin siRNA delivery reagent from Integrated DNA Technologies. Briefly, for a 100 mm plate of cells, a reaction mixed 36 μl of 40 μM siRNA in Dharmacon siRNA buffer (300mM KCl, 30mM HEPES-pH 7.5, 1.0mM MgCl2, B-002000-UB-100) with 8 μl of 200 μM Transductin and 164 μl PBS-10% glycerol on ice for 15 min. Cells were washed with serum free media 2x, and then 4 ml of 10% “Q-serum media” was added to each plate. The Q-serum media were prepared as following: 5 ml FBS + 1 ml Source 30Q resin (Amersham Bioscience) was tumbled for 30 minutes at room temperature, was then spun for 5 minutes at 2000rpm, followed by syringe filtration through a 0.22 μm filter. The transfection mixture was diluted 1:5 in 10% Q-serum media and added to the plate for followed by a 6h incubation with final siRNA concentrations at 40 nM. After 6h, the transfection mixture was removed and replaced with 10% FBS media. Cells were analyzed 72 hours post-delivery for invasion capacity or for β-arrestin protein levels by immunobloting probed with an antibody against β-arrestins (A1CT, which recognizes both β-arrestins (10, 19)).
Statistical analysis
Data are presented as mean ± SEM and analyzed by Student’s two- tailed t-test and ANOVA followed by Tukey-Kramer honestly significant difference post hoc test. Statistical difference of survival curves was analyzed by log-rank test. A P value < 0.05 is considered statistically significant.
Acknowledgments
Funding: This study was supported by NIH grants HL060539 (P.W.N.), HL077291 (P.W.N.), HL16037 (R.J.L.), HL70631 (R.J.L.), and HL016347 (W.M.F.). R.J.L. is an Investigator with the Howard Hughes Medical Institute.
Footnotes
Competing interests: R.J.L. is a founder and member of the Scientific Advisory Board for Trevena, Inc., a company that discovers and develops novel G protein-coupled receptor-targeted medicines.
Author contributions: A.K.L. conceived, designed, performed, and analyzed most experiments and wrote the manuscript. J.J.K. conceived and performed the gene expression experiments, analyzed the data, and wrote the manuscript. E.N.P. performed the lung mechanics experiments. T.X. performed the knockdown experiments and analyzed the data. Y.L. developed the invasion assay protocol that was used. J.L. helped with the invasion experiments. E.B.M. purified human lung fibroblasts from IPF patients. W.M.F. in whose laboratory the lung mechanics experiments were performed provided funding for those experiments. D.J. conceived experiments, analyzed the data, and wrote the manuscript. R.J.L. in whose laboratory the gene expression experiments were conceived and performed, provided the knockout animals, provided funding for experiments, and wrote the manuscript. P.W.N. conceived and provided funding for all experiments and wrote the manuscript.
REFERENCES AND NOTES
- 1.Gross TJ, Hunninghake GW. Idiopathic pulmonary fibrosis. N Engl J Med. 2001;345:517–525. doi: 10.1056/NEJMra003200. [DOI] [PubMed] [Google Scholar]
- 2.Nava S, Rubini F. Lung and chest wall mechanics in ventilated patients with end stage idiopathic pulmonary fibrosis. Thorax. 1999;54:390–395. doi: 10.1136/thx.54.5.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Selman M, King TE, Pardo A. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med. 2001;134:136–151. doi: 10.7326/0003-4819-134-2-200101160-00015. [DOI] [PubMed] [Google Scholar]
- 4.Kang HR, Cho SJ, Lee CG, Homer RJ, Elias JA. Transforming growth factor (TGF)-beta1 stimulates pulmonary fibrosis and inflammation via a Bax-dependent, bid-activated pathway that involves matrix metalloproteinase-12. J Biol Chem. 2007;282:7723–7732. doi: 10.1074/jbc.M610764200. [DOI] [PubMed] [Google Scholar]
- 5.Bonniaud P, Kolb M, Galt T, Robertson J, Robbins C, Stampfli M, Lavery C, Margetts PJ, Roberts AB, Gauldie J. Smad3 null mice develop airspace enlargement and are resistant to TGF-beta-mediated pulmonary fibrosis. J Immunol. 2004;173:2099–2108. doi: 10.4049/jimmunol.173.3.2099. [DOI] [PubMed] [Google Scholar]
- 6.Bonniaud P, Margetts PJ, Kolb M, Schroeder JA, Kapoun AM, Damm D, Murphy A, Chakravarty S, Dugar S, Higgins L, Protter AA, Gauldie J. Progressive transforming growth factor beta1-induced lung fibrosis is blocked by an orally active ALK5 kinase inhibitor. Am J Respir Crit Care Med. 2005;171:889–898. doi: 10.1164/rccm.200405-612OC. [DOI] [PubMed] [Google Scholar]
- 7.Birkedal-Hansen H, Moore WG, Bodden MK, Windsor LJ, Birkedal-Hansen B, DeCarlo A, Engler JA. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med. 1993;4:197–250. doi: 10.1177/10454411930040020401. [DOI] [PubMed] [Google Scholar]
- 8.Murphy G, Gavrilovic J. Proteolysis and cell migration: creating a path? Curr Opin Cell Biol. 1999;11:614–621. doi: 10.1016/s0955-0674(99)00022-8. [DOI] [PubMed] [Google Scholar]
- 9.DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. Beta-arrestins and cell signaling. Annu Rev Physiol. 2007;69:483–510. doi: 10.1146/annurev.physiol.69.022405.154749. [DOI] [PubMed] [Google Scholar]
- 10.Kovacs JJ, Whalen EJ, Liu R, Xiao K, Kim J, Chen M, Wang J, Chen W, Lefkowitz RJ. Beta-arrestin-mediated localization of smoothened to the primary cilium. Science. 2008;320:1777–1781. doi: 10.1126/science.1157983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kovacs JJ, Hara MR, Davenport CL, Kim J, Lefkowitz RJ. Arrestin development: emerging roles for beta-arrestins in developmental signaling pathways. Dev Cell. 2009;17:443–458. doi: 10.1016/j.devcel.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walter N, Collard HR, King TE., Jr Current perspectives on the treatment of idiopathic pulmonary fibrosis. Proc Am Thorac Soc. 2006;3:330–338. doi: 10.1513/pats.200602-016TK. [DOI] [PubMed] [Google Scholar]
- 13.Walker JK, Fong AM, Lawson BL, Savov JD, Patel DD, Schwartz DA, Lefkowitz RJ. Beta-arrestin-2 regulates the development of allergic asthma. J Clin Invest. 2003;112:566–574. doi: 10.1172/JCI17265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosano L, Cianfrocca R, Masi S, Spinella F, Di Castro V, Biroccio A, Salvati E, Nicotra MR, Natali PG, Bagnato A. Beta-arrestin links endothelin A receptor to beta-catenin signaling to induce ovarian cancer cell invasion and metastasis. Proc Natl Acad Sci U S A. 2009;106:2806–2811. doi: 10.1073/pnas.0807158106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adamson IY, Bowden DH. The pathogenesis of bleomycin-induced pulmonary fibrosis in mice. Am J Pathol. 1974;77:185–197. [PMC free article] [PubMed] [Google Scholar]
- 16.Snider GL, Celli BR, Goldstein RH, O’Brien JJ, Lucey EC. Chronic interstitial pulmonary fibrosis produced in hamsters by endotracheal bleomycin. Lung volumes, volume-pressure relations, carbon monoxide uptake, and arterial blood gas studied. Am Rev Respir Dis. 1978;117:289–297. doi: 10.1164/arrd.1978.117.2.289. [DOI] [PubMed] [Google Scholar]
- 17.Lovgren AK, Jania LA, Hartney JM, Parsons KK, Audoly LP, Fitzgerald GA, Tilley SL, Koller BH. COX-2-derived prostacyclin protects against bleomycin-induced pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2006;291:L144–156. doi: 10.1152/ajplung.00492.2005. [DOI] [PubMed] [Google Scholar]
- 18.Shen AS, Haslett C, Feldsien DC, Henson PM, Cherniack RM. The intensity of chronic lung inflammation and fibrosis after bleomycin is directly related to the severity of acute injury. Am Rev Respir Dis. 1988;137:564–571. doi: 10.1164/ajrccm/137.3.564. [DOI] [PubMed] [Google Scholar]
- 19.Chen W, Kirkbride KC, How T, Nelson CD, Mo J, Frederick JP, Wang XF, Lefkowitz RJ, Blobe GC. Beta-arrestin 2 mediates endocytosis of type III TGF-beta receptor and down-regulation of its signaling. Science. 2003;301:1394–1397. doi: 10.1126/science.1083195. [DOI] [PubMed] [Google Scholar]
- 20.Tager AM, LaCamera P, Shea BS, Campanella GS, Selman M, Zhao Z, Polosukhin V, Wain J, Karimi-Shah BA, Kim ND, Hart WK, Pardo A, Blackwell TS, Xu Y, Chun J, Luster AD. The lysophosphatidic acid receptor LPA1 links pulmonary fibrosis to lung injury by mediating fibroblast recruitment and vascular leak. Nat Med. 2008;14:45–54. doi: 10.1038/nm1685. [DOI] [PubMed] [Google Scholar]
- 21.White ES, Thannickal VJ, Carskadon SL, Dickie EG, Livant DL, Markwart S, Toews GB, Arenberg DA. Integrin alpha4beta1 regulates migration across basement membranes by lung fibroblasts: a role for phosphatase and tensin homologue deleted on chromosome 10. Am J Respir Crit Care Med. 2003;168:436–442. doi: 10.1164/rccm.200301-041OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kesteloot F, Desmouliere A, Leclercq I, Thiry M, Arrese JE, Prockop DJ, Lapiere CM, Nusgens BV, Colige A. ADAM metallopeptidase with thrombospondin type 1 motif 2 inactivation reduces the extent and stability of carbon tetrachloride-induced hepatic fibrosis in mice. Hepatology. 2007;46:1620–1631. doi: 10.1002/hep.21868. [DOI] [PubMed] [Google Scholar]
- 23.Kyriakides TR, Zhu YH, Smith LT, Bain SD, Yang Z, Lin MT, Danielson KG, Iozzo RV, LaMarca M, McKinney CE, Ginns EI, Bornstein P. Mice that lack thrombospondin 2 display connective tissue abnormalities that are associated with disordered collagen fibrillogenesis, an increased vascular density, and a bleeding diathesis. J Cell Biol. 1988;140:419–430. doi: 10.1083/jcb.140.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zanetti M, Braghetta P, Sabatelli P, Mura I, Doliana R, Colombatti A, Volpin D, Bonaldo P, Bressan GM. EMILIN-1 deficiency induces elastogenesis and vascular cell defects. Mol Cell Biol. 2004;24:638–650. doi: 10.1128/MCB.24.2.638-650.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vancheri C, Failla M, Crimi N, Raghu G. Idiopathic pulmonary fibrosis: a disease with similarities and links to cancer biology. Eur Respir J. 2010;35:496–504. doi: 10.1183/09031936.00077309. [DOI] [PubMed] [Google Scholar]
- 26.Cool CD, Groshong SD, Rai PR, Henson PM, Stewart JS, Brown KK. Fibroblast foci are not discrete sites of lung injury or repair: the fibroblast reticulum. Am J Respir Crit Care Med. 2006;174:654–658. doi: 10.1164/rccm.200602-205OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Woodhouse EC, Chuaqui RF, Liotta LA. General mechanisms of metastasis. Cancer. 1997;80:1529–1537. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1529::aid-cncr2>3.3.co;2-#. [DOI] [PubMed] [Google Scholar]
- 28.Boire A, Covic L, Agarwal A, Jacques S, Sherifi S, Kuliopulos A. PAR1 is a matrix metalloprotease-1 receptor that promotes invasion and tumorigenesis of breast cancer cells. Cell. 2005;120:303–313. doi: 10.1016/j.cell.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 29.Huntington JT, Shields JM, Der CJ, Wyatt CA, Benbow U, Slingluff CL, Jr, Brinckerhoff CE. Overexpression of collagenase 1 (MMP-1) is mediated by the ERK pathway in invasive melanoma cells: role of BRAF mutation and fibroblast growth factor signaling. J Biol Chem. 2004;279:33168–33176. doi: 10.1074/jbc.M405102200. [DOI] [PubMed] [Google Scholar]
- 30.Sunami E, Tsuno N, Osada T, Saito S, Kitayama J, Tomozawa S, Tsuruo T, Shibata Y, Muto T, Nagawa H. MMP-1 is a prognostic marker for hematogenous metastasis of colorectal cancer. Oncologist. 2000;5:108–114. doi: 10.1634/theoncologist.5-2-108. [DOI] [PubMed] [Google Scholar]
- 31.Gill SE, Huizar I, Bench EM, Sussman SW, Wang Y, Khokha R, Parks WC. Tissue inhibitor of metalloproteinases 3 regulates resolution of inflammation following acute lung injury. Am J Pathol. 2010;176:64–73. doi: 10.2353/ajpath.2010.090158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schade B, Lam SH, Cernea D, Sanguin-Gendreau V, Cardiff RD, Jung BL, Hallett M, Muller WJ. Distinct ErbB-2 coupled signaling pathways promote mammary tumors with unique pathologic and transcriptional profiles. Cancer Res. 2007;67:7579–7588. doi: 10.1158/0008-5472.CAN-06-4724. [DOI] [PubMed] [Google Scholar]
- 33.Wlazlinski A, Engers R, Hoffmann MJ, Hader C, Jung V, Muller M, Schulz WA. Downregulation of several fibulin genes in prostate cancer. Prostate. 2007;67:1770–1780. doi: 10.1002/pros.20667. [DOI] [PubMed] [Google Scholar]
- 34.Haaland CM, Heaphy CM, Butler KS, Fischer EG, Griffith JK, Bisoffi M. Differential gene expression in tumor adjacent histologically normal prostatic tissue indicates field cancerization. Int J Oncol. 2009;35:537–546. doi: 10.3892/ijo_00000365. [DOI] [PubMed] [Google Scholar]
- 35.Prasad NB, Somervell H, Tufano RP, Dackiw AP, Marohn MR, Califano JA, Wang Y, Westra WH, Clark DP, Umbricht CB, Libutti SK, Zeiger MA. Identification of genes differentially expressed in benign versus malignant thyroid tumors. Clin Cancer Res. 2008;14:3327–3337. doi: 10.1158/1078-0432.CCR-07-4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tian J, Pecaut MJ, Slater JM, Gridley DS. Spaceflight modulates expression of extracellular matrix, adhesion, and profibrotic molecules in mouse lung. J Appl Physiol. 2010;108:162–171. doi: 10.1152/japplphysiol.00730.2009. [DOI] [PubMed] [Google Scholar]
- 37.Seki N, Kodama J, Hashimoto I, Hongo A, Yoshinouchi M, Kudo T. Thrombospondin-1 and -2 messenger RNA expression in normal and neoplastic endometrial tissues: correlation with angiogenesis and prognosis. Int J Oncol. 2001;19:305–310. doi: 10.3892/ijo.19.2.305. [DOI] [PubMed] [Google Scholar]
- 38.Kodama J, Hashimoto I, Seki N, Hongo A, Yoshinouchi M, Okuda H, Kudo T. Thrombospondin-1 and -2 messenger RNA expression in epithelial ovarian tumor. Anticancer Res. 2001;21:2983–2987. [PubMed] [Google Scholar]
- 39.Segal E, Sirlin CB, Ooi C, Adler AS, Gollub J, Chen X, Chan BK, Matcuk GR, Barry CT, Chang HY, Kuo MD. Decoding global gene expression programs in liver cancer by noninvasive imaging. Nat Biotechnol. 2007;25:675–680. doi: 10.1038/nbt1306. [DOI] [PubMed] [Google Scholar]
- 40.Farrow B, Berger DH, Rowley D. Tumor-derived pancreatic stellate cells promote pancreatic cancer cell invasion through release of thrombospondin-2. J Surg Res. 2009;156:155–160. doi: 10.1016/j.jss.2009.03.040. [DOI] [PubMed] [Google Scholar]
- 41.Scrideli CA, Carlotti CG, Jr, Okamoto OK, Andrade VS, Cortez MA, Motta FJ, Lucio-Eterovic AK, Neder L, Rosemberg S, Oba-Shinjo SM, Marie SK, Tone LG. Gene expression profile analysis of primary glioblastomas and non-neoplastic brain tissue: identification of potential target genes by oligonucleotide microarray and real-time quantitative PCR. J Neurooncol. 2008;88:281–291. doi: 10.1007/s11060-008-9579-4. [DOI] [PubMed] [Google Scholar]
- 42.Nakahara H, Nomizu M, Akiyama SK, Yamada Y, Yeh Y, Chen WT. A mechanism for regulation of melanoma invasion. Ligation of alpha6beta1 integrin by laminin G peptides. J Biol Chem. 1996;271:27221–27224. doi: 10.1074/jbc.271.44.27221. [DOI] [PubMed] [Google Scholar]
- 43.Sathyanarayana UG, Padar A, Huang CX, Suzuki M, Shigematsu H, Bekele BN, Gazdar AF. Aberrant promoter methylation and silencing of laminin-5-encoding genes in breast carcinoma. Clin Cancer Res. 2003;9:6389–6394. [PubMed] [Google Scholar]
- 44.Akashi T, Ito E, Eishi Y, Koike M, Nakamura K, Burgeson RE. Reduced expression of laminin alpha 3 and alpha 5 chains in non-small cell lung cancers. Jpn J Cancer Res. 2001;92:293–301. doi: 10.1111/j.1349-7006.2001.tb01094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Su Y, Raghuwanshi SK, Yu Y, Nanney LB, Richardson RM, Richmond A. Altered CXCR2 signaling in beta-arrestin-2-deficient mouse models. J Immunol. 2005;175:5396–5402. doi: 10.4049/jimmunol.175.8.5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Denton CP, Khan K, Hoyles RK, Shiwen X, Leoni P, Chen Y, Eastwood M, Abraham DJ. Inducible lineage-specific deletion of TbetaRII in fibroblasts defines a pivotal regulatory role during adult skin wound healing. J Invest Dermatol. 2009;129:194–204. doi: 10.1038/jid.2008.171. [DOI] [PubMed] [Google Scholar]
- 47.Li J, Ghio AJ, Cho SH, Brinckerhoff CE, Simon SA, Liedtke W. Diesel exhaust particles activate the matrix-metalloproteinase-1 gene in human bronchial epithelia in a beta-arrestin-dependent manner via activation of RAS. Environ Health Perspect. 2009;117:400–409. doi: 10.1289/ehp.0800311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Conner DA, Mathier MA, Mortensen RM, Christe M, Vatner SF, Seidman CE, Seidman JG. beta-Arrestin1 knockout mice appear normal but demonstrate altered cardiac responses to beta-adrenergic stimulation. Circ Res. 1997;81:1021–1026. doi: 10.1161/01.res.81.6.1021. [DOI] [PubMed] [Google Scholar]
- 49.Bohn LM, Lefkowitz RJ, Gainetdinov RR, Peppel K, Caron MG, Lin FT. Enhanced morphine analgesia in mice lacking beta-arrestin 2. Science. 1999;286:2495–2498. doi: 10.1126/science.286.5449.2495. [DOI] [PubMed] [Google Scholar]
- 50.Gharaee-Kermani M, Ullenbruch M, Phan SH. Animal models of pulmonary fibrosis. Methods Mol Med. 2005;117:251–259. doi: 10.1385/1-59259-940-0:251. [DOI] [PubMed] [Google Scholar]
- 51.Huszar G, Maiocco J, Naftolin F. Monitoring of collagen and collagen fragments in chromatography of protein mixtures. Anal Biochem. 1980;105:424–429. doi: 10.1016/0003-2697(80)90481-9. [DOI] [PubMed] [Google Scholar]
- 52.Jiang D, Liang J, Hodge J, Lu B, Zhu Z, Yu S, Fan J, Gao Y, Yin Z, Homer R, Gerard C, Noble PW. Regulation of pulmonary fibrosis by chemokine receptor CXCR3. J Clin Invest. 2004;114:291–299. doi: 10.1172/JCI16861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jiang D, Liang J, Campanella GS, Guo R, Yu S, Xie T, Liu N, Jung Y, Homer R, Meltzer EB, Li Y, Tager AM, Goetinck PF, Luster AD, Noble PW. Inhibition of pulmonary fibrosis in mice by CXCL10 requires glycosaminoglycan binding and syndecan-4. J Clin Invest. 2010;120:2049–2057. doi: 10.1172/JCI38644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.American Thoracic Society. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS) Am J Respir Crit Care Med. 2000;161:646–664. doi: 10.1164/ajrccm.161.2.ats3-00. [DOI] [PubMed] [Google Scholar]
- 55.Teder P, Vandivier RW, Jiang D, Liang J, Cohn L, Pure E, Henson PM, Noble PW. Resolution of lung inflammation by CD44. Science. 2002;296:155–158. doi: 10.1126/science.1069659. [DOI] [PubMed] [Google Scholar]
- 56.Tager AM, Kradin RL, LaCamera P, Bercury SD, Campanella GS, Leary CP, Polosukhin V, Zhao LH, Sakamoto H, Blackwell TS, Luster AD. Inhibition of pulmonary fibrosis by the chemokine IP-10/CXCL10. Am J Respir Cell Mol Biol. 2004;31:395–404. doi: 10.1165/rcmb.2004-0175OC. [DOI] [PubMed] [Google Scholar]